The Modification of Offspring Stress-Related Behavior and the Expression of Drd1, Drd2, and Nr3c1 by a Western-Pattern Diet in Mus Musculus
Abstract
:1. Introduction
Current Study
2. Materials and Methods
2.1. Animals
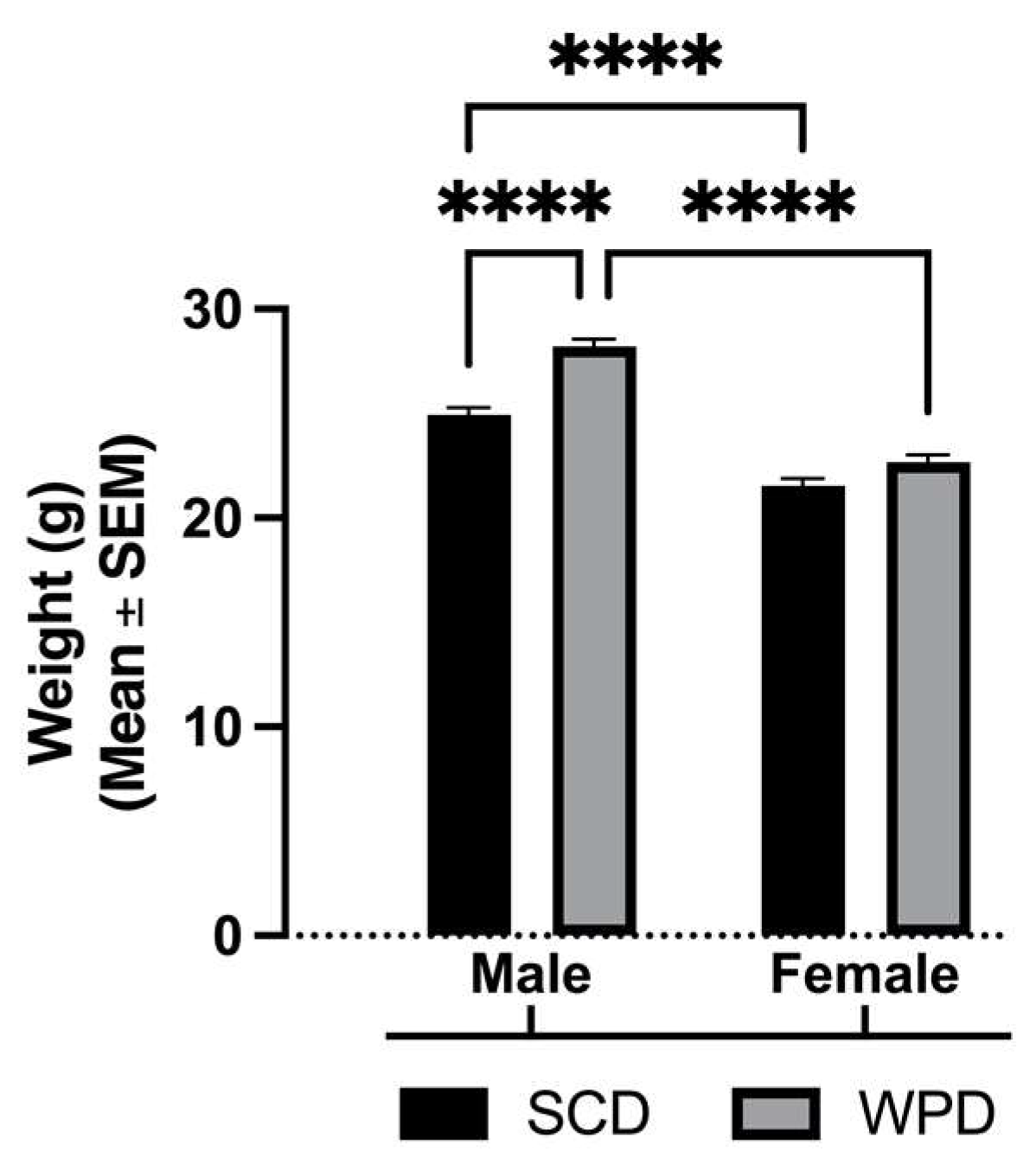
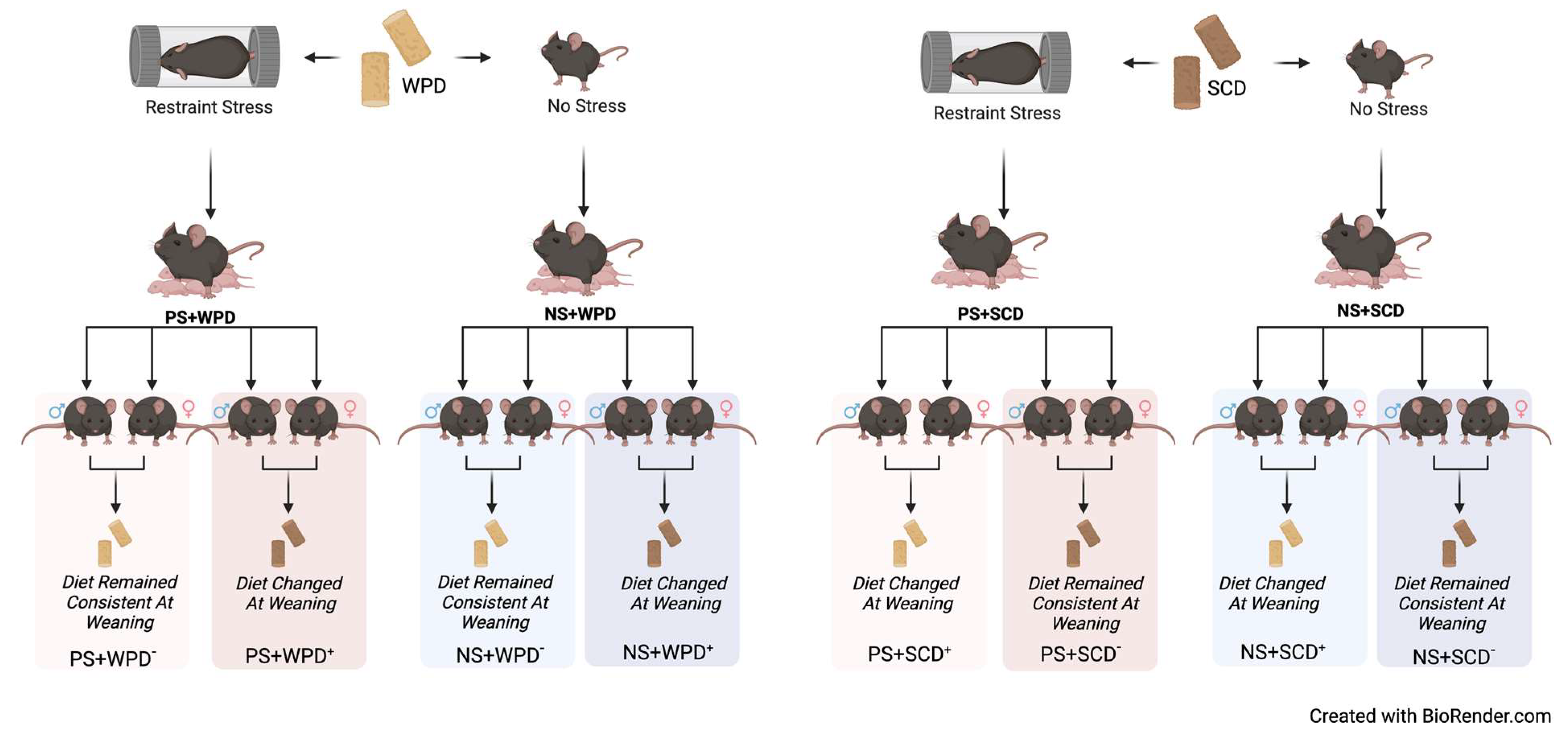
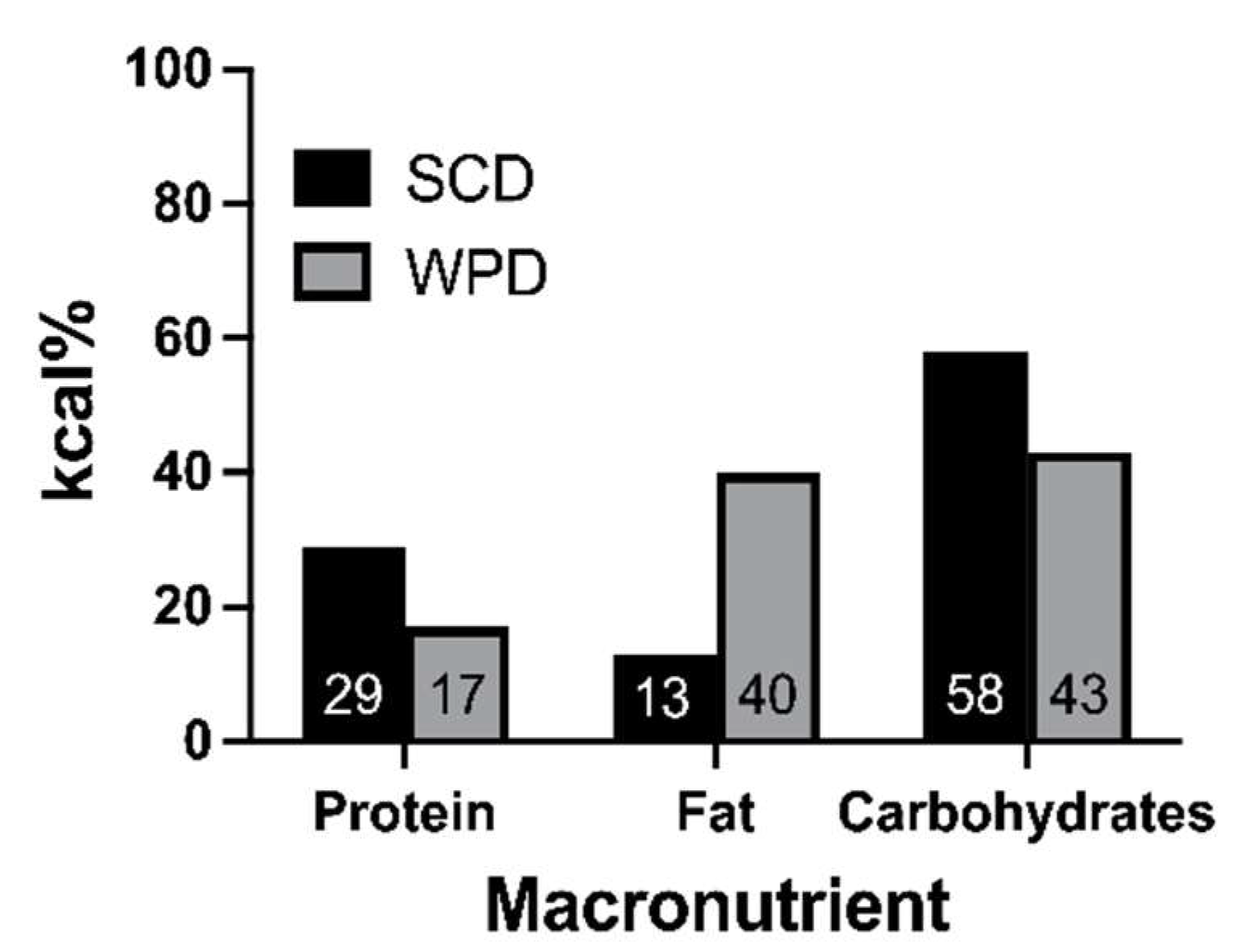
2.2. Experimental Design and Treatment Groups
2.3. Maternal Behavior
2.4. Offspring Behavior
2.5. Neural Tissue Collection
2.6. RNA Extraction and Quantitative Real-Time PCR
3. Results
3.1. Maternal Behavior
3.1.1. SCD-Fed Stressed Dams Spend Less Time in Nest with Pups Than WPD-Fed Stressed Dams and Non-Stressed Dams
3.1.2. Stressed Dams Display a Higher Latency to Retrieve Displaced Pups, but a WPD Significantly Decreases Latency to Retrieve Pups
3.2. Open-Field Behavior in Adult Offspring
3.2.1. The Offspring of WPD-Fed Stressed Dams Exhibit More Locomotor Activity Than the Off-Spring of SCD-Fed Stressed Dams
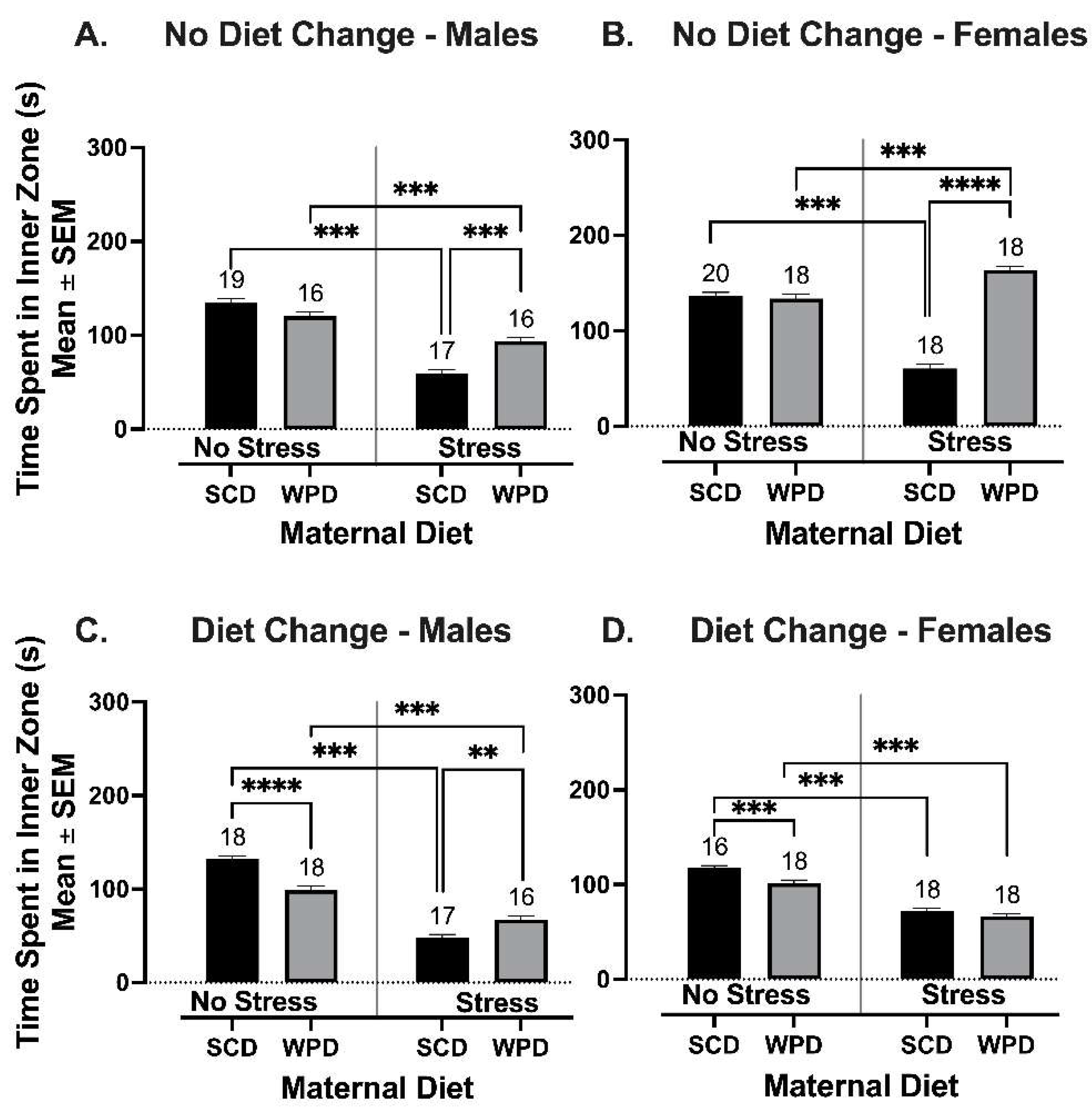
3.2.2. The WPD-Fed Offspring of Stressed Dams Spend More Time in the Inner Zone of the OF than the SCD-Fed Offspring of Stressed Dams When Diet Remains Consistent, but a Diet Change at Weaning Reduces This Effect
3.2.3. The Offspring of WPD-Fed Stressed Dams Spend Less Time Freezing Than the Offspring of SCD-Fed Stressed Dams, a Diet Change at Weaning Reverses This Effect in Females
3.3. Neural Gene Expression
3.3.1. The Effects of PS on Drd1 Expression in the Nucleus Accumbens Depend on Both Maternal and Post-Weaning Diet, and Are More Pronounced in Female Offspring
3.3.2. The Offspring of Stressed Dams Have Decreased Drd2 Expression in the Ventral Tegmental Area When Exposed to a Maternal and/or Postweaning WPD, and Increased Expression When Maternal and Postweaning Diets Are SCD
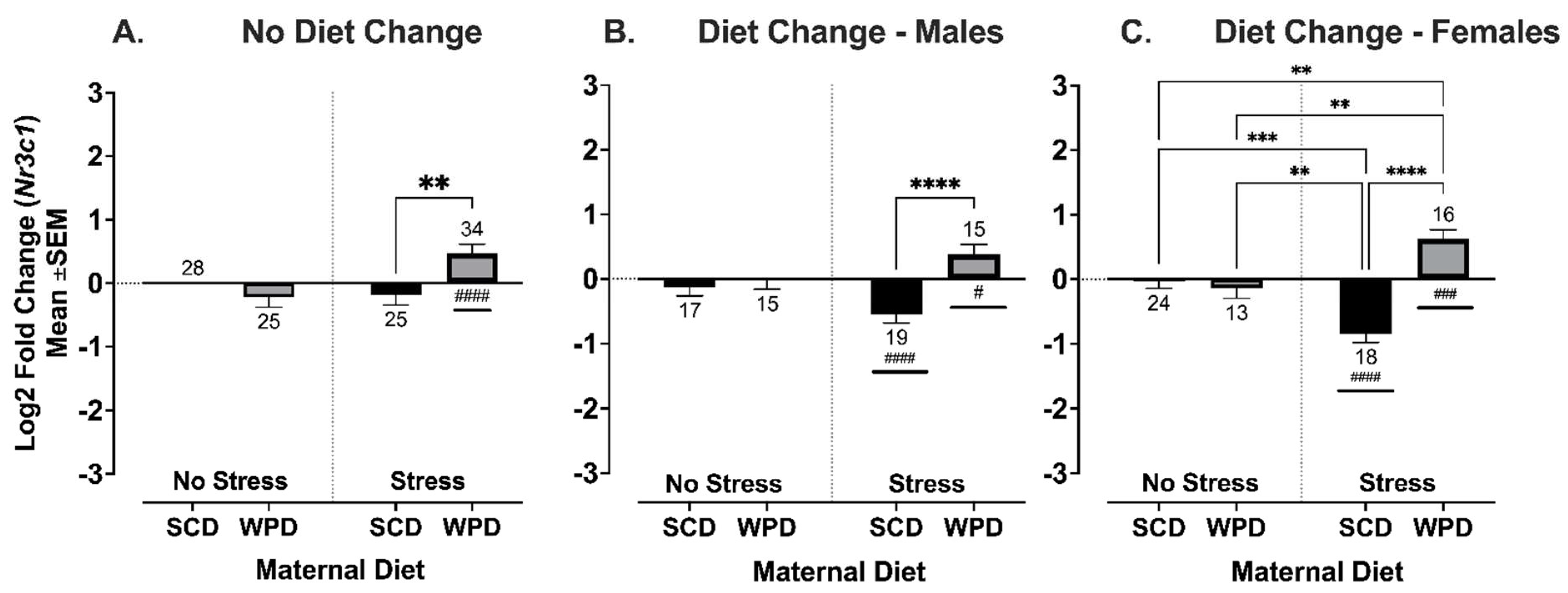
3.3.3. The Offspring of WPD-Fed Stressed Dams Have Increased Expression of Glucocorticoid Receptor, Nr3c1, in the Hippocampus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kuzawa, C.W.; Quinn, E.A. Developmental Origins of Adult Function and Health: Evolutionary Hypotheses. Annu. Rev. Anthropol. 2009, 38, 131–147. [Google Scholar] [CrossRef]
