A Unique Enhancement of Propionibacterium freudenreichii’s Ability to Remove Pb(II) from Aqueous Solution by Tween 80 Treatment
Abstract
:1. Introduction
2. Results
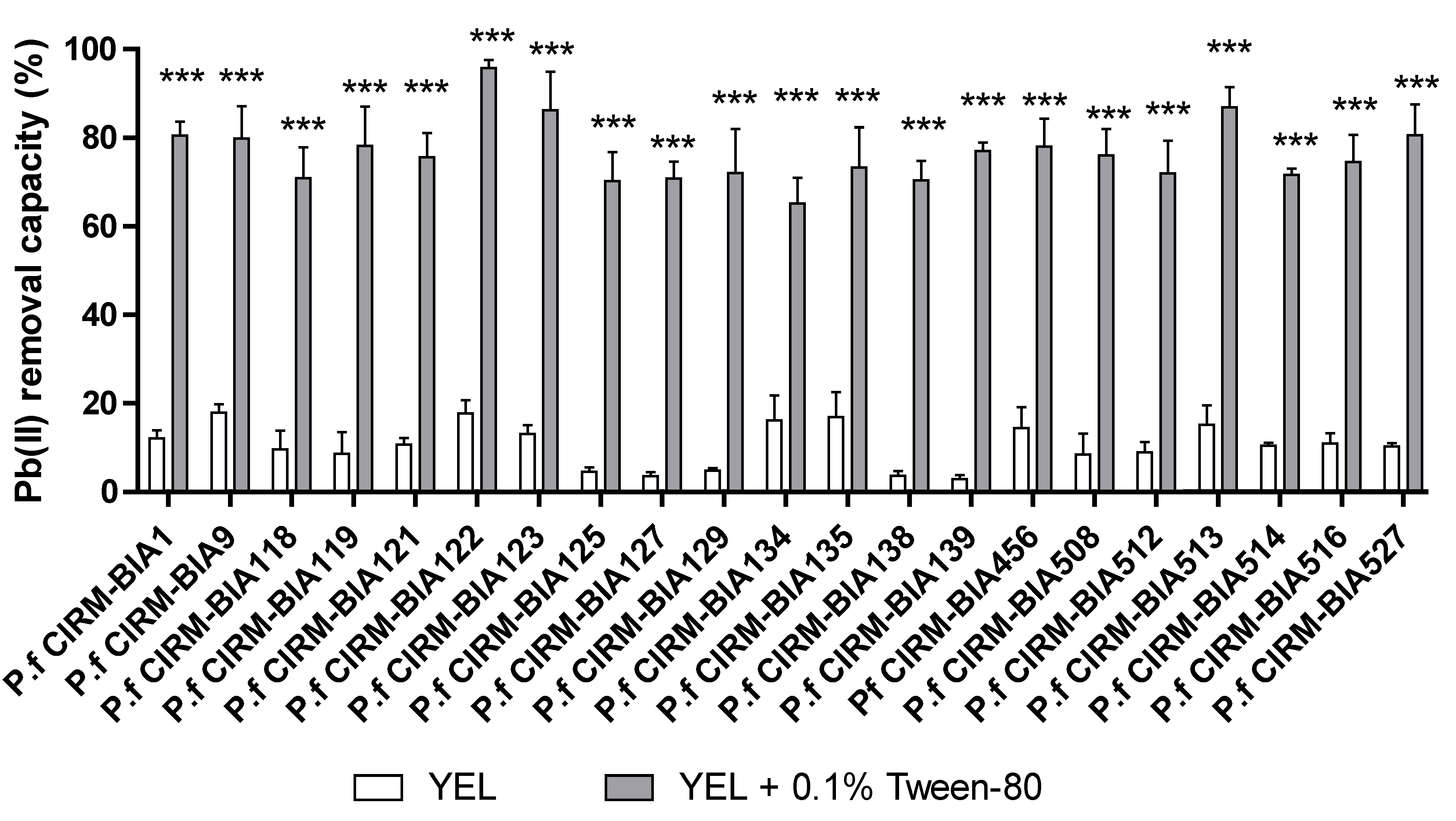
2.1. Tween® 80 Markedly Enhances P. freudenreichii’s Pb(II) Removal
2.2. Tween® 80 Prior Pb(II) Exposure Is Sufficient to Enhance P. freudenreichii’s Pb(II) Removal
2.3. Influence of the Tween® 80 Concentration, the Pb Concentration, and the Bacteria-Tween® 80 Contact Time
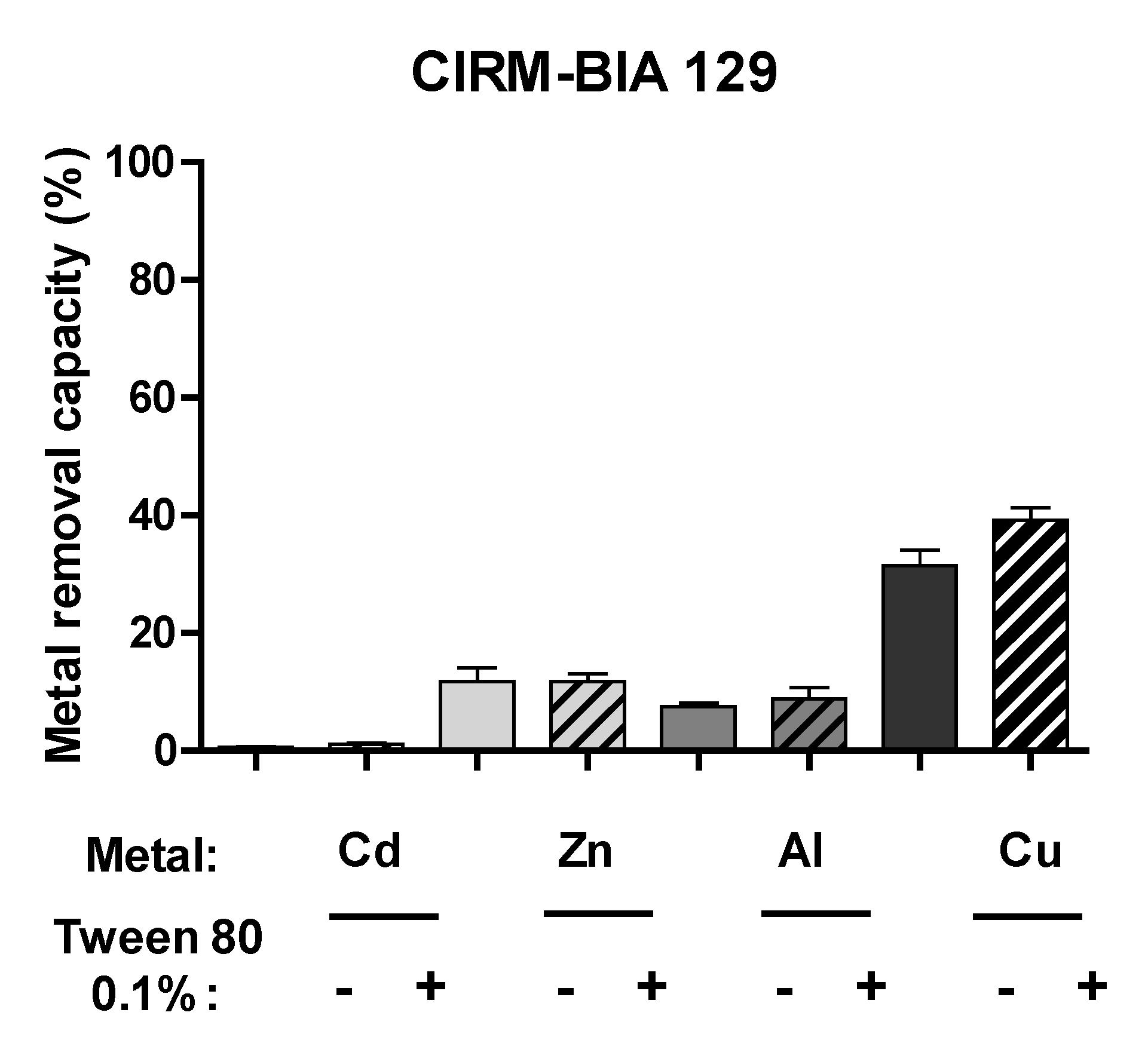
2.4. Tween® 80 Has No Impact on Other Metals Removal by P. freudenreichii’s
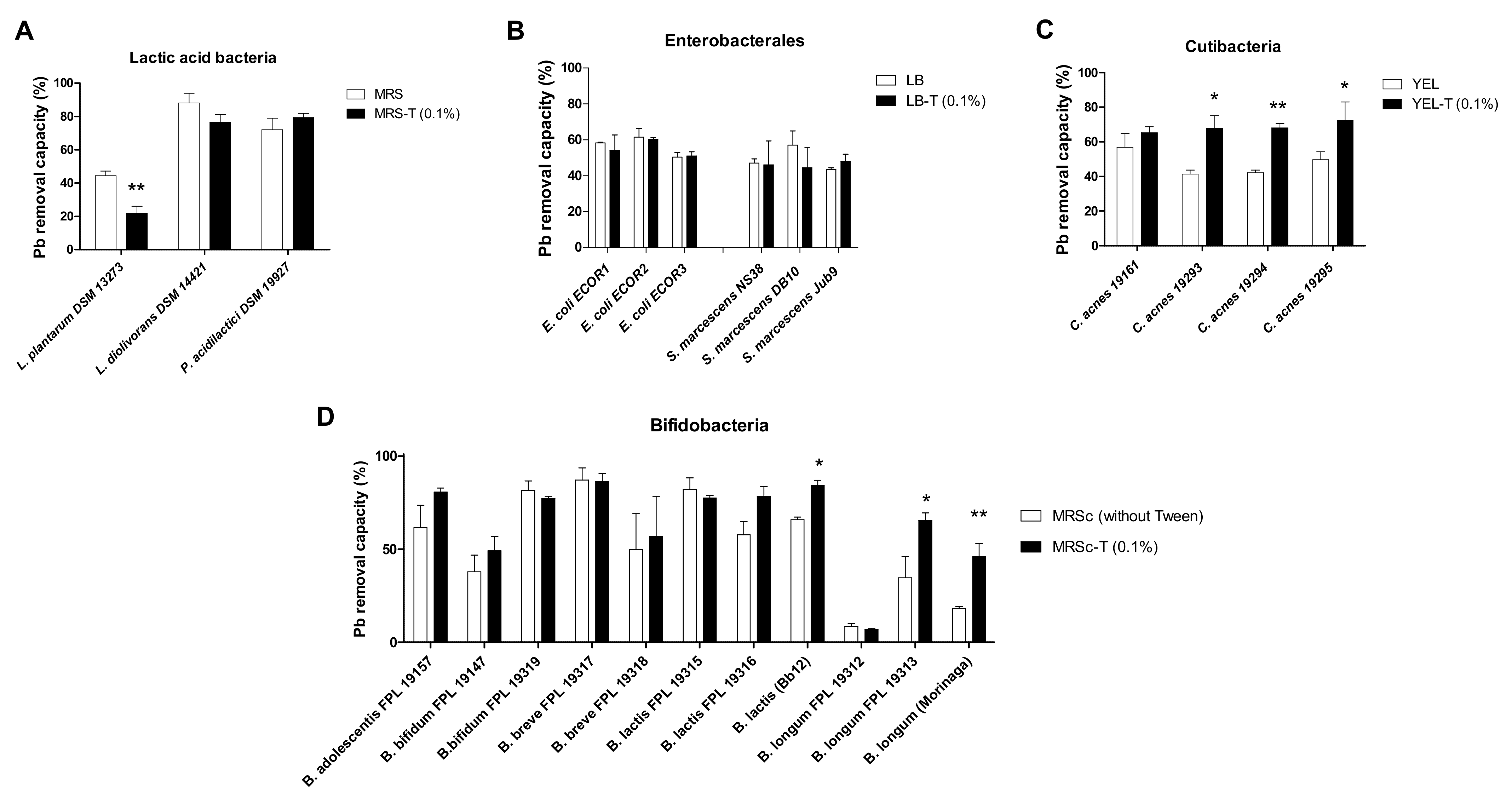
2.5. Tween® 80 Impact on Pb(II) Removal Is Bacterium Specific
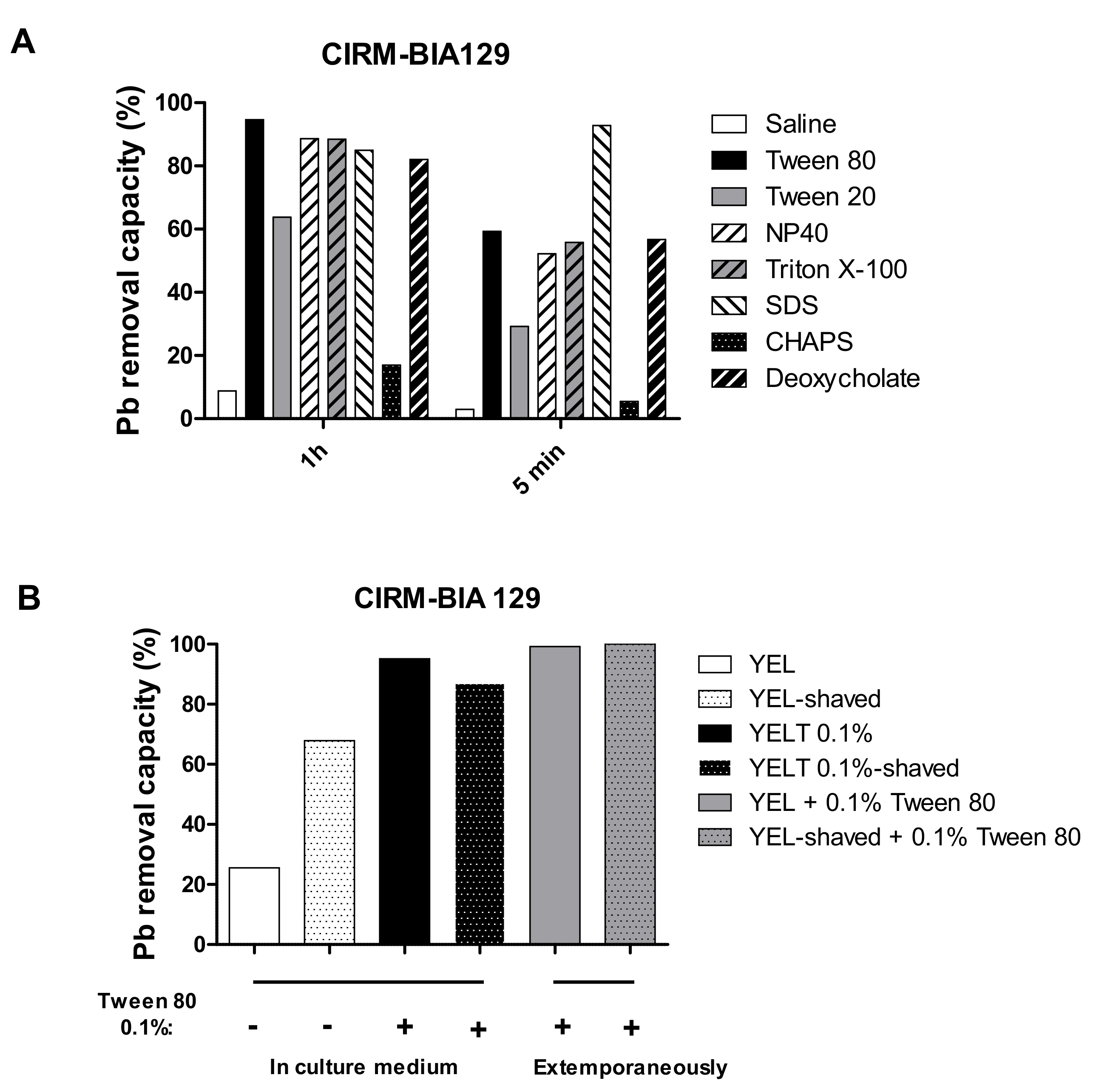
2.6. Other Detergents Differ in Their Effects on Pb(II) Removal by P. freudenreichii
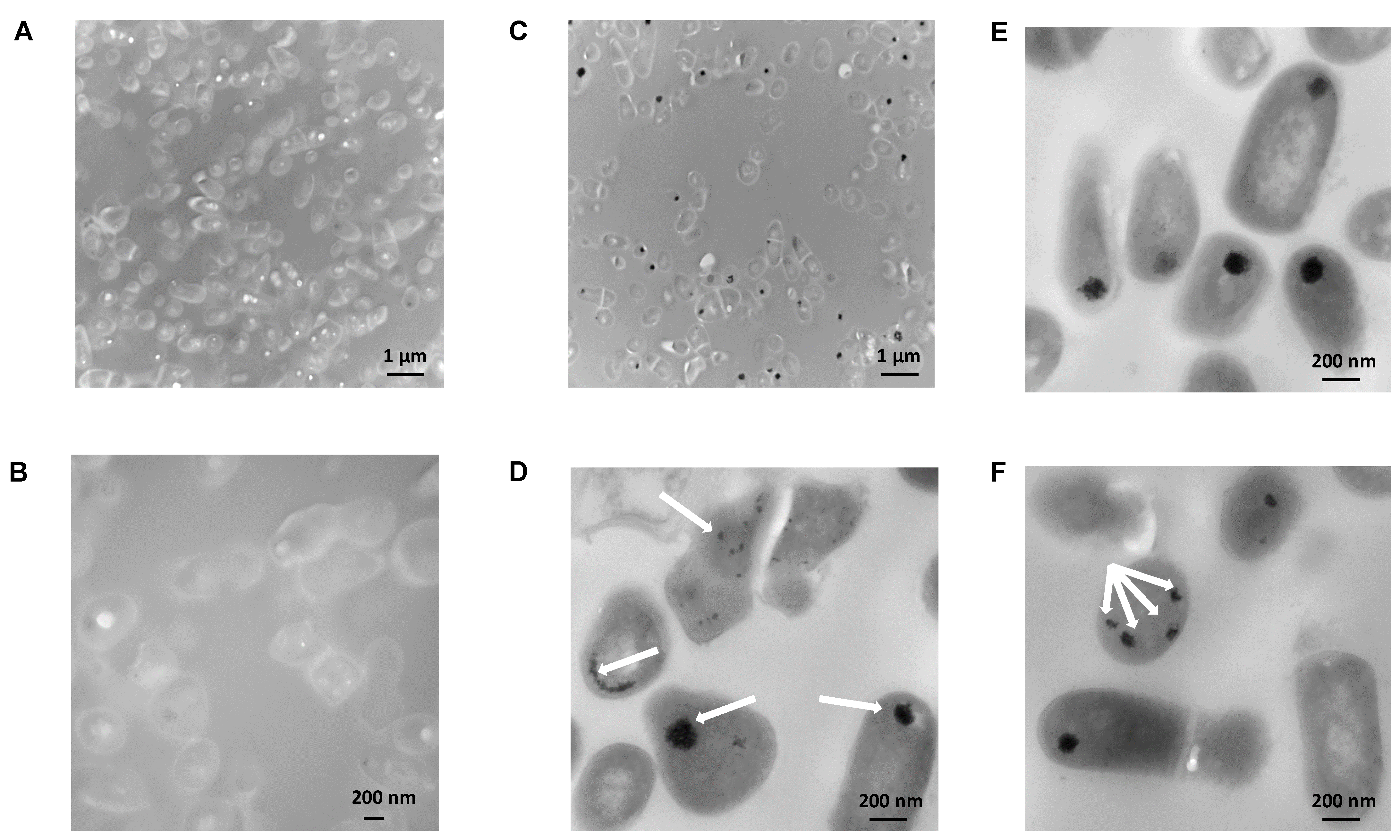
2.7. Contribution of Cell Surface Integrity to the Pb(II) Removal Mechanism
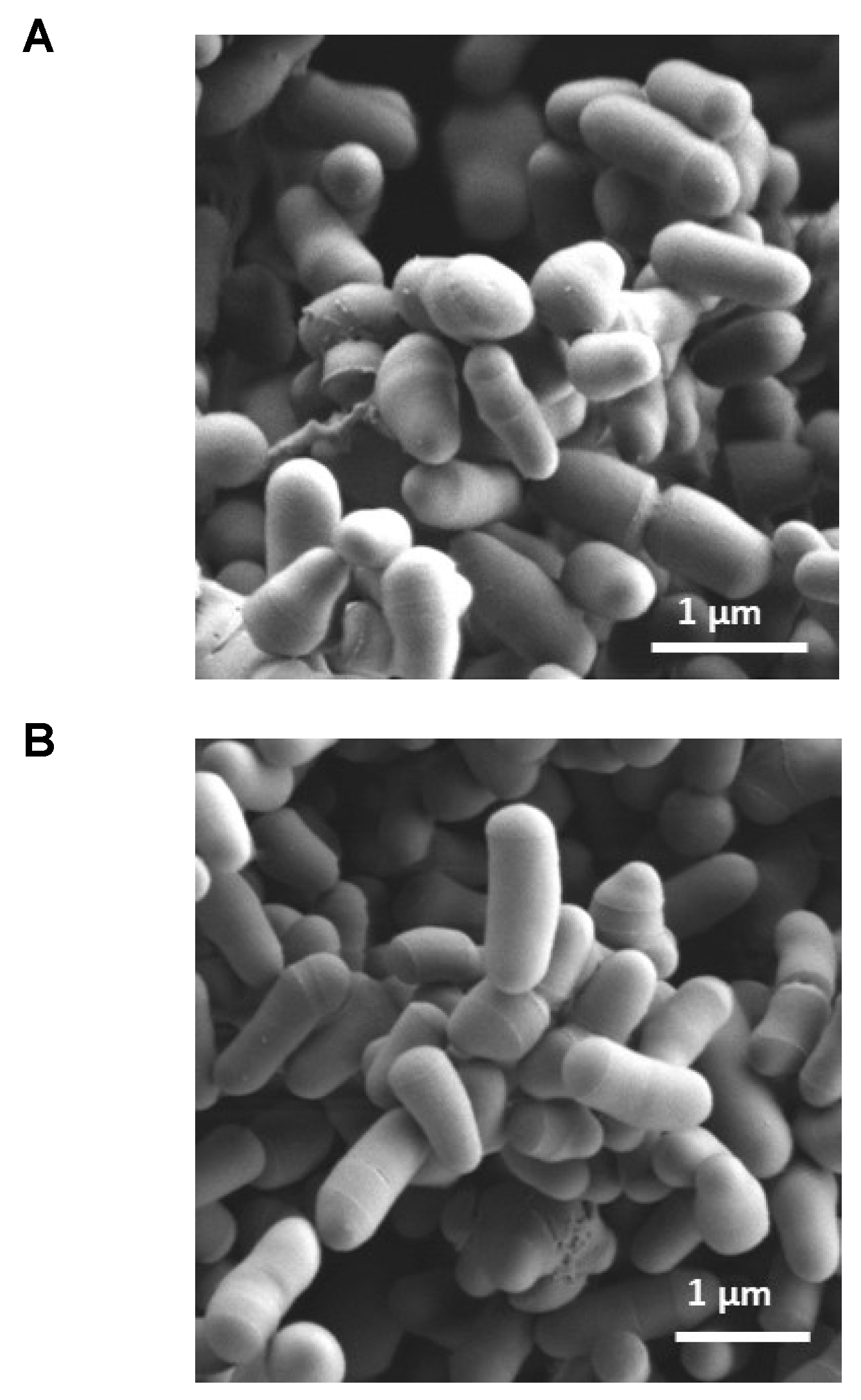
2.8. Morphological Appearance of Bacteria Exposed to Tween® 80
3. Discussion
3.1. General Overview
3.2. Mechanisms Involved
3.3. Possible Applications
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Bacterial Strains and Culture Conditions
4.3. Metal Removal Assays
4.4. Electrophoretic Mobility (Zeta Potential) Analysis
4.5. Enzymatic Shaving of Surface Proteins
4.6. Electron Microscopy
4.6.1. Scanning Electron Microscopy
4.6.2. Transmission Electron Microscopy (TEM)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watt, G.C.; Britton, A.; Gilmour, H.G.; Moore, M.R.; Murray, G.D.; Robertson, S.J. Public Health Implications of New Guidelines for Lead in Drinking Water: A Case Study in an Area with Historically High Water Lead Levels. Food Chem. Toxicol. 2000, 38, S73–S79. [Google Scholar] [CrossRef]
