Cannabinoids and Chronic Liver Diseases
Abstract
:1. Introduction
2. Cannabinoids and the Endocannabinoid System (eCBS)
2.1. The eCBS
2.2. Phytocannabinoids
3. The Endocannabinoid System in Chronic Liver Diseases
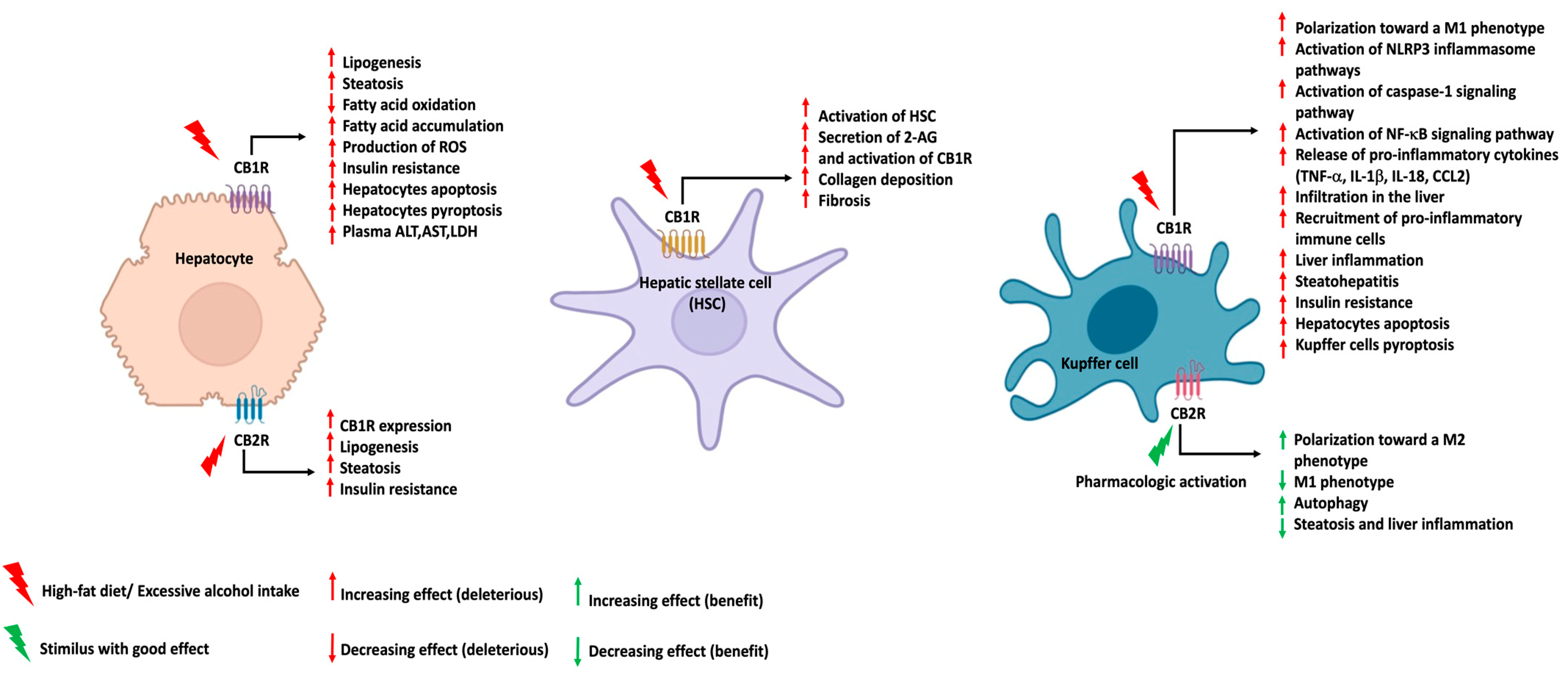
3.1. Hepatic Endocannabinoid System in Normal Physiology
3.2. Hepatic Endocannabinoid System in NAFLD and NASH
3.3. Hepatic Endocannabinoid System in ALD
3.4. The Hepatic Endocannabinoid System in Chronic Viral Hepatitis
4. Impact of Cannabis Intake and Cannabinoid-Based Medicine on NAFLD, NASH, ALD, and HCV-Induced Liver Disorders: Evidence from Preclinical, Observational, and Clinical-Trial Studies
4.1. Cannabis Use and CLD
4.1.1. Cannabis Use and NAFLD and NASH
4.1.2. Cannabis Use and ALD
4.1.3. Cannabis Use and HCV/HBV-Associated CLD
4.2. Cannabinoid-Based Medicine and CLDs
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makri, E.; Goulas, A.; Polyzos, S.A. Epidemiology, Pathogenesis, Diagnosis and Emerging Treatment of Nonalcoholic Fatty Liver Disease. Arch. Med. Res. 2021, 52, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Choi, S.E.; Jeong, W.I. Hepatic Cannabinoid Signaling in the Regulation of Alcohol-Associated Liver Disease. Alcohol Res. 2021, 41, 12. [Google Scholar] [CrossRef]
- WHO. Hepatitis. WHO (World Health Organization). 2021. Available online: https://www.who.int/health-topics/hepatitis#tab=tab_1 (accessed on 24 April 2022).
- Lim, H.K.; Jeffrey, G.P.; Ramm, G.A.; Soekmadji, C. Pathogenesis of Viral Hepatitis-Induced Chronic Liver Disease: Role of Extracellular Vesicles. Front. Cell. Infect. Microbiol. 2020, 10, 587628. [Google Scholar] [CrossRef]
- Chopra, S.L.M. Management of Nonalcoholic Fatty Liver Disease in Adults. Up To Date. 2022. Available online: https://www.uptodate.com/contents/management-of-nonalcoholic-fatty-liver-disease-in-adults (accessed on 15 July 2022).
