Dentin Particulate for Bone Regeneration: An In Vitro Study
Abstract
:1. Introduction
2. Results
2.1. Characterization of DPSCs by Flow Cytometry
2.2. Cell Proliferation
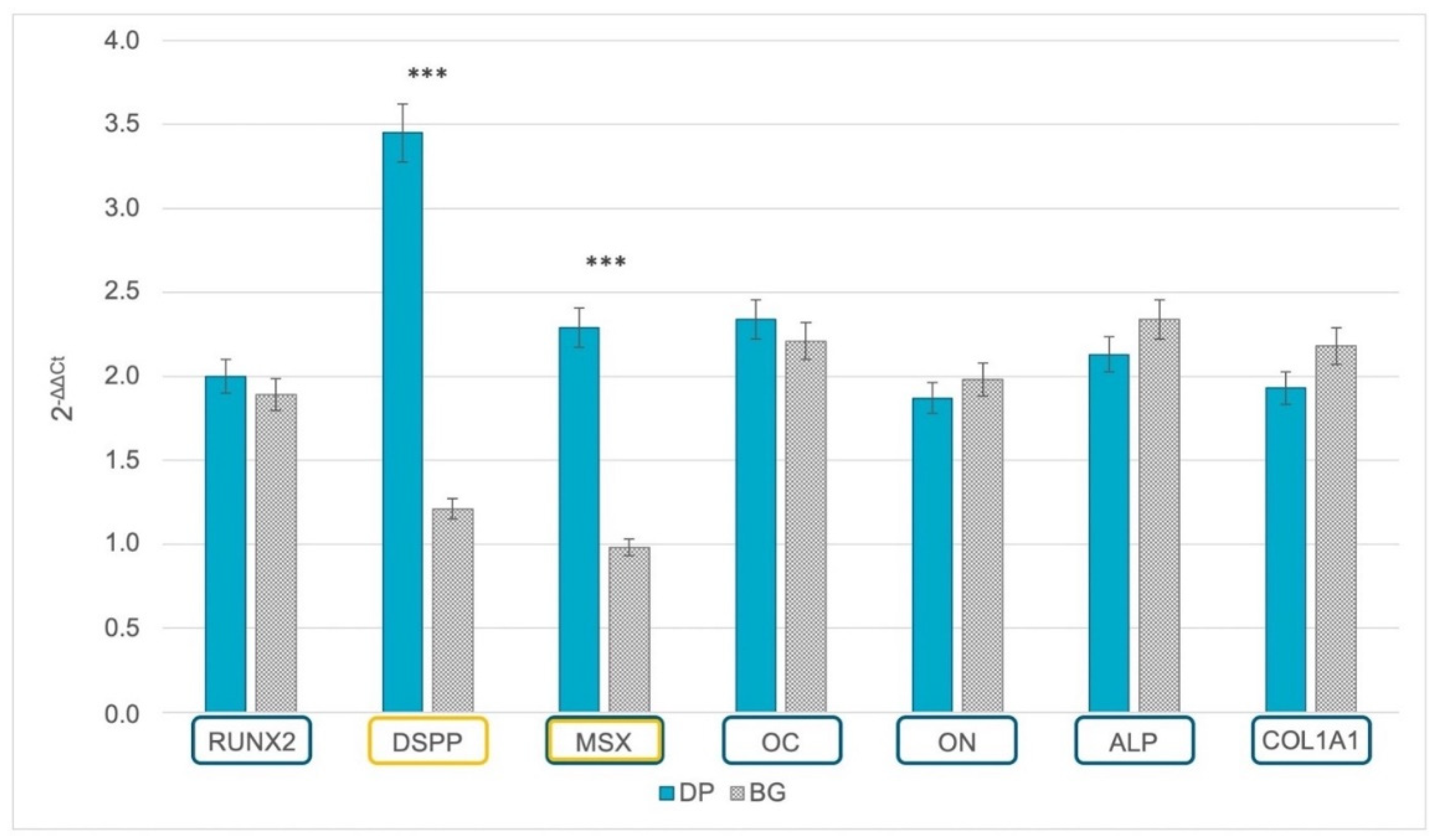
2.3. Gene Expression of Odontoblastic and Osteoblastic Markers
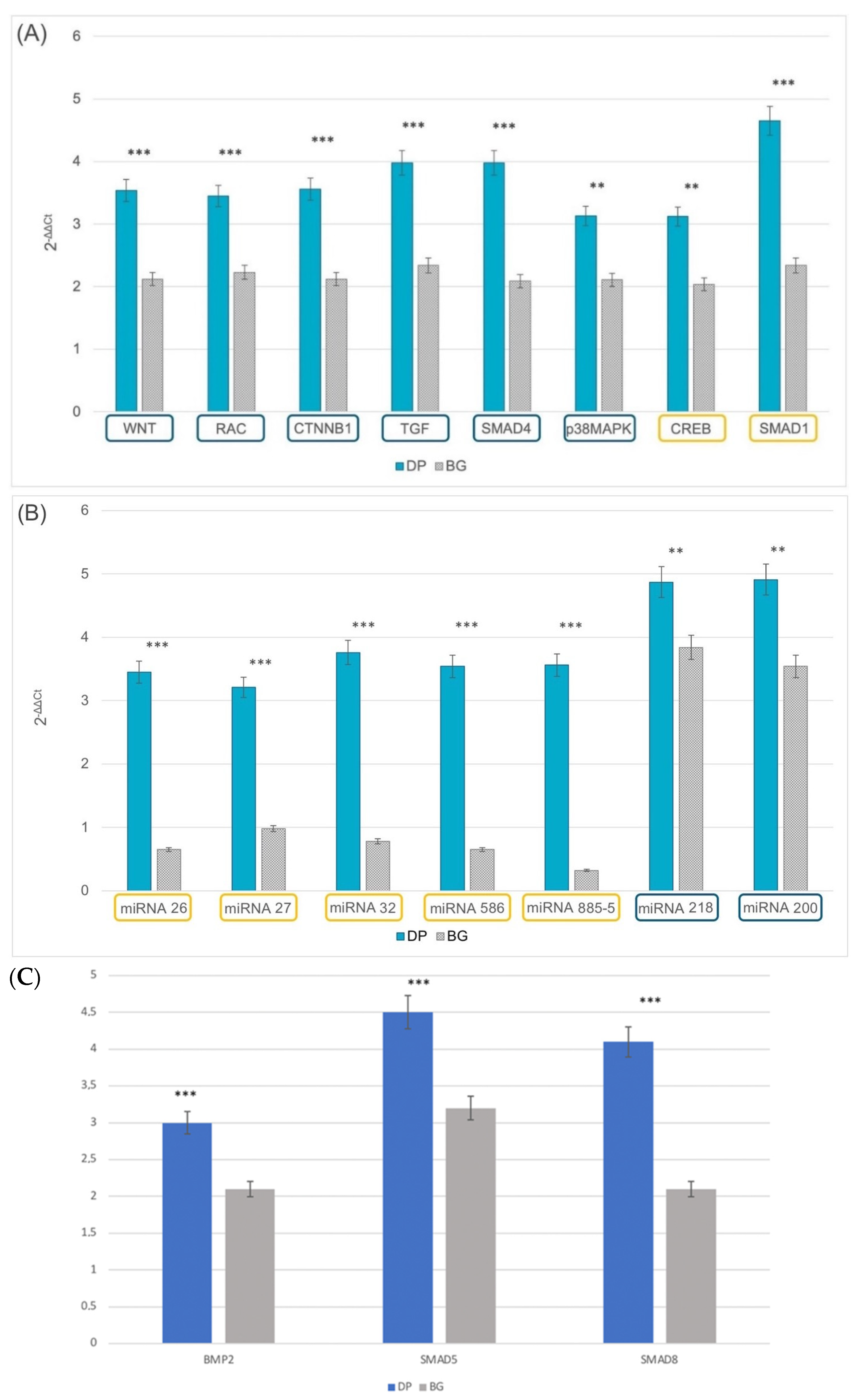
