Vascular Reactions of the Diving Reflex in Men and Women Carrying Different ADRA1A Genotypes
Abstract
1. Introduction
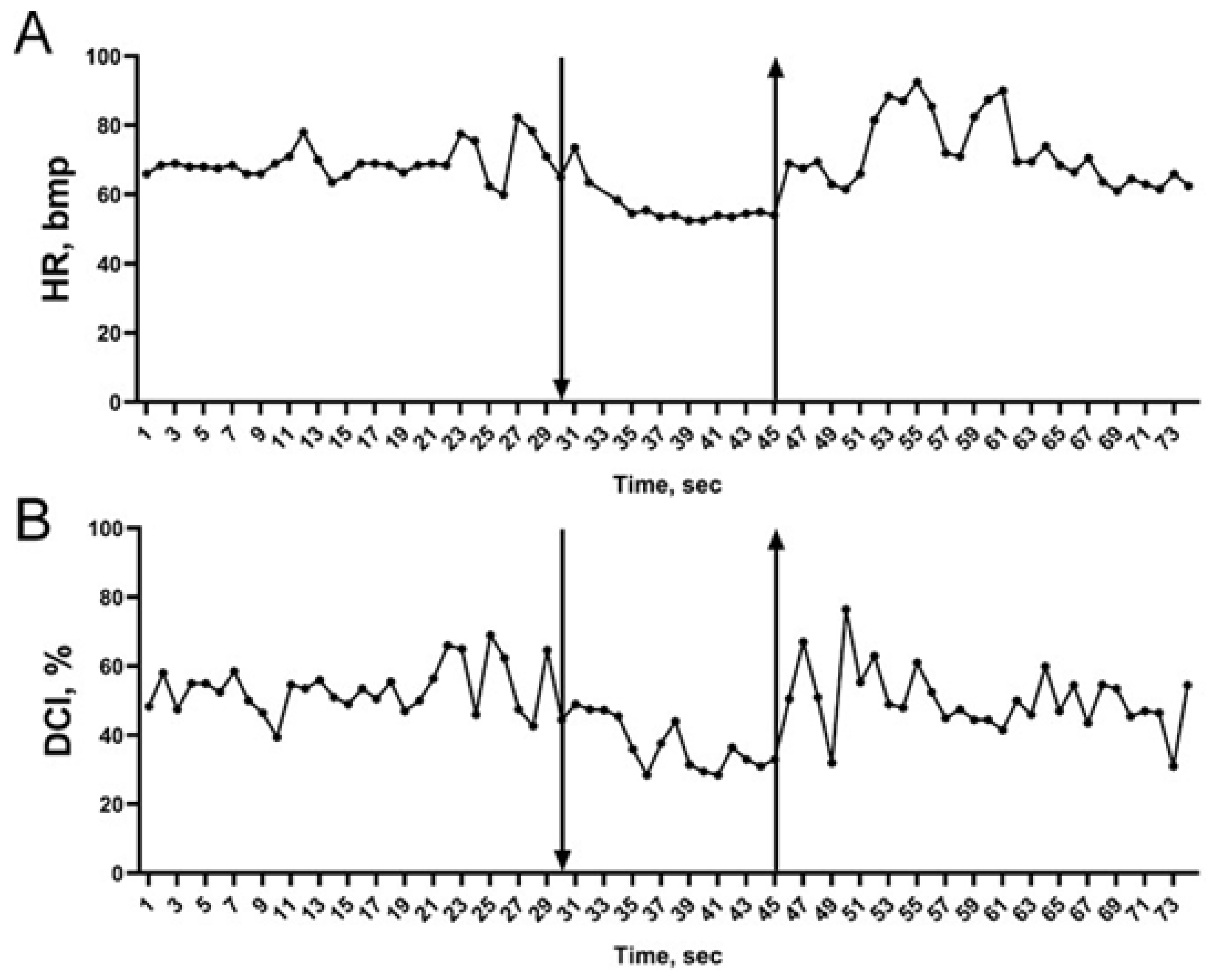
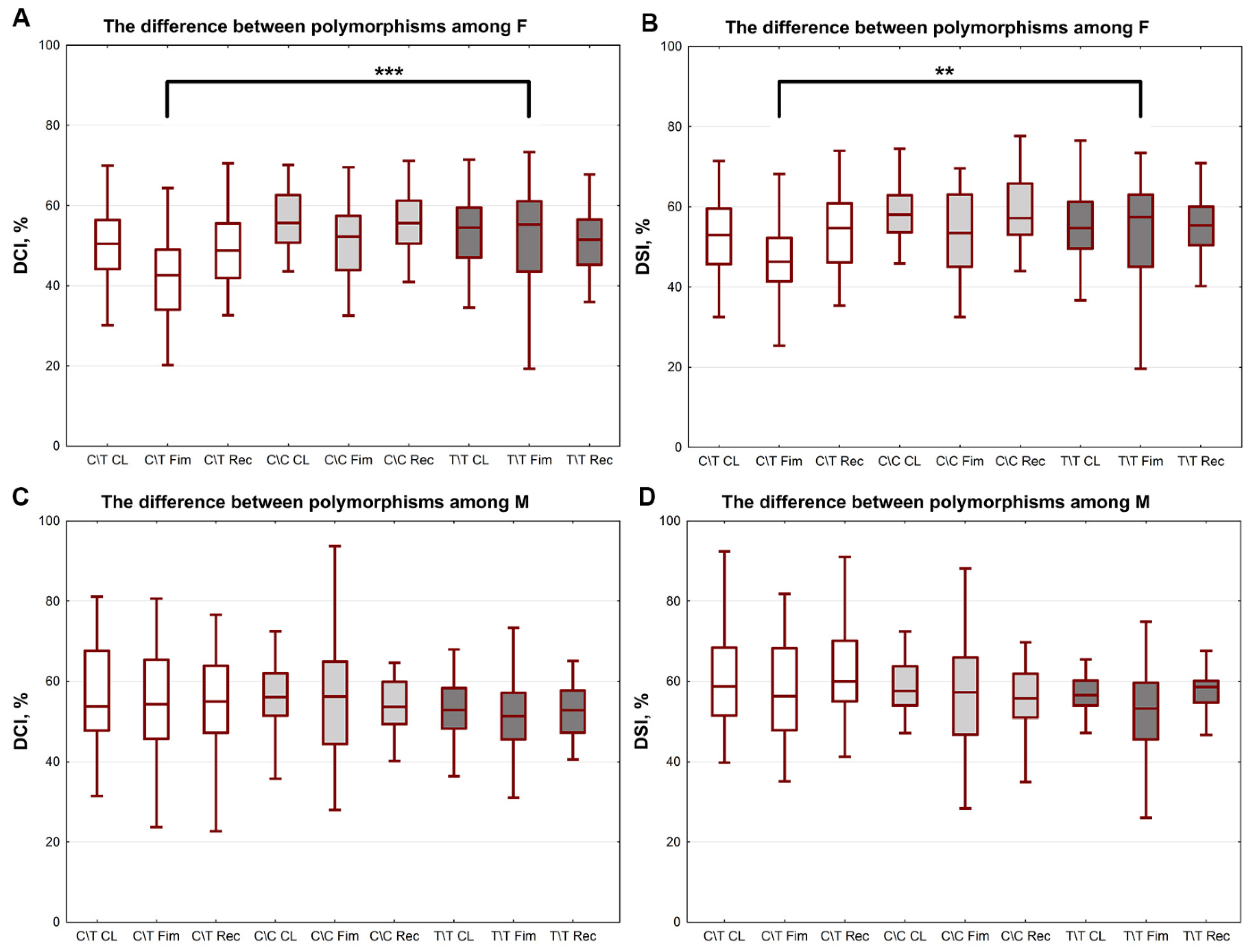
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fitz-Clarke, J.R. Breath-hold diving. Compr. Physiol. 2018, 8, 585–630. [Google Scholar] [CrossRef]
- Campbell, L.B.; Gooden, B.A.; Horowitz, J.D. Cardiovascular responses to partial and total immersion in man. J. Physiol. 1969, 202, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Elsner, R.; Gooden, B. Diving and Asphyxia: A Comparative Study of Animals and Man; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar] [CrossRef]
- Andersson, J.P.A.; Linér, M.H.; Fredsted, A.; Schagatay, E.K.A. Cardiovascular and respiratory responses to apneas with and without face immersion in exercising humans. J. Appl. Physiol. 2004, 96, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Galantsev, V.P. Adaptation of the Cardiovascular System of Diving Animals; LSU: Leningrad, Soviet Union, 1988. [Google Scholar]
- Butler, P.J. Metabolic regulation in diving birds and mammals. Respir. Physiol. Neurobiol. 2004, 141, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Espersen, K.; Frandsen, H.; Lorentzen, T.; Kanstrup, I.-L.; Christensen, N.J. The human spleen as an erythrocyte reservoir in diving-related interventions. J. Appl. Physiol. 2002, 92, 2071–2079. [Google Scholar] [CrossRef]
- Schaefer, K.E.; Allison, R.D.; Dougherty, J.H.; Carey, C.R.; Walker, R.; Yost, F.; Parker, D. Pulmonary and circulatory adjustments determining the limits of depths in breathhold diving. Science 1968, 162, 1020–1023. [Google Scholar] [CrossRef]
- Kollias, J.; Van Derveer, D.; Dorchak, K.J.; Greenleaf, J.E. Physiologic Responses to Water Immersion in Man: A Compendium of Research. Technical Memorandum (No. NASA-TM-X-3308); NASA Ames Research Center: Mountain View, CA, USA, 1976; pp. 1–87.
- Linér, M.H. Cardiovascular and pulmonary responses to breath-hold diving in humans. Acta Physiol. Scand. Suppl. 1994, 620, 1–32. [Google Scholar]
- Lindholm, P.; Nyrén, S. Studies on inspiratory and expiratory glossopharyngeal breathing in breath-hold divers employing magnetic resonance imaging and spirometry. Eur. J. Appl. Physiol. 2005, 94, 646–651. [Google Scholar] [CrossRef]
