Intermittent Hypoxia on the Attenuation of Induced Nasal Allergy and Allergic Asthma by MAPK Signaling Pathway Downregulation in a Mice Animal Model
Abstract
:1. Introduction
2. Results
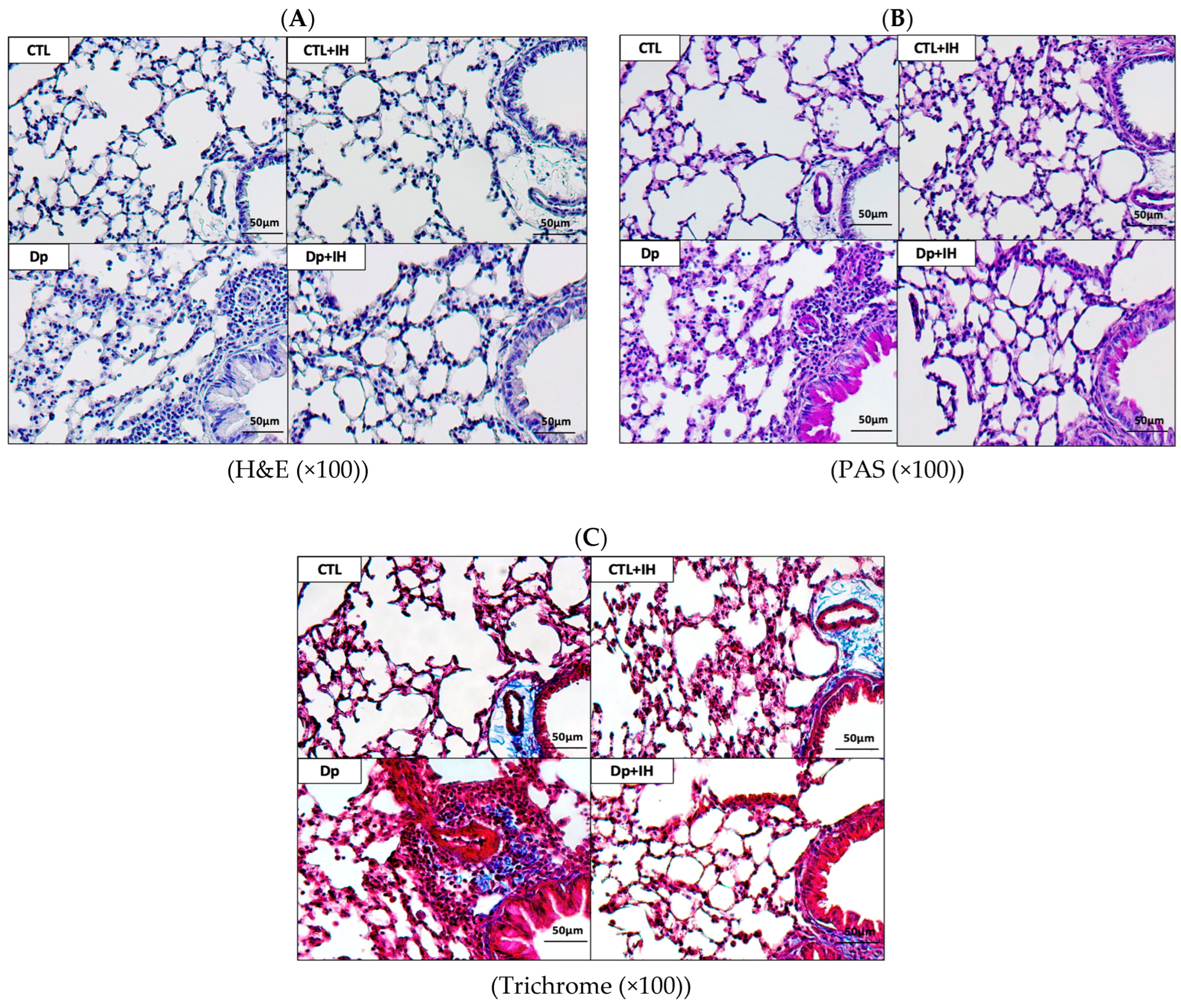
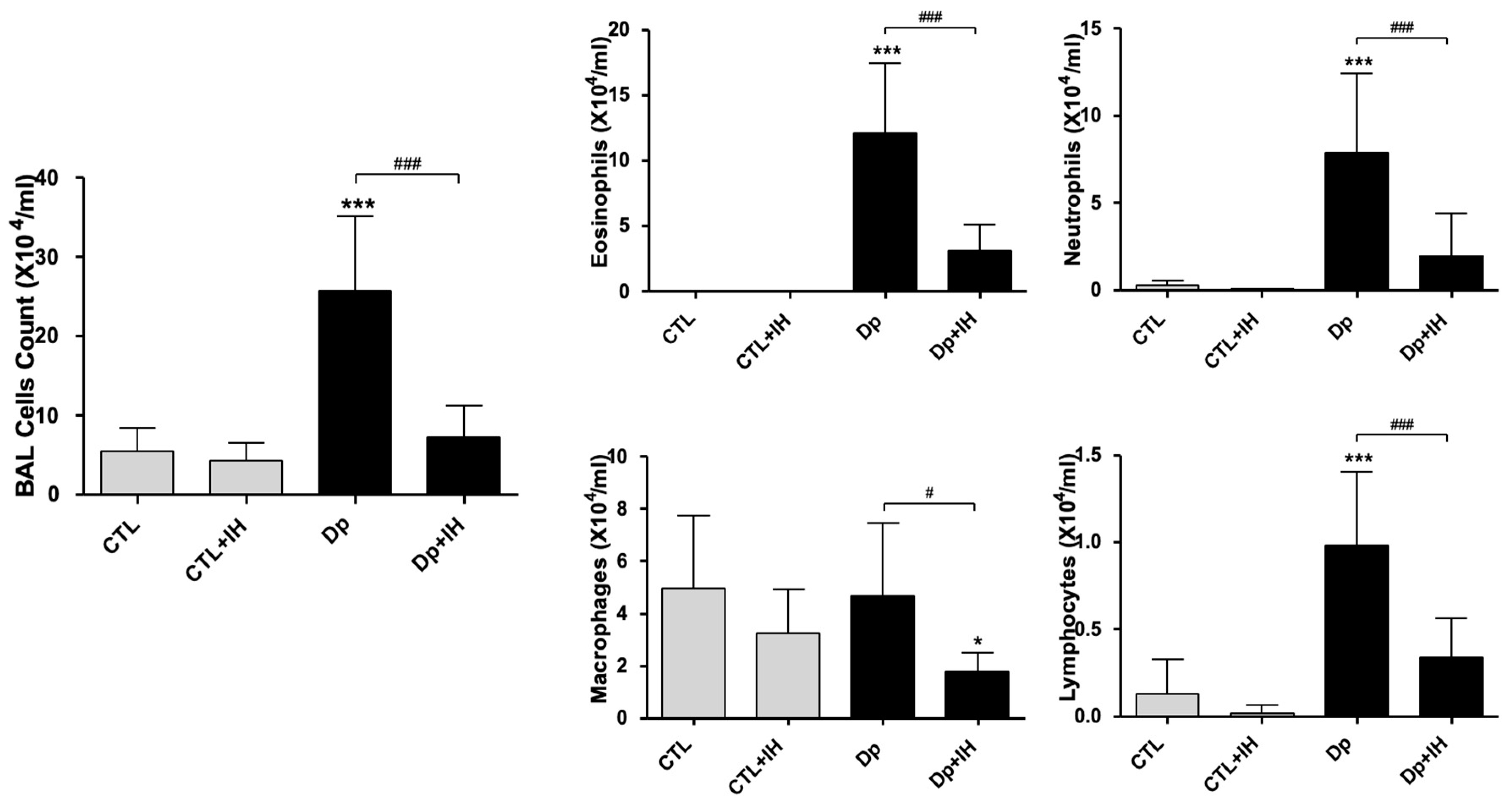
2.1. IH Reduced Airway Inflammation in Mice with Dp-Induced Allergic Asthma
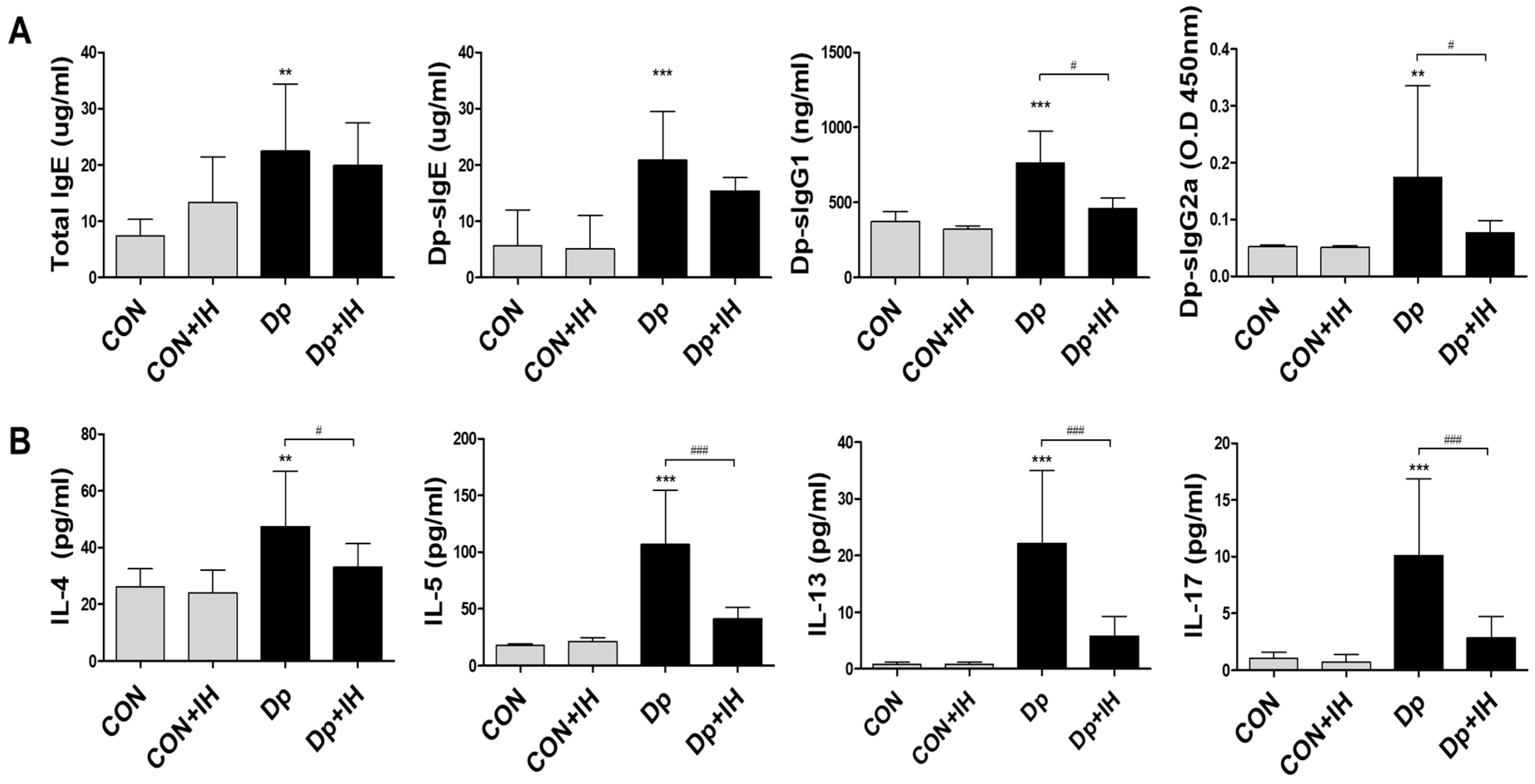
2.2. Intermittent Hypoxia Inhibited Cytokine Production in Allergic-Induced Asthma Model
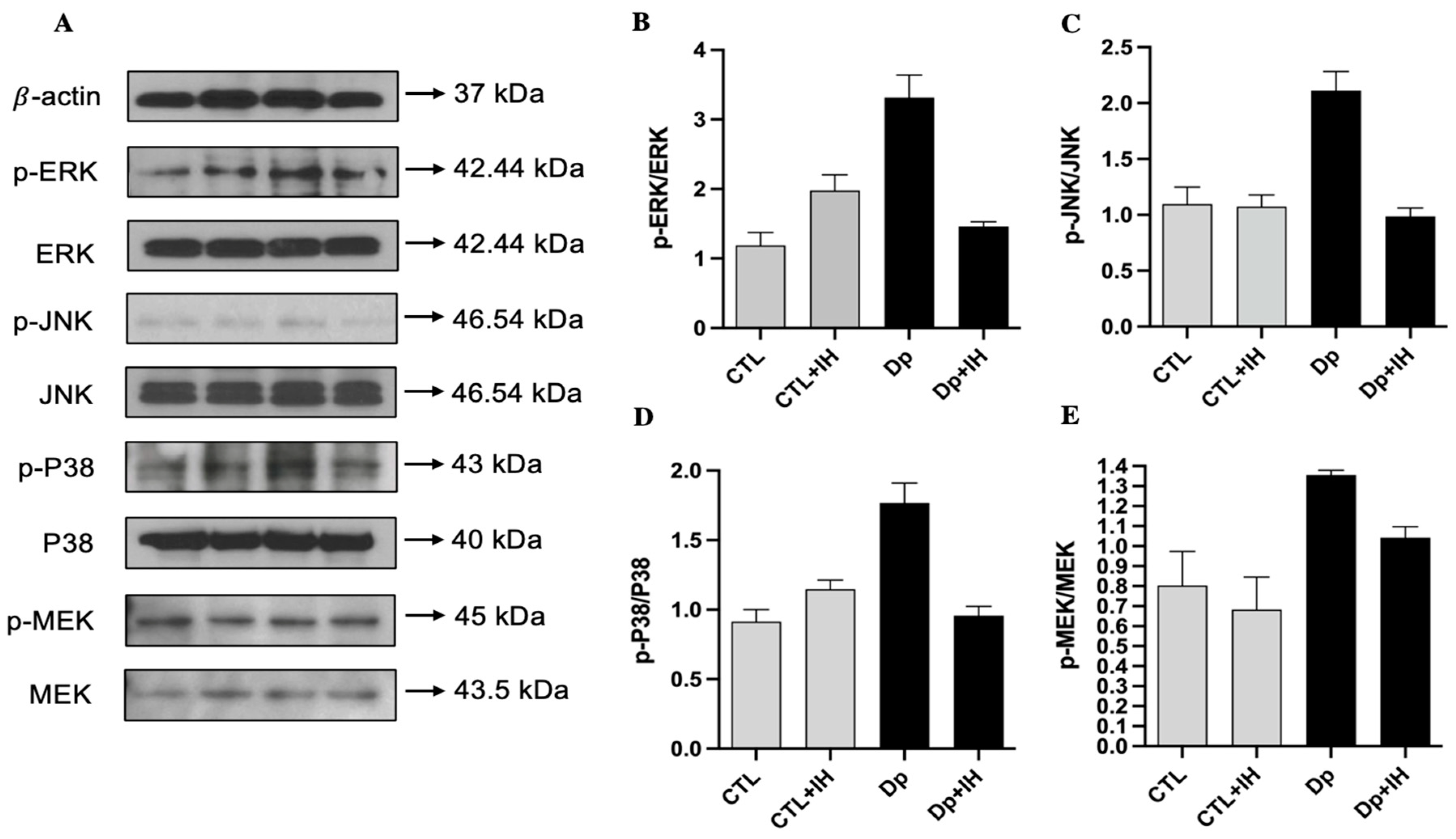
2.3. IH Inactivated the MAPK Pathway in Dp-Induced Asthma
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Induction of Allergic Asthma
4.3. Intermittent Hypoxia Condition
4.4. BALF Analysis
4.5. ELISA
4.6. Histopathology
4.7. Protein Extraction and Western Blot
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Athari, S.S. Targeting cell signaling in allergic asthma. Signal Transduct. Target. Ther. 2019, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Kumar, M.; Mabalirajan, U.; Pattnaik, B.; Aggarwal, S.; Singh, R.; Singh, S.; Mukerji, M.; Ghosh, B.; Agrawal, A. Hypoxia Response in Asthma Differential Modulation on Inflammation and