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Berk, K.; Bzdega, W.; Konstantynowicz-Nowicka, K.; Charytoniuk, T.; Zywno, H.; Chabowski, A. Phytocannabinoids-A Green Approach toward Non-Alcoholic Fatty Liver Disease Treatment. J. Clin. Med. 2021, 10, 393. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bab, I.; Bíró, T.; Cabral, G.A.; Dey, S.K.; Di Marzo, V.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296. [Google Scholar] [CrossRef] [Green Version]
- Iannotti, F.A.; Di Marzo, V.; Petrosino, S. Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. Prog. Lipid Res. 2016, 62, 107–128. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Vitale, R.M. The Endocannabinoid System and PPARs: Focus on Their Signalling Crosstalk, Action and Transcriptional Regulation. Cells 2021, 10, 586. [Google Scholar] [CrossRef]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; De Petrocellis, L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: A further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr. Med. Chem. 2010, 17, 1430–1449. [Google Scholar] [CrossRef] [PubMed]
- Pistis, M.; Melis, M. From surface to nuclear receptors: The endocannabinoid family extends its assets. Curr. Med. Chem. 2010, 17, 1450–1467. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Picardi, P.; Pallottini, V.; Martini, C.; Petrosino, S.; Proto, M.C.; Vitale, M.; Laezza, C.; Gazzerro, P.; Di Marzo, V.; et al. Anandamide drives cell cycle progression through CB1 receptors in a rat model of synchronized liver regeneration. J. Cell Physiol. 2015, 230, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, S.V.; Schwabe, R.F. Endocannabinoids and liver disease. II. Endocannabinoids in the pathogenesis and treatment of liver fibrosis. Am. J. Physiol. Gastrointest Liver Physiol. 2008, 294, G357–G362. [Google Scholar] [CrossRef] [PubMed]
- Patsenker, E.; Stickel, F. Cannabinoids in liver diseases. Clin. Liver Dis. 2016, 7, 21–25. [Google Scholar] [CrossRef]
- Silvestri, C.; Di Marzo, V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013, 17, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Mai, P.; Yang, L.; Tian, L.; Wang, L.; Jia, S.; Zhang, Y.; Liu, X.; Yang, L.; Li, L. Endocannabinoid System Contributes to Liver Injury and Inflammation by Activation of Bone Marrow-Derived Monocytes/Macrophages in a CB1-Dependent Manner. J. Immunol. 2015, 195, 3390–3401. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Mukhopadhyay, P.; Cao, Z.; Wang, H.; Feng, D.; Haskó, G.; Mechoulam, R.; Gao, B.; Pacher, P. Cannabidiol attenuates alcohol-induced liver steatosis, metabolic dysregulation, inflammation and neutrophil-mediated injury. Sci. Rep. 2017, 7, 12064. [Google Scholar] [CrossRef] [Green Version]
- Julien, B.; Grenard, P.; Teixeira-Clerc, F.; Van Nhieu, J.T.; Li, L.; Karsak, M.; Zimmer, A.; Mallat, A.; Lotersztajn, S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 2005, 128, 742–755. [Google Scholar] [CrossRef]
- Mallat, A.; Lotersztajn, S. Endocannabinoids as novel mediators of liver diseases. J. Endocrinol. Investig. 2006, 29, 58–65. [Google Scholar]
- Kunos, G. Interactions Between Alcohol and the Endocannabinoid System. Alcohol Clin. Exp. Res. 2020, 44, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; De Petrocellis, L.; Di Marzo, V. From Phytocannabinoids to Cannabinoid Receptors and Endocannabinoids: Pleiotropic Physiological and Pathological Roles Through Complex Pharmacology. Physiol. Rev. 2016, 96, 1593–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopustinskiene, D.M.; Masteikova, R.; Lazauskas, R.; Bernatoniene, J. Cannabis sativa L. Bioactive Compounds and Their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Zimmermann-Klemd, A.M.; Fiebich, B.L.; Heinrich, M.; Gründemann, C.; Steinberger, P.; Kowarschik, S.; Huber, R. Immunosuppressive activity of non-psychoactive Cannabis sativa L. extract on the function of human T lymphocytes. Int. Immunopharmacol. 2022, 103, 108448. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.L.; Springs, A.E.; Kaminski, N.E. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochem. Pharmacol. 2008, 76, 726–737. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, P.; Rajesh, M.; Horváth, B.; Bátkai, S.; Park, O.; Tanchian, G.; Gao, R.Y.; Patel, V.; Wink, D.A.; Liaudet, L.; et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic. Biol. Med. 2011, 50, 1368–1381. [Google Scholar] [CrossRef] [Green Version]
- Lim, M.P.; Devi, L.A.; Rozenfeld, R. Cannabidiol causes activated hepatic stellate cell death through a mechanism of endoplasmic reticulum stress-induced apoptosis. Cell Death Dis. 2011, 2, e170. [Google Scholar] [CrossRef]
- Yang, L.; Rozenfeld, R.; Wu, D.; Devi, L.A.; Zhang, Z.; Cederbaum, A. Cannabidiol protects liver from binge alcohol-induced steatosis by mechanisms including inhibition of oxidative stress and increase in autophagy. Free Radic. Biol. Med. 2014, 68, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, C.; Paris, D.; Martella, A.; Melck, D.; Guadagnino, I.; Cawthorne, M.; Motta, A.; Di Marzo, V. Two non-psychoactive cannabinoids reduce intracellular lipid levels and inhibit hepatosteatosis. J. Hepatol. 2015, 62, 1382–1390. [Google Scholar] [CrossRef]
- Huang, Y.; Wan, T.; Pang, N.; Zhou, Y.; Jiang, X.; Li, B.; Gu, Y.; Huang, Y.; Ye, X.; Lian, H.; et al. Cannabidiol protects livers against nonalcoholic steatohepatitis induced by high-fat high cholesterol diet via regulating NF-κB and NLRP3 inflammasome pathway. J. Cell Physiol. 2019, 234, 21224–21234. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Gu, Y.; Huang, Y.; Zhou, Y.; Pang, N.; Luo, J.; Tang, Z.; Zhang, Z.; Yang, L. CBD Alleviates Liver Injuries in Alcoholics With High-Fat High-Cholesterol Diet Through Regulating NLRP3 Inflammasome-Pyroptosis Pathway. Front. Pharmacol. 2021, 12, 724747. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, K.A.; Ratcliffe, S.H.; Barrett, D.A.; Thomas, E.L.; Stott, C.; Bell, J.D.; O’Sullivan, S.E.; Tan, G.D. Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes Care 2016, 39, 1777–1786. [Google Scholar] [CrossRef] [Green Version]
- Clinicaltrials.gov. Study to Assess the Effect of Cannabidiol on Liver Fat Levels in Subjects with Fatty Liver Disease. 2018. Available online: https://clinicaltrials.gov/ct2/show/results/NCT01284634 (accessed on 23 May 2022).
