Meier–Gorlin Syndrome: Clinical Misdiagnosis, Genetic Testing and Functional Analysis of ORC6 Mutations and the Development of a Prenatal Test
Abstract
:1. Introduction
2. Results
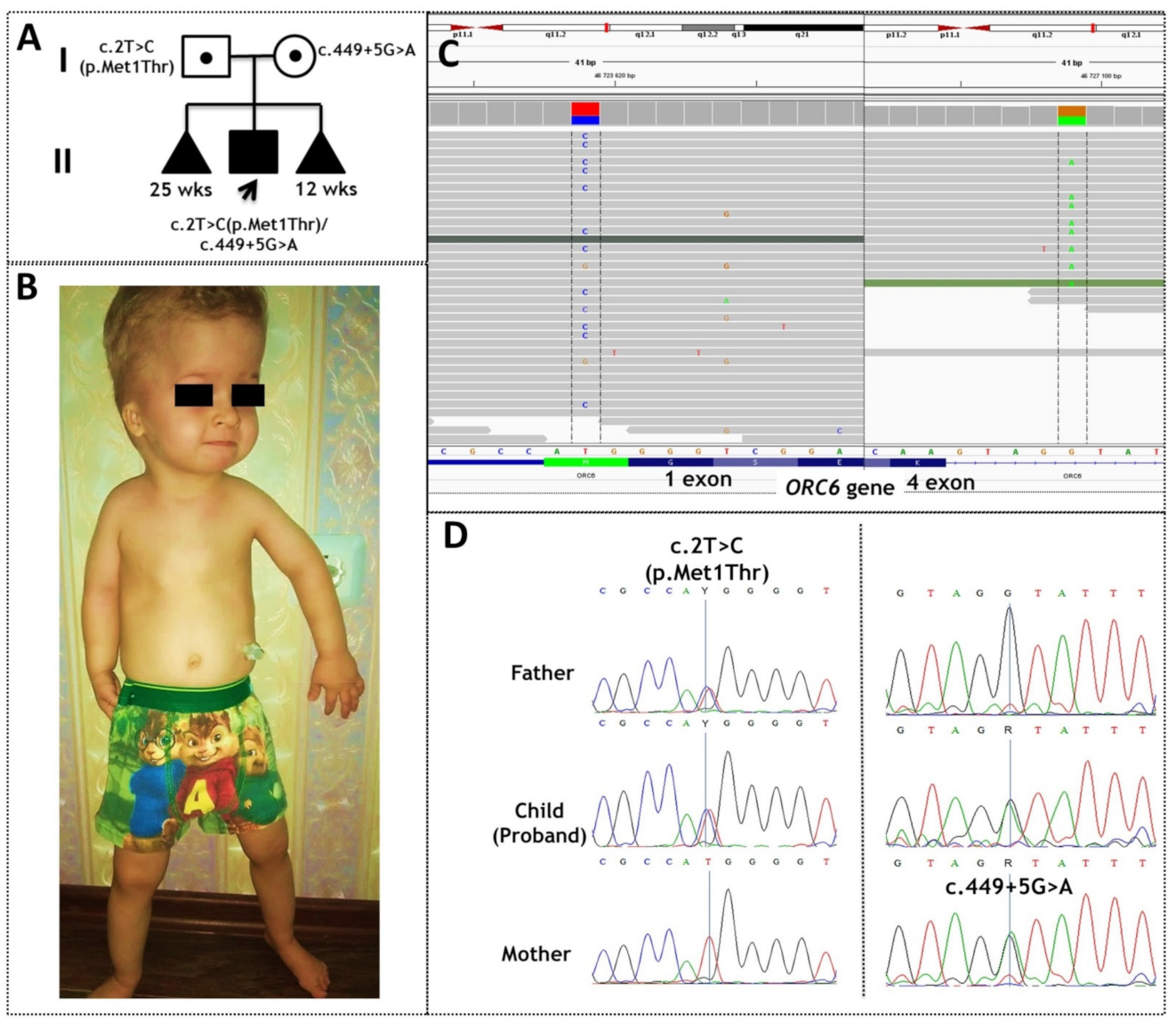
2.1. Case Presentation
2.2. In Silico Analysis of Mutations c.2T>C(p.Met1Thr) and c.449+5G>A of ORC6
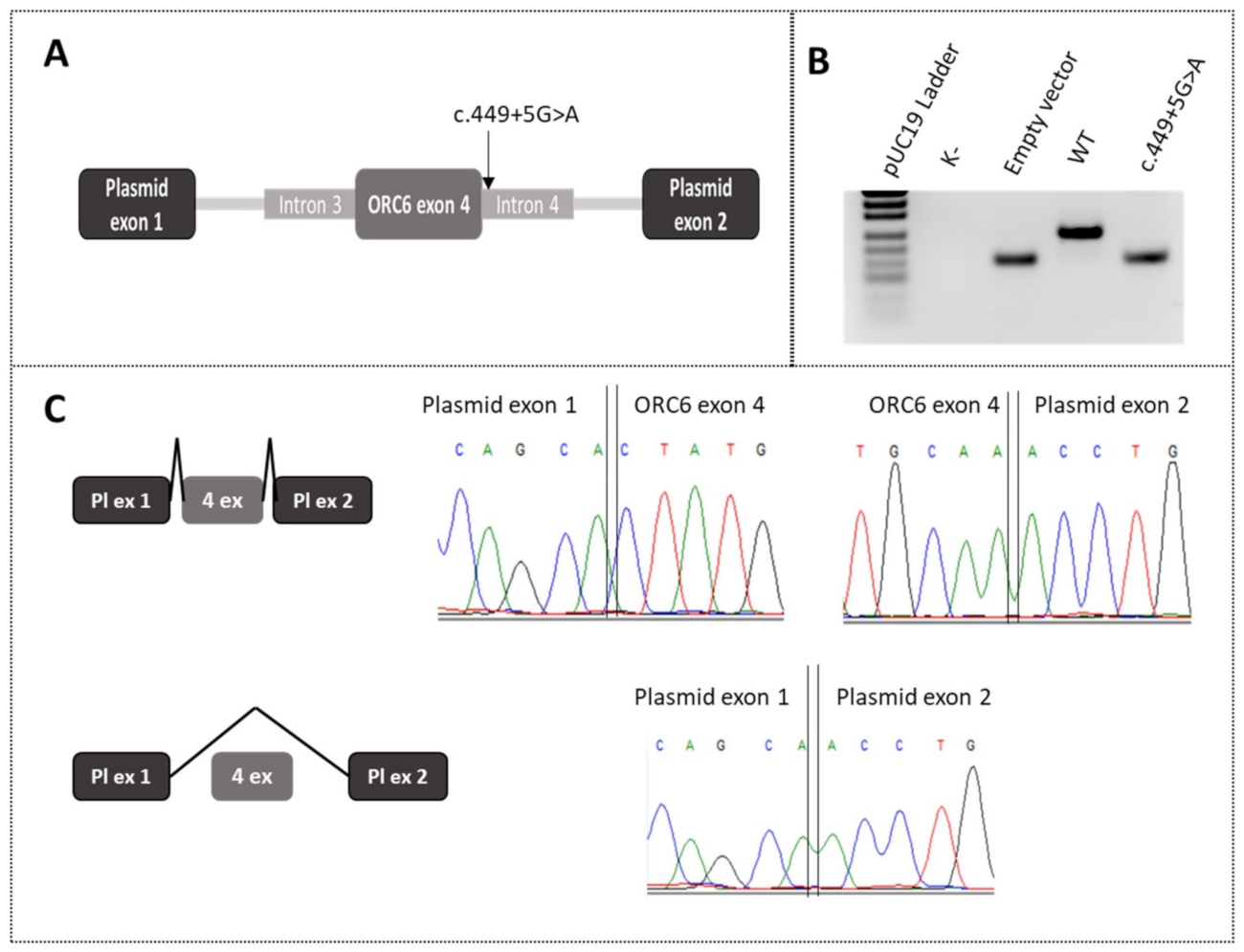
2.3. In Vitro Minigene Assays of the c.449+5G>A Mutation in the ORC6 Gene
2.4. A Prenatal Genetic Diagnostic System
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. The Diagnostic Genetic Testing
4.2.1. Genomic-DNA Preparation
4.2.2. Library Preparation, Clinical Exome Sequencing and Bioinformatic Analysis
4.2.3. PCR and Sanger Sequencing
4.3. In Silico Analysis
4.4. The In Vitro Minigene Assay
4.4.1. Cell Culture
4.4.2. Minigene Constructs
4.4.3. The Splicing Assay
4.5. The Prenatal Genetic Diagnostic System
4.5.1. PCR-RFLP and PAGE
4.5.2. PCR and Fragment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Munnik, S.A.; Hoefsloot, E.H.; Roukema, J.; Schoots, J.; Knoers, N.V.; Brunner, H.G.; Jackson, A.P.; Bongers, E.M. Meier-Gorlin syndrome. Orphanet J. Rare Dis. 2015, 10, 114. [Google Scholar] [CrossRef]
- Shalev, S.A.; Khayat, M.; Etty, D.S.; Elpeleg, O. Further insight into the phenotype associated with a mutation in the ORC6 gene, causing Meier-Gorlin syndrome 3. Am. J. Med. Genet. A 2015, 167, 607–611. [Google Scholar] [CrossRef]
- Li, J.; Ding, Y.; Chang, G.; Cheng, Q.; Li, X.; Wang, J.; Wang, X.; Shen, Y. A boy with Meier-Gorlin syndrome carrying a novel ORC6 mutation and uniparental disomy of chromosome 16. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2017, 34, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, L.S.; Bongers, E.M.; Leitch, A.; Brown, S.; Schoots, J.; Harley, M.E.; Aftimos, S.; Al-Aama, J.Y.; Bober, M.; Brown, P.A.; et al. Mutations in the pre-replication complex cause Meier-Gorlin syndrome. Nat. Genet. 2011, 43, 356–359. [Google Scholar] [CrossRef] [PubMed]
- de Munnik, S.A.; Bicknell, L.S.; Aftimos, S.; Al-Aama, J.Y.; van Bever, Y.; Bober, M.B.; Clayton-Smith, J.; Edrees, A.Y.; Feingold, M.; Fryer, A.; et al. Meier-Gorlin syndrome genotype-phenotype studies: 35 individuals with pre-replication complex gene mutations and 10 without molecular diagnosis. Eur. J. Hum. Genet. 2012, 20, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Nabais, S.M.J.; Miller, K.A.; McQuaid, M.; Koelling, N.; Wilkie, A.O.M.; Wurtele, H.; de Brouwer, A.P.M.; Oliveira, J. Biallelic GINS2 variant p.(Arg114Leu) causes Meier-Gorlin syndrome with craniosynostosis. J. Med. Genet. 2021, 59, 776–780, jmedgenet-2020-107572. [Google Scholar] [CrossRef] [PubMed]
- Nerakh, G.; Vineeth, V.S.; Tallapaka, K.; Nair, L.; Dalal, A.; Aggarwal, S. Microcephalic primordial dwarfism with predominant Meier-Gorlin phenotype, ichthyosis, and multiple joint deformities-Further expansion of DONSON Cell Cycle-opathy phenotypic spectrum. Am. J. Med. Genet. A 2022, 188, 2139–2146. [Google Scholar] [CrossRef]
- Knapp, K.M.; Jenkins, D.E.; Sullivan, R.; Harms, F.L.; von Elsner, L.; Ockeloen, C.W.; de Munnik, S.; Bongers, E.M.H.F.; Murray, J.; Pachter, N.; et al. MCM complex members MCM3 and MCM7 are associated with a phenotypic spectrum from Meier-Gorlin syndrome to lipodystrophy and adrenal insufficiency. Eur. J. Hum. Genet. 