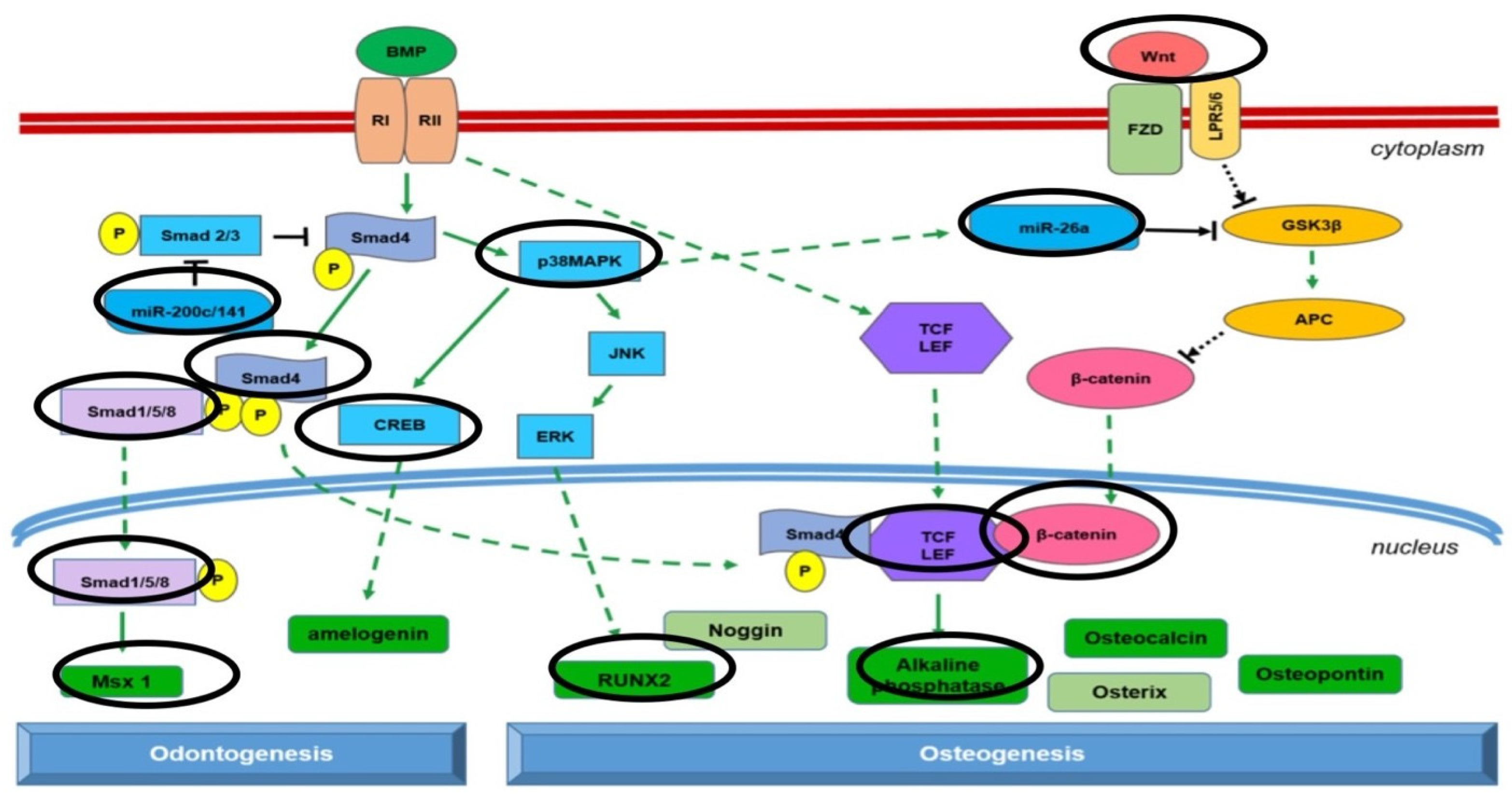
2.4. Epigenetics
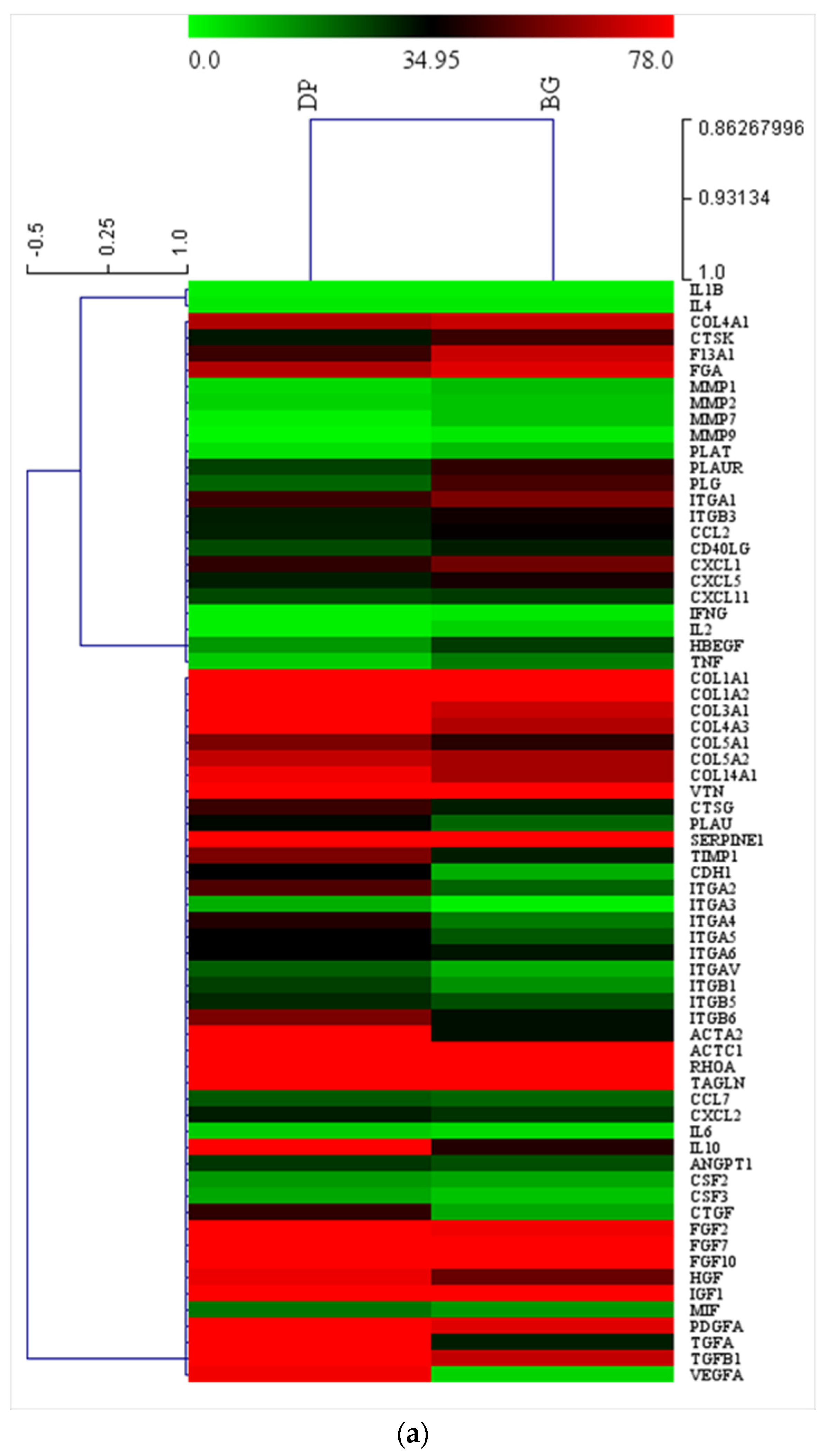
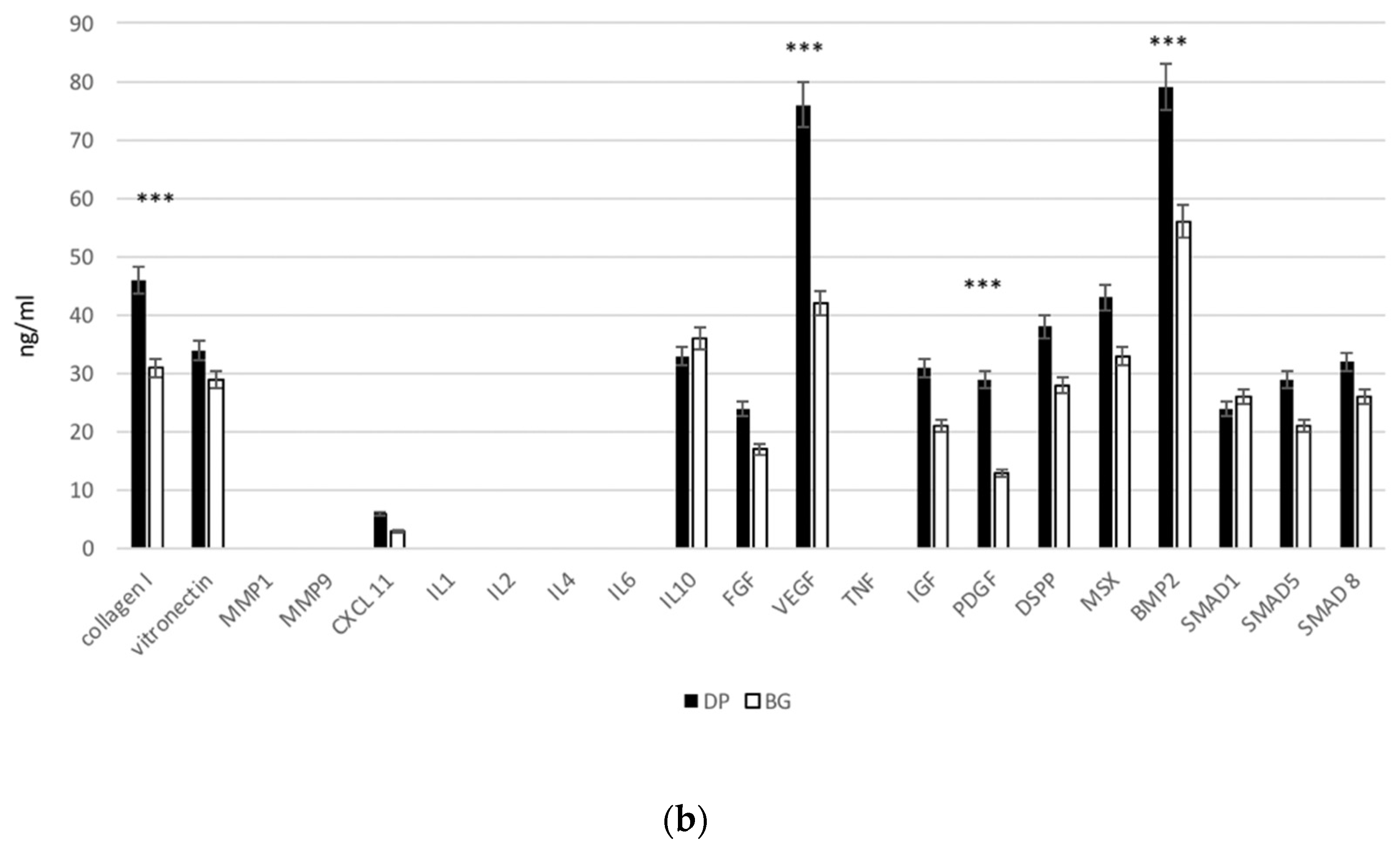
2.5. Extracellular Matrix and Adhesion Proteins
2.6. Intracellular ALP Activity
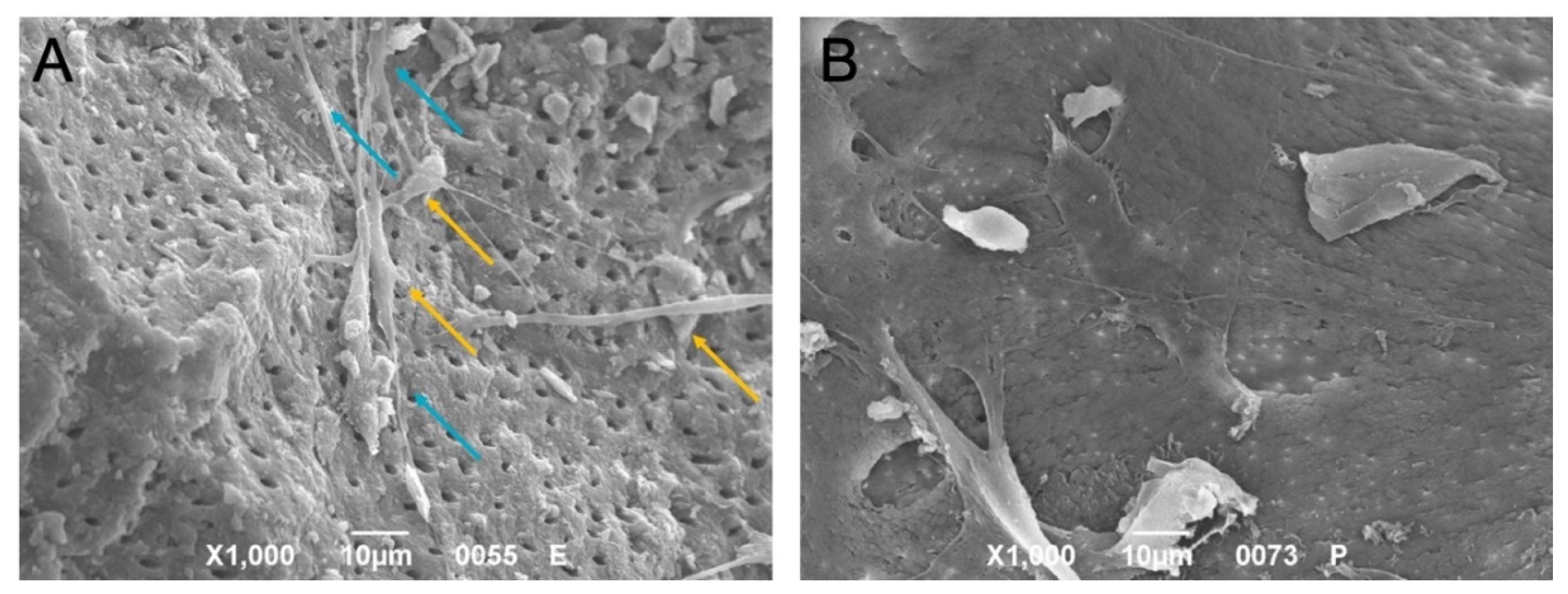
2.7. Morphology
2.8. Compressive Properties of Extracellular Matrix
3. Discussion
4. Materials and Methods
4.1. Isolation of Dental Pulp Stem Cells
4.2. Characterization of DPSCs by Flow Cytometry
4.3. Sample Preparation with Kometa Bio®
- Remove crowns and fillings of any kind, dental plaque, discolored dentin, decay, and periodontal ligament, and rinse with a sterile physiological saline;
- Crush tooth fragments with the SDG (Kometa Bio®) to obtain and collect DP with a size between 300 and 1200 μm and with a porosity of 2–14 μm;
- Wash the DP for 10 min in a sterilized glass container with a 0.5 molar solution of NaOH with 20% ethanol, followed by 10 min in a sterile saline solution.