- Eichinger, M.; Walterspacher, S.; Scholz, T.; Tetzlaff, K.; Röcker, K.; Muth, C.-M.; Puderbach, M.; Kauczor, H.-U.; Sorichter, S. Lung hyperinflation: Foe or friend? Eur. Respir. J. 2008, 32, 1113–1116. [Google Scholar] [CrossRef]
- Seccombe, L.M.; Chung, S.C.S.; Jenkins, C.R.; Frater, C.J.; Mackey, D.W.J.; Pearson, M.A.; Emmett, L.; Peters, M.J. Lung perfusion and chest wall configuration is altered by glossopharyngeal breathing. Eur. Respir. J. 2009, 36, 151–156. [Google Scholar] [CrossRef]
- Schagatay, E.; Andersson, J. Diving response and apneic time in humans. Undersea Hyperb. Med. 1998, 25, 13–19. [Google Scholar] [PubMed]
- Zemlyanukhina, T.A.; Rybyakova, T.V.; Podyacheva, E.Y.; Baranova, T.I. Features of diving reflex in synchronized swimmers. Theory Pract. Phys. Cult. 2020, 6, 13–15. [Google Scholar]
- Suresh, K.; Shimoda, L.A. Lung Circulation. Compr. Physiol. 2016, 6, 897–943. [Google Scholar] [CrossRef] [PubMed]
- Podyacheva, E.Y.; Zemlyanukhina, T.; Shadrin, L.; Baranova, T. Features of hemodynamics of pulmonary circulation during the diving reflex. Biol. Commun. 2020, 65, 244–251. [Google Scholar] [CrossRef]
- Minneman, K.P. Alpha 1-adrenergic receptor subtypes, inositol phosphates, and sources of cell Ca2+. Pharmacol. Rev. 1988, 40, 87–119. [Google Scholar] [PubMed]
- Liu, S.F.; Barnes, P.J. Neural control of pulmonary vascular tone. In The Lung: Scientific Foundations, 2nd ed.; Crystal, R.G., West, J.B., Weibel, E.R., Barnes, P.J., Eds.; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1997; pp. 1457–1472. [Google Scholar]
- A Oriowo, M.; Chandrasekhar, B.; A Kadavil, E. α1-adrenoceptor subtypes mediating noradrenaline-induced contraction of pulmonary artery from pulmonary hypertensive rats. Eur. J. Pharmacol. 2003, 482, 255–263. [Google Scholar] [CrossRef]
- Leblais, V.; Delannoy, E.; Fresquet, F.; Bégueret, H.; Bellance, N.; Banquet, S.; Allières, C.; Leroux, L.; Desgranges, C.; Gadeau, A.; et al. β-adrenergic relaxation in pulmonary arteries: Preservation of the endothelial nitric oxide-dependent 2 component in pulmonary hypertension. Cardiovasc. Res. 2007, 77, 202–210. [Google Scholar] [CrossRef]
- Baranova, T.I.; Berlov, D.N.; Glotov, O.S.; Korf, E.A.; Minigalin, A.D.; Mitrofanova, A.V.; Ahmetov, I.I.; Glotov, A.S. Genetic determination of the vascular reactions in humans in response to the diving reflex. Am. J. Physiol. Circ. Physiol. 2017, 312, H622–H631. [Google Scholar] [CrossRef]