- Sinclair, K.D.; Lea, R.G.; Rees, W.D.; Young, L.E. The Developmental Origins of Health and Disease: Current Theories and Epigenetic Mechanisms. Soc. Reprod. Fertil. Suppl. 2007, 64, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, B.R.H.; Mulder, E.J.H.; Mennes, M.; Glover, V. Antenatal Maternal Anxiety and Stress and the Neurobehavioural Development of the Fetus and Child: Links and Possible Mechanisms. A Review. Neurosci. Biobehav. Rev. 2005, 29, 237–258. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, P.D.; Entringer, S.; Buss, C.; Lu, M.C. The Contribution of Maternal Stress to Preterm Birth: Issues and Considerations. Clin. Perinatol. 2011, 38, 351–384. [Google Scholar] [CrossRef]
- Champagne, F.A.; Meaney, M.J. Stress During Gestation Alters Postpartum Maternal Care and the Development of the Offspring in a Rodent Model. Biol. Psychiatry 2006, 59, 1227–1235. [Google Scholar] [CrossRef]
- Heiming, R.S.; Bodden, C.; Jansen, F.; Lewejohann, L.; Kaiser, S.; Lesch, K.-P.; Palme, R.; Sachser, N. Living in a Dangerous World Decreases Maternal Care: A Study in Serotonin Transporter Knockout Mice. Horm. Behav. 2011, 60, 397–407. [Google Scholar] [CrossRef]
- Meaney, M.J. Maternal Care, Gene Expression, and the Transmission of Individual Differences in Stress Reactivity across Generations. Annu. Rev. Neurosci. 2001, 24, 1161–1192. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Holmes, A.; Wellman, C.L. Stress-Induced Prefrontal Reorganization and Executive Dysfunction in Rodents. Neurosci. Biobehav. Rev. 2009, 33, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Mitra, R.; Shankaranarayana Rao, B.S.; Chattarji, S. Chronic Stress Induces Contrasting Patterns of Dendritic Remodeling in Hippocampal and Amygdaloid Neurons. J. Neurosci. 2002, 22, 6810–6818. [Google Scholar] [CrossRef]
- Maccari, S.; Darnaudery, M.; Morley-Fletcher, S.; Zuena, A.R.; Cinque, C.; Van Reeth, O. Prenatal Stress and Long-Term Consequences: Implications of Glucocorticoid Hormones. Neurosci. Biobehav. Rev. 2003, 27, 119–127. [Google Scholar] [CrossRef]