- Riedel, G.; Fadda, P.; McKillop-Smith, S.; Pertwee, R.G.; Platt, B.; Robinson, L. Synthetic and plant-derived cannabinoid receptor antagonists show hypophagic properties in fasted and non-fasted mice. Br. J. Pharmacol. 2009, 156, 1154–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bátkai, S.; Mukhopadhyay, P.; Horváth, B.; Rajesh, M.; Gao, R.Y.; Mahadevan, A.; Amere, M.; Battista, N.; Lichtman, A.H.; Gauson, L.A.; et al. Δ8-Tetrahydrocannabivarin prevents hepatic ischaemia/reperfusion injury by decreasing oxidative stress and inflammatory responses through cannabinoid CB2 receptors. Br. J. Pharmacol. 2012, 165, 2450–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wargent, E.T.; Zaibi, M.S.; Silvestri, C.; Hislop, D.C.; Stocker, C.J.; Stott, C.G.; Guy, G.W.; Duncan, M.; Di Marzo, V.; Cawthorne, M.A. The cannabinoid Δ(9)-tetrahydrocannabivarin (THCV) ameliorates insulin sensitivity in two mouse models of obesity. Nutr. Diabetes 2013, 3, e68. [Google Scholar] [CrossRef] [PubMed]
- Palomares, B.; Ruiz-Pino, F.; Garrido-Rodriguez, M.; Eugenia Prados, M.; Sánchez-Garrido, M.A.; Velasco, I.; Vazquez, M.J.; Nadal, X.; Ferreiro-Vera, C.; Morrugares, R.; et al. Tetrahydrocannabinolic acid A (THCA-A) reduces adiposity and prevents metabolic disease caused by diet-induced obesity. Biochem. Pharmacol. 2020, 171, 113693. [Google Scholar] [CrossRef]
- Carmona-Hidalgo, B.; González-Mariscal, I.; García-Martín, A.; Prados, M.E.; Ruiz-Pino, F.; Appendino, G.; Tena-Sempere, M.; Muñoz, E. Δ9-Tetrahydrocannabinolic Acid markedly alleviates liver fibrosis and inflammation in mice. Phytomedicine 2021, 81, 153426. [Google Scholar] [CrossRef]
- Romero-Zerbo, S.Y.; García-Fernández, M.; Espinosa-Jiménez, V.; Pozo-Morales, M.; Escamilla-Sánchez, A.; Sánchez-Salido, L.; Lara, E.; Cobo-Vuilleumier, N.; Rafacho, A.; Olveira, G.; et al. The Atypical Cannabinoid Abn-CBD Reduces Inflammation and Protects Liver, Pancreas, and Adipose Tissue in a Mouse Model of Prediabetes and Non-alcoholic Fatty Liver Disease. Front. Endocrinol. 2020, 11, 103. [Google Scholar] [CrossRef]
- Louvet, A.; Teixeira-Clerc, F.; Chobert, M.N.; Deveaux, V.; Pavoine, C.; Zimmer, A.; Pecker, F.; Mallat, A.; Lotersztajn, S. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology 2011, 54, 1217–1226. [Google Scholar] [CrossRef]
- Melgar-Lesmes, P.; Perramon, M.; Jiménez, W. Roles of the Hepatic Endocannabinoid and Apelin Systems in the Pathogenesis of Liver Fibrosis. Cells 2019, 8, 1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegmund, S.V.; Wojtalla, A.; Schlosser, M.; Zimmer, A.; Singer, M.V. Fatty acid amide hydrolase but not monoacyl glycerol lipase controls cell death induced by the endocannabinoid 2-arachidonoyl glycerol in hepatic cell populations. Biochem. Biophys. Res. Commun. 2013, 437, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, B.; Cinar, R.; Yin, S.; Liu, J.; Tam, J.; Godlewski, G.; Harvey-White, J.; Mordi, I.; Cravatt, B.F.; Lotersztajn, S.; et al. Hyperactivation of anandamide synthesis and regulation of cell-cycle progression via cannabinoid type 1 (CB1) receptors in the regenerating liver. Proc. Natl. Acad. Sci. USA 2011, 108, 6323–6328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.Y.; Alexa, K.; Cortes, M.; Schatzman-Bone, S.; Kim, A.J.; Mukhopadhyay, B.; Cinar, R.; Kunos, G.; North, T.E.; Goessling, W. Cannabinoid receptor signaling regulates liver development and metabolism. Development 2016, 143, 609–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Schmidt, S.F.; Friedman, J.M. Developmental role for endocannabinoid signaling in regulating glucose metabolism and growth. Diabetes 2013, 62, 2359–2367. [Google Scholar] [CrossRef] [Green Version]
- De Gottardi, A.; Spahr, L.; Ravier-Dall’Antonia, F.; Hadengue, A. Cannabinoid receptor 1 and 2 agonists increase lipid accumulation in hepatocytes. Liver. Int. 2010, 30, 1482–1489. [Google Scholar] [CrossRef]
- Bazwinsky-Wutschke, I.; Zipprich, A.; Dehghani, F. Endocannabinoid System in Hepatic Glucose Metabolism, Fatty Liver Disease, and Cirrhosis. Int. J. Mol. Sci. 2019, 20, 2516. [Google Scholar] [CrossRef] [Green Version]
- Chanda, D.; Kim, Y.H.; Kim, D.K.; Lee, M.W.; Lee, S.Y.; Park, T.S.; Koo, S.H.; Lee, C.H.; Choi, H.S. Activation of cannabinoid receptor type 1 (Cb1r) disrupts hepatic insulin receptor signaling via cyclic AMP-response element-binding protein H (Crebh)-mediated induction of Lipin1 gene. J. Biol. Chem. 2012, 287, 38041–38049. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Hong, W.; Lu, S.; Li, Y.; Guan, Y.; Weng, X.; Feng, Z. The NLRP3 Inflammasome in Non-Alcoholic Fatty Liver Disease and Steatohepatitis: Therapeutic Targets and Treatment. Front. Pharmacol. 2022, 13, 780496. [Google Scholar] [CrossRef]
- Mallat, A.; Lotersztajn, S. Cannabinoid receptors as novel therapeutic targets for the management of non-alcoholic steatohepatitis. Diabetes Metab. 2008, 34, 680–684. [Google Scholar] [CrossRef] [Green Version]
- Parfieniuk, A.; Flisiak, R. Role of cannabinoids in chronic liver diseases. World J. Gastroenterol. 2008, 14, 6109–6114. [Google Scholar] [CrossRef] [PubMed]
- Cota, D.; Genghini, S.; Pasquali, R.; Pagotto, U. Antagonizing the cannabinoid receptor type 1: A dual way to fight obesity. J. Endocrinol. Investig. 2003, 26, 1041–1044. [Google Scholar] [CrossRef]