- Al Osman, M.; Yang, F.; Massey, I.Y. Exposure Routes and Health Effects of Heavy Metals on Children. Biometals 2019, 32, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of Lead: A Review with Recent Updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Guallar, E.; Silbergeld, E.K.; Rothenberg, S.J. Lead Exposure and Cardiovascular Disease—A Systematic Review. Environ. Health Perspect. 2007, 115, 472–482. [Google Scholar] [CrossRef]
- Breton, J.; Daniel, C.; Vignal, C.; Body-Malapel, M.; Garat, A.; Plé, C.; Foligné, B. Does Oral Exposure to Cadmium and Lead Mediate Susceptibility to Colitis? The Dark-and-Bright Sides of Heavy Metals in Gut Ecology. Sci. Rep. 2016, 6, 19200. [Google Scholar] [CrossRef]
- Liu, W.; Feng, H.; Zheng, S.; Xu, S.; Massey, I.Y.; Zhang, C.; Wang, X.; Yang, F. Pb Toxicity on Gut Physiology and Microbiota. Front. Physiol. 2021, 12, 574913. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Gupta, A.; Joia, J. Microbes as Potential Tool for Remediation of Heavy Metals: A Review. J. Microb. Biochem. Technol. 2016, 8, 364–372. [Google Scholar] [CrossRef]
- Tiquia-Arashiro, S.M. Lead Absorption Mechanisms in Bacteria as Strategies for Lead Bioremediation. Appl. Microbiol. Biotechnol. 2018, 102, 5437–5444. [Google Scholar] [CrossRef]
- Arun, K.B.; Madhavan, A.; Sindhu, R.; Emmanual, S.; Binod, P.; Pugazhendhi, A.; Sirohi, R.; Reshmy, R.; Awasthi, M.K.; Gnansounou, E.; et al. Probiotics and Gut Microbiome—Prospects and Challenges in Remediating Heavy Metal Toxicity. J. Hazard. Mater. 2021, 420, 126676. [Google Scholar] [CrossRef]
