1. Introduction
A cell needs two basic elements—mass and energy. The mass consists mainly of amino acids. They enable basic structures and functions in the form of proteins and enzymes and constitute the largest component of a cell’s mass [
1]. For energy, the most important representatives are ATP and NAD(P)H, which are essentially obtained from carbohydrates and lipids. Glycolysis, and with it glucose, is established as one of the oldest reaction pathways for energy production, with the help of which ATP and NADH can be produced very fast [
2]. For this reason, and due to its high water solubility, glucose is the central energy resource for the cells of an organism.
Mass and energy are essential for the maintenance of the cell and, to an even greater extent, for proliferation. In the case of neoplastic cells, defined by space-occupying proliferation that is almost unlimited, they, therefore, play an even greater role. For this reason, different forms of restriction are potential strategies for tumour therapy. What they all have in common is that a resource is limited to such an extent that it is no longer sufficiently available to supply the tumour cells. In the case of glucose, the approach is based on the so-called Warburg effect, which was described by Warburg and colleagues in 1924 [
3]. They were able to show that tumour cells take up more glucose under aerobic conditions and metabolise it to lactate. This metabolic pathway is normally used only under an oxygen deficiency, i.e., under anaerobic conditions. At first glance, this seems inefficient, since glycolysis provides only two ATP as a net gain, while complete degradation to CO
2 provides another 32 ATP. Although the causes of the Warburg effect are not yet understood in detail, there are a number of possibilities. For example, aerobic glycolysis enables the very rapid synthesis of ATP to maintain a high ATP level, increased synthesis of NADPH, which is increasingly needed for the biosynthesis of lipids, among other molecules, and the acidification of the tumour microenvironment, which can suppress the immune response to the tumour [
4]. It is now known that the Warburg effect is generally characteristic of proliferating cells, but it plays a quantitatively and qualitatively greater role in neoplastic cells due to the nonlimited proliferation. For this reason, glucose restriction, which prevents the tumour from using glucose in any way, offers a fundamental approach to tumour therapy [
5].
However, the possibilities for attacking tumours with the help of restriction have expanded considerably in the past 10 years. Although glucose is one of the central molecules in metabolism, especially in tumours, the restriction of energy and/or mass also offers a very good approach. Energy can be reduced with the help of calorie restriction, and mass can be reduced by amino acid or protein restriction [
6,
7,
8,
9]. Central to all forms of restriction is the induction of a low-energy metabolism (LEM), which is significantly mediated by the protein complex mTOR. The intracellular activity of the mTOR determines proliferation and growth. A multitude of sensors pass information about the energy and mass content to the mTOR switch point. For example, the energy equivalents ATP and NADH can be measured via the protein AMPK (5′ AMP-activated protein kinase) and with the help of sirtuins [
10]. A lack of energy leads to the inhibition of the mTOR via the two proteins mentioned and results in a halt to proliferation and the induction of autophagy for the purpose of energy recycling. The content of selected amino acids is measured with the help of protein complexes; in the case of methionine, SAMTOR plays a decisive role. Here, methionine is not measured directly, but an intermediate product, S-adenosylmethionine (SAM), whose content is an indicator of the intracellular methionine concentration. However, a low methionine concentration also leads to the inhibition of the mTOR [
11].
In the long term, all forms of restriction lead to astonishing results in almost all organism types, from yeast to nematodes to Drosophila to humans. In addition to the extension of the absolute lifespan, the prevention of heart and circulatory diseases, type II diabetes and cancer are prominent [
7,
12,
13]. The restriction of glucose in the form of a reduced increase (low carb) or as absolute an avoidance as possible (ketogenic) results in similar mechanisms to calorie restriction [
14].