- Liu, J.; Cinar, R.; Xiong, K.; Godlewski, G.; Jourdan, T.; Lin, Y.; Ntambi, J.M.; Kunos, G. Monounsaturated fatty acids generated via stearoyl CoA desaturase-1 are endogenous inhibitors of fatty acid amide hydrolase. Proc. Natl. Acad. Sci. USA 2013, 110, 18832–18837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osei-Hyiaman, D.; DePetrillo, M.; Pacher, P.; Liu, J.; Radaeva, S.; Bátkai, S.; Harvey-White, J.; Mackie, K.; Offertáler, L.; Wang, L.; et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Investig. 2005, 115, 1298–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osei-Hyiaman, D.; Liu, J.; Zhou, L.; Godlewski, G.; Harvey-White, J.; Jeong, W.I.; Bátkai, S.; Marsicano, G.; Lutz, B.; Buettner, C.; et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J. Clin. Investig. 2008, 118, 3160–3169. [Google Scholar] [CrossRef]
- Dibba, P.; Li, A.; Cholankeril, G.; Iqbal, U.; Gadiparthi, C.; Khan, M.A.; Kim, D.; Ahmed, A. Mechanistic Potential and Therapeutic Implications of Cannabinoids in Nonalcoholic Fatty Liver Disease. Medicines 2018, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Laudermilk, L.; Ware, J.; Rosa, T.; Mathews, K.; Gay, E.; Amato, G.; Maitra, R. Peripherally Selective CB1 Receptor Antagonist Improves Symptoms of Metabolic Syndrome in Mice. ACS Pharmacol. Transl. Sci. 2021, 4, 757–764. [Google Scholar] [CrossRef]
- Tam, J.; Vemuri, V.K.; Liu, J.; Bátkai, S.; Mukhopadhyay, B.; Godlewski, G.; Osei-Hyiaman, D.; Ohnuma, S.; Ambudkar, S.V.; Pickel, J.; et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J. Clin. Investig. 2010, 120, 2953–2966. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Tian, L.; Zhang, Z.; Zhou, X.; Ji, X.; Liu, F.; Dong, C.; Hou, L.; Zhao, X.; Chang, N.; et al. Cannabinoid Receptor 1/miR-30b-5p Axis Governs Macrophage NLRP3 Expression and Inflammasome Activation in Liver Inflammatory Disease. Mol. Ther. Nucleic Acids 2020, 20, 725–738. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Q.; Liang, G.; Franks, T.; Boucher, M.; Bence, K.K.; Lu, M.; Castorena, C.M.; Zhao, S.; Elmquist, J.K.; et al. Cannabinoid receptor 1 signaling in hepatocytes and stellate cells does not contribute to NAFLD. J. Clin. Investig. 2021, 131, e152242. [Google Scholar] [CrossRef]
- Kunos, G.; Jourdan, T.; Tam, J. Do endocannabinoids acting via hepatic CB-1 contribute to NAFLD and hepatic insulin resistance? J. Clin. Investig. 2022, 132, e155330. [Google Scholar] [CrossRef]
- Deveaux, V.; Cadoudal, T.; Ichigotani, Y.; Teixeira-Clerc, F.; Louvet, A.; Manin, S.; Nhieu, J.T.; Belot, M.P.; Zimmer, A.; Even, P.; et al. Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis. PLoS ONE 2009, 4, e5844. [Google Scholar] [CrossRef] [PubMed]
- Agudo, J.; Martin, M.; Roca, C.; Molas, M.; Bura, A.S.; Zimmer, A.; Bosch, F.; Maldonado, R. Deficiency of CB2 cannabinoid receptor in mice improves insulin sensitivity but increases food intake and obesity with age. Diabetologia 2010, 53, 2629–2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldassarre, M.; Giannone, F.A.; Napoli, L.; Tovoli, A.; Ricci, C.S.; Tufoni, M.; Caraceni, P. The endocannabinoid system in advanced liver cirrhosis: Pathophysiological implication and future perspectives. Liver Int. 2013, 33, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Kamikubo, R.; Kai, K.; Tsuji-Naito, K.; Akagawa, M. β-Caryophyllene attenuates palmitate-induced lipid accumulation through AMPK signaling by activating CB2 receptor in human HepG2 hepatocytes. Mol. Nutr. Food Res. 2016, 60, 2228–2242. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.V.; Matyas, C.; Erdelyi, K.; Cinar, R.; Nieri, D.; Chicca, A.; Nemeth, B.T.; Paloczi, J.; Lajtos, T.; Corey, L.; et al. β-Caryophyllene protects against alcoholic steatohepatitis by attenuating inflammation and metabolic dysregulation in mice. Br. J. Pharmacol. 2018, 175, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Meng, N.; Chang, Y.; Tang, W. Endocannabinoids signaling: Molecular mechanisms of liver regulation and diseases. Front. Biosci. 2016, 21, 1488–1501. [Google Scholar] [CrossRef] [Green Version]
- Lavanco, G.; Castelli, V.; Brancato, A.; Tringali, G.; Plescia, F.; Cannizzaro, C. The endocannabinoid-alcohol crosstalk: Recent advances on a bi-faceted target. Clin. Exp. Pharmacol. Physiol. 2018, 45, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Patsenker, E.; Stoll, M.; Millonig, G.; Agaimy, A.; Wissniowski, T.; Schneider, V.; Mueller, S.; Brenneisen, R.; Seitz, H.K.; Ocker, M.; et al. Cannabinoid receptor type I modulates alcohol-induced liver fibrosis. Mol. Med. 2011, 17, 1285–1294. [Google Scholar] [CrossRef]
- Jeong, W.I.; Osei-Hyiaman, D.; Park, O.; Liu, J.; Bátkai, S.; Mukhopadhyay, P.; Horiguchi, N.; Harvey-White, J.; Marsicano, G.; Lutz, B.; et al. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab. 2008, 7, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Amato, G.S.; Manke, A.; Harris, D.L.; Wiethe, R.W.; Vasukuttan, V.; Snyder, R.W.; Lefever, T.W.; Cortes, R.; Zhang, Y.; Wang, S.; et al. Blocking Alcoholic Steatosis in Mice with a Peripherally Restricted Purine Antagonist of the Type 1 Cannabinoid Receptor. J. Med. Chem. 2018, 61, 4370–4385. [Google Scholar] [CrossRef] [PubMed]
- Denaës, T.; Lodder, J.; Chobert, M.N.; Ruiz, I.; Pawlotsky, J.M.; Lotersztajn, S.; Teixeira-Clerc, F. The Cannabinoid Receptor 2 Protects Against Alcoholic Liver Disease Via a Macrophage Autophagy-Dependent Pathway. Sci. Rep. 2016, 6, 28806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trebicka, J.; Racz, I.; Siegmund, S.V.; Cara, E.; Granzow, M.; Schierwagen, R.; Klein, S.; Wojtalla, A.; Hennenberg, M.; Huss, S.; et al. Role of cannabinoid receptors in alcoholic hepatic injury: Steatosis and fibrogenesis are increased in CB2 receptor-deficient mice and decreased in CB1 receptor knockouts. Liver Int. 2011, 31, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.J.; Yu, J.W.; Wan, L.; Zhang, X.Y.; Shi, Y.G.; Chen, M.Y. Endocannabinoid system activation contributes to glucose metabolism disorders of hepatocytes and promotes hepatitis C virus replication. Int. J. Infect. Dis. 2014, 23, 75–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyoda, M.; Kitaoka, A.; Machida, K.; Nishinakagawa, T.; Yada, R.; Kohjima, M.; Kato, M.; Kotoh, K.; Sakamoto, N.; Shiota, G.; et al. Association between lipid accumulation and the cannabinoid system in Huh7 cells expressing HCV genes. Int. J. Mol. Med. 2011, 27, 619–624. [Google Scholar] [CrossRef] [PubMed]