- George, F.; Daniel, C.; Thomas, M.; Singer, E.; Guilbaud, A.; Tessier, F.J.; Revol-Junelles, A.-M.; Borges, F.; Foligné, B. Occurrence and Dynamism of Lactic Acid Bacteria in Distinct Ecological Niches: A Multifaceted Functional Health Perspective. Front. Microbiol. 2018, 9, 2899. [Google Scholar] [CrossRef] [PubMed]
- Monachese, M.; Burton, J.P.; Reid, G. Bioremediation and Tolerance of Humans to Heavy Metals through Microbial Processes: A Potential Role for Probiotics? Appl. Environ. Microbiol. 2012, 78, 6397–6404. [Google Scholar] [CrossRef] [PubMed]
- Halttunen, T.; Salminen, S.; Tahvonen, R. Rapid Removal of Lead and Cadmium from Water by Specific Lactic Acid Bacteria. Int. J. Food Microbiol. 2007, 114, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, J.N.; Ohnishi, K.; Munekage, Y.; Iwasaki, K.; Wei, M.Q. Characterization of Lactic Acid Bacteria-Based Probiotics as Potential Heavy Metal Sorbents. J. Appl. Microbiol. 2012, 112, 1193–1206. [Google Scholar] [CrossRef]
- Tian, F.; Zhai, Q.; Zhao, J.; Liu, X.; Wang, G.; Zhang, H.; Zhang, H.; Chen, W. Lactobacillus plantarum CCFM8661 Alleviates Lead Toxicity in Mice. Biol. Trace Elem. Res. 2012, 150, 264–271. [Google Scholar] [CrossRef]
- Kinoshita, H.; Sohma, Y.; Ohtake, F.; Ishida, M.; Kawai, Y.; Kitazawa, H.; Saito, T.; Kimura, K. Biosorption of Heavy Metals by Lactic Acid Bacteria and Identification of Mercury Binding Protein. Res. Microbiol. 2013, 164, 701–709. [Google Scholar] [CrossRef]