- Baranova, T.I.; Berlov, D.N.; Zavarina, L.B.; Minigalin, A.D.; Smith, N.Y.; Xu, S.; Yanvareva, I.N. The function of the heart changes in implementation of the diving reactions in humans. Ross. Fiziol. Zh. Im. I. M. Sechenova 2015, 101, 337–348. [Google Scholar]
- Baranova, T.I.; Berlov, D.N.; Yanvareva, I.N. Changes in cerebral blood flow on performance of a diving reaction in humans. Neurosci. Behav. Physiol. 2015, 46, 36–41. [Google Scholar] [CrossRef]
- Baranova, T.I.; Berlov, D.N.; Glotov, A.S.; Glotov, O.S.; Zavarina, L.B.; Kachanova, T.A.; Podyacheva, E.Y.; Shleikina, A.V. Genetic determinants of adaptive cardiovascular reactions in imitation of diving in humans. Russ. J. Physiol. 2018, 104, 845–855. (In Russian) [Google Scholar] [CrossRef]
- Shibata, K.; Hirasawa, A.; Moriyama, N.; Kawabe, K.; Ogawa, S.; Tsujimoto, G. α1a-adrenoceptor polymorphism: Pharmacological characterization and association with benign prostatic hypertrophy. Br. J. Pharmacol. 1996, 118, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Morris, D.P.; Smith, M.P.; Svetkey, L.P.; Newman, M.F.; Rotter, J.I.; Buchanan, T.A.; Beckstrom-Sternberg, S.M.; Green, E.D.; Schwinn, D.A. Novel human α1a-adrenoceptor single nucleotide polymorphisms alter receptor pharmacology and biological function. Naunyn Schmiedeberg Arch. Pharmacol. 2005, 371, 229–239. [Google Scholar] [CrossRef]
- Xie, A.; Skatrud, J.B.; Puleo, D.S.; Morgan, B.J. Arousal from sleep shortens sympathetic burst latency in humans. J. Physiol. 1999, 515, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Ge, D.; Snieder, H.; He, J.; Chen, S.; Huang, J.; Li, B.; Chen, R.; Qiang, B. Association of α1A adrenergic receptor gene variants on chromosome 8p21 with human stage 2 hypertension. J. Hypertens. 2006, 24, 1049–1056. [Google Scholar] [CrossRef]
- Miller, V.M.; Duckles, S.P. Vascular actions of estrogens: Functional implications. Pharmacol. Rev. 2008, 60, 210–241. [Google Scholar] [CrossRef]
- McConnaughey, M.M.; Iams, S.G. Sex hormones change adrenoceptors in blood vessels of the spontaneously hypertensive rat. Clin. Exp. Hypertens. 1993, 15, 153–170. [Google Scholar] [CrossRef]