4.4. Deproteinized Bovine Bone Matrix
4.5. Cell Culture
4.6. Cell Proliferation
4.7. Real-Time Polymerase Chain Reaction (RT-PCR)
4.8. Intracellular ALP Activity Assay
4.9. Scanning Electron Microscopy (SEM)
4.10. Compressive Properties of Extracellular Matrix
4.11. ELISA Assay
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Suzuki, O.; Shiwaku, Y.; Hamai, R. Octacalcium phosphate bone substitute materials: Comparison between properties of biomaterials and other calcium phosphate materials. Dent. Mater. J. 2020, 39, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Moussa, N.T.; Dym, H. Maxillofacial Bone Grafting Materials. Dent. Clin. North Am. 2020, 64, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Brunello, G.; Panda, S.; Schiavon, L.; Sivolella, S.; Biasetto, L.; Del Fabbro, M. The Impact of Bioceramic Scaffolds on Bone Regeneration in Preclinical In Vivo Studies: A Systematic Review. Materials 2020, 13, 1500. [Google Scholar] [CrossRef] [PubMed]
- Yahav, A.; Kurtzman, G.M.; Katzap, M.; Dudek, D.; Baranes, D. Bone Regeneration: Properties and clinical applications of biphasic calcium sulfate. Dent. Clin. N. Am. 2020, 64, 453–472. [Google Scholar] [CrossRef]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piattelli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-Based Nanomaterials for Tissue Engineering in the Dental Field. Nanomaterials 2018, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, A.; Sahin, D.; Sader, R.; Becker, J.; Schwarz, F. Efficacy of autogenous teeth for the reconstruction of alveolar ridge deficiencies: A systematic review. Clin. Oral Investig. 2019, 23, 4263–4287. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, J.; Um, I.-W.; Kim, K.-W.; Murata, M.; Akazawa, T.; Mitsugi, M. Tooth-derived bone graft material. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 103–111. [Google Scholar] [CrossRef]
- Gual-Vaques, P.; Polis-Yanes, C.; Estrugo-Devesa, A.; Ayuso-Montero, R.; Mari-Roig, A.; Lopez-Lopez, J. Autogenous teeth used for bone grafting: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2017, 23, e112–e119. [Google Scholar] [CrossRef] [PubMed]
- Khanijou, M.; Seriwatanachai, D.; Boonsiriseth, K.; Suphangul, S.; Pairuchvej, V.; Srisatjaluk, R.L.; Wongsirichat, N. Bone graft material derived from extracted tooth: A review literature. J. Oral Maxillofac. Surg. Med. Pathol. 2018, 31, 1–7. [Google Scholar] [CrossRef]
- Becker, K.; Drescher, D.; Hönscheid, R.; Golubovic, V.; Mihatovic, I.; Schwarz, F. Biomechanical, micro-computed tomographic and immunohistochemical analysis of early osseous integration at titanium implants placed following lateral ridge augmentation using extracted tooth roots. Clin. Oral Implant. Res. 2017, 28, 334–340. [Google Scholar] [CrossRef]
- Parvini, P.; Schwarz, F.; Dmd, M.K.H.; Rauch, N.; Nienkemper, M.; Becker, K. Microstructural volumetric analysis of vertical alveolar ridge augmentation using autogenous tooth roots. Clin. Implant Dent. Relat. Res. 2020, 22, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Jandik, K.; Stauber, M.; Mihatovic, I.; Drescher, D.; Schwarz, F. Microstructural volumetric analysis of lateral ridge augmentation using differently conditioned tooth roots. Clin. Oral Investig. 2018, 23, 3063–3071. [Google Scholar] [CrossRef] [PubMed]
- Pohl, S.; Binderman, I.; Tomac, J. Maintenance of Alveolar Ridge Dimensions Utilizing an Extracted Tooth Dentin Particulate Autograft and Platelet-Rich fibrin: A Retrospective Radiographic Cone-Beam Computed Tomography Study. Materials 2020, 13, 1083. [Google Scholar] [CrossRef]
- Kuperschlag, A.; Keršytė, G.; Kurtzman, G.M.; Horowitz, R.A. Autogenous Dentin Grafting of Osseous Defects Distal to Mandibular Second Molars After Extraction of Impacted Third Molars. Compend. Contin. Educ. Dent. 2020, 41, 76. [Google Scholar] [PubMed]
- Andrade, J.C.; Camino, M.; Nally, M.; Quirynen, B.; Martínez, N. Pinto, Combining autologous particulate dentin, L-PRF, and fibrinogen to create a matrix for predictable ridge preservation: A pilot clinical study. Clin. Oral Investig. 2020, 24, 1151–1160. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Nevins, M.; Schupbach, P. New Bone Formation Using an Extracted Tooth as a Biomaterial: A Case Report with Histologic Evidence. Int. J. Periodontics Restor. Dent. 2019, 39, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Del Canto-Diaz, A.; De Elio-Oliveros, J.; Alobera-Gracia, M.; Del Canto-Pingarron, M.; Martinez-Gonzalez, J. Use of autologous tooth-derived graft material in the post-extraction dental socket. Pilot Study Med. Oral Patol. Oral Cir. Bucal. 2018, 24, e53–e60. [Google Scholar] [CrossRef]
- Brunello, G.; Zanotti, F.; Trentini, M.; Zanolla, I.; Pishavar, E.; Favero, V.; Favero, R.; Favero, L.; Bressan, E.; Bonora, M.; et al. Exosomes Derived from Dental Pulp Stem Cells Show Different Angiogenic and Osteogenic Properties in Relation to the Age of the Donor. Pharmaceutics 2022, 14, 908. [Google Scholar] [CrossRef]
- Memarian, P.; Pishavar, E.; Zanotti, F.; Trentini, M.; Camponogara, F.; Soliani, E.; Gargiulo, P.; Isola, M.; Zavan, B. Active Materials for 3D Printing in Small Animals: Current Modalities and Future Directions for Orthopedic Applications. Int. J. Mol. Sci. 2022, 23, 1045. [Google Scholar] [CrossRef]
- Valdec, S.; Pasic, P.; Soltermann, A.; Thoma, D.; Stadlinger, B.; Rücker, M. Alveolar ridge preservation with autologous particulated dentin—A case series. Int. J. Implant Dent. 2017, 3, 12. [Google Scholar] [CrossRef]
- Binderman, G.I.; Hallel, C.; Nardy, A.; Yaffe, L. Sapoznikov, A novel procedure to process extracted teeth for immediate grafting of autogenous dentin. J. Interdiscipl. Med. Dent. Sci. 2014, 2, 154. [Google Scholar] [CrossRef]