- Ameen, F.A.; Hamdan, A.M.; El-Naggar, M.Y. Assessment of the Heavy Metal Bioremediation Efficiency of the Novel Marine Lactic Acid Bacterium, Lactobacillus Plantarum MF042018. Sci. Rep. 2020, 10, 314. [Google Scholar] [CrossRef]
- Lin, D.; Ji, R.; Wang, D.; Xiao, M.; Zhao, J.; Zou, J.; Li, Y.; Qin, T.; Xing, B.; Chen, Y.; et al. The Research Progress in Mechanism and Influence of Biosorption between Lactic Acid Bacteria and Pb(II): A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 395–410. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Wang, Q.; Li, D.; Han, X.; Yu, H. A Facile Approach for Surface Alteration of Pseudomonas putida I3 by Supplying K2SO4 into Growth Medium: Enhanced Removal of Pb(II) from Aqueous Solution. Bioresour. Technol. 2017, 232, 79–86. [Google Scholar] [CrossRef]
- Cousin, F.J.; Mater, D.D.G.; Foligne, B.; Jan, G. Dairy Propionibacteria as Human Probiotics: A Review of Recent Evidence. Dairy Sci. Technol. 2011, 91, 1–26. [Google Scholar] [CrossRef]
- Poonam; Pophaly, S.D.; Tomar, S.K.; De, S.; Singh, R. Multifaceted Attributes of Dairy Propionibacteria: A Review. World J. Microbiol. Biotechnol. 2012, 28, 3081–3095. [Google Scholar] [CrossRef] [PubMed]
- Altieri, C. Dairy Propionibacteria as Probiotics: Recent Evidences. World J. Microbiol. Biotechnol. 2016, 32, 172. [Google Scholar] [CrossRef] [PubMed]
- Rabah, H.; Rosa do Carmo, F.L.; Jan, G. Dairy Propionibacteria: Versatile Probiotics. Microorganisms 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.; Halttunen, T.; Tahvonen, R.; Salminen, S. Probiotic Bacteria as Potential Detoxification Tools: Assessing Their Heavy Metal Binding Isotherms. Can. J. Microbiol. 