- van der Poorten, D.; Shahidi, M.; Tay, E.; Sesha, J.; Tran, K.; McLeod, D.; Milliken, J.S.; Ho, V.; Hebbard, L.W.; Douglas, M.W.; et al. Hepatitis C virus induces the cannabinoid receptor 1. PLoS ONE 2010, 5, e12841. [Google Scholar] [CrossRef] [Green Version]
- Patsenker, E.; Sachse, P.; Chicca, A.; Gachet, M.S.; Schneider, V.; Mattsson, J.; Lanz, C.; Worni, M.; de Gottardi, A.; Semmo, M.; et al. Elevated levels of endocannabinoids in chronic hepatitis C may modulate cellular immune response and hepatic stellate cell activation. Int. J. Mol. Sci. 2015, 16, 7057–7076. [Google Scholar] [CrossRef] [Green Version]
- Coppola, N.; Zampino, R.; Bellini, G.; Macera, M.; Marrone, A.; Pisaturo, M.; Boemio, A.; Nobili, B.; Pasquale, G.; Maione, S.; et al. Association between a polymorphism in cannabinoid receptor 2 and severe necroinflammation in patients with chronic hepatitis C. Clin. Gastroenterol. Hepatol. 2014, 12, 334–340. [Google Scholar] [CrossRef]
- Sagnelli, C.; Uberti-Foppa, C.; Hasson, H.; Bellini, G.; Minichini, C.; Salpietro, S.; Messina, E.; Barbanotti, D.; Merli, M.; Punzo, F.; et al. Cannabinoid receptor 2-63 RR variant is independently associated with severe necroinflammation in HIV/HCV coinfected patients. PLoS ONE 2017, 12, e0181890. [Google Scholar] [CrossRef] [Green Version]
- Adejumo, A.C.; Alliu, S.; Ajayi, T.O.; Adejumo, K.L.; Adegbala, O.M.; Onyeakusi, N.E.; Akinjero, A.M.; Durojaiye, M.; Bukong, T.N. Cannabis use is associated with reduced prevalence of non-alcoholic fatty liver disease: A cross-sectional study. PLoS ONE 2017, 12, e0176416. [Google Scholar] [CrossRef]
- Penner, E.A.; Buettner, H.; Mittleman, M.A. The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am. J. Med. 2013, 126, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Le Strat, Y.; Le Foll, B. Obesity and cannabis use: Results from 2 representative national surveys. Am. J. Epidemiol. 2011, 174, 929–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshaarawy, O.; Anthony, J.C. Are cannabis users less likely to gain weight? Results from a national 3-year prospective study. Int. J. Epidemiol. 2019, 48, 1695–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidot, D.C.; Prado, G.; Hlaing, W.M.; Florez, H.J.; Arheart, K.L.; Messiah, S.E. Metabolic Syndrome Among Marijuana Users in the United States: An Analysis of National Health and Nutrition Examination Survey Data. Am. J. Med. 2016, 129, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Rajavashisth, T.B.; Shaheen, M.; Norris, K.C.; Pan, D.; Sinha, S.K.; Ortega, J.; Friedman, T.C. Decreased prevalence of diabetes in marijuana users: Cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open 2012, 2, e000494. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Kim, W.; Kwak, M.S.; Chung, G.E.; Yim, J.Y.; Ahmed, A. Inverse association of marijuana use with nonalcoholic fatty liver disease among adults in the United States. PLoS ONE 2017, 12, e0186702. [Google Scholar] [CrossRef] [Green Version]
- Adejumo, A.C.; Ajayi, T.O.; Adegbala, O.M.; Adejumo, K.L.; Alliu, S.; Akinjero, A.M.; Onyeakusi, N.E.; Ojelabi, O.; Bukong, T.N. Cannabis use is associated with reduced prevalence of progressive stages of alcoholic liver disease. Liver Int. 2018, 38, 1475–1486. [Google Scholar] [CrossRef]
- Fuster, D.; So-Armah, K.; Cheng, D.M.; Coleman, S.M.; Gnatienko, N.; Lioznov, D.; Krupitsky, E.M.; Freiberg, M.S.; Samet, J.H. Lack of Association Between Recent Cannabis Use and Advanced Liver Fibrosis Among HIV-positive Heavy Drinkers. Curr. HIV Res. 2021, 19, 324–331. [Google Scholar] [CrossRef]
- Barré, T.; Rojas Rojas, T.; Lacombe, K.; Protopopescu, C.; Poizot-Martin, I.; Nishimwe, M.L.; Zucman, D.; Esterle, L.; Billaud, E.; Aumaitre, H.; et al. Cannabis use and reduced risk of elevated fatty liver index in HIV-HCV co-infected patients: A longitudinal analysis (ANRS CO13 HEPAVIH). Expert Rev. Anti Infect. Ther. 2021, 19, 1147–1156. [Google Scholar] [CrossRef]
- Barré, T.; Pol, S.; Ramier, C.; Di Beo, V.; Carrat, F.; Bureau, M.; Bourlière, M.; Dorival, C.; Serfaty, L.; Asselah, T.; et al. Cannabis Use Is Inversely Associated with Overweight and Obesity in Hepatitis B Virus-Infected Patients (ANRS CO22 Hepather Cohort). Cannabis Cannabinoid Res. 2021. [Google Scholar] [CrossRef]