All forms of restriction have much in common, as the reactions either use basic mechanisms of the cell or rely on evolutionarily conserved mechanisms. However, there will be equally striking differences due to the complexity. Especially with regard to the evaluation of the advantages and disadvantages of individual forms of restriction, the level of scientific knowledge is very low. This is mainly because scientific working groups usually focus only on one particular form of restriction, and thus, it is very difficult to compare the individual forms of restriction. In the case of glucose, this is further complicated by the fact that many studies do not work with a constant glucose concentration. Physiologically, in mammals, the glucose concentration in the blood is kept at a certain threshold value, which it does not fall below—in humans, approximately 3 mM, and in mice, approximately 6 mM [
15]. Many experiments with glucose carried out in cell culture start with a concentration that is in the low-carb range. However, this value is not static, but continues to drop over the course of the experiment. This can lead to inconsistent results that are difficult to interpret. Experiments cannot always be carried out over a longer period of time in this way, as cells undergo apoptosis or simply die if the glucose deficiency remains below the physiological threshold [
16].
The aim of our research group is to enable the comparison of individual restriction forms at the molecular level. First, we use the murine model system L929, which enables the comparison of different restrictions in one and the same system [
17,
18,
19]. Second, we use closed, circular perfusion cultures that allow metabolite concentrations to be kept constant over longer periods of time. In this work, we used liquid chromatography mass spectrometry (LC-MS) to analyse the effect of the glycolysis inhibitor 2-deoxy-glucose (2-DG) at time points 2, 5, 8, 24, 48, and 72 h under proliferative conditions in our L929 model system. In addition, we compared methionine restriction (MetR) with glucose restriction under LowCarb conditions (3 mM) in a perfusion culture over a period of 7 days.
3. Discussion
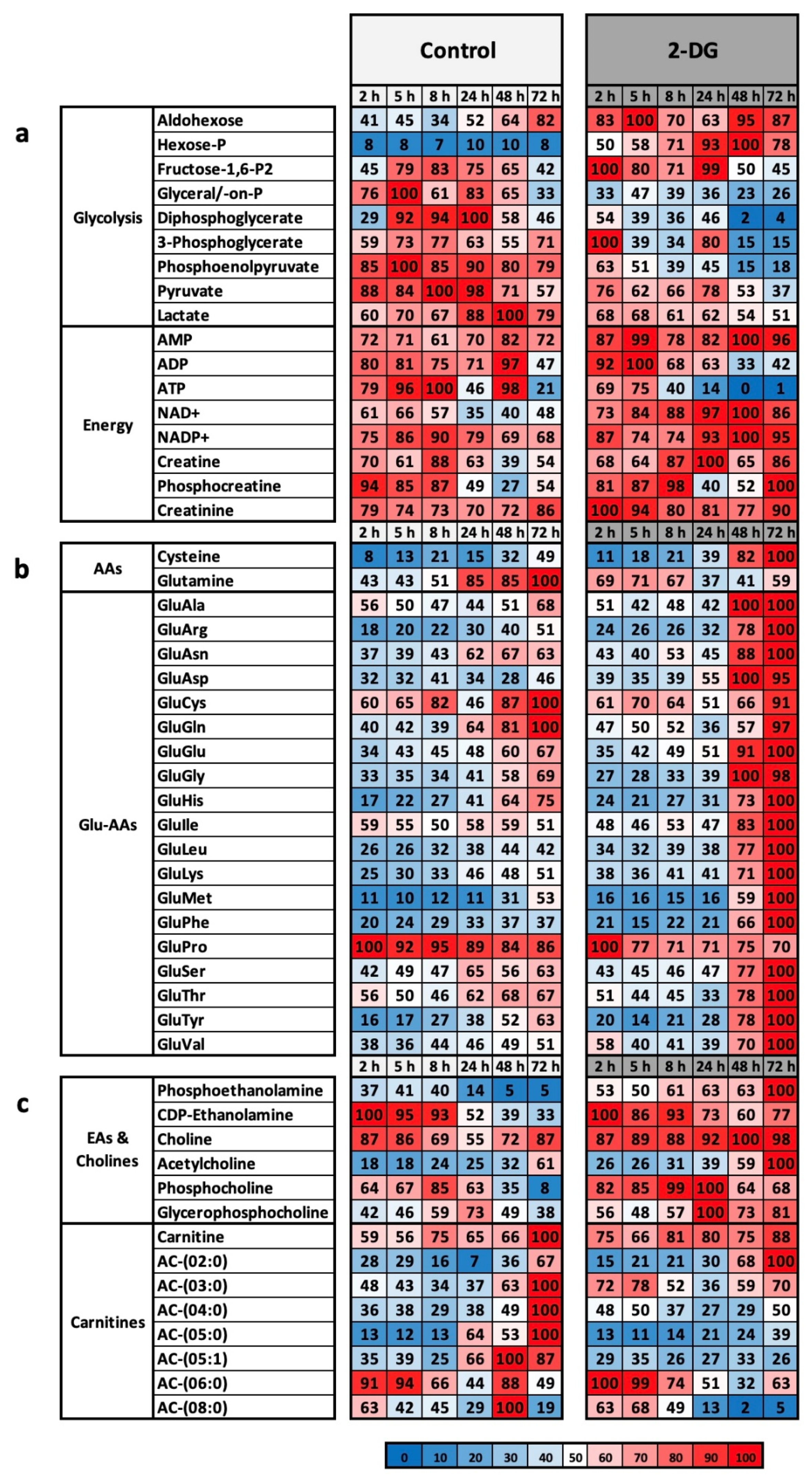
In this work, we used liquid chromatography mass spectrometry to analyse the influence of glucose restriction using the glycolysis inhibitor 2-DG in our L929 model system to demonstrate that the system is suitable for analyses in the LowCarb condition. In a further analysis, we then compared the metabolome analyses of the two restriction conditions MetR and LowCarb in a perfusion system.