- López-Canales, O.; Castillo-Hernández, M.D.C.; Vargas-Robles, H.; Rios, A.; Canales, J.S.L.; Escalante, B. Androgens mediate β-adrenergic vasorelaxation impairment using adenylyl cyclase. J. Cardiovasc. Pharmacol. 2018, 71, 147–154. [Google Scholar] [CrossRef]
- Riedel, K.; Deussen, A.J.; Tolkmitt, J.; Weber, S.; Schlinkert, P.; Zatschler, B.; Friebel, C.; Müller, B.; El-Armouche, A.; Morawietz, H.; et al. Estrogen determines sex differences in adrenergic vessel tone by regulation of endothelial β-adrenoceptor expression. Am. J. Physiol. Circ. Physiol. 2019, 317, H243–H254. [Google Scholar] [CrossRef]
- Saleh, T.M.; Connell, B.J. Centrally mediated effect of 17β-estradiol on parasympathetic tone in male rats. Am. J. Physiol. Integr. Comp. Physiol. 1999, 276, R474–R481. [Google Scholar] [CrossRef]
- Hammoud, A.; Tikhomirov, A.; Myasishcheva, G.; Shaheen, Z.; Volkov, A.; Briko, A.; Shchukin, S. Multi-channel bioimpedance system for detecting vascular tone in human limbs: An approach. Sensors 2022, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Gooden, B.A. Mechanism of the human diving response. Integr. Psychol. Behav. Sci. 1994, 29, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Baranova, T.I. Ob osobennostiakh serdechno-sosudistoĭ sistemy pri nyriatel’noĭ reaktsii u cheloveka [Characteristics of the human cardiovascular system in the human diving response]. Ross. Fiziol. Zh. Im. I. M. Sechenova 2004, 90, 20–31. [Google Scholar] [PubMed]
- McMahon, T.J.; Hood, J.S.; Kadowitz, P.J. Pulmonary vasodilator response to vagal stimulation is blocked by N omega-nitro-L-arginine methyl ester in the cat. Circ. Res. 1992, 70, 364–369. [Google Scholar] [CrossRef]
- Ding, X.; Murray, P.A. Regulation of pulmonary venous tone in response to muscarinic receptor activation. Am. J. Physiol. Cell. Mol. Physiol. 2005, 288, L131–L140. [Google Scholar] [CrossRef]
- Moretta, D.; Papamatheakis, D.G.; Morris, D.P.; Giri, P.C.; Blood, Q.; Murray, S.; Ramzy, M.; Romero, M.; Vemulakonda, S.; Lauw, S.; et al. Long-term high-altitude hypoxia and alpha adrenoceptor-dependent pulmonary arterial contractions in fetal and adult sheep. Front. Physiol. 2019, 10, 1032. [Google Scholar] [CrossRef]
- Bartelink, M.L.; De Wit, A.; Wollersheim, H.; Theeuwes, A.; Thien, T. Skin vascular reactivity in healthy subjects: Influence of hormonal status. J. Appl. Physiol. 1993, 74, 727–732. [Google Scholar] [CrossRef]
