Salicylic Acid Regulates Root Gravitropic Growth via Clathrin-Independent Endocytic Trafficking of PIN2 Auxin Transporter in Arabidopsis thaliana
Abstract
:1. Introduction
2. Results
2.1. High-Concentration SA Application Affects Root Gravitropic Growth in a Dose-Dependent Manner
2.2. SA Reduces Auxin Accumulation and Distribution in the Root Apical Meristem
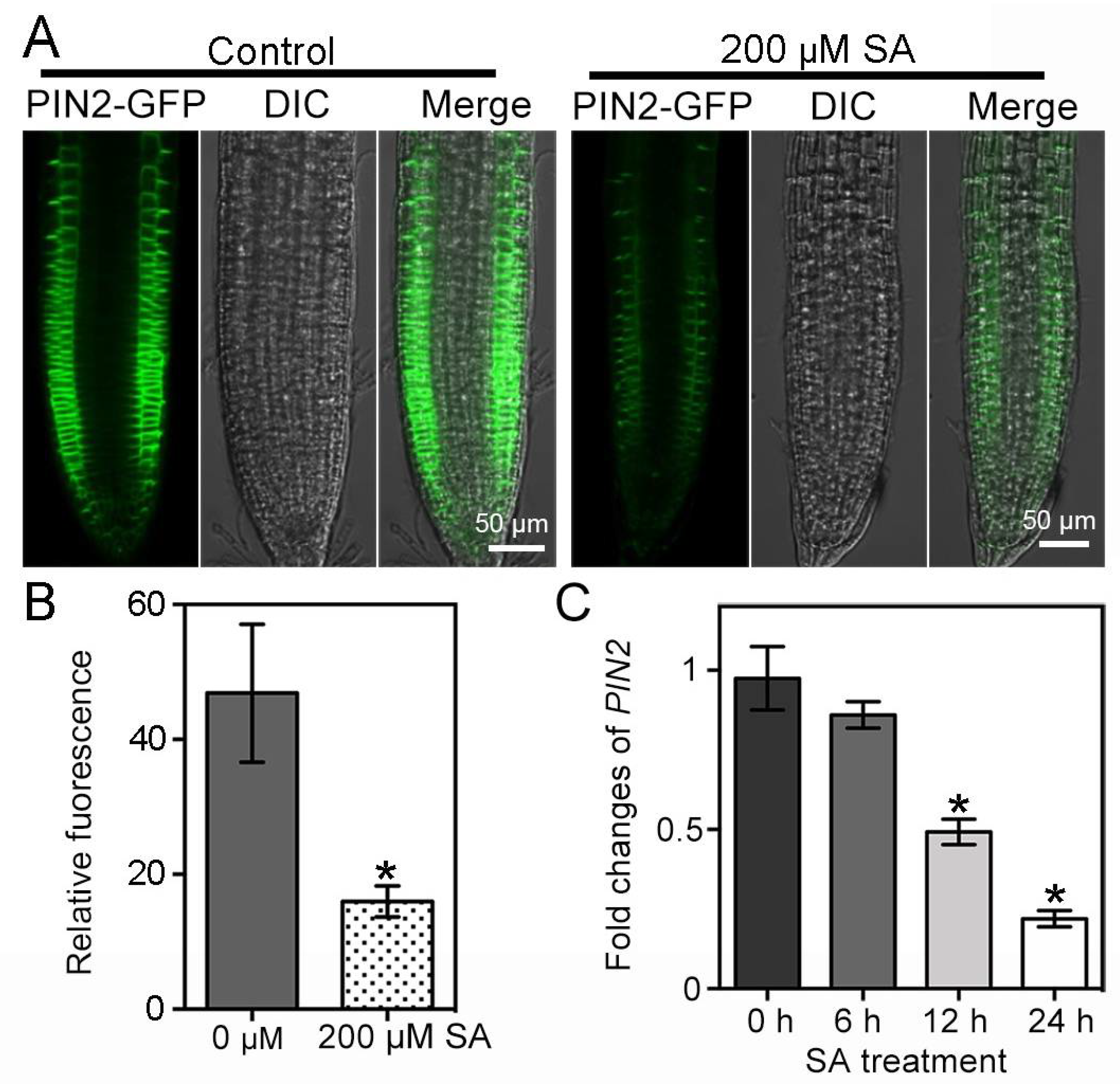
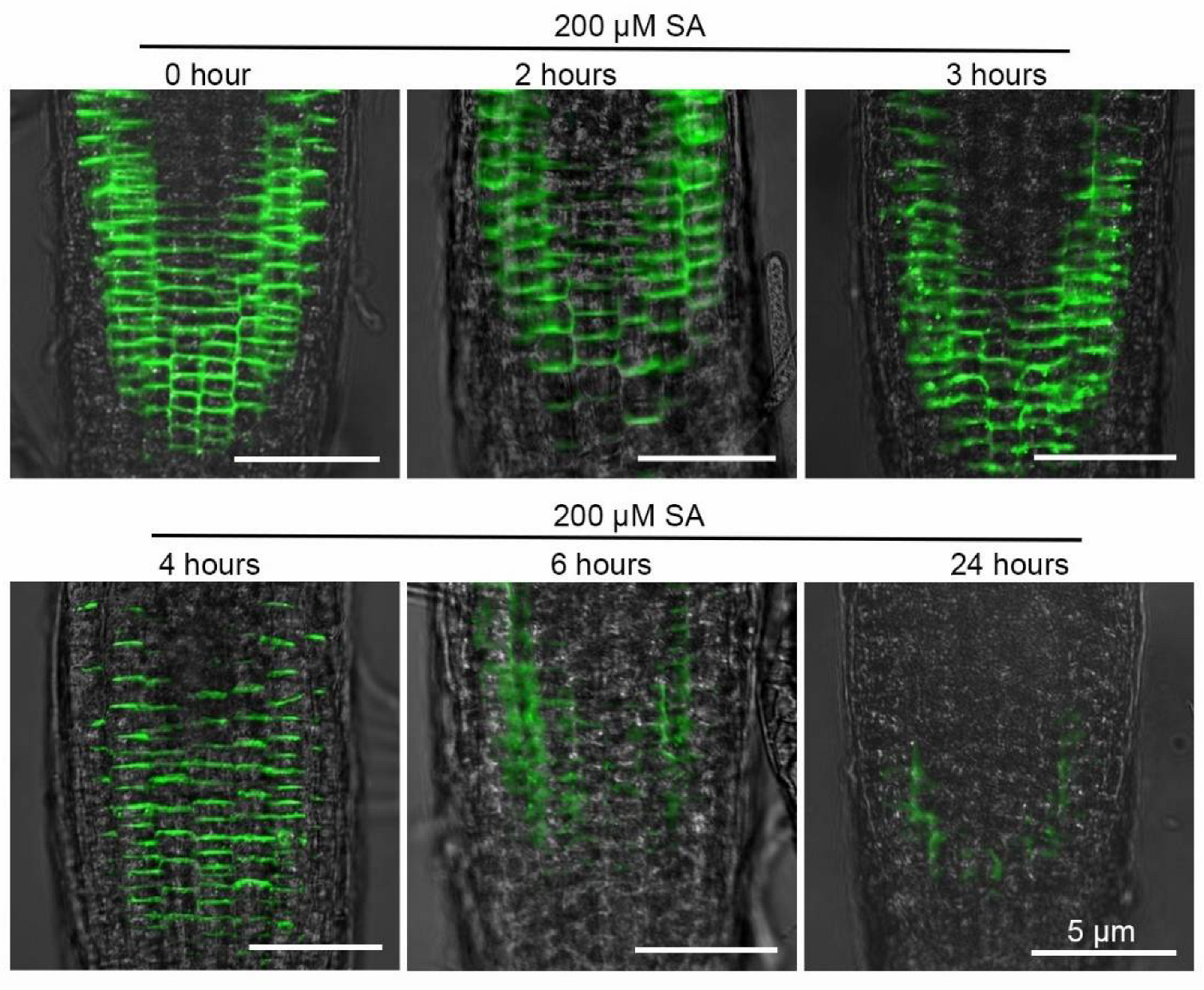
2.3. SA Reduces PIN2 Accumulation and Distribution in the Roots
2.4. SA-Induced PIN2 Internalization Is Independent of Clathrin-Mediated Endocytosis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Growth Condition
4.3. Root Gravitropism Assay
4.4. Confocal Microscopy Analysis
4.5. Quantitative Real-Time PCR (qRT-PCR)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic Acid as a Safe Plant Protector and Growth Regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Peñuelas, J. Photo- and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta 2003, 217, 758–766. [Google Scholar] [CrossRef]
- Chini, A.; Grant, J.J.; Seki, M.; Shinozaki, K.; Loake, G.J. Drought tolerance established by enhanced expression of the CC-NBS-LRR gene, ADR1, requires salicylic acid, EDS1 and ABI1. Plant J. 2004, 38, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Borsani, O.; Valpuesta, V.; Botella, M.A. Evidence for a Role of Salicylic Acid in the Oxidative Damage Generated by NaCl and Osmotic Stress in Arabidopsis Seedlings. Plant Physiol. 2001, 126, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Janda, T.; Szalai, G.; Tari, I.; Páldi, E. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 1999, 208, 175–180. [Google Scholar] [CrossRef]
- Kang, H.-M.; Saltveit, M.E. Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol. Plant. 2002, 115, 571–576. [Google Scholar] [CrossRef]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.-J. Salicylic Acid Alleviates the Cadmium Toxicity in Barley Seedlings. Plant Physiol. 2003, 132, 272–281. [Google Scholar] [CrossRef]
- Yang, Z.-M.; Wang, J.; Wang, S.-H.; Xu, L.-L. Salicylic acid-induced aluminum tolerance by modulation of citrate efflux from roots of Cassia tora L. Planta 2003, 217, 168–174. [Google Scholar] [CrossRef]