- Groesz, L.M.; McCoy, S.; Carl, J.; Saslow, L.; Stewart, J.; Adler, N.; Laraia, B.; Epel, E. What Is Eating You? Stress and the Drive to Eat. Appetite 2012, 58, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Y.; Chrétien, P.; Meaney, M.J.; Gratton, A. Influence of Naturally Occurring Variations in Maternal Care on Prepulse Inhibition of Acoustic Startle and the Medial Prefrontal Cortical Dopamine Response to Stress in Adult Rats. J. Neurosci. 2005, 25, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Weaver, I.C.G.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic Programming by Maternal Behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef]
- Foster, M.T.; Warne, J.P.; Ginsberg, A.B.; Horneman, H.F.; Pecoraro, N.C.; Akana, S.F.; Dallman, M.F. Palatable Foods, Stress, and Energy Stores Sculpt Corticotropin-Releasing Factor, Adrenocorticotropin, and Corticosterone Concentrations after Restraint. Endocrinology 2009, 150, 2325–2333. [Google Scholar] [CrossRef]
- la Fleur, S.E.; Houshyar, H.; Roy, M.; Dallman, M.F. Choice of Lard, But Not Total Lard Calories, Damps Adrenocorticotropin Responses to Restraint. Endocrinology 2005, 146, 2193–2199. [Google Scholar] [CrossRef]
- Pecoraro, N.; Reyes, F.; Gomez, F.; Bhargava, A.; Dallman, M.F. Chronic Stress Promotes Palatable Feeding, Which Reduces Signs of Stress: Feedforward and Feedback Effects of Chronic Stress. Endocrinology 2004, 145, 3754–3762. [Google Scholar] [CrossRef]
- Tomiyama, A.J.; Dallman, M.F.; Epel, E.S. Comfort Food Is Comforting to Those Most Stressed: Evidence of the Chronic Stress Response Network in High Stress Women. Psychoneuroendocrinology 2011, 36, 1513–1519. [Google Scholar] [CrossRef]
- Masi, F.; Scheggi, S.; Mangiavacchi, S.; Tolu, P.; Tagliamonte, A.; Graziella De Montis, M.; Gambarana, C. Dopamine Output in the Nucleus Accumbens Shell Is Related to the Acquisition and the Retention of a Motivated Appetitive Behavior in Rats. Brain Res. 2001, 903, 102–109. [Google Scholar] [CrossRef]