- Phillippe, M.; Saunders, T.; Bangalore, S. A mechanism for testosterone modulation of alpha-1 adrenergic receptor expression in the DDT1 MF-2 smooth muscle myocyte. Mol. Cell. Biochem. 1991, 100, 79–90. [Google Scholar] [CrossRef]
- Maclusky, N.J.; Naftolin, F.; Krey, L.C.; Franks, S. The catechol estrogens. J. Steroid Biochem. 1981, 15, 111–124. [Google Scholar] [CrossRef]
- Arnold, A.P.; Gorski, R.A. Gonadal steroid induction of structural sex differences in the central nervous system. Annu. Rev. Neurosci. 1984, 7, 413–442. [Google Scholar] [CrossRef]
- Dergacheva, O. Chronic intermittent hypoxia alters neurotransmission from lateral paragigantocellular nucleus to parasympathetic cardiac neurons in the brain stem. J. Neurophysiol. 2015, 113, 380–389. [Google Scholar] [CrossRef]
- Rainbow, T.C.; Degroff, V.; Luine, V.N.; McEwen, B.S. Estradiol 17β increases the number of muscarinic receptors in hypothalamic nuclei. Brain Res. 1980, 198, 239–243. [Google Scholar] [CrossRef]
- Olsen, K.; Edwards, E.; Schechter, N.; Whalen, R. Muscarinic receptors in preoptic area and hypothalamus: Effects of cyclicity, sex and estrogen treatment. Brain Res. 1988, 448, 223–229. [Google Scholar] [CrossRef]
- Huang, A.; Koller, A. Endothelin and prostaglandin H2 enhance arteriolar myogenic tone in hypertension. Hypertension 1997, 30, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.M.; Connell, B.J. 17β-estradiol modulates baroreflex sensitivity and autonomic tone of female rats. J. Auton. Nerv. Syst. 2000, 80, 148–161. [Google Scholar] [CrossRef]
- Qiao, G.-F.; Li, B.-Y.; Lu, Y.-J.; Fu, Y.-L.; Schild, J.H. 17β-estradiol restores excitability of a sexually dimorphic subset of myelinated vagal afferents in ovariectomized rats. Am. J. Physiol. Physiol. 2009, 297, C654–C664. [Google Scholar] [CrossRef]
- Ciriello, J.; Caverson, M.M. Effect of estrogen on vagal afferent projections to the brainstem in the female. Brain Res. 2016, 1636, 21–42. [Google Scholar] [CrossRef]
- Becker, J.B.; Prendergast, B.J.; Liang, J.W. Female rats are not more variable than male rats: A meta-analysis of neuroscience studies. Biol. Sex Differ. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Huang, J.; Li, Y.; Thijs, L.; Wang, Z.; Lu, X.; Cao, K.; Xie, S.; Staessen, J.A.; et al. Cardiovascular and metabolic phenotypes in relation to the ADRA2B insertion/deletion polymorphism in a Chinese population. J. Hypertens. 2005, 23, 2201–2207. [Google Scholar] [CrossRef][Green Version]