- Barré, T.; Nishimwe, M.L.; Protopopescu, C.; Marcellin, F.; Carrat, F.; Dorival, C.; Delarocque-Astagneau, E.; Larrey, D.; Bourlière, M.; Petrov-Sanchez, V.; et al. Cannabis use is associated with a lower risk of diabetes in chronic hepatitis C-infected patients (ANRS CO22 Hepather cohort). J. Viral Hepat. 2020, 27, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, S.; Vilotitch, A.; Roux, P.; Esterle, L.; Spire, B.; Marcellin, F.; Salmon-Ceron, D.; Dabis, F.; Chas, J.; Rey, D.; et al. Daily cannabis and reduced risk of steatosis in human immunodeficiency virus and hepatitis C virus-co-infected patients (ANRS CO13-HEPAVIH). J. Viral Hepat. 2018, 25, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.M.; Dodge, J.L.; Sarkar, M.; French, A.L.; Tien, P.C.; Glesby, M.J.; Golub, E.T.; Augenbraun, M.; Plankey, M.; Peters, M.G. Marijuana Use Is Not Associated With Progression to Advanced Liver Fibrosis in HIV/Hepatitis C Virus-coinfected Women. Clin. Infect. Dis. 2016, 63, 512–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrieri, M.P.; Serfaty, L.; Vilotitch, A.; Winnock, M.; Poizot-Martin, I.; Loko, M.A.; Lions, C.; Lascoux-Combe, C.; Roux, P.; Salmon-Ceron, D.; et al. Cannabis Use and Reduced Risk of Insulin Resistance in HIV-HCV Infected Patients: A Longitudinal Analysis (ANRS CO13 HEPAVIH). Clin. Infect. Dis. 2015, 61, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Hézode, C.; Roudot-Thoraval, F.; Nguyen, S.; Grenard, P.; Julien, B.; Zafrani, E.S.; Pawlotsky, J.M.; Dhumeaux, D.; Lotersztajn, S.; Mallat, A. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology 2005, 42, 63–71. [Google Scholar] [CrossRef]
- Hézode, C.; Zafrani, E.S.; Roudot-Thoraval, F.; Costentin, C.; Hessami, A.; Bouvier-Alias, M.; Medkour, F.; Pawlostky, J.M.; Lotersztajn, S.; Mallat, A. Daily cannabis use: A novel risk factor of steatosis severity in patients with chronic hepatitis C. Gastroenterology 2008, 134, 432–439. [Google Scholar] [CrossRef]
- Vázquez-Bourgon, J.; Setién-Suero, E.; Pilar-Cuéllar, F.; Romero-Jiménez, R.; Ortiz-García de la Foz, V.; Castro, E.; Crespo-Facorro, B. Effect of cannabis on weight and metabolism in first-episode non-affective psychosis: Results from a three-year longitudinal study. J. Psychopharmacol. 2019, 33, 284–294. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Liu, W. Does Cannabis Intake Protect Against Non-alcoholic Fatty Liver Disease? A Two-Sample Mendelian Randomization Study. Front. Genet. 2020, 11, 949. [Google Scholar] [CrossRef]
- Whitfield, J.B.; Masson, S.; Liangpunsakul, S.; Mueller, S.; Aithal, G.P.; Eyer, F.; Gleeson, D.; Thompson, A.; Stickel, F.; Soyka, M.; et al. Obesity, Diabetes, Coffee, Tea, and Cannabis Use Alter Risk for Alcohol-Related Cirrhosis in 2 Large Cohorts of High-Risk Drinkers. Am. J. Gastroenterol. 2021, 116, 106–115. [Google Scholar] [CrossRef]
- Ishida, J.H.; Peters, M.G.; Jin, C.; Louie, K.; Tan, V.; Bacchetti, P.; Terrault, N.A. Influence of cannabis use on severity of hepatitis C disease. Clin. Gastroenterol. Hepatol. 2008, 6, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Brunet, L.; Moodie, E.E.; Rollet, K.; Cooper, C.; Walmsley, S.; Potter, M.; Klein, M.B. Marijuana smoking does not accelerate progression of liver disease in HIV-hepatitis C coinfection: A longitudinal cohort analysis. Clin. Infect. Dis. 2013, 57, 663–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Howell, G.T.; Turner, L.; Corace, K.; Garber, G.; Cooper, C. Marijuana use in hepatitis C infection does not affect liver biopsy histology or treatment outcomes. Can. J. Gastroenterol. Hepatol. 2014, 28, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Costiniuk, C.T.; Mills, E.; Cooper, C.L. Evaluation of oral cannabinoid-containing medications for the management of interferon and ribavirin-induced anorexia, nausea and weight loss in patients treated for chronic hepatitis C virus. Can. J. Gastroenterol. 2008, 22, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre, D.L.; Clements, B.J.; Malibu, Y. Cannabis use improves retention and virological outcomes in patients treated for hepatitis C. Eur. J. Gastroenterol. Hepatol. 2006, 18, 1057–1063. [Google Scholar] [CrossRef]
- Costiniuk, C.T.; Jenabian, M.A. Reply to: Benefits of cannabis use for metabolic disorders and survival in people living with HIV with or without hepatitis C. Aids 2020, 34, 955–956. [Google Scholar] [CrossRef]
- Andrews, C.N.; Devlin, S.M.; Le Foll, B.; Fischer, B.; Tse, F.; Storr, M.; Congly, S.E. Canadian Association of Gastroenterology Position Statement: Use of Cannabis in Gastroenterological and Hepatic Disorders. J. Can. Assoc. Gastroenterol. 2019, 2, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Atakan, Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2020, 2, 241–254. [Google Scholar] [CrossRef] [Green Version]
- De Ternay, J.; Naassila, M.; Nourredine, M.; Louvet, A.; Bailly, F.; Sescousse, G.; Maurage, P.; Cottencin, O.; Carrieri, P.M.; Rolland, B. Therapeutic Prospects of Cannabidiol for Alcohol Use Disorder and Alcohol-Related Damages on the Liver and the Brain. Front. Pharmacol. 2019, 10, 627. [Google Scholar] [CrossRef]
- Koch, J.E.; Matthews, S.M. Delta9-tetrahydrocannabinol stimulates palatable food intake in Lewis rats: Effects of peripheral and central administration. Nutr. Neurosci. 2001, 4, 179–187. [Google Scholar] [CrossRef]
- Wiley, J.L.; Burston, J.J.; Leggett, D.C.; Alekseeva, O.O.; Razdan, R.K.; Mahadevan, A.; Martin, B.R. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br. J. Pharmacol. 2005, 145, 293–300. [Google Scholar] [CrossRef] [Green Version]
- McIntosh, A.L.; Martin, G.G.; Huang, H.; Landrock, D.; Kier, A.B.; Schroeder, F. Δ(9)-Tetrahydrocannabinol induces endocannabinoid accumulation in mouse hepatocytes: Antagonism by Fabp1 gene ablation. J. Lipid Res. 2018, 59, 646–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caraceni, P.; Viola, A.; Piscitelli, F.; Giannone, F.; Berzigotti, A.; Cescon, M.; Domenicali, M.; Petrosino, S.; Giampalma, E.; Riili, A.; et al. Circulating and hepatic endocannabinoids and endocannabinoid-related molecules in patients with cirrhosis. Liver Int. 2010, 30, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertwee, R. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakotoarivelo, V.; Sihag, J.; Flamand, N. Role of the Endocannabinoid System in the Adipose Tissue with Focus on Energy Metabolism. Cells 2021, 10, 1279. [Google Scholar] [CrossRef]