- Teegarden, S.L.; Nestler, E.J.; Bale, T.L. ΔFosB-Mediated Alterations in Dopamine Signaling Are Normalized by a Palatable High-Fat Diet. Biol. Psychiatry 2008, 64, 941–950. [Google Scholar] [CrossRef]
- MacDonald, A.F.; Billington, C.J.; Levine, A.S. Alterations in Food Intake by Opioid and Dopamine Signaling Pathways between the Ventral Tegmental Area and the Shell of the Nucleus Accumbens. Brain Res. 2004, 1018, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, R.J.; Williams, J.T.; Neve, K.A. Dopamine Receptor Signaling: Intracellular Pathways to Behavior. In The Dopamine Receptors; Neve, K.A., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 137–173. ISBN 978-1-60327-332-9. [Google Scholar]
- Kenny, P.J. Common Cellular and Molecular Mechanisms in Obesity and Drug Addiction. Nat. Rev. Neurosci. 2011, 12, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, L.A.; Grosenick, L.; Finkelstein, J.C.; Kauvar, I.V.; Fenno, L.E.; Adhikari, A.; Lammel, S.; Mirzabekov, J.J.; Airan, R.D.; Zalocusky, K.A.; et al. Natural Neural Projection Dynamics Underlying Social Behavior. Cell 2014, 157, 1535–1551. [Google Scholar] [CrossRef]
- Francis, T.C.; Chandra, R.; Friend, D.M.; Finkel, E.; Dayrit, G.; Miranda, J.; Brooks, J.M.; Iñiguez, S.D.; O’Donnell, P.; Kravitz, A.; et al. Nucleus Accumbens Medium Spiny Neuron Subtypes Mediate Depression-Related Outcomes to Social Defeat Stress. Biol. Psychiatry 2015, 77, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Mood and Anxiety Related Phenotypes in Mice; Gould, T.D., Ed.; Neuromethods; Humana Press: Totowa, NJ, USA, 2009; Volume 42, pp. 1–20. ISBN 978-1-60761-302-2. [Google Scholar]
- Zapala, M.A.; Hovatta, I.; Ellison, J.A.; Wodicka, L.; Del Rio, J.A.; Tennant, R.; Tynan, W.; Broide, R.S.; Helton, R.; Stoveken, B.S.; et al. Adult Mouse Brain Gene Expression Patterns Bear an Embryologic Imprint. Proc. Natl. Acad. Sci. USA 2005, 102, 10357–10362. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B.J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, 5th ed.; Elsevier/Academic Press: London, UK; San Diego, CA, USA; Cambridge, UK; MA Kidlington: Oxford, UK, 2019; ISBN 978-0-12-816157-9. [Google Scholar]