2006, 52, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Halttunen, T.; Collado, M.C.; El-Nezami, H.; Meriluoto, J.; Salminen, S. Combining Strains of Lactic Acid Bacteria May Reduce Their Toxin and Heavy Metal Removal Efficiency from Aqueous Solution. Lett. Appl. Microbiol. 2008, 46, 160–165. [Google Scholar] [CrossRef]
- George, F.; Mahieux, S.; Daniel, C.; Titécat, M.; Beauval, N.; Houcke, I.; Neut, C.; Allorge, D.; Borges, F.; Jan, G.; et al. Assessment of Pb(II), Cd(II), and Al(III) Removal Capacity of Bacteria from Food and Gut Ecological Niches: Insights into Biodiversity to Limit Intestinal Biodisponibility of Toxic Metals. Microorganisms 2021, 9, 456. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Growth of Probiotic Lactobacilli in the Presence of Oleic Acid Enhances Subsequent Survival in Gastric Juice. Microbiology 2007, 153, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Reitermayer, D.; Kafka, T.A.; Lenz, C.A.; Vogel, R.F. Interrelation between Tween and the Membrane Properties and High Pressure Tolerance of Lactobacillus plantarum. BMC Microbiol. 2018, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Menberu, M.A.; Hayes, A.J.; Liu, S.; Psaltis, A.J.; Wormald, P.-J.; Vreugde, S. Tween 80 and Its Derivative Oleic Acid Promote the Growth of Corynebacterium accolens and Inhibit Staphylococcus aureus Clinical Isolates. Int. Forum Allergy Rhinol. 2021, 11, 810–813. [Google Scholar] [CrossRef]
- Di Stefano, M.; Pagani, E.; Pesatori, E.V.; Bergonzi, M.; Figura, N.; Corazza, G.R.; Di Sabatino, A. Polysorbate 80 Add-on Therapy in the Treatment of Helicobacter pylori Infection: Polysorbate 80 and HP Antibiotic Resistance. Clin. Nutr. ESPEN 2019, 34, 101–103. [Google Scholar] [CrossRef]