2021, 29, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Knapp, K.M.; Fellows, B.; Aggarwal, S.; Dalal, A.; Bicknell, L.S. A synonymous variant in a non-canonical exon of CDC45 disrupts splicing in two affected sibs with Meier-Gorlin syndrome with craniosynostosis. Eur. J. Med. Genet. 2021, 64, 104182. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Reddy, C.; Saini, A.G.; Vyas, S. Meier-Gorlin syndrome presenting as early infantile epileptic encephalopathy. BMJ Case Rep. 2020, 13, e235468. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, A.L.; Kliszczak, M.; Cooper, F.; Murray, J.; Sanchez-Pulido, L.; Twigg, S.R.; Goriely, A.; McGowan, S.J.; Miller, K.A.; Taylor, I.B.; et al. Mutations in CDC45, Encoding an Essential Component of the Pre-initiation Complex, Cause Meier-Gorlin Syndrome and Craniosynostosis. Am. J. Hum. Genet. 2016, 99, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Burrage, L.C.; Charng, W.L.; Eldomery, M.K.; Willer, J.R.; Davis, E.E.; Lugtenberg, D.; Zhu, W.; Leduc, M.S.; Akdemir, Z.C.; Azamian, M.; et al. De Novo GMNN Mutations Cause Autosomal-Dominant Primordial Dwarfism Associated with Meier-Gorlin Syndrome. Am. J. Hum. Genet. 2015, 97, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Bellelli, R.; Boulton, S.J. Spotlight on the Replisome: Aetiology of DNA Replication-Associated Genetic Diseases. Trends Genet. 2021, 37, 317–336. [Google Scholar] [CrossRef]
- Schmit, M.; Bielinsky, A.K. Congenital Diseases of DNA Replication: Clinical Phenotypes and Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 911. [Google Scholar] [CrossRef] [PubMed]
- ORC6—Origin Recognition Complex Subunit 6—Homo Sapiens (Human)—ORC6 Gene & Protein. Available online: https://www.uniprot.org/uniprot/Q9Y5N6 (accessed on 22 April 2022).