Analysis of the glycolysis inhibitor 2-DG showed a clear antiproliferative effect on L929 cells (
Figure 2). In addition, as expected, the inhibitor was shown to slow glycolysis at the metabolic level downstream of the actual target, glucose-6-phosphate isomerase (
Figure 3) [
26]. The clearest marker for the effectiveness of 2-DG is the extreme drop in the intracellular ATP concentration to 1% (
Figure 3) [
27]. The conclusion is that the cell line L929 is very well suited for analysing glycolysis metabolism.
Using perfusion culture, the two restriction conditions MetR and LowCarb were compared over a period of 7 days in a closed perfusion culture. Analysis of the metabolome showed that both restriction conditions cause observable changes at the molecular level. In the case of MetR, in addition to the low methionine and GluMet contents, it is particularly noticeable that some amino acids are increasingly secreted. This is a phenomenon that we have already defined in an earlier paper as characteristic of L929 under MetR [
18]. Glucose restriction was also shown to be specific at the molecular level and clearly differed from the effects induced by 2-DG, as here the concentration of the first two metabolites of glycolysis—hexose phosphate and fructose 1,6-bisphosphate—strongly decreased and then accumulated to a greater degree than 3-phosphoglycerate. The decrease in UDP-glucose, a product that, as already mentioned, is strongly dependent on the turnover rate of glycolysis, also clearly showed the changes induced by LowCarb.
Nevertheless, it is noticeable that the vast majority of the metabolites remained at similar levels, even compared to the control. However, this is also evidence of the quality of the analysis. For example, if one analyses gene expression across the transcriptome of 10,000 genes, the absolute majority of genes will not be differentially regulated. If, for example, 9000 genes were differentially regulated, this would be a strong indication of a faulty analysis. Thus, on the one hand, the many metabolites at the same level show the quality of the analysis and the stability of the perfusion culture, but on the other hand, the question arises as to what extent the different forms of restriction have been implemented consistently? In the following discussion, we want to assess to what extent the results reflect the implementation of the two restriction forms MetR and LowCarb in our system, which factors have a decisive influence on the profile, and which further analyses are useful.