- Freitas, S.R.; Pereira, A.C.; Floriano, M.S.; Mill, J.G.; Krieger, J.E. Association of alpha1a-adrenergic receptor polymorphism and blood pressure phenotypes in the Brazilian population. BMC Cardiovasc. Disord. 2008, 8, 40–47. [Google Scholar] [CrossRef]
- Brylske, A. The next generation: What you need to know about kid divers. In The Complete Diver: The History, Science and Practice of Scuba Diving; Dive Training LLC: Parkville, MO, USA, 2012; pp. 155–161. [Google Scholar]
- Tishchenko, M.I. Stroke volume measurement by integral reography of human body. Physiol. J. USSR [Fiziol. Zhurnal SSSR] 1973, 59, 1216–1224. (In Russian) [Google Scholar]
- Palko, T. Impedance rheography for systemic and pulmonary circulation study and clinical application. In Proceedings of the 13th International Conference on Electrical Bioimpedance and the 8th Conference on Electrical Impedance Tomography, IFMBE, Graz, Austria, 29 August–2 September 2007; Scharfetter, H., Merwa, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 17. [Google Scholar] [CrossRef]
- Zenkov, L.R.; Ronkin, M.A. Functional Diagnostics of Nervous Diseases; Medicine: Moscow, Russia, 1991; ISBN 5-225-01170-5. [Google Scholar]
- Korpas, D.; Hálek, J.; Doležal, L. Parameters describing the pulse wave. Physiol. Res. 2009, 58, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Alian, A.A.; Shelley, K.H. Photoplethysmography. Best Pract. Res. Clin. Anaesthesiol. 2014, 28, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.P.; Maniatis, T. Molecular Cloning: A Laboratory Manual. Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]

| Parameter | Men (n = 52) | Women (n = 50) |
|---|---|---|
| Weight, kg | 73.4 ± 10.7 | 58.6 ± 8.5 |
| Height, cm | 178.9 ± 6.2 | 165.7 ± 6.2 |
| BMI | 22.8 ± 2.5 | 21.4 ± 3.1 |
| Age, yr | 24.2± 4.7 | 21.9 ± 2.9 |
| Parameter | Attempt #1 | Attempt #2 | Attempt #3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CL | FIm | Rec | CL | FIm | Rec | CL | FIm | Rec | |
| HR, bmp | 74.3 ± 1.2 | 65.3 ± 1.3 *** | 68.4 ± 1.2 | 72.9 ± 1.1 | 63.5 ± 1.4 *** | 67.9 ± 1.1 | 73.3 ± 1.2 | 63.5 ± 1.2 *** | 67.8 ± 1.1 |