- Tran, H.L.B.; Doan, V.N. Human dental pulp stem cells cultured onto dentin derived scaffold can regenerate dentin-like tissue in vivo. Cell Tissue Bank 2015, 16, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xie, C.; Ren, N.; Bai, S.; Zhao, Y. Human Freeze-dried Dentin Matrix as a Biologically Active Scaffold for Tooth Tissue Engineering. J. Endod. 2019, 45, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Schwartz-Dabney, C.; Dechow, P. Variations in cortical material properties throughout the human dentate mandible. Am. J. Phys. Anthr. 2003, 120, 252–277. [Google Scholar] [CrossRef]
- Rieger, M.; Fareed, K.; Adams, W.; Tanquist, R. Bone stress distribution for three endosseous implants. J. Prosthet. Dent. 1989, 61, 223–228. [Google Scholar] [CrossRef]
- Stanford, C.M. Biomechanical and functional behavior of implants. Adv. Dent. Res. 1999, 13, 88–92. [Google Scholar] [CrossRef]
- Sperling, I.; Itzkowitz, D.; Kaufman, A.Y.; Binderman, I. A new treatment of heterotransplanted teeth to prevent progression of root resorption. Dent. Traumatol. 1986, 2, 117–120. [Google Scholar] [CrossRef]
- Andersson, L.; Bodin, I.; Sorensen, S. Progression of root resorption following replantation of human teeth after extended extraoral storage. Dent. Traumatol. 1989, 5, 38–47. [Google Scholar] [CrossRef]
- Joshi, C.P.; Dani, N.H.; Khedkar, S.U. Alveolar ridge preservation using autogenous tooth graft versus beta-tricalcium phosphate alloplast: A randomized, controlled, prospective, clinical pilot study. J. Indian Soc. Periodontol. 2016, 20, 429–434. [Google Scholar] [CrossRef]
- Pohl, V.; Schuh, C.; Fischer, M.B.; Haas, R. A New Method Using Autogenous Impacted Third Molars for Sinus Augmentation to Enhance Implant Treatment: Case Series with Preliminary Results of an Open, Prospective Longitudinal Study. Int. J. Oral Maxillofac. Implant. 2016, 31, 622–630. [Google Scholar] [CrossRef]
- Minamizato, T.; Koga, I.; Takashi, Y.; Nakatani, M.; Umebayashi, Y.; Sumita, T.; Ikeda, I. Clinical application of autogenous partially demineralized dentin matrix prepared immediately after extraction for alveolar bone regeneration in implant dentistry: A pilot study. Int. J. Oral Maxillofac. Surg. 2018, 47, 125–132. [Google Scholar] [CrossRef]
- Kim, Y.-K. Bone graft material using teeth. J. Korean Assoc. Oral Maxillofac. Surg. 2012, 38, 134–138. [Google Scholar] [CrossRef]
- Park, S.-M.; Um, I.-W.; Kim, Y.-K.; Kim, K.-W. Clinical application of auto-tooth bone graft material. J. Korean Assoc. Oral Maxillofac. Surg. 2012, 38, 2–8. [Google Scholar] [CrossRef]
- Ravindran, S.; George, A. Dentin Matrix Proteins in Bone Tissue Engineering. Adv. Exp. Med. Biol. 2015, 881, 129–142. [Google Scholar] [CrossRef]
- Ghensi, P.; Bressan, E.; Gardin, C.; Ferroni, L.; Ruffato, L.; Caberlotto, M.; Soldini, C.; Zavan, B. Osteo Growth Induction titanium surface treatment reduces ROS production of mesenchymal stem cells increasing their osteogenic commitment. Mater. Sci. Eng. C 2017, 74, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Hadden, W.J.; Choi, Y.S. The extracellular microscape governs mesenchymal stem cell fate. J. Biol. Eng. 2016, 10, 16. [Google Scholar] [CrossRef]
- Saha, S.; Ji, L.; de Pablo, J.J.; Palecek, S.P. Inhibition of human embryonic stem cell differentiation by mechanical strain. J. Cell. Physiol. 2005, 206, 126–137. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Y.-K.; Yang, M.T.; Desai, R.; Yu, X.; Liu, Z.; Chen, C. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat. Methods 2010, 7, 733–736. [Google Scholar] [CrossRef]
- Becquart, P.; Cruel, M.; Hoc, T.; Sudre, L.; Pernelle, K.; Bizios, R.; Logeart-Avramoglou, D.; Petite, H.; Bensidhoum, M. Human mesenchymal stem cell responses to hydrostatic pressure and shear stress. Eur. Cells Mater. 2016, 31, 160–173. [Google Scholar] [CrossRef]
- Sharp, L.A.; Lee, Y.W.; Goldstein, A.S. Effect of Low-Frequency Pulsatile Flow on Expression of Osteoblastic Genes by Bone Marrow Stromal Cells. Ann. Biomed. Eng. 2009, 37, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Zavan, B.; Giorgi, C.; Bagnara, G.P.; Vindigni, V.; Abatangelo, G.; Cortivo, R. Osteogenic and chondrogenic differentiation: Comparison of human and rat bone marrow mesenchymal stem cells cultured into polymeric scaffolds. Eur. J. Histochem. 2007, 51 (Suppl. 1), 1–8. [Google Scholar] [PubMed]
- Gardin, C.; Bressan, E.; Ferroni, L.; Nalesso, E.; Vindigni, V.; Stellini, E.; Pinton, P.; Sivolella, S.; Zavan, B. In Vitro Concurrent Endothelial and Osteogenic Commitment of Adipose-Derived Stem Cells and Their Genomical Analyses Through Comparative Genomic Hybridization Array: Novel Strategies to Increase the Successful Engraftment of Tissue-Engineered Bone Grafts. Stem. Cells Dev. 2012, 21, 767–777. [Google Scholar] [CrossRef]
- Gardin, C.; Ferroni, L.; Bressan, E.; Guirado, J.L.C.; Degidi, M.; Piattelli, A.; Zavan, B. Adult Stem Cells Properties in Terms of Commitment, Aging and Biological Safety of Grit-Blasted and Acid-Etched Ti Dental Implants Surfaces. Int. J. Mol. Cell. Med. 2014, 3, 225–236. [Google Scholar] [PubMed]
- Tatullo, M.; Marrelli, M.; Falisi, G.; Rastelli, C.; Palmieri, F.; Gargari, M.; Zavan, B.; Paduano, F.; Benagiano, V. Mechanical influence of tissue culture plates and extracellular matrix on mesenchymal stem cell behavior: A topical review. Int. J. Immunopathol. Pharmacol. 2015, 29, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, M.S. Mechanism of Tissue Reconstruction by Dissociated Cells, II: Time-Course of Events. Science 1962, 137, 762–763. [Google Scholar] [CrossRef]
- Lo, C.-M.; Wang, H.-B.; Dembo, M.; Wang, Y.-L. Cell Movement Is Guided by the Rigidity of the Substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef]
- Choi, Y.S.; Vincent, L.G.; Lee, A.R.; Dobke, M.K.; Engler, A.J. Mechanical derivation of functional myotubes from adipose-derived stem cells. Biomaterials 2012, 33, 2482–2491. [Google Scholar] [CrossRef]
- Engler, A.; Griffin, M.A.; Sen, S.; Bonnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef]
- Yang, M.T.; Fu, J.; Wang, Y.-K.; Desai, R.A.; Chen, C.S. Assaying stem cell mechanobiology on microfabricated elastomeric substrates with geometrically modulated rigidity. Nat. Protoc. 2011, 6, 187–213. [Google Scholar] [CrossRef]