- Freeman, J.L.; Garcia, D.; Kim, D.; Hopf, A.; Salt, D.E. Constitutively Elevated Salicylic Acid Signals Glutathione-Mediated Nickel Tolerance in Thlaspi Nickel Hyperaccumulators. Plant Physiol. 2005, 137, 1082–1091. [Google Scholar] [CrossRef]
- Larkindale, J.; Knight, M.R. Protection against Heat Stress-Induced Oxidative Damage in Arabidopsis Involves Calcium, Abscisic Acid, Ethylene, and Salicylic Acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [CrossRef]
- Larkindale, J.; Hall, J.D.; Knight, M.; Vierling, E. Heat Stress Phenotypes of Arabidopsis Mutants Implicate Multiple Signaling Pathways in the Acquisition of Thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed]
- Paciorek, T.; Zažímalová, E.; Ruthardt, N.; Petrášek, J.; Stierhof, Y.-D.; Kleine-Vehn, J.; Morris, D.A.; Emans, N.; Jürgens, G.; Geldner, N.; et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 2005, 435, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Bagautdinova, Z.Z.; Omelyanchuk, N.; Tyapkin, A.V.; Kovrizhnykh, V.V.; Lavrekha, V.V.; Zemlyanskaya, E.V. Salicylic Acid in Root Growth and Development. Int. J. Mol. Sci. 2022, 23, 2228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, J.; Yuan, J.; Wang, X.-L.; Zhao, Q.P.; Kong, P.T.; Zhang, X. NITRIC OXIDE-ASSOCIATED PROTEIN1 (At NOA 1) is essential for salicylic acid-induced root waving in Arabidopsis thaliana. New Phytol. 2015, 207, 211–224. [Google Scholar] [CrossRef]
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Mironova, V.V. Salicylic Acid Affects Root Meristem Patterning via Auxin Distribution in a Concentration-Dependent Manner. Plant Physiol. 2019, 180, 1725–1739. [Google Scholar] [CrossRef]
- Armengot, L.; Marquès-Bueno, M.M.; Soria-Garcia, A.; Müller, M.; Munné-Bosch, S.; Martínez, M.C. Functional interplay between protein kinase CK2 and salicylic acid sustains PIN transcriptiona expression and root development. Plant J. 2014, 78, 411–423. [Google Scholar] [CrossRef]
- Agtuca, B.; Rieger, E.; Hilger, K.; Song, L.; Robert, C.; Erb, M.; Karve, A.; Ferrieri, R.A. Carbon-11 Reveals Opposing Roles of Auxin and Salicylic Acid in Regulating Leaf Physiology, Leaf Metabolism, and Resource Allocation Patterns that Impact Root Growth in Zea mays. J. Plant Growth Regul. 2014, 33, 328–339. [Google Scholar] [CrossRef]