- Al Farraj, D.A.; Alkufeidy, R.M.; Alkubaisi, N.A.; Alshammari, M.K. Polynuclear Aromatic Anthracene Biodegradation by Psychrophilic Sphingomonas Sp., Cultivated with Tween-80. Chemosphere 2021, 263, 128115. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Di Donato, P.; Nicolaus, B.; Di Marco, G.; Michaud, L.; Lo Giudice, A. Extracellular Polymeric Substances with Metal Adsorption Capacity Produced by Pseudoalteromonas Sp. MER144 from Antarctic Seawater. Environ. Sci. Pollut. Res. Int. 2018, 25, 4667–4677. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tong, M.; Sun, X.; Li, B. Cystine-Modified Biomass for Cd(II) and Pb(II) Biosorption. J. Hazard. Mater. 2007, 143, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Zoghi, A.; Khosravi-Darani, K.; Sohrabvandi, S. Surface Binding of Toxins and Heavy Metals by Probiotics. MRMC 2014, 14, 84–98. [Google Scholar] [CrossRef]
- Mishra, S.; Lin, Z.; Pang, S.; Zhang, Y.; Bhatt, P.; Chen, S. Biosurfactant Is a Powerful Tool for the Bioremediation of Heavy Metals from Contaminated Soils. J. Hazard. Mater. 2021, 418, 126253. [Google Scholar] [CrossRef]
- Göksungur, Y.; Uren, S.; Güvenç, U. Biosorption of Cadmium and Lead Ions by Ethanol Treated Waste Baker’s Yeast Biomass. Bioresour. Technol. 2005, 96, 103–109. [Google Scholar] [CrossRef]
- Deutsch, S.-M.; Parayre, S.; Bouchoux, A.; Guyomarc’h, F.; Dewulf, J.; Dols-Lafargue, M.; Baglinière, F.; Cousin, F.J.; Falentin, H.; Jan, G.; et al. Contribution of Surface β-Glucan Polysaccharide to Physicochemical and Immunomodulatory Properties of Propionibacterium freudenreichii. Appl. Environ. Microbiol. 2012, 78, 1765–1775. [Google Scholar] [CrossRef]