- GTEx Portal: ORC6. Available online: https://www.gtexportal.org/home/gene/ORC6 (accessed on 22 April 2022).
- Gene: ORC6 (ENSG00000091651)—Splice Variants—Homo_Sapiens—Ensembl Genome Browser 106. Available online: http://www.ensembl.org/Homo_sapiens/Gene/Splice?db=core;g=ENSG00000091651;r=16:46689643-46698394 (accessed on 22 April 2022).
- gnomAD SV: ORC6. Available online: https://gnomad.broadinstitute.org/gene/ORC6?dataset=gnomad_sv_r2_1 (accessed on 22 April 2022).
- gnomAD v3.1: ORC6. Available online: https://gnomad.broadinstitute.org/gene/ENSG00000091651?dataset=gnomad_r3 (accessed on 22 April 2022).
- ClinVar. Available online: https://www.ncbi.nlm.nih.gov/clinvar (accessed on 22 April 2022).
- Xu, N.; You, Y.; Liu, C.; Balasov, M.; Lun, L.T.; Geng, Y.; Fung, C.P.; Miao, H.; Tian, H.; Choy, T.T.; et al. Structural basis of DNA replication origin recognition by human Orc6 protein binding with DNA. Nucleic Acids Res. 2020, 48, 11146–11161. [Google Scholar] [CrossRef] [PubMed]
- Balasov, M.; Akhmetova, K.; Chesnokov, I. Humanized Drosophila Model of the Meier-Gorlin Syndrome Reveals Conserved and Divergent Features of the Orc6 Protein. Genetics 2020, 216, 995–1007. [Google Scholar] [CrossRef]
- De Vries, J.; Yntema, J.L.; van Die, C.E.; Crama, N.; Cornelissen, E.A.M.; Hamel, B.C.J. Jeune syndrome: Description of 13 cases and a proposal for follow-up protocol. Eur. J. Pediatr. 2009, 169, 77–88. [Google Scholar] [CrossRef]
- Balasov, M.; Akhmetova, K.; Chesnokov, I. Drosophila model of Meier-Gorlin syndrome based on the mutation in a conserved C-Terminal domain of Orc6. Am. J Med. Genet. A 2015, 167, 2533–2540. [Google Scholar] [CrossRef]
- Bell, S.P.; Stillman, B. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature 1992, 357, 128–134. [Google Scholar] [CrossRef] [PubMed]
- DePamphilis, M.L.; Blow, J.J.; Ghosh, S.; Saha, T.; Noguchi, K.; Vassilev, A. Regulating the licensing of DNA replication origins in metazoa. Curr. Opin. Cell Biol. 2006, 18, 231–239. [Google Scholar] [CrossRef]
- Siddiqui, K.; Stillman, B. ATP-dependent assembly of the human origin recognition complex. J. Biol. Chem. 2007, 282, 32370–32383. [Google Scholar] [CrossRef] [PubMed]
- Balasov, M.; Huijbregts, R.P.; Chesnokov, I. Functional analysis of an Orc6 mutant in Drosophila. Proc. Natl. Acad. Sci. USA 2009, 106, 10672–10677. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, S.G.; Prasanth, K.V.; Stillman, B. Orc6 involved in DNA replication, chromosome segregation, and cytokinesis. Science 2002, 297, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Alver, R.C.; Chadha, G.S.; Blow, J.J. The contribution of dormant origins to genome stability: From cell biology to human genetics. DNA Repair 2014, 19, 182–189. [Google Scholar] [CrossRef]
- Stiff, T.; Alagoz, M.; Alcantara, D.; Outwin, E.; Brunner, H.G.; Bongers, E.M.; O’Driscoll, M.; Jeggo, P.A. Deficiency in origin licensing proteins impairs cilia formation: Implications for the aetiology of Meier-Gorlin syndrome. PLoS Genet. 2013, 9, e1003360. [Google Scholar] [CrossRef]
- Maerz, L.D.; Casar, T.T.; Gerhards, J.; Donow, C.; Jeggo, P.A.; Philipp, M. Analysis of cilia dysfunction phenotypes in zebrafish embryos depleted of Origin recognition complex factors. Eur. J. Hum. Genet. 2019, 27, 772–782. [Google Scholar] [CrossRef]