- Gutiérrez-Coronado, M.A.; Trejo-López, C.; Larqué-Saavedra, A. Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol. Biochem. 1998, 36, 563–565. [Google Scholar] [CrossRef]
- San-Miguel, R.; Gutiérrez, M.; Larqué-Saavedra, A. Salicylic Acid Increases the Biomass Accumulation of Pinus patula. South. J. Appl. For. 2003, 27, 52–54. [Google Scholar] [CrossRef]
- Du, Y.; Tejos, R.; Beck, M.; Himschoot, E.; Li, H.; Robatzek, S.; Vanneste, S.; Friml, J. Salicylic acid interferes with clathrin-mediated endocytic protein trafficking. Proc. Natl. Acad. Sci. USA 2013, 110, 7946–7951. [Google Scholar] [CrossRef] [PubMed]
- Cholodny, N. Beitrage zur Analyse der geotropischen Reaktion. Jahrb. Wiss. Bot. 1926, 65, 447–459. [Google Scholar]
- Went, F. On growth-accelerating substances in the coleoptile of Avena sativa. Proc. Kon. Akad. Wetensch. Amst. 1926, 30, 10–19. [Google Scholar]
- Young, L.M.; Evans, M.L.; Hertel, R. Correlations between Gravitropic Curvature and Auxin Movement across Gravistimulated Roots of Zea mays. Plant Physiol. 1990, 92, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Dolan, L. Pointing roots in the right direction: The role of auxin transport in response to gravity. Genes Dev. 1998, 12, 2091–2095. [Google Scholar] [CrossRef]
- Rosen, E.; Chen, R.; Masson, P.H. Root gravitropism: A complex response to a simple stimulus? Trends Plant Sci. 1999, 4, 407–412. [Google Scholar] [CrossRef]
- Joo, J.H.; Bae, Y.S.; Lee, J.S. Role of Auxin-Induced Reactive Oxygen Species in Root Gravitropism. Plant Physiol. 2001, 126, 1055–1060. [Google Scholar] [CrossRef]
- Müller, A.; Guan, C.; Gälweiler, L.; Tänzler, P.; Huijser, P.; Marchant, A.; Parry, G.; Bennett, M.; Wisman, E.; Palme, K. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998, 17, 6903–6911. [Google Scholar] [CrossRef]
- Geisler, M.; Wang, B.; Zhu, J. Auxin transport during root gravitropism: Transporters and techniques. Plant Biol. 2014, 16, 50–57. [Google Scholar] [CrossRef]