Epithelial Injury. Am. J. Respir. Cell Mol. Biol. 2012, 47, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Yepez, S.; Baay-Guzman, G.J.; Bebenek, I.G.; Hernandez-Pando, R.; Vega, M.I.; Chi, L.; Riedl, M.; Diaz-Sanchez, D.; Kleerup, E.; Tashkin, D.P.; et al. Hypoxia Inducible Factor promotes murine allergic airway inflammation and is increased in asthma and rhinitis. Allergy 2011, 66, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Dale, E.A.; Mabrouk, F.B.; Mitchell, G.S. Unexpected Benefits of Intermittent Hypoxia: Enhanced Respiratory and Nonrespiratory Motor Function. Physiology 2014, 29, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Opazo, A.; Mitchell, G.S. Therapeutic potential of intermittent hypoxia: A matter of dose. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014, 307, R1181–R1197. [Google Scholar] [CrossRef] [PubMed]
- In, S.M.; Park, D.Y.; Lee, K.I.; Gu, G.; Kim, H.J. The effects of intermittent hypoxia on human nasal mucosa. Sleep Breath 2021, 25, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecinska, A.; Budzinska, A.; Mojzych, M.; Kontek, R. Metastasis and MAPK Pathways. Int. J. Mol. Sci. 2022, 23, 3847. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Gu, X.; Shen, H.J.; Shi, Y.H.; Li, Y.; Zhang, J.; Chen, Y.Y.; Chen, Z.H.; Ma, J.Y.; Li, Q.Y. Chronic Intermittent Hypoxia Reduces the Effects of Glucosteroid in Asthma via Activating the p38 MAPK Signaling Pathway. Front. Physiol. 2021, 12, 703281. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Fields, R.D.; Baker, T.; Fletcher, E.C. Intermittent hypoxia: Cell to system. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2001, 281, L524–L528. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta-Mol. Cell Res. 2011, 1813, 1619–1633. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2012, 76, 496. [Google Scholar] [CrossRef]
- McCain, J. The MAPK (ERK) Pathway: Investigational Combinations for the Treatment of BRAF-Mutated Metastatic Melanoma. Pharm. Ther. 2013, 38, 96–108. [Google Scholar]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [PubMed]
- Safa, A.; Abak, A.; Shoorei, H.; Taheri, M.; Ghafouri-Fard, S. MicroRNAs as regulators of ERK/MAPK pathway: A comprehensive review. Biomed. Pharm. 2020, 132, 110853. [Google Scholar] [CrossRef]
- Verweij, M.M.; De Knop, K.J.; Bridts, C.H.; De Clerck, L.S.; Stevens, W.J.; Ebo, D.G. P38 mitogen-activated protein kinase signal transduction in the diagnosis and follow up of immunotherapy of wasp venom allergy. Cytom. B Clin. Cytom. 2010, 78, 302–307. [Google Scholar] [CrossRef]
- Sang, N.; Stiehl, D.P.; Bohensky, J.; Leshchinsky, I.; Srinivas, V.; Caro, J. MAPK signaling up-regulates the activity of hypoxia-inducible factors by its effects on p300. J. Biol. Chem. 2003, 278, 14013–14019. [Google Scholar] [CrossRef] [PubMed]
- Low, T.; Lin, T.Y.; Lin, J.Y.; Lai, C.J. Airway hyperresponsiveness induced by intermittent hypoxia in rats. Respir. Physiol. Neurobiol. 2022, 295, 103787. [Google Scholar] [CrossRef] [PubMed]
- Zeyen, L.; Seternes, O.M.; Mikkola, I. Crosstalk between p38 MAPK and GR Signaling. Int. J. Mol. Sci. 2022, 23, 3322. [Google Scholar] [CrossRef] [PubMed]
- Broytman, O.; Braun, R.K.; Morgan, B.J.; Pegelow, D.F.; Hsu, P.N.; Mei, L.S.; Koya, A.K.; Eldridge, M.; Teodorescu, M. Effects of chronic intermittent hypoxia on allergen-induced airway inflammation in rats. Am. J. Respir. Cell Mol. Biol. 2015, 52, 162–170. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultonov, D.; Kim, Y.H.; Park, H.; Kim, K.