- Brass, K.E.; Herndon, N.; Gardner, S.A.; Grindstaff, J.L.; Campbell, P. Intergenerational Effects of Paternal Predator Cue Exposure on Behavior, Stress Reactivity, and Neural Gene Expression. Horm. Behav. 2020, 124, 104806. [Google Scholar] [CrossRef]
- Henry, C.; Kabbaj, M.; Simon, H.; Moal, M.; Maccari, S. Prenatal Stress Increases the Hypothalamo-Pituitary-Adrenal Axis Response in Young and Adult Rats. J. Neuroendocrinol. 1994, 6, 341–345. [Google Scholar] [CrossRef]
- Henry, C.; Guegant, G.; Cador, M.; Arnauld, E.; Arsaut, J.; Moal, M.L.; Demotes-Mainard, J. Prenatal Stress in Rats Facilitates Amphetamine-Induced Sensitization and Induces Long-Lasting Changes in Dopamine Receptors in the Nucleus Accumbens. Brain Res. 1995, 685, 179–186. [Google Scholar] [CrossRef]
- Speight, A.; Davey, W.G.; McKenna, E.; Voigt, J.-P.W. Exposure to a Maternal Cafeteria Diet Changes Open-Field Behaviour in the Developing Offspring. Int. J. Dev. Neurosci. 2017, 57, 34–40. [Google Scholar] [CrossRef]
- Gawlińska, K.; Gawliński, D.; Korostyński, M.; Borczyk, M.; Frankowska, M.; Piechota, M.; Filip, M.; Przegaliński, E. Maternal Dietary Patterns Are Associated with Susceptibility to a Depressive-like Phenotype in Rat Offspring. Dev. Cogn. Neurosci. 2021, 47, 100879. [Google Scholar] [CrossRef]
- Sasaki, A.; de Vega, W.C.; St-Cyr, S.; Pan, P.; McGowan, P.O. Perinatal High Fat Diet Alters Glucocorticoid Signaling and Anxiety Behavior in Adulthood. Neuroscience 2013, 240, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.D.; Tsang, V. Enduring Consequences of Maternal Obesity for Brain Inflammation and Behavior of Offspring. FASEB J. 2010, 24, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.; Langley-Evans, S.C.; Voigt, J.-P. The Impact of Maternal Cafeteria Diet on Anxiety-Related Behaviour and Exploration in the Offspring. Physiol. Behav. 