- Lin, D.; Yao, H.; Jia, L.; Tan, J.; Xu, Z.; Zheng, W.; Xue, H. Phospholipase D-derived phosphatidic acid promotes root hair development under phosphorus deficiency by suppressing vacuolar degradation of PIN-FORMED2. New Phytol. 2020, 226, 142–155. [Google Scholar] [CrossRef]
- Sasayama, D.; Ganguly, A.; Park, M.; Cho, H.-T. The M3 phosphorylation motif has been functionally conserved for intracellular trafficking of long-looped PIN-FORMEDs in the Arabidopsis root hair cell. BMC Plant Biol. 2013, 13, 189. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Ma, Z.; Wang, D.; Sun, Y.; Wen, C.; Huang, D.; Chen, Z.; Yang, L.; Tan, S.; Li, R.; et al. Salicylic acid regulates PIN2 auxin transporter hyperclustering and root gravitropic growth via Remorin-dependent lipid nanodomain organisation in Arabidopsis thaliana. New Phytol. 2021, 229, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Philosoph-Hadas, S.; Friedman, H.; Meir, S. Gravitropic Bending and Plant Hormones. Vitam. Horm. 2005, 72, 31–78. [Google Scholar] [CrossRef]
- Tan, S.; Abas, M.; Verstraeten, I.; Glanc, M.; Molnár, G.; Hajný, J.; Lasák, P.; Petřík, I.; Russinova, E.; Petrášek, J.; et al. Salicylic Acid Targets Protein Phosphatase 2A to Attenuate Growth in Plants. Curr. Biol. 2020, 30, 381–395. [Google Scholar] [CrossRef]
- Ottenschläger, I.; Wolff, P.; Wolverton, C.; Bhalerao, R.P.; Sandberg, G.; Ishikawa, H.; Evans, M.; Palme, K. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc. Natl. Acad. Sci. USA 2003, 100, 2987–2991. [Google Scholar] [CrossRef]
- Brunoud, G.; Wells, D.; Oliva, M.; Larrieu, A.; Mirabet, V.; Burrow, A.H.; Beeckman, T.; Kepinski, S.; Traas, J.; Bennett, M.; et al. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 2012, 482, 103–106. [Google Scholar] [CrossRef]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Yang, Y.; Li, R.; He, Q.; Fang, X.; Luu, D.-T.; Maurel, C.; Lin, J. Single-Molecule Analysis of PIP2;1 Dynamics and Partitioning Reveals Multiple Modes of Arabidopsis Plasma Membrane Aquaporin Regulation. Plant Cell 2011, 23, 3780–3797. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Tang, Y.; Tang, R.; Jing, Y.; Zhang, C.; Zhang, B.; Li, X.; Cui, Y.; Zhang, C.; et al. Arabidopsis choline transporter-like 1 (CTL1) regulates secretory trafficking of auxin transporters to control seedling growth. PLoS Biol. 2017, 15, e2004310. [Google Scholar] [CrossRef]