The incubation period has a significant influence on the metabolic profile. It is possible that the system must be incubated for a longer period than 7 days so that the specific restriction profiles can be more extreme. On the other hand, the different forms of restriction also have many common molecular mechanisms, as already described [
14]. Thus, the results can also be interpreted as a slow but sure alignment of the metabolites over time, while in the short term, the differences are much larger. Looking at the overall profile of the 2-DG analysis (
Supplement File S1), for example, it is noticeable that at the early time points within the first 8 h, most of the metabolites are significantly different compared to those in the control.
The metabolites selected for analysis also play a role. In LC-MS analysis, we chose a setup to analyse characteristic metabolites of individual metabolic pathways, such as glycolysis or the TCA cycle. However, these approximately 170 metabolites represent only a small part of the real existing metabolites of a cell. Thus, it is possible that other metabolites not used in this analysis show a much more differentiated picture than the products we selected. Again, the argument applies that the most important metabolic pathways align over time.
The growth conditions can also influence the results. In this work, we decided to start the analyses under confluent conditions to obtain sufficient cell material for the LC-MS analyses. Furthermore, we know from previous work that the low energy status under MetR is essentially independent of proliferation or cell contact inhibition [
17].
Figure 6 clearly shows that the cells continued to grow steadily within the perfusion culture. We could not observe this effect in the static cell culture. However, we assume that this effect has no negative influence on the LC-MS analyses. Again, the already mentioned argument applies that the high number of metabolites at similar levels is a quality criterion. If proliferation affected the results, the differences when comparing MetR to the control and LowCarb should be much more striking. However, the question arises as to why the cells manage to continue growing even under the LowCarb condition.
Figure 2a shows the analyses performed to determine the glucose concentration used in perfusion culture. Under static conditions, the proliferation rate decreased dramatically after 96 h with an initial concentration of 3 mM glucose, but under these experimental conditions, the glucose level also continued to decrease during the experimental period. Basically, this experiment served to confirm that we can work with a level of 3 mM glucose in the perfusion culture without running the risk of cell death. After 96 h at 3 mM, the cells were observed to be alive under a microscope and proliferated significantly better than under even lower glucose concentrations. We used a medium tank in the perfusion culture that provided 60 times the volume of the contents of the Petri dish. We assumed that these conditions were suitable for L929 cells and that the cells were well supplied over this period. However, we did not want to lower the glucose concentration below 3 mM because this concentration does not correspond to the physiological conditions of the blood glucose level. In mice, 3 mM is already a very low value. Therefore, in the perfusion culture, the constant supply of fresh medium seems to be sufficient for the cells to grow steadily both in the control and under LowCarb.
The closed perfusion culture is another factor that has a strong influence on the metabolome. Basically, the closed version offers the advantage that the medium is conditioned over the experimental period. Thus, on the one hand, the medium can be included in the LC-MS analysis; on the other hand, secreted messengers may well be essential to implement the individual restriction forms. However, the exact opposite can also occur. The active substances in the medium influence the cellular events more than MetR or LowCarb. Analysis of the supernatants showed that a large number of metabolites accumulated over the experimental period. Thus, it is possible that the concentration of the secreted metabolites influences the metabolism and overlaps both MetR and LowCarb in the metabolic profile, leading to an alignment of the LC-MS profiles.
However, perfusion culture offers extreme advantages. Basically, the main advantage lies in the simulation of the blood flow of an organism, in that, on the one hand, fresh nutrients can be continuously supplied and, on the other hand, waste and possibly toxins can be removed. The differences between open and closed perfusion systems will be shown in further studies.
In principle, L929 cells are also suitable for analysing glucose metabolism. They react to 2-DG inhibition accordingly. The cells are also suitable for perfusion culture, and we were able to show that after 7 days in a closed system, the cells implemented a MetR- or LowCarb-specific metabolism. However, a large number of metabolites were at similar levels even compared to the control. For this reason, the question arises as to whether the restriction forms result in a largely similar metabolism or whether the profile is influenced more significantly by certain factors than by the restriction itself. Based on the above arguments, in future work, the cells should be analysed over a longer period of time (up to 21 days) to determine whether the metabolism continues to differentiate or remains similar. Furthermore, the experiments should be conducted in an open perfusion system to limit the effect on the restrictive factors methionine and glucose. We are sure that future analyses using the perfusion culture technique will reveal fundamental insights and mechanisms under the different forms of restriction.
