Translating Material Science into Bone Regenerative Medicine Applications: State-of-The Art Methods and Protocols
Abstract
:1. Introduction
2. Bone Scaffold Architecture: Characterizing Scaffold Internal Structure and Composition through Spectroscopy, Microscopy, and Mechanical Testing
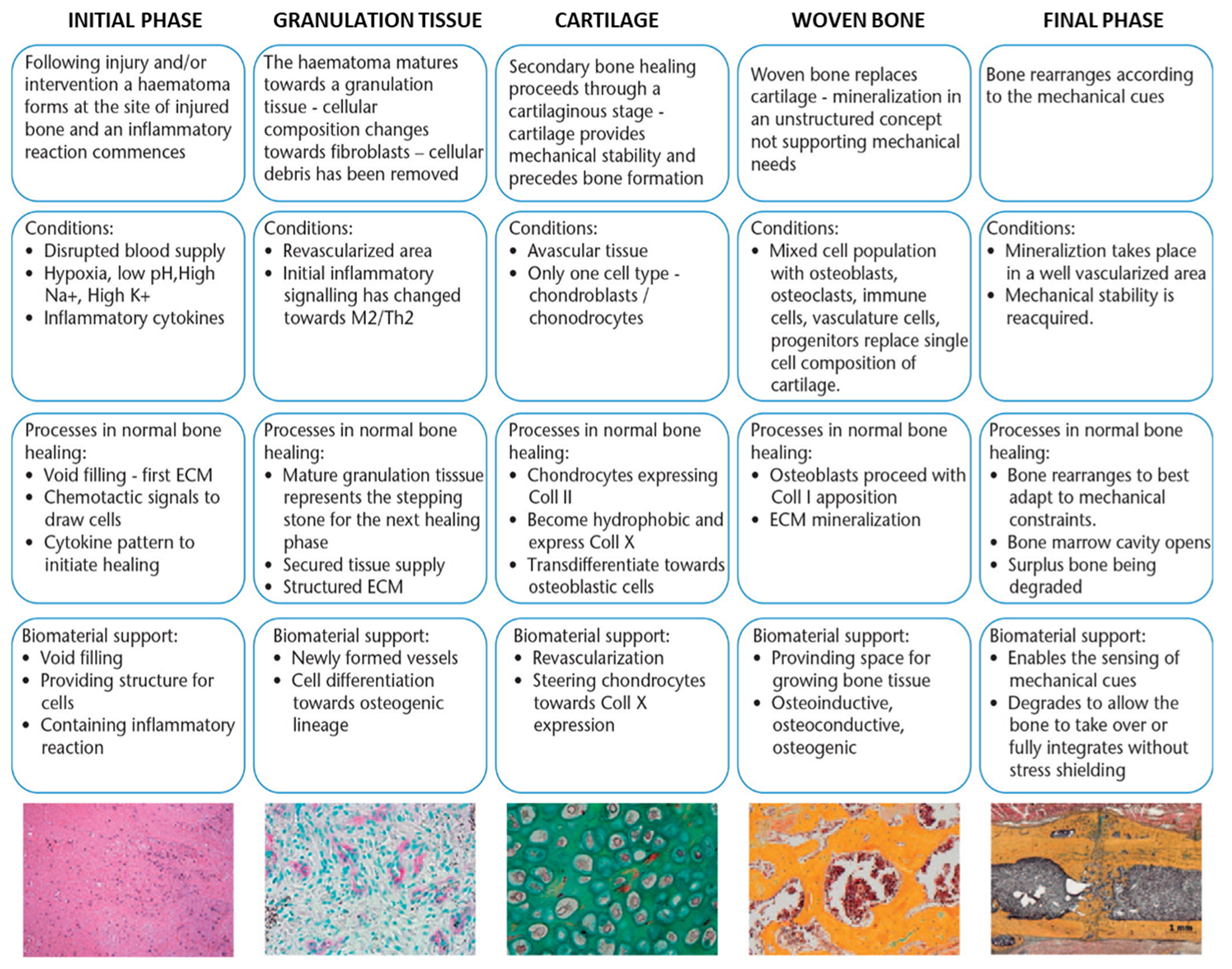
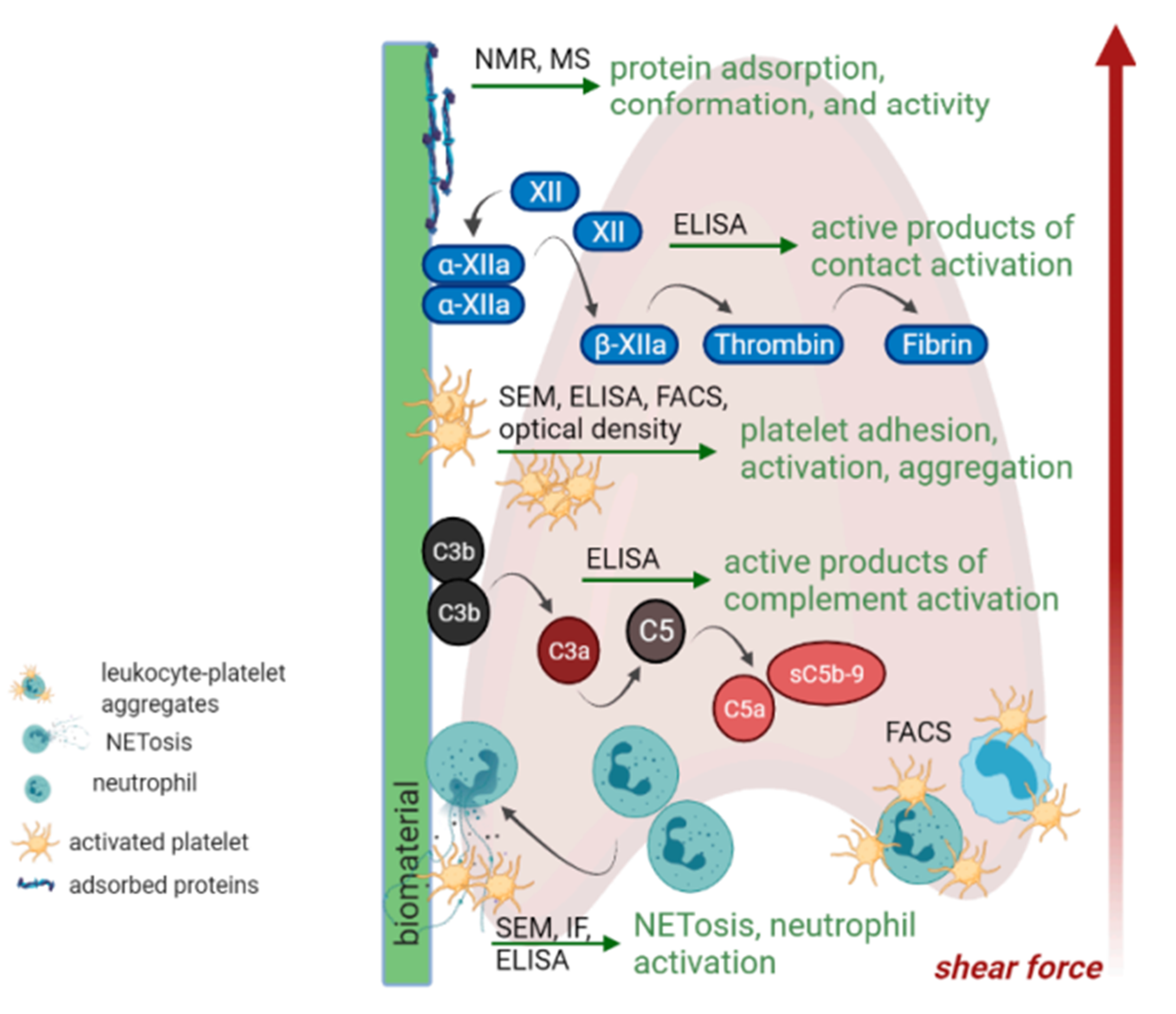
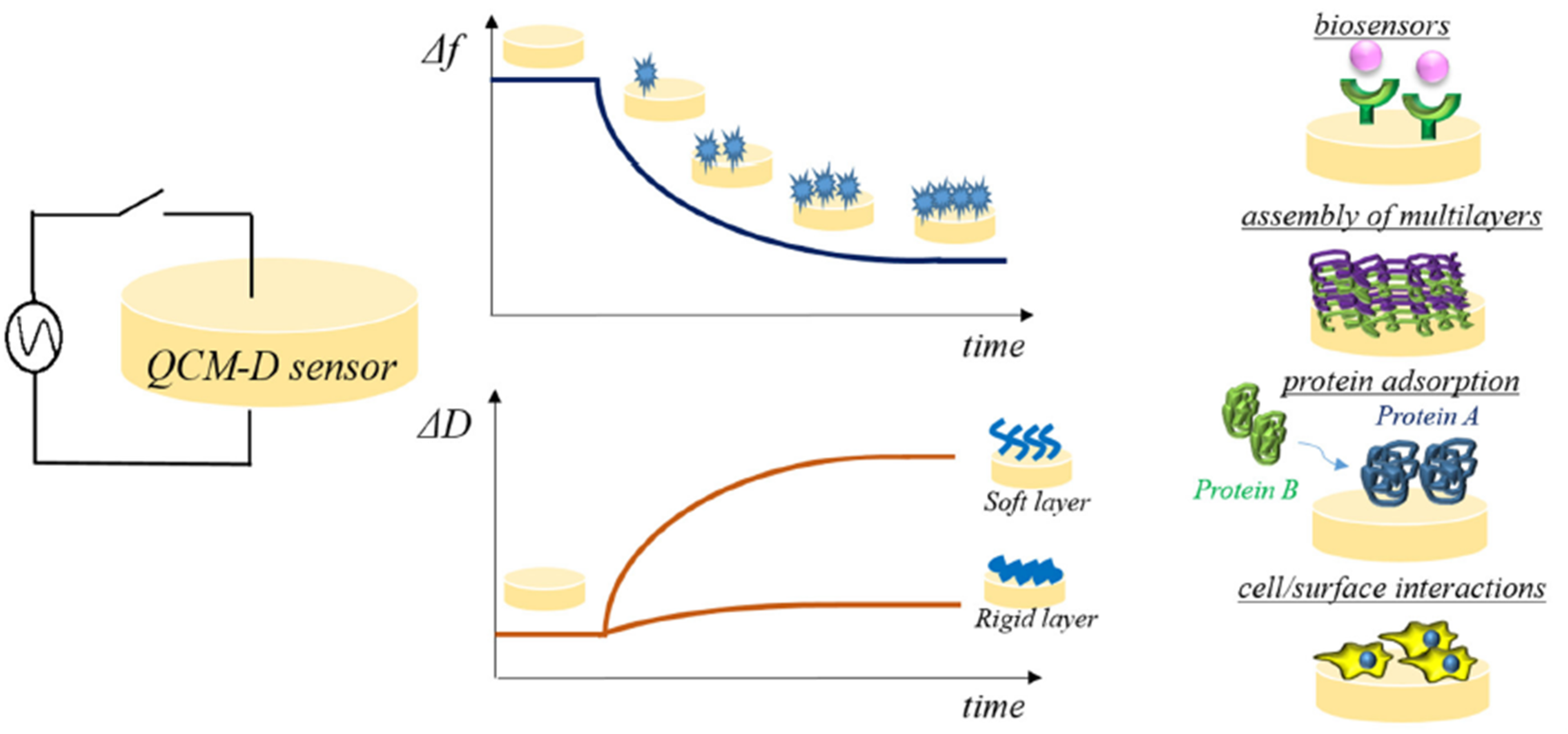
3. Biomaterial-Tissue Interface