2011, 103, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Adam, T.C.; Epel, E.S. Stress, Eating and the Reward System. Physiol. Behav. 2007, 91, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, L.K.; Kaplan, A.R.; Bock, R.; Phamluong, K.; Shin, J.H.; Bocarsly, M.E.; Eberhart, L.; Ron, D.; Alvarez, V.A. D1 Receptor Hypersensitivity in Mice with Low Striatal D2 Receptors Facilitates Select Cocaine Behaviors. Neuropsychopharmacology 2019, 44, 805–816. [Google Scholar] [CrossRef]
- de Jong, J.W.; Roelofs, T.J.M.; Mol, F.M.U.; Hillen, A.E.J.; Meijboom, K.E.; Luijendijk, M.C.M.; van der Eerden, H.A.M.; Garner, K.M.; Vanderschuren, L.J.M.J.; Adan, R.A.H. Reducing Ventral Tegmental Dopamine D2 Receptor Expression Selectively Boosts Incentive Motivation. Neuropsychopharmacology 2015, 40, 2085–2095. [Google Scholar] [CrossRef]
- Collins, G.T.; Chen, Y.; Tschumi, C.; Rush, E.L.; Mensah, A.; Koek, W.; France, C.P. Effects of Consuming a Diet High in Fat and/or Sugar on the Locomotor Effects of Acute and Repeated Cocaine in Male and Female C57BL/6J Mice. Exp. Clin. Psychopharmacol. 2015, 23, 228–237. [Google Scholar] [CrossRef]
- Gawliński, D.; Gawlińska, K.; Frankowska, M.; Filip, M. Maternal High-sugar Diet Changes Offspring Vulnerability to Reinstatement of Cocaine-seeking Behavior: Role of Melanocortin-4 Receptors. FASEB J. 2020, 34, 9192–9206. [Google Scholar] [CrossRef]
- Levay, E.A.; Paolini, A.G.; Govic, A.; Hazi, A.; Penman, J.; Kent, S. Anxiety-like Behaviour in Adult Rats Perinatally Exposed to Maternal Calorie Restriction. Behav. Brain Res. 2008, 191, 164–172. [Google Scholar] [CrossRef]
- Purcell, R.H.; Sun, B.; Pass, L.L.; Power, M.L.; Moran, T.H.; Tamashiro, K.L.K. Maternal Stress and High-Fat Diet Effect on Maternal Behavior, Milk Composition, and Pup Ingestive Behavior. Physiol. Behav. 2011, 104, 474–479. [Google Scholar] [CrossRef]
- Liu, D.; Diorio, J.; Tannenbaum, B.; Caldji, C.; Francis, D.; Freedman, A.; Sharma, S.; Pearson, D.; Plotsky, P.M.; Meaney, M.J. Maternal Care, Hippocampal Glucocorticoid Receptors, and Hypothalamic-Pituitary-Adrenal Responses to Stress. Science 1997, 277, 1659–1662. [Google Scholar] [CrossRef] [PubMed]