4. Materials and Methods
4.1. Cell Culture
The murine fibroblast cell line L929 was purchased from the Leibniz Institute, DSMZ-German Collection of Microorganisms and Cell Cultures GmbH (Braunschweig, Germany). Cells were cultured in RPMI 1640 medium (Gibco, Life Technologies; Darmstadt, Germany) with 10% FCS (Sigma-Aldrich, Darmstadt, Germany) and 1% penicillin/streptomycin (P/S; 100 U/mL penicillin and 100 µg/mL streptomycin (Thermo Fisher Scientific, Darmstadt, Germany)) at 37 °C in a humidified atmosphere containing 5% CO2.
4.2. ImageXpress Pico Automated Cell Imaging System—Digital Microscopy (Pico Assay)
Cells were seeded at 10,000 cells in 100 µL of culture medium per well of a 96-well plate and incubated overnight. The following day, the cells were incubated in complete, methionine-free, cysteine-free, or methionine- and cysteine-free medium. The incubation time is stated in the corresponding figure legend. For staining, 10 µL of Hoechst staining solution (1:200 dilution of Hoechst 33342 (Thermo Fisher, Darmstadt, Germany) (10 mg/mL in H2O) in medium) was added to each well. After a 20–30-min incubation period, wells were analysed with an ImageXpress Pico automated cell imaging system (Molecular Devices, San Jose, CA, USA) via automated digital microscopy. The cells were analysed with transmitted light in the DAPI channel at 4x magnification. The complete area of every well was screened. The focus and exposure time were set via an auto setup and controlled by analysing 3–4 test wells. Finally, every result was confirmed visually, and 95% of the cells were counted and analysed.
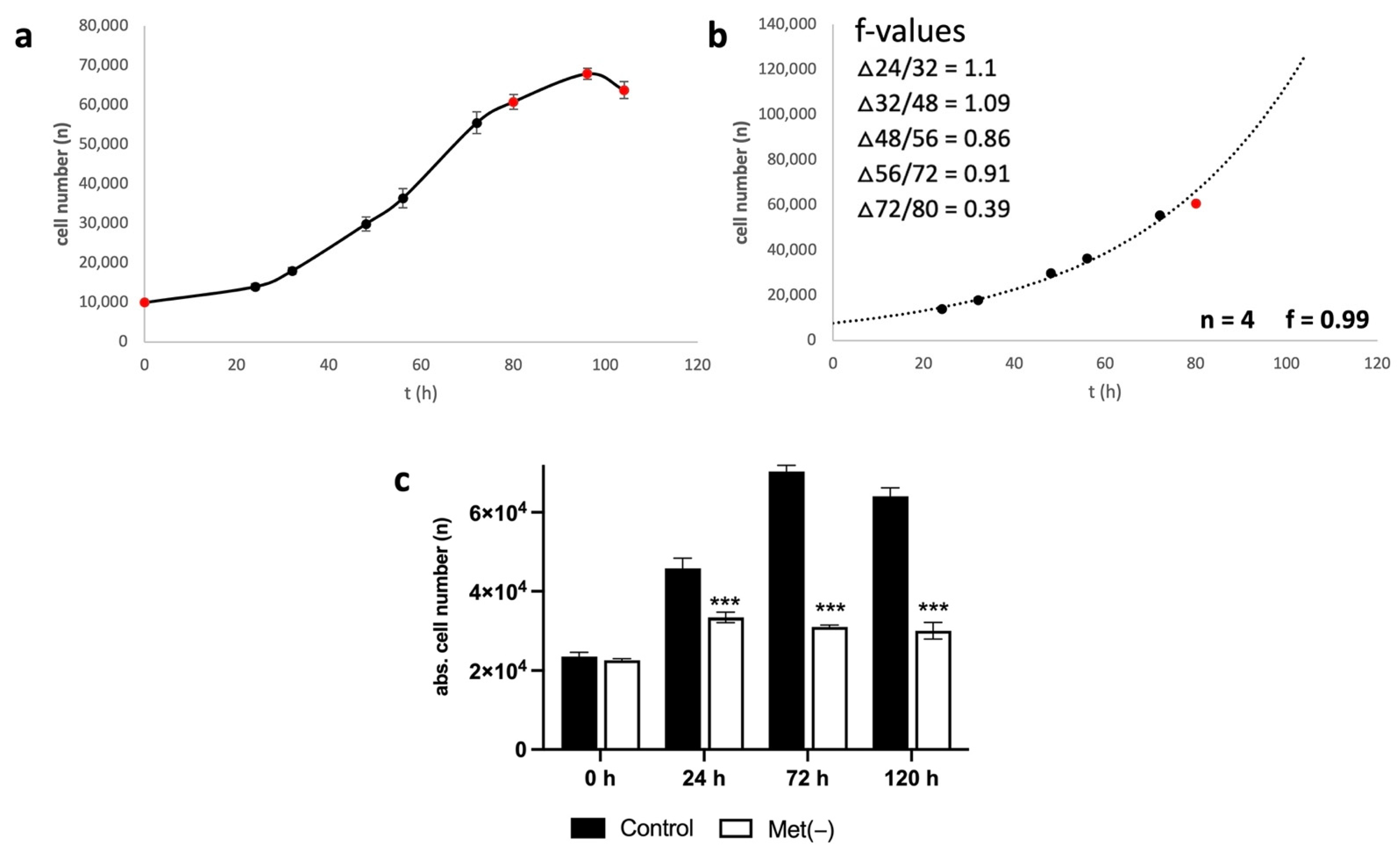
4.3. Analysis of the Cell Progression Rate Using the Pico Assay
Cells were seeded at 10,000 cells in 100 µL of culture medium per well in a 96-well plate. After 24, 32, 48, 56, 72, 80, 96, 104, and 120 h, cell numbers were measured with six values for every time point, as described under Pico Assay (
Section 4.2). The growth of a cell population can be described with the following formula:
(Nt = cell number at time t; N0 = cell number at time 0; t = time in days (d); f = cell division frequency (1/d)).
To determine f, the formula is rearranged as follows:
To obtain an overview, the measured values were first plotted as a simple diagram. From this, it was possible to see at what point the growth entered the plateau phase. Then, the values were plotted as an exponential curve, and only values in the exponential growth phase were used to determine f. In the case of L929, these were the time points 24, 32, 48, 56, and 72 h. From these values, the individual f values were calculated for the intermediate periods (Δ24/32, Δ32/48, Δ48/56, and Δ56/72). The total value f was then calculated as the mean of the four Δf values.
4.4. Experiments under 2-DG for LC-MS
L929 cells were seeded in 20 mL of medium in 15 cm Petri dishes and incubated overnight. A total of 2 × 106 cells/Petri dish were seeded under proliferative conditions for 2, 5, and 8 h, and 1 × 106 cells were seeded at 24, 48, and 72 h to prevent confluence during the test period. Every value was measured in triplicate. All media contained 10% FCS and 1% P/S [100 U/mL penicillin and 100 µg/mL streptomycin (Thermo Fisher Scientific, Darmstadt, Germany)]. After seeding, the cells were incubated with 20 mL of complete RPMI medium or 20 mL of RPMI medium with 625 µM 2-deoxy-D-glucose (2-DG) (Sigma-Aldrich, Darmstadt, Germany). Before harvesting, 1 mL of the supernatant was stored for analysis. The remaining medium was then removed, and the cells were washed with 10 mL of PBS and detached with 3 mL of trypsin/EDTA (Thermo Fisher Scientific, Darmstadt, Germany). After the addition of 7 mL of the appropriate medium, the absolute cell number in the suspensions was analysed with the automated cell counter EVETM [NanoEntek (VWR, Darmstadt, Germany)]. Each sample was measured three times, and the mean value was calculated to obtain an accurate result. Pellets with 1 × 106 cells were produced by centrifugation (5 min at 1200 rpm at RT). Until the LC-MS analysis, all samples were stored at −20 °C.