| SO, mL | 91.9 ± 2.1 | 93.9 ± 2.4 | 95.8 ± 2.2 | 93.1 ± 2.2 | 95.2 ± 2.33 | 96.4 ± 2.1 | 95.1 ± 2.1 | 96.4 ± 2.2 | 97.0 ± 2.1 |
| PWA, pm | 0.92 ± 0.09 | 0.39 ± 0.03 *** | 1.09 ± 0.10 | 0.93 ± 0.09 | 0.41 ± 0.04 *** | 1.06 ± 0.10 | 0.80 ± 0.08 | 0.36 ± 0.03 *** | 0.98 ± 0.09 |
| RI, Ohm | 0.30 ± 0.014 | 0.30 ± 0.018 | 0.29 ± 0.009 | 0.29 ± 0.009 | 0.28 ± 0.008 | 0.30 ± 0.009 | 0.29 ± 0.008 | 0.27 ± 0.008 | 0.28 ± 0.008 |
| DCI, % | 56.16 ± 1.03 | 51.41 ± 1.4 *** | 53.68 ± 0.96 | 55.31 ± 0.9 | 50.3 ± 1.4 ** | 54.21 ± 0.94 | 54.74 ± 0.94 | 52.32 ± 1.4 | 53.27 ± 0.99 |
| DSI, % | 58.09 ± 0.97 | 52.26 ± 1.4 *** | 58.21 ± 0.9 | 57.92 ± 0.9 | 52.87 ± 1.4 ** | 59.16 ± 0.94 | 57.57 ± 0.93 | 54.88 ± 1.4 | 58.50 ± 1.0 |
| SBP, mmHg | 115.1 ± 0.99 | 129.4 ± 1.63 *** | 125.9 ± 1.08 | 116.3 ± 0.96 | 129.5 ± 1.61 *** | 117.3 ± 0.92 | 117.9 ± 0.89 | 127.9 ± 1.51 *** | 116.4 ± 1.04 |
| DBP, mmHg | 69.41 ± 0.63 | 83.64 ± 1.09 *** | 69.84 ± 0.63 | 68.7 ± 0.92 | 82.89 ± 1.06 *** | 70.63 ± 0.60 | 70.8 ± 0.68 | 83.32 ± 1.08 *** | 70.51 ± 0.67 |
| Parameter | Women (n = 50) | Men (n = 52) | ||||
|---|---|---|---|---|---|---|
| CL | FIm | Rec | CL | FIm | Rec | |
| HR, bmp | 73.75 ± 0.98 | 63.39 ± 1.13 | 68.22 ± 0.88 | 74.23 ± 1.29 | 66.02 ± 1.21 | 69.51 ± 1.30 |
| SO, mL | 79.06 ± 1.15 | 79.69 ± 1.29 | 82.20 ± 1.05 | 107.10 ± 1.45 *** | 109.70 ± 1.57 *** | 109.80 ± 1.53 *** |
| PWA, pm | 0.58 ± 0.05 | 0.3 ± 0.03 | 0.7 ± 0.06 | 1.13 ± 0.09 *** | 0.45 ± 0.03 ** | 1.28 ± 0.09 *** |
| RI, Ohm | 0.31 ± 0.01 *** | 0.32 ± 0.01 *** | 0.31 ± 0.01 *** | 0.26 ± 0.01 | 0.24 ± 0.01 | 0.27 ± 0.01 |
| DCI, % | 52.99 ± 0.78 | 47.70 ± 1.05 | 51.27 ± 0.76 | 55.03 ± 0.82 | 53.52 ± 1.23 *** | 53.62 ± 0.85 |
| DSI, % | 55.34 ± 0.78 | 50.54 ± 1.06 | 55.77 ± 0.75 | 58.06 ± 0.7673 | 56.32 ± 1.22 *** | 58.83 ± 0.81 |
| SBP, mmHg | 110.2 ± 1.4 | 125.2 ± 2.1 | 112.0 ± 1.3 | 119.5 ± 1.2 ** | 133.7 ± 2.6 ** | 119.0 ± 1.1 * |
| DBP, mmHg | 68.57 ± 0.96 | 83.26 ± 1.48 | 69.50 ± 0.97 | 70.02 ± 0.85 | 83.70 ± 1.71 | 70.02 ± 0.86 |
| ADRA1A (rs1048101) | C/T | T/T | C/C |
|---|---|---|---|
| Total (n = 102) | 47 | 23 | 32 |
| Women (n = 50) | 21 | 12 | 17 |
| Men (n = 52) | 26 | 11 | 15 |
| Genotype | Women (n = 50) | Men (n = 52) | ||||
|---|---|---|---|---|---|---|
| CL | FIm | Rec | CL | FIm | Rec | |
| C/T | 0.315 ± 0.012 ** | 0.313 ± 0.013 *** | 0.308 ± 0.011 * | 0.257 ± 0.008 | 0.246 ± 0.008 | 0.264 ± 0.009 |