- Caldji, C.; Tannenbaum, B.; Sharma, S.; Francis, D.; Plotsky, P.M.; Meaney, M.J. Maternal Care during Infancy Regulates the Development of Neural Systems Mediating the Expression of Fearfulness in the Rat. Proc. Natl. Acad. Sci. USA 1998, 95, 5335–5340. [Google Scholar] [CrossRef] [PubMed]
- Hodes, G.E.; Epperson, C.N. Sex Differences in Vulnerability and Resilience to Stress Across the Life Span. Biol. Psychiatry 2019, 86, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Rincel, M.; Lépinay, A.L.; Delage, P.; Fioramonti, J.; Théodorou, V.S.; Layé, S.; Darnaudéry, M. Maternal High-Fat Diet Prevents Developmental Programming by Early-Life Stress. Transl. Psychiatry 2016, 6, e966. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, B.; Blom, W.A.; Koolhaas, J.M.; van Dijk, G. Behavioral and Physiological Responses to Stress Are Affected by High-Fat Feeding in Male Rats. Physiol. Behav. 2001, 73, 371–377. [Google Scholar] [CrossRef]
- Connor, K.L.; Vickers, M.H.; Beltrand, J.; Meaney, M.J.; Sloboda, D.M. Nature, Nurture or Nutrition? Impact of Maternal Nutrition on Maternal Care, Offspring Development and Reproductive Function: Nutritional Compromise, Maternal Care and Offspring Development. J. Physiol. 2012, 590, 2167–2180. [Google Scholar] [CrossRef]
- Ferreira, A.; Castro, J.P.; Andrade, J.P.; Dulce Madeira, M.; Cardoso, A. Cafeteria-Diet Effects on Cognitive Functions, Anxiety, Fear Response and Neurogenesis in the Juvenile Rat. Neurobiol. Learn. Mem. 2018, 155, 197–207. [Google Scholar] [CrossRef]
- Graf, A.E.; Lallier, S.W.; Waidyaratne, G.; Thompson, M.D.; Tipple, T.E.; Hester, M.E.; Trask, A.J.; Rogers, L.K. Maternal High Fat Diet Exposure Is Associated with Increased Hepcidin Levels, Decreased Myelination, and Neurobehavioral Changes in Male Offspring. Brain Behav. Immun. 2016, 58, 369–378. [Google Scholar] [CrossRef]
- Peleg-Raibstein, D.; Luca, E.; Wolfrum, C. Maternal High-Fat Diet in Mice Programs Emotional Behavior in Adulthood. Behav. Brain Res. 2012, 233, 398–404. [Google Scholar] [CrossRef]