- Jiang, R.; Wu, X.; Xiao, Y.; Kong, D.; Li, Y.; Wang, H. Tween 20 Regulate the Function and Structure of Transmembrane Proteins of Bacillus Cereus: Promoting Transmembrane Transport of Fluoranthene. J. Hazard. Mater. 2021, 403, 123707. [Google Scholar] [CrossRef]
- Zoghi, A.; Massoud, R.; Todorov, S.D.; Chikindas, M.L.; Popov, I.; Smith, S.; Khosravi-Darani, K. Role of the Lactobacilli in Food Bio-Decontamination: Friends with Benefits. Enzyme Microb. Technol. 2021, 150, 109861. [Google Scholar] [CrossRef]
- Abdel-Megeed, R.M. Probiotics: A Promising Generation of Heavy Metal Detoxification. Biol. Trace Elem. Res. 2021, 199, 2406–2413. [Google Scholar] [CrossRef]
- Foligné, B.; Breton, J.; Mater, D.; Jan, G. Tracking the Microbiome Functionality: Focus on Propionibacterium Species. Gut 2013, 62, 1227–1228. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, S.-M.; Mariadassou, M.; Nicolas, P.; Parayre, S.; Le Guellec, R.; Chuat, V.; Peton, V.; Le Maréchal, C.; Burati, J.; Loux, V.; et al. Identification of Proteins Involved in the Anti-Inflammatory Properties of Propionibacterium freudenreichii by Means of a Multi-Strain Study. Sci. Rep. 2017, 7, 46409. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Harris, H.M.B.; McCann, A.; Guo, C.; Argimón, S.; Zhang, W.; Yang, X.; Jeffery, I.B.; Cooney, J.C.; Kagawa, T.F.; et al. Expanding the Biotechnology Potential of Lactobacilli through Comparative Genomics of 213 Strains and Associated Genera. Nat. Commun. 2015, 6, 8322. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.C.; Reinbold, G.W.; Vedamuthu, E.R. An Evaluation of the Taxonomy of Propionibacterium. Can. J. Microbiol. 1968, 14, 1185–1191. [Google Scholar] [CrossRef]