-S. Intermittent Hypoxia on the Attenuation of Induced Nasal Allergy and Allergic Asthma by MAPK Signaling Pathway Downregulation in a Mice Animal Model. Int. J. Mol. Sci. 2022, 23, 9235. https://doi.org/10.3390/ijms23169235
Sultonov D, Kim YH, Park H, Kim K-S. Intermittent Hypoxia on the Attenuation of Induced Nasal Allergy and Allergic Asthma by MAPK Signaling Pathway Downregulation in a Mice Animal Model. International Journal of Molecular Sciences. 2022; 23(16):9235. https://doi.org/10.3390/ijms23169235
Chicago/Turabian StyleSultonov, Doston, Young Hyo Kim, Hyelim Park, and Kyu-Sung Kim. 2022. "Intermittent Hypoxia on the Attenuation of Induced Nasal Allergy and Allergic Asthma by MAPK Signaling Pathway Downregulation in a Mice Animal Model" International Journal of Molecular Sciences 23, no. 16: 9235. https://doi.org/10.3390/ijms23169235
APA StyleSultonov, D., Kim, Y. H., Park, H., & Kim, K.-S. (2022). Intermittent Hypoxia on the Attenuation of Induced Nasal Allergy and Allergic Asthma by MAPK Signaling Pathway Downregulation in a Mice Animal Model. International Journal of Molecular Sciences, 23(16), 9235. https://doi.org/10.3390/ijms23169235