4.5. Closed Perfusion Culture Experiments for LC-MS
For perfusion culture (
Figure 7), we used RPMI 1640 (Genaxxon Bioscience, Ulm, Germany), which lacks methionine and glucose, as the basal medium. Every medium was prepared from the basal medium. All media contained 10% FCS (Sigma-Aldrich, Darmstadt, Germany) and 1% P/S [100 U/mL penicillin and 100 µg/mL streptomycin (Thermo Fisher Scientific, Darmstadt, Germany)]. For the control (complete medium), the amino acid methionine (Sigma-Aldrich, Darmstadt, Germany) was added at 15 mg/L, and glucose (Sigma-Aldrich, Darmstadt, Germany) was added at 11.1 mM. For MetR medium, glucose was added at the same concentration, and for LowCarb medium, methionine was added at 15 mg/L and glucose was added at a final concentration of 3 mM.
A total of 1 × 10
6 cells were seeded in 35 mm dishes (Greiner Bio-One, Frickenhausen, Germany). After 48 h, cells reached confluence, the medium was removed, and the cells were stimulated with complete medium (control), MetR, or LowCarb medium. Petri dishes were incubated in a bioreactor at 37 °C in a humidified atmosphere containing 5% CO
2. Every medium tank contained 180 mL (60-fold excess compared to the contents of the Petri dish (3 mL)). The flow rate was 0.8 mL/min, with the inflow being slower than the outflow to prevent the Petri dish from overflowing. During incubation, the Petri dish was supplied with medium using a dual perfusion set for 35 mm petri dishes (PeCon GmbH, Erbach, Germany). This consisted of a perfusion ring, which had an inlet and outlet for the medium, and a metal lid with a glass interior to prevent contamination
Figure 1a–c. After 7 days, 1 mL of the supernatant was removed before harvesting and stored for analysis. The remaining medium was then removed, and the cells were washed with 10 mL of PBS and detached with 3 mL of trypsin/EDTA (Thermo Fisher Scientific, Darmstadt, Germany). After the addition of 7 mL of the appropriate medium, the absolute cell number in the suspensions was analysed with the automated cell counter EVE
TM [NanoEntek (VWR, Darmstadt, Germany)]. Each sample was measured four times, and the mean value was calculated to obtain an accurate result. Pellets with 1 × 10
6 cells were produced by centrifugation (5 min at 1200 rpm at RT). Until the LC-MS analysis, all samples were stored at −20 °C.
4.6. LC-MS
Analysis of water-soluble metabolites in cell extracts and culture media.
4.6.1. Cells
After the addition of 0.5 mL of MeOH/CH3CN/H2O (50/30/20, v/v/v) containing 10 µM lamivudine, cell pellets were homogenised by ultrasound treatment (10 × 1 s, 250 W output energy). Medium: One hundred microlitres of culture medium was combined with 0.4 mL of MeOH/CH3CN (50/30, v/v) containing 10 µM lamivudine. The external standard lamivudine was not used for absolute metabolite quantification, but was used as a quality control to compensate for eventually occurring technical issues. As quality control and for the determination of the corresponding retention times, most of the annotated metabolites (which are commercially available) were run as mixtures of pure compounds under identical experimental conditions. General procedure: The resulting suspension was centrifuged (20 kRCF for 2 min in an Eppendorf centrifuge 5424), and the supernatant was applied to a C18-SPE column that was activated with 0.5 mL of CH3CN and equilibrated with 0.5 mL of MeOH/CH3CN/H2O (50/30/20, v/v/v). The SPE eluate was evaporated in a vacuum concentrator. The resulting pellet was dissolved in 50 µL (cell extracts) or 500 µL (media extracts) of 5 mM NH4OAc in CH3CN/(25%/75%, v/v).