- Di Luca, A.; Ostrowska, B.; Lorenzo-Moldero, I.; Lepedda, A.J.; Swieszkowski, W.; Van Blitterswijk, C.; Moroni, L. Gradients in pore size enhance the osteogenic differentiation of human mesenchymal stromal cells in three-dimensional scaffolds. Sci. Rep. 2016, 6, 22898. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, T.J.; Voisin, M.; Niebur, G.; McNamara, L.M. Multiscale Modeling of Trabecular Bone Marrow: Understanding the Micromechanical Environment of Mesenchymal Stem Cells During Osteoporosis. J. Biomech. Eng. 2015, 137, 011003. [Google Scholar] [CrossRef] [PubMed]
- Hart, N.H.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R.U. Mechanical basis of bone strength: In-fluence of bone material, bone structure and muscle action. J. Musculoskelet. Neuronal Interact. 2017, 17, 114–139. [Google Scholar] [PubMed]
- Foessl, I.; Kotzbeck, P.; Obermayer-Pietsch, B. miRNAs as novel biomarkers for bone related diseases. J. Lab. Precis. Med. 2019, 4, 2. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Liang, C.; Chen, L.; Zhang, G.; Qian, A. Mechanosensitive miRNAs and Bone Formation. Int. J. Mol. Sci. 2017, 18, 1684. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, L.; Tong, X.; Zhang, M.; Zhao, Y.; Guo, J.; Lei, L.; Chen, X.; Tickner, J.; Xu, J.; et al. Mechanical Stress Regulates Bone Metabolism Through MicroRNAs. J. Cell. Physiol. 2016, 232, 1239–1245. [Google Scholar] [CrossRef]
- Baba, O.; Qin, C.; Brunn, J.C.; Wygant, J.N.; McIntyre, B.W.; Butler, W.T. Colocalization of dentin matrix protein 1 and dentin sialoprotein at late stages of rat molar development. Matrix Biol. 2004, 23, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Baba, O.; Brunn, J.C.; McKee, M.D.; Bonewald, L.; Butler, W.T. Dentin matrix protein 1 (DMP1) and dentin sialo-phosphoprotein (DSPP) share unique properties including tissue localization, proteolytic processing, and high molecular weight forms. In Proceedings of the 8th ICCBMT, Banff, AB, Canada, 17–22 October 2004; Sodek, J., Landis, W., Eds.; University of Toronto Press: Toronto, ON, Canada; Banff, AB, Canada, 2005; pp. 74–177. [Google Scholar]
- Suzuki, S.; Sreenath, T.; Haruyama, N.; Honeycutt, C.; Terse, A.; Cho, A.; Kohler, T.; Müller, R.; Goldberg, M.; Kulkarni, A.B. Dentin sialoprotein and dentin phosphoprotein have distinct roles in dentin mineralization. Matrix Biol. 2009, 28, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Song, J.; Zhang, H.; Huang, E.; Song, D.; Tollemar, V.; Wang, J.; Wang, J.; Mohammed, M.; Wei, Q.; et al. Wnt and BMP signaling crosstalk in regulating dental stem cells: Implications in dental tissue engineering. Genes Dis. 2016, 3, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.R.; Burr, D.B. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: So many hypotheses, so few data. J. Oral Maxillofac. Surg. 2009, 67 (Suppl. 5), 61–70. [Google Scholar] [CrossRef] [PubMed]
- Grawish, M.E.; Grawish, L.M.; Grawish, H.M.; Grawish, M.M.; Holiel, A.A.; Sultan, N.; El-Negoly, S.A. Demineralized Dentin Matrix for Dental and Alveolar Bone Tissues Regeneration: An Innovative Scope Review. Tissue Eng. Regen. Med. 2022, 19, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Murata, M.; Shakya, M.; Yamada, K.; Akazawa, T. Bio-Absorption of Human Dentin-Derived Biomaterial in Sheep Critical-Size Iliac Defects. Materials 2021, 14, 223. [Google Scholar] [CrossRef]
- El Halaby, H.M.; Abu-Seida, A.M.; Fawzy, M.I.; Farid, M.H.; Bastawy, H.A. Evaluation of the regenerative potential of dentin conditioning and naturally derived scaffold for necrotic immature permanent teeth in a dog model. Int. J. Exp. Pathol. 2020, 101, 264–276. [Google Scholar] [CrossRef]
- Imhof, T.; Korkmaz, Y.; Koch, M.; Sengle, G. Schiavinato A EMILIN proteins are novel extracellular constituents of the den-tin-pulp complex. Sci. Rep. 2020, 10, 15320. [Google Scholar] [CrossRef] [PubMed]
- Athirasala, A.; Tahayeri, A.; Thrivikraman, G.; França, C.M.; Monteiro, N.; Tran, V.; Ferracane, J.; Bertassoni, L.E. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication 2018, 10, 024101. [Google Scholar] [CrossRef]
- Marshall, G.W., Jr.; Wu-Magidi, I.C.; Watanabe, L.G.; Inai, N.; Balooch, M.; Kinney, J.H.; Marshall, S.J. Effect of citric acid concentration on dentin demineralization, dehydration, and rehydration: Atomic force microscopy study. J. Biomed. Mater. Res. 1998, 42, 500–507. [Google Scholar] [CrossRef]
- Linde, A. Dentin mineralization and the role of odontoblasts in calcium transport. Connect. Tissue Res. 1995, 33, 163–170. [Google Scholar] [CrossRef]
- Nakashima, M. Dentin induction by implants of autolyzed antigen-extracted allogeneic dentin on amputated pulps of dogs. Endod. Dent. Traumatol. 1989, 5, 279–286. [Google Scholar] [CrossRef]
- Hussain, A.; Tebyaniyan, H.; Khayatan, D. The Role of Epigenetic in Dental and Oral Regenerative Medicine by Different Types of Dental Stem Cells: A Comprehensive Overview. Stem Cells Int. 2022, 2022, 5304860. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, S.; Huang, C.C.; Kang, M.; Lu, Y.; Ravindran, S.; Cooper, L.F. The importance of cellular and exosomal miRNAs in mesenchymal stem cell osteoblastic differentiation. Sci. Rep. 2021, 11, 5953. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Huang, C.-C.; Gajendrareddy, P.; Lu, Y.; Shirazi, S.; Ravindran, S.; Cooper, L.F. Extracellular Vesicles From TNFα Preconditioned MSCs: Effects on Immunomodulation and Bone Regeneration. Front. Immunol. 2022, 13, 878194. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Miya, K.M.; Ravindran, S. 3D Encapsulation and tethering of functionally engineered extracellular vesi-cles to hydrogels. Acta Biomater. 2021, 126, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Controversial Topics in Orthodontics: Can We Reach Consensus? Shroff, Bhavna. 2021. Available online: https://deepblue.lib.umich.edu/handle/2027.42/166464 (accessed on 2 August 2022).