- Sze, A.; Erickson, D.; Ren, L.; Li, D. Zeta-Potential Measurement Using the Smoluchowski Equation and the Slope of the Current-Time Relationship in Electroosmotic Flow. J. Colloid Interface Sci. 2003, 261, 402–410. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
George, F.; Titécat, M.; Barois, N.; Daniel, C.; Garat, A.; Jan, G.; Foligné, B. A Unique Enhancement of Propionibacterium freudenreichii’s Ability to Remove Pb(II) from Aqueous Solution by Tween 80 Treatment. Int. J. Mol. Sci. 2022, 23, 9207. https://doi.org/10.3390/ijms23169207
George F, Titécat M, Barois N, Daniel C, Garat A, Jan G, Foligné B. A Unique Enhancement of Propionibacterium freudenreichii’s Ability to Remove Pb(II) from Aqueous Solution by Tween 80 Treatment. International Journal of Molecular Sciences. 2022; 23(16):9207. https://doi.org/10.3390/ijms23169207
Chicago/Turabian StyleGeorge, Fanny, Marie Titécat, Nicolas Barois, Catherine Daniel, Anne Garat, Gwénaël Jan, and Benoît Foligné. 2022. "A Unique Enhancement of Propionibacterium freudenreichii’s Ability to Remove Pb(II) from Aqueous Solution by Tween 80 Treatment" International Journal of Molecular Sciences 23, no. 16: 9207. https://doi.org/10.3390/ijms23169207
APA StyleGeorge, F., Titécat, M., Barois, N., Daniel, C., Garat, A., Jan, G., & Foligné, B. (2022). A Unique Enhancement of Propionibacterium freudenreichii’s Ability to Remove Pb(II) from Aqueous Solution by Tween 80 Treatment. International Journal of Molecular Sciences, 23(16), 9207. https://doi.org/10.3390/ijms23169207