- Zhang, J.; Mazur, E.; Balla, J.; Gallei, M.; Kalousek, P.; Medveďová, Z.; Li, Y.; Wang, Y.; Prát, T.; Vasileva, M.; et al. Strigolactones inhibit auxin feedback on PIN-dependent auxin transport canalization. Nat. Commun. 2020, 11, 3508. [Google Scholar] [CrossRef]
- Koltai, H. Cellular events of strigolactone signalling and their crosstalk with auxin in roots. J. Exp. Bot. 2015, 66, 4855–4861. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Dempsey, D.M.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Essemine, J.; Pang, X.; Chen, H.; Jin, J.; Cai, W. Abscisic Acid Regulates the Root Growth Trajectory by Reducing Auxin Transporter PIN2 Protein Levels in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 632676. [Google Scholar] [CrossRef] [PubMed]
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 603–612. [Google Scholar] [CrossRef]
- Retzer, K.; Akhmanova, M.; Konstantinova, N.; Malínská, K.; Leitner, J.; Petrasek, J.; Luschnig, C. Brassinosteroid signaling delimits root gravitropism via sorting of the Arabidopsis PIN2 auxin transporter. Nat. Commun. 2019, 10, 5516. [Google Scholar] [CrossRef]
- Sandvig, K.; Kavaliauskiene, S.; Skotland, T. Clathrin-independent endocytosis: An increasing degree of complexity. Histochem. Cell Biol. 2018, 150, 107–118. [Google Scholar] [CrossRef]
- Wu, Y.; Chang, Y.; Luo, L.; Tian, W.; Gong, Q.; Liu, X. Abscisic acid employs NRP-dependent PIN2 vacuolar degradation to suppress auxin-mediated primary root elongation in Arabidopsis. New Phytol. 2022, 233, 297–312. [Google Scholar] [CrossRef]
- Zwiewka, M.; Bielach, A.; Tamizhselvan, P.; Madhavan, S.; Ryad, E.E.; Tan, S.; Hrtyan, M.; Dobrev, P.; Vanková, R.; Friml, J.; et al. Root Adaptation to H2O2-Induced Oxidative Stress by ARF-GEF BEN1- and Cytoskeleton-Mediated PIN2 Trafficking. Plant Cell Physiol. 2019, 60, 255–273. [Google Scholar] [CrossRef]
- Ki, D.; Sasayama, D.; Cho, H.-T. The M3 Phosphorylation Site Is Required for Trafficking and Biological Roles of PIN-FORMED1, 2, and 7 in Arabidopsis. Front. Plant Sci. 2016, 7, 1479. [Google Scholar] [CrossRef]