- Sullivan, E.L.; Nousen, E.K.; Chamlou, K.A. Maternal High Fat Diet Consumption during the Perinatal Period Programs Offspring Behavior. Physiol. Behav. 2014, 123, 236–242. [Google Scholar] [CrossRef]
- Winther, G.; Elfving, B.; Müller, H.K.; Lund, S.; Wegener, G. Maternal High-Fat Diet Programs Offspring Emotional Behavior in Adulthood. Neuroscience 2018, 388, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Zeeni, N.; Bassil, M.; Fromentin, G.; Chaumontet, C.; Darcel, N.; Tome, D.; Daher, C.F. Environmental Enrichment and Cafeteria Diet Attenuate the Response to Chronic Variable Stress in Rats. Physiol. Behav. 2015, 139, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Tomiyama, A.J.; Dallman, M.F. Stress and Reward. In Food and Addiction; Brownell, K.D., Gold, M.S., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 266–272. ISBN 978-0-19-973816-8. [Google Scholar]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. N. Am. 2018, 102, 183–197. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clauss, N.; Allen, K.B.; Billings, K.D.; Tolliver, M.D.M.; Garza, R.; Byrd-Craven, J.; Campbell, P. The Modification of Offspring Stress-Related Behavior and the Expression of Drd1, Drd2, and Nr3c1 by a Western-Pattern Diet in Mus Musculus. Int. J. Mol. Sci. 2022, 23, 9245. https://doi.org/10.3390/ijms23169245
Clauss N, Allen KB, Billings KD, Tolliver MDM, Garza R, Byrd-Craven J, Campbell P. The Modification of Offspring Stress-Related Behavior and the Expression of Drd1, Drd2, and Nr3c1 by a Western-Pattern Diet in Mus Musculus. International Journal of Molecular Sciences. 2022; 23(16):9245. https://doi.org/10.3390/ijms23169245
Chicago/Turabian StyleClauss, Nikki, Kelsey Brass Allen, Katie D. Billings, Mikayla D. M. Tolliver, Ray Garza, Jennifer Byrd-Craven, and Polly Campbell. 2022. "The Modification of Offspring Stress-Related Behavior and the Expression of Drd1, Drd2, and Nr3c1 by a Western-Pattern Diet in Mus Musculus" International Journal of Molecular Sciences 23, no. 16: 9245. https://doi.org/10.3390/ijms23169245