- Thornton, G.K.; Woods, C.G. Primary microcephaly: Do all roads lead to Rome? Trends Genet. 2009, 25, 501–510. [Google Scholar] [CrossRef]
- Gabriel, E.; Ramani, A.; Altinisik, N.; Gopalakrishnan, J. Human Brain Organoids to Decode Mechanisms of Microcephaly. Front. Cell. Neurosci. 2020, 14, 115. [Google Scholar] [CrossRef]
- Nigg, E.A.; Raff, J.W. Centrioles, centrosomes, and cilia in health and disease. Cell 2009, 139, 663–678. [Google Scholar] [CrossRef]
- Schock, E.N.; Brugmann, S.A. Discovery, Diagnosis, and Etiology of Craniofacial Ciliopathies. Cold Spring Harb. Perspect. Biol. 2017, 9, a028258. [Google Scholar] [CrossRef]
- Tabler, J.M.; Rice, C.P.; Liu, K.J.; Wallingford, J.B. A novel ciliopathic skull defect arising from excess neural crest. Dev. Biol. 2016, 417, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Barrell, W.B.; Al-Lami, H.A.; Goos, J.A.C.; Swagemakers, S.M.A.; van Dooren, M.; Torban, E.; van der Spek, P.J.; Mathijssen, I.M.J.; Liu, K.J. Identification of a novel variant of the ciliopathic gene FUZZY associated with craniosynostosis. Eur. J. Hum. Genet. 2022, 30, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Balk, K.; Biesecker, L.G. The clinical atlas of Greig cephalopolysyndactyly syndrome. Am. J. Med. Genet. A 2008, 146, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.A.; Jenkins, D.; Vasudevan, P.C.; Kirchhoff, M.; Skovby, F.; Rieubland, C.; Gallati, S.; Rittinger, O.; Kroisel, P.M.; Johnson, D.; et al. Metopic and sagittal synostosis in Greig cephalopolysyndactyly syndrome: Five cases with intragenic mutations or complete deletions of GLI3. Eur. J. Hum. Genet. 2011, 19, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Elson, E.; Perveen, R.; Donnai, D.; Wall, S.; Black, G.C. De novo GLI3 mutation in acrocallosal syndrome: Broadening the phenotypic spectrum of GLI3 defects and overlap with murine models. J. Med. Genet. 2002, 39, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Serpieri, V.; D’Abrusco, F.; Dempsey, J.C.; Cheng, Y.H.; Arrigoni, F.; Baker, J.; Battini, R.; Bertini, E.S.; Borgatti, R.; Christman, A.K.; et al. SUFU haploinsufficiency causes a recognisable neurodevelopmental phenotype at the mild end of the Joubert syndrome spectrum. J. Med. Genet. 2021, 1–7. [Google Scholar] [CrossRef]
- Barba, M.; Di, P.L.; Massimi, L.; Geloso, M.C.; Frassanito, P.; Caldarelli, M.; Michetti, F.; Della, L.S.; Romitti, P.A.; Di, R.C.; et al. BBS9 gene in nonsyndromic craniosynostosis: Role of the primary cilium in the aberrant ossification of the suture osteogenic niche. Bone 2018, 112, 58–70. [Google Scholar] [CrossRef]
- Justice, C.M.; Musolf, A.M.; Cuellar, A.; Lattanzi, W.; Simeonov, E.; Kaneva, R.; Paschall, J.; Cunningham, M.; Wilkie, A.O.M.; Wilson, A.F.; et al. Targeted Sequencing of Candidate Regions Associated with Sagittal and Metopic Nonsyndromic Craniosynostosis. Genes 2022, 13, 816. [Google Scholar] [CrossRef]
- Hartill, V.; Szymanska, K.; Sharif, S.M.; Wheway, G.; Johnson, C.A. Meckel-Gruber Syndrome: An Update on Diagnosis, Clinical Management, and Research Advances. Front. Pediatr. 2017, 5, 244. [Google Scholar] [CrossRef]