4.6.2. LC Parameters
Mobile phase A consisted of 5 mM NH4OAc in CH3CN/H2O (5/95, v/v), and mobile phase B consisted of 5 mM NH4OAc in CH3CN/H2O (95/5, v/v).
After application of 3 µL of the sample to a ZIC-HILIC column (at 30 °C), the LC gradient programme was as follows: 100% solvent B for 2 min, a linear decrease to 40% solvent B over 16 min, maintenance at 40% solvent B for 9 min, and an increase to 100% solvent B over 1 min. The column was maintained at 100% solvent B for 5 min for column equilibration before each injection. The flow rate was maintained at 200 μL/min. The eluent was directed to the ESI source of the QE-MS from 1.85 min to 20.0 min after sample injection.
The MS parameters were as follows: scan type, full MS in positive-and-negative mode (alternating); scan range, 69–1000 m/z; resolution, 70,000; AGC-target, 3E6; maximum injection time, 200 ms; sheath gas, 30; auxiliary gas, 10; sweep gas, 3; spray voltage, 3.6 kV (positive mode) or 2.5 kV (negative mode); capillary temperature, 320 °C; S-lens RF level, 55.0; and auxiliary gas heater temperature, 120 °C. Annotation and data evaluation: peaks corresponding to the calculated monoisotopic masses (MIM +/− H+ ± 2 mMU) were integrated using TraceFinder software (Thermo Scientific, Bremen, Germany). Materials: Ultrapure water was obtained from a Millipore water purification system (Milli-Q Merck Millipore, Darmstadt, Germany). HPLC–MS solvents, LC–MS NH4OAc, and lamivudine were purchased from Merck (Darmstadt, Germany). The RP18-SPE columns were 50 mg of Strata C18-E (55 µm) in 1-mL tubes (Phenomenex, Aschaffenburg, Germany). The sonifier was a Branson Ultrasonics 250 equipped with a 13-mm sonotrode (Thermo Scientific, Bremen, Germany).
4.6.3. LC-MS System
A Thermo Scientific Dionex UltiMate 3000 UHPLC system linked to a Q Exactive mass spectrometer (QE-MS) equipped with a HESI probe (Thermo Scientific, Bremen, Germany) was used. The samples were analysed with a high-resolution mass spectrometer, allowing the generation of XIC data that were analysed by applying a very narrow m/z margin (+/−3 mMU). The particle filter was a Javelin filter with an ID of 2.1 mm (Thermo Scientific, Bremen, Germany). The UPLC-precolumn was a SeQuant ZIC-HILIC column (5-μm particles, 20 × 2 mm) (Merck, Darmstadt, Germany). The UPLC column was a SeQuant ZIC-HILIC column (3.5-μm particles, 100 × 2.1 mm) (Merck, Darmstadt, Germany).
Raw data analysis and value generation (in short):
LC-MS analyses were carried out in four independent experiments at 24 h, 48 h, 72 h, 96 h, and 120 h, with each value obtained from triplicate measurements. Metabolites were quantified in cell pellets and corresponding supernatants (media) under methionine-supplemented and methionine-free conditions (12 samples per time point in total). The resulting peak areas were normalised against that of lamivudine as an external standard. From this, the mean value and standard deviation were calculated for each triplicate. For better comparisons, the values were converted to percentages. For the values of the media, the control measurement of the medium used was defined as 100%. For the cell pellets, the highest measured value in each test series within an experiment was defined as 100%. From these values, the average mean values from the four experiments were then summarised in the individual tables. For a better overview, the results were rounded to natural numbers and shown as a heatmap. The corresponding colour range is indicated individually under each table. The raw data and results for the two profiles are provided in the
Supplementary Files S1 and S2.
4.7. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA). One-way ANOVA was used to compare and analyse the data of different groups, followed by the Tukey–Kramer multiple comparison test (ns; nonsignificant; * p < 0.05, *** p < 0.001).