| T/T | 0.307 ± 0.013 * | 0.3438 ± 0.03 *** | 0.3101 ± 0.01 * | 0.2536 ± 0.014 | 0.2211 ± 0.009 | 0.2598 ± 0.01 |
| C/C | 0.3484 ± 0.038 | 0.3245 ± 0.016 | 0.3296 ± 0.02 | 0.2876 ± 0.01 | 0.2631 ± 0.01 | 0.2835 ± 0.01 |
| Genotype | Women (n = 50) | Men (n = 52) | ||||
|---|---|---|---|---|---|---|
| CL | FIm | Rec | CL | FIm | Rec | |
| C/T | 50.49 ± 1.13 | 43.36 ± 1.58 | 49.13 ± 1.11 | 56.28 ± 1.29 * | 55.00 ± 1.67 *** | 54.98 ± 1.37 * |
| T/T | 54.3 ± 1.21 | 51.8 ± 1.79 | 51.97 ± 1.24 | 51.61 ± 1.21 | 48.78 ± 2.10 | 51.44 ± 1.01 |
| C/C | 55.93 ± 1.89 | 49.76 ± 1.88 | 54.47 ± 1.69 | 56.23 ± 1.47 | 55.78 ± 2.94 | 53.04 ± 1.70 |
| Genotype | Women (n = 50) | Men (n = 52) | ||||
|---|---|---|---|---|---|---|
| CL | FIm | Rec | CL | FIm | Rec | |
| C/T | 53.71 ± 1.22 | 47.39 ± 1.62 | 54.6 ± 1.23 | 59.58 ± 1.25 * | 58.1 ± 1.61 *** | 61.13 ± 1.24 * |
| T/T | 55.89 ± 1.13 | 53.91 ± 1.79 | 55.78 ± 1.06 | 54.98 ± 0.97 | 51.87 ± 2.08 | 57.4 ± 1.01 |
| C/C | 57.76 ± 1.83 | 51.4 ± 2.00 | 58.14 ± 1.69 | 58.21 ± 1.36 | 57.52 ± 3.11 | 55.09 ± 1.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranova, T.; Podyacheva, E.; Zemlyanukhina, T.; Berlov, D.; Danilova, M.; Glotov, O.; Glotov, A. Vascular Reactions of the Diving Reflex in Men and Women Carrying Different ADRA1A Genotypes. Int. J. Mol. Sci. 2022, 23, 9433. https://doi.org/10.3390/ijms23169433
Baranova T, Podyacheva E, Zemlyanukhina T, Berlov D, Danilova M, Glotov O, Glotov A. Vascular Reactions of the Diving Reflex in Men and Women Carrying Different ADRA1A Genotypes. International Journal of Molecular Sciences. 2022; 23(16):9433. https://doi.org/10.3390/ijms23169433
Chicago/Turabian StyleBaranova, Tatyana, Ekaterina Podyacheva, Tatyana Zemlyanukhina, Dmitrii Berlov, Maria Danilova, Oleg Glotov, and Andrey Glotov. 2022. "Vascular Reactions of the Diving Reflex in Men and Women Carrying Different ADRA1A Genotypes" International Journal of Molecular Sciences 23, no. 16: 9433. https://doi.org/10.3390/ijms23169433
APA StyleBaranova, T., Podyacheva, E., Zemlyanukhina, T., Berlov, D., Danilova, M., Glotov, O., & Glotov, A. (2022). Vascular Reactions of the Diving Reflex in Men and Women Carrying Different ADRA1A Genotypes. International Journal of Molecular Sciences, 23(16), 9433. https://doi.org/10.3390/ijms23169433