- Vogelezang, S.; Bradfield, J.P.; Grant, S.F.A.; Felix, J.F.; Jaddoe, V.W.V. Genetics of early-life head circumference and genetic correlations with neurological, psychiatric and cognitive outcomes. BMC Med. Genom. 2022, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Banne, E.; Atawneh, O.; Henneke, M.; Brockmann, K.; Gärtner, J.; Elpeleg, O.; Edvardson, S. West syndrome, microcephaly, grey matter heterotopia and hypoplasia of corpus callosum due to a novel ARFGEF2 mutation. J. Med. Genet. 2013, 50, 772–775. [Google Scholar] [CrossRef]
- Yilmaz, S.; Gokben, S.; Serdaroglu, G.; Eraslan, C.; Mancini, G.M.; Tekin, H.; Tekgul, H. The expanding phenotypic spectrum of ARFGEF2 gene mutation: Cardiomyopathy and movement disorder. Brain Dev. 2016, 38, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishna, U.; Bornholdt, D.; Scott, H.S.; Patel, U.C.; Rossier, C.; Engel, H.; Bottani, A.; Chandal, D.; Blouin, J.L.; Solanki, J.V.; et al. The phenotypic spectrum of GLI3 morphopathies includes autosomal dominant preaxial polydactyly type-IV and postaxial polydactyly type-A/B.; No phenotype prediction from the position of GLI3 mutations. Am. J. Hum. Genet. 1999, 65, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.P.; Connor, E.C.; Veltmaat, J.M.; Lana-Elola, E.; Veistinen, L.; Tanimoto, Y.; Bellusci, S.; Rice, R. Gli3Xt-J/Xt-J mice exhibit lambdoid suture craniosynostosis which results from altered osteoprogenitor proliferation and differentiation. Hum. Mol. Genet. 2010, 19, 3457–3467. [Google Scholar] [CrossRef]
- Veistinen, L.; Takatalo, M.; Tanimoto, Y.; Kesper, D.A.; Vortkamp, A.; Rice, D.P. Loss-of-Function of Gli3 in Mice Causes Abnormal Frontal Bone Morphology and Premature Synostosis of the Interfrontal Suture. Front. Physiol. 2012, 3, 121. [Google Scholar] [CrossRef]
- Tamayo-Orrego, L.; Gallo, D.; Racicot, F.; Bemmo, A.; Mohan, S.; Ho, B.; Salameh, S.; Hoang, T.; Jackson, A.P.; Brown, G.W.; et al. Sonic hedgehog accelerates DNA replication to cause replication stress promoting cancer initiation in medulloblastoma. Nat. Cancer 2020, 1, 840–854. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Isolation and Quantification of DNA. Cold Spring Harb. Protoc. 2018, 6, pdb-top093336. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Barbitoff, Y.A.; Khmelkova, D.N.; Pomerantseva, E.A.; Slepchenkov, A.V.; Zubashenko, N.A.; Mironova, I.V.; Kaimonov, V.S.; Polev, D.E.; Tsay, V.V.; Glotov, A.S.; et al. Expanding the Russian allele frequency reference via cross-laboratory data integration: Insights from 6,096 exome samples. medRxiv 2021. [Google Scholar] [CrossRef]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Aguilera, M.A.; Meyer, R.; Massouras, A. VarSome: The human genomic variant search engine. Bioinformatics 2018, 35, 1978–1980. [Google Scholar] [CrossRef] [PubMed]
- Reese, M.G.; Eeckman, F.H.; Kulp, D.; Haussler, D. Improved Splice Site Detection in Genie. J. Comput. Biol. 1997, 4, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Yeo, G.; Burge, C.B. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J. Comput. Biol. 2004, 11, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Desmet, F.O.; Hamroun, D.; Lalande, M.; Collod-Béroud, G.; Claustres, M.; Béroud, C. Human Splicing Finder: An online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009, 37, e67. [Google Scholar] [CrossRef]
- varSEAK—Shared Experience And Knowledge. Available online: https://varseak.bio (accessed on 22 April 2022).