- Kang, M.; Thalji, G.; Huang, C.-C.; Shirazi, S.; Lu, Y.; Ravindran, S.; Cooper, L.F. Macrophage Control of Incipient Bone Formation in Diabetic Mice. Front. Cell Dev. Biol. 2021, 8, 596622. [Google Scholar] [CrossRef]
- Kang, M.; Huang, C.-C.; Lu, Y.; Shirazi, S.; Gajendrareddy, P.; Ravindran, S.; Cooper, L.F. Bone regeneration is mediated by macrophage extracellular vesicles. Bone 2020, 141, 115627. [Google Scholar] [CrossRef]
- Huang, C.; Miya, K.M.; Ravindran, S. Functionally engineered extracellular vesicles improve bone regeneration. Acta Biomater. 2020, 109, 182–194. [Google Scholar] [CrossRef]
- Atsawasuwan, P.; Shirazi, S. Advances in Orthodontic Tooth Movement: Gene Therapy and Molecular Biology Aspect. In Current Approaches in Orthodontics; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; D’Amora, U.; Calzà, L.; Ronca, A.; Tremoli, E.; Ambrosio, L.; Zavan, B. Exosomes of mesenchymal stem cells delivered from methacrylated hyaluronic acid patch improve the regenerative properties of endothelial and dermal cells. Biomater. Adv. 2022, 139, 213000. [Google Scholar] [CrossRef]
- Trentini, M.; Zanotti, F.; Tiengo, E.; Camponogara, F.; Degasperi, M.; Licastro, D.; Lovatti, L.; Zavan, B. An Apple a Day Keeps the Doctor Away: Potential Role of miRNA 146 on Macrophages Treated with Exosomes Derived from Apples. Biomedicines 2022, 10, 415. [Google Scholar] [CrossRef]
- Gardin, C.; Ferroni, L.; Erdoğan, Y.K.; Zanotti, F.; De Francesco, F.; Trentini, M.; Brunello, G.; Ercan, B.; Zavan, B. Nanostructured Modifications of Titanium Surfaces Improve Vascular Regenerative Properties of Exosomes Derived from Mesenchymal Stem Cells: Preliminary In Vitro Results. Nanomaterials 2021, 11, 3452. [Google Scholar] [CrossRef] [PubMed]
- Chachques, J.C.; Gardin, C.; Lila, N.; Ferroni, L.; Migonney, V.; Falentin-Daudre, C.; Zanotti, F.; Trentini, M.; Brunello, G.; Rocca, T.; et al. Elastomeric Cardiowrap Scaffolds Functionalized with Mesenchymal Stem Cells-Derived Exosomes Induce a Positive Modulation in the Inflammatory and Wound Healing Response of Mesenchymal Stem Cell and Macro-phage. Biomedicines 2021, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, B.; Palmieri, F.; Amantea, M.; Nuzzolese, M.; Valletta, R.; Zavan, B.; Vito, D. Promising Scaffold-Free Ap-proaches in Translational Dentistry. Int. J. Environ. Res. Public Health 2020, 17, 3001. [Google Scholar] [CrossRef] [PubMed]




| Gene Symbol | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Product Length (bp) |
|---|---|---|---|
| ALP | TCAGAGGGAAGGAGATAGAGAGTC | AGCCAGAAACCATATGTCAAGAGA | 171 |
| COL1A1 | GAGCCAGCAGATCGAGA | ACCAGTCTCCATGTTGCAGA | 178 |
| DSPP | AGATCCGCACGCAGTAT GAG | AGGTCGCAGGTCAAGGA | 178 |
| MSX | CAGGAGATCACAGAGTATGCCAA | AGATGCGGTGGCTAAAGGTC | 179 |
| OC | TGCATGTGTCTTAGTCTTAGTCACCGCTA | ACTTAGTGCTTACAGGAACCA | 167 |
| ON | TGCATGTGTCTTAGTCTTAGTCACC | GCTAACTTAGTGCTTACAGGAACCA | 186 |
| RUNX2 | TCTTAGGCAGCTCTTTGGGA | TCCCTTGTCATGAAGCCTTGG | 182 |
| CTNNB1 | TAATAAACAGCTCTAAGCCCA | ATCCTCTACATTTAGCCTAGA | 176 |
| CREB | CGTTAGGCGGCTCAATGGGA | TAACTCTTCCGGAAGCCTAAG | 178 |
| p38MAPK | GTTCCGGCTTGCTCTTTGCTA | TCCAATGACATCCGAGCCTAGA | 181 |
| RAC | AACTCATTCCTTTGCCTT | TCAATGATGTTCCCTCCAG | 184 |
| SMAD1 | GGATAGAGGCTTTGGGACCT | TCCTCTTGAAGGGTCCTTGCA | 178 |
| SMAD4 | TGCATTACGATCAAGGCTG | TATTGGATTGGAAGCTGCCCTTG | 167 |
| TGF | CCGGGCAGAATTTGA | TGGTCAATGTGAAGAA GG | 185 |
| WNT | GCTCTTCAAAGGTATTTG | TGGTCCAACCCGTCATC CT | 185 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunello, G.; Zanotti, F.; Scortecci, G.; Sapoznikov, L.; Sivolella, S.; Zavan, B. Dentin Particulate for Bone Regeneration: An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 9283. https://doi.org/10.3390/ijms23169283
Brunello G, Zanotti F, Scortecci G, Sapoznikov L, Sivolella S, Zavan B. Dentin Particulate for Bone Regeneration: An In Vitro Study. International Journal of Molecular Sciences. 2022; 23(16):9283. https://doi.org/10.3390/ijms23169283
Chicago/Turabian StyleBrunello, Giulia, Federica Zanotti, Gerard Scortecci, Lari Sapoznikov, Stefano Sivolella, and Barbara Zavan. 2022. "Dentin Particulate for Bone Regeneration: An In Vitro Study" International Journal of Molecular Sciences 23, no. 16: 9283. https://doi.org/10.3390/ijms23169283
APA StyleBrunello, G., Zanotti, F., Scortecci, G., Sapoznikov, L., Sivolella, S., & Zavan, B. (2022). Dentin Particulate for Bone Regeneration: An In Vitro Study. International Journal of Molecular Sciences, 23(16), 9283. https://doi.org/10.3390/ijms23169283