- Ortiz-Morea, F.A.; Savatin, D.V.; Dejonghe, W.; Kumar, R.; Luo, Y.; Adamowski, M.; Van den Begin, J.; Dressano, K.; de Oliveira, G.P.; Zhao, X.; et al. Danger-associated peptide signaling in Arabidopsis requires clathrin. Proc. Natl. Acad. Sci. USA 2016, 113, 11028–11033. [Google Scholar] [CrossRef]
- Jing, Y.; Zheng, X.; Zhang, D.; Shen, N.; Wang, Y.; Yang, L.; Fu, A.; Shi, J.; Zhao, F.; Lan, W.; et al. Danger-Associated Peptides Interact with PIN-Dependent Local Auxin Distribution to Inhibit Root Growth in Arabidopsis. Plant Cell 2019, 31, 1767–1787. [Google Scholar] [CrossRef]
- Puri, V.; Watanabe, R.; Singh, R.D.; Dominguez, M.; Brown, J.C.; Wheatley, C.L.; Marks, D.L.; Pagano, R.E. Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J. Cell Biol. 2001, 154, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Lamaze, C. Clathrin-Dependent or Not: Is It Still the Question? Traffic 2002, 3, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.; Gong, C.; Patel, S.; Lee, J.-K. Regulation of Plant Mineral Nutrition by Signal Molecules. Microorganisms 2021, 9, 774. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Ge, H.; Chen, J.; Li, X.; Yang, L.; Zhang, H.; Wang, Y. Salicylic Acid Regulates Root Gravitropic Growth via Clathrin-Independent Endocytic Trafficking of PIN2 Auxin Transporter in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 9379. https://doi.org/10.3390/ijms23169379
Zhou H, Ge H, Chen J, Li X, Yang L, Zhang H, Wang Y. Salicylic Acid Regulates Root Gravitropic Growth via Clathrin-Independent Endocytic Trafficking of PIN2 Auxin Transporter in Arabidopsis thaliana. International Journal of Molecular Sciences. 2022; 23(16):9379. https://doi.org/10.3390/ijms23169379
Chicago/Turabian StyleZhou, Houjun, Haiman Ge, Jiahong Chen, Xueqin Li, Lei Yang, Hongxia Zhang, and Yuan Wang. 2022. "Salicylic Acid Regulates Root Gravitropic Growth via Clathrin-Independent Endocytic Trafficking of PIN2 Auxin Transporter in Arabidopsis thaliana" International Journal of Molecular Sciences 23, no. 16: 9379. https://doi.org/10.3390/ijms23169379