- Laun, A.S.; Shrader, S.H.; Brown, K.J.; Song, Z.H. GPR3, GPR6, and GPR12 as novel molecular targets: Their biological functions and interaction with cannabidiol. Acta Pharmacol. Sin. 2019, 40, 300–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, H.I.; Toyang, N.J.; McLaughlin, W. Potential of Cannabidiol for the Treatment of Viral Hepatitis. Pharmacogn. Res. 2017, 9, 116–118. [Google Scholar] [CrossRef] [Green Version]
- Walsh, K.B.; McKinney, A.E.; Holmes, A.E. Minor Cannabinoids: Biosynthesis, Molecular Pharmacology and Potential Therapeutic Uses. Front. Pharmacol. 2021, 12, 777804. [Google Scholar] [CrossRef]
- Millar, S.A.; Stone, N.L.; Bellman, Z.D.; Yates, A.S.; England, T.J.; O’Sullivan, S.E. A systematic review of cannabidiol dosing in clinical populations. Br. J. Clin. Pharmacol. 2019, 85, 1888–1900. [Google Scholar] [CrossRef]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef] [Green Version]
- Kiriiri, G.K.; Njogu, P.M.; Mwangi, A.N. Exploring different approaches to improve the success of drug discovery and development projects: A review. Future J. Pharm. Sci. 2020, 6, 27. [Google Scholar] [CrossRef]
- Nielsch, U.; Fuhrmann, U.; Jaroch, S. New Approaches to Drug Discovery; Springer Nature: New York, NY, USA, 2016. [Google Scholar]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef] [PubMed]
- Rong, C.; Carmona, N.E.; Lee, Y.L.; Ragguett, R.M.; Pan, Z.; Rosenblat, J.D.; Subramaniapillai, M.; Shekotikhina, M.; Almatham, F.; Alageel, A.; et al. Drug-drug interactions as a result of co-administering Δ(9)-THC and CBD with other psychotropic agents. Expert Opin. Drug Saf. 2018, 17, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Peltekian, K.M. Cannabis and the liver: Things you wanted to know but were afraid to ask. Can. Liver J. 2019, 2, 51–57. [Google Scholar] [CrossRef]
- Stout, S.M.; Cimino, N.M. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: A systematic review. Drug Metab. Rev. 2014, 46, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.; Yamaori, S.; Okamoto, Y.; Yamamoto, I.; Watanabe, K. Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab. Pharm. 2013, 28, 332–338. [Google Scholar] [CrossRef] [Green Version]
| Main Outcomes and Conclusions | Study Design and Methodology | References |
|---|---|---|
| Cannabidiol | ||
| In vivo mice model of segmental hepatic ischemia. | [29] |
| In vitro and in vivo models of hepatic fibrosis. | [30] |
| In vitro and in vivo models of alcohol-induced liver steatosis. | [31] |
| In vitro and in vivo models of hepatosteatosis. Transcriptional, posttranslational, and metabolomic assays. | [32] |
|
| [33] |
| Mice-liver-injury model induced by ethanol plus high-fat high-cholesterol diet (EHFD) for 8 weeks. | [34] |
| Randomized double-blind placebo-controlled study: 5 treatment arms: CBD alone (100 mg twice daily); THCV alone (5 mg twice daily); 1:1 ratio of CBD and THCV (5 mg/5 mg, twice daily); 20:1 ratio of CBD and THCV (100 mg/5 mg, twice daily); or matched placebo for 13 weeks, on 62 subjects with noninsulin-treated type 2 diabetes. | [35] |
| Randomized partially blind placebo-controlled dose-ranging phase 2 clinical study: 200/400/800 mg of CBD daily or placebo for 8 weeks on 25 participants with NAFLD. | [36] |
| Tetrahydrocannabivarin | ||
| In vivo model of fasting and feeding mice. | [37] |
| In vitro and in vivo mice models of hepatic ischemia. | [38] |
| In vivo mice model of diet-induced obesity (DIO) treated with regimens of increasing doses of THCV:
| [39] |
| In vitro and in vivo models of hepatosteatosis. Transcriptional, posttranslational, and metabolomic assays. | [32] |
| Randomized double-blind placebo-controlled study: 5 treatment arms: CBD alone (100 mg twice daily); THCV alone (5 mg twice daily); 1:1 ratio of CBD and THCV (5 mg/5 mg, twice daily); 20:1 ratio of CBD and THCV (100 mg/5 mg, twice daily); matched placebo for 13 weeks, on 62 subjects with noninsulin-treated type 2 diabetes. | [35] |
| Tetrahydrocannabinolic acid | ||
| In vitro functional assay and in vivo mice model of high fat diet (HFD)-induced obesity. | [40] |
| In vitro model of liver fibrosis and in vivo ice model of nonalcoholic liver fibrosis induced by CCl4 treatment of 23-weeks of high-fat-diet (HFD) feeding. | [41] |
| Atypical Cannabinoid Abn-CBD | ||
| Diet-induced obese mouse model of prediabetes and nonalcoholic fatty liver disease (NAFLD). | [42] |
| Main Outcome | Study Design | Participant Characteristics | Method to Assess Outcome | Reference |
|---|---|---|---|---|
| NAFLD/NASH | ||||
| Cannabis use is associated with lower prevalence of NAFLD/NASH. | Population-based case–control study | A total of 5,950,391 patients, 18 years and older, in three groups: noncannabis users (98.04%), nondependent cannabis users (1.74%), and dependent cannabis users (0.22%). | Multivariate logistic regression to determine the odds of developing NAFLD with respect to cannabis use. | [83] |
| Cannabis use is associated with lower levels of fasting insulin and insulin resistance. | Examination survey | 4657 adults aged 18 years and older | Fasting insulin and glucose measured via blood samples after a 9 h fast, and HOMA-IR calculated to evaluate insulin resistance. Multiple linear regression to determine associations. | [84] |