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef]

| Locus, Distance from Gene, Repeat | Child (Proband) 1 | Father 1 | Mother 1 | |
|---|---|---|---|---|
| STR1 | 0.01 Mbp, (AC)n | 308/310 | 308/308 | 308/310 |
| ORC6 | mutations | c.2T>C/c.449+5G>A | c.2T>C/N | N/c.449+5G>A |
| Exon 7, (AGAT)n | 267/271 | 267/263 | 279/271 | |
| STR2 | 0.52 Mbp, (AGAT)n | 99/103 | 99/90 | 107/103 |
| Features | ORC6-Based MGS | Jeune Syndrome | |
|---|---|---|---|
| Our Patient 1 (Male, 3 y.o.) | 7 Patients 1 (5 Males and 2 Females; 3 y 10 m to 15 y 5 m) [5] | 13 Patients 1 (8 Males and 5 Females; 5 Weeks to 22 y.o.) [23] | |
| Main skeletal features | intrauterine growth retardation; short stature; tower head; short neck; short limbs; narrow chest; brachydactyly. | intrauterine growth retardation; short stature; patellar aplasia/hypoplasia; slender ribs and long bones; microcephaly; clinodactyly; contractures/clubfeet. | short stature; short limbs; short-rib dysplasia with narrow chest (persistent respiratory manifestations); unusually shaped pelvis; extra fingers and/or toes. |
| Facial features (change with age) | bilateral microtia; high forehead; downslanted palpebral fissures; narrow nose; micrognathia. | bilateral microtia; malformed ears; high forehead; downslanted palpebral fissures; micrognathism with full lips and small mouth; accentuated nasolabial folds; convex nasal profile. | no. |
| Additional clinical features | respiratory and feeding problems during infancy and gastrostomy; normal intellect; muscular hypotonia; moderate internal hydrocephalus; enlargement of subarachnoid spaces of frontal lobes. | respiratory and feeding problems during infancy; nasogastric feeding/gastrostomy; normal intellect but delayed motor or speech development; abnormal genitalia (cryptorchidism or small testes/mammary hypoplasia or hypoplastic labia minora/majora). | renal insufficiency; abnormality of retinal pigmentation; abnormality of the liver. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarenko, M.S.; Viakhireva, I.V.; Skoblov, M.Y.; Soloveva, E.V.; Sleptcov, A.A.; Nazarenko, L.P. Meier–Gorlin Syndrome: Clinical Misdiagnosis, Genetic Testing and Functional Analysis of ORC6 Mutations and the Development of a Prenatal Test. Int. J. Mol. Sci. 2022, 23, 9234. https://doi.org/10.3390/ijms23169234
Nazarenko MS, Viakhireva IV, Skoblov MY, Soloveva EV, Sleptcov AA, Nazarenko LP. Meier–Gorlin Syndrome: Clinical Misdiagnosis, Genetic Testing and Functional Analysis of ORC6 Mutations and the Development of a Prenatal Test. International Journal of Molecular Sciences. 2022; 23(16):9234. https://doi.org/10.3390/ijms23169234
Chicago/Turabian StyleNazarenko, Maria S., Iuliia V. Viakhireva, Mikhail Y. Skoblov, Elena V. Soloveva, Aleksei A. Sleptcov, and Ludmila P. Nazarenko. 2022. "Meier–Gorlin Syndrome: Clinical Misdiagnosis, Genetic Testing and Functional Analysis of ORC6 Mutations and the Development of a Prenatal Test" International Journal of Molecular Sciences 23, no. 16: 9234. https://doi.org/10.3390/ijms23169234
APA StyleNazarenko, M. S., Viakhireva, I. V., Skoblov, M. Y., Soloveva, E. V., Sleptcov, A. A., & Nazarenko, L. P. (2022). Meier–Gorlin Syndrome: Clinical Misdiagnosis, Genetic Testing and Functional Analysis of ORC6 Mutations and the Development of a Prenatal Test. International Journal of Molecular Sciences, 23(16), 9234. https://doi.org/10.3390/ijms23169234