| Prevalence of obesity is lower in cannabis users than in nonusers. | Cross-sectional data on 2 population-based nationally representative studies. | 52,375 adults aged 18 years or older | Logistic regression model with obesity as a categorical outcome, and the frequency of cannabis use in the past year as the primary association. | [85] |
| Inverse association between cannabis use and obesity. | Population-based 3-year prospective study. | 43,093 adults aged 18 years or older | General linear modeling yields estimates for change in body-mass index regressed on cannabis-use status. | [86] |
| Current marijuana use is associated with lower odds of metabolic syndrome across emerging and middle-aged adults. | Population-based 5-year prospective study | 8478 adults, 20–59 years old | Metabolic syndrome was defined as ≥ 3 of the following: elevated fasting glucose, high triglycerides, low high-density-lipoprotein cholesterol, elevated systolic/diastolic blood pressure, and increased waist circumference. An age-stratified analysis was conducted to examine the relationship between marijuana use and metabolic syndrome among emerging adults (20–30 years), adults (31–44 years), and middle-aged adults (45–59 years). | [87] |
| Marijuana use was independently associated with a lower prevalence of diabetes mellitus. | Cross-sectional study | 10,896 adults, 20–59 years old | Univariate and multivariate logistic regression analyses were used to determine the relationship between diabetes mellitus and marijuana use. | [88] |
| Active marijuana use provided a protective effect against NAFLD independent of known metabolic risk factors. | Cross-sectional data from 2 National Health and Nutrition Examination Surveys | 22,366 | NAFLD was defined either by a serum alanine aminotransferase (ALT) that was >30 IU/L for men and >19 IU/L for women in the absence of other liver diseases, or based on ultrasonography. | [89] |
| ALD | ||||
| Among alcohol users, cannabis use was associated with significantly lower odds of developing alcoholic steatosis, steatohepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma. | Cross-sectional data from National Health and Nutrition Examination Survey | 319,514 adults 18 years and older | Univariate and multivariate logistic regression analyses were used to determine the relationship between alcoholic steatosis, steatohepatitis, fibrosis, cirrhosis, hepatocellular carcinoma, and cannabis use. | [90] |
| No association between cannabis use and advanced liver fibrosis in heavy alcohol drinkers. | Cross-sectional study | 248 HIV-positive individuals with heavy alcohol use | Transient elastography was used to detect advanced liver fibrosis among participants. | [91] |
| HCV/HBV infection | ||||
| Regular or daily cannabis use was associated with a reduced risk of an elevated fatty liver index in HIV–HCV-coinfected individuals. | 5-year longitudinal study | 997 HIV–HCV-coinfected individuals | Mixed-effects multivariable logistic and linear regression models | [92] |
| Cannabis use was associated with lower risks of obesity in chronically HBV-infected individuals. | Cross-sectional study | 3706 chronically HBV-infected individuals | Logistic and multinomial regression analyses | [93] |
| Cannabis use was independently associated with a lower risk of diabetes in chronically HCV-infected individuals. | Cross-sectional study | 10,445 chronically HCV-infected individuals | Multivariate logistic regression analyses | [94] |
| Daily cannabis use was independently associated with a reduced prevalence of steatosis. | Cross-sectional study in a nationwide multicenter cohort | 838 adults, HIV–HCV-coinfected individuals | A logistic regression model was used to evaluate the association between cannabis use and steatosis. | [95] |
| THC-rich cannabis use was not associated with progression to significant liver fibrosis. | 11-year longitudinal study | 575 HIV–HCV-coinfected women | Cox proportional hazards regression analysis | [96] |
| Cannabis use is associated with a lower insulin-resistance risk in HIV–HCV-coinfected individuals. | 60-month longitudinal study | 703 HIV–HCV-coinfected individuals | A mixed-effects multivariable logistic regression model | [97] |
| Daily cannabis smoking is significantly associated with fibrosis progression during chronic hepatitis C virus infection. | Cross-sectional study | 270 untreated chronically hepatitis-infected individuals | Multivariate stepwise logistic regression analyses | [98] |
| Daily cannabis smoking as a novel independent predictor of steatosis severity during chronic hepatitis C virus infection. | Cross-sectional study | 315 untreated chronically hepatitis-infected individuals | Multivariate stepwise logistic regression analyses | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mboumba Bouassa, R.-S.; Sebastiani, G.; Di Marzo, V.; Jenabian, M.-A.; Costiniuk, C.T. Cannabinoids and Chronic Liver Diseases. Int. J. Mol. Sci. 2022, 23, 9423. https://doi.org/10.3390/ijms23169423
Mboumba Bouassa R-S, Sebastiani G, Di Marzo V, Jenabian M-A, Costiniuk CT. Cannabinoids and Chronic Liver Diseases. International Journal of Molecular Sciences. 2022; 23(16):9423. https://doi.org/10.3390/ijms23169423
Chicago/Turabian StyleMboumba Bouassa, Ralph-Sydney, Giada Sebastiani, Vincenzo Di Marzo, Mohammad-Ali Jenabian, and Cecilia T. Costiniuk. 2022. "Cannabinoids and Chronic Liver Diseases" International Journal of Molecular Sciences 23, no. 16: 9423. https://doi.org/10.3390/ijms23169423
APA StyleMboumba Bouassa, R.-S., Sebastiani, G., Di Marzo, V., Jenabian, M.-A., & Costiniuk, C. T. (2022). Cannabinoids and Chronic Liver Diseases. International Journal of Molecular Sciences, 23(16), 9423. https://doi.org/10.3390/ijms23169423