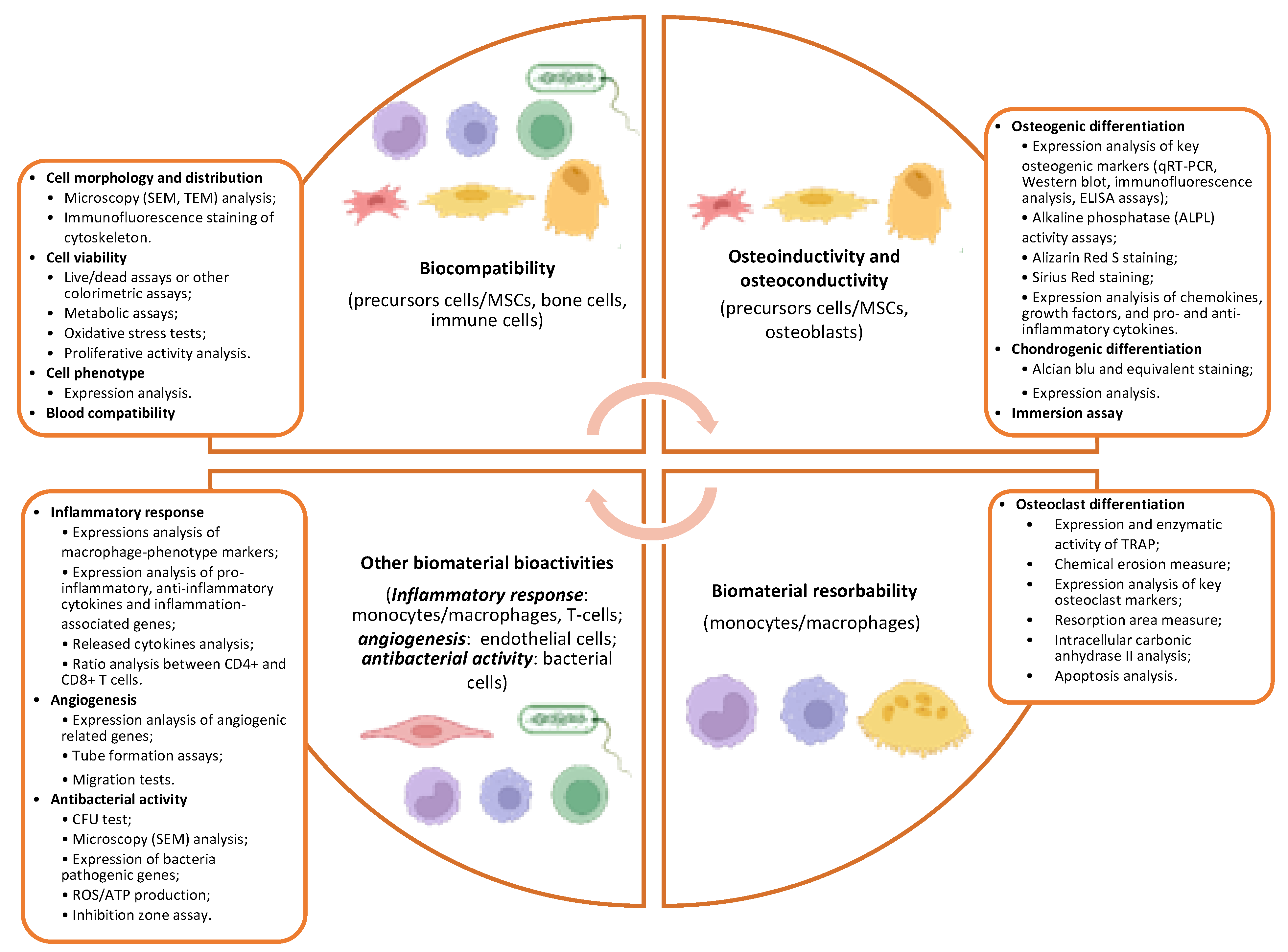
4. Biological Characterization of Bone Substitute Materials
5. Biomaterial Biocompatibility
6. Biomaterial Osteoinductivity and Osteoconductivity
6.1. Types of Cells
6.2. Osteogenesis
6.3. Chondrogenesis
7. Biomaterial Resorbability
8. Other Biomaterial Bioactivities
8.1. Inflammatory Response
8.2. Angiogenesis
8.3. Antimicrobial Properties
9. Animal Models for In Vivo Biomaterial Testing
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Fracture Collaborators. Global, Regional, and National Burden of Bone Fractures in 204 Countries and Territories, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet Health Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Smakaj, A.; De Mauro, D.; Rovere, G.; Pietramala, S.; Maccauro, G.; Parolini, O.; Lattanzi, W.; Liuzza, F. Clinical Application of Adipose Derived Stem Cells for the Treatment of Aseptic Non-Unions: Current Stage and Future Perspectives—Systematic Review. Int. J. Mol. Sci. 2022, 23, 3057. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, Osteoconduction and Osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, V.; Lattanzi, W.; Perini, G.; Augello, A.; Papi, M.; De Spirito, M. 3D-Printed Graphene for Bone Reconstruction. 2D Mater. 2020, 7, 022004. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture Healing: The Diamond Concept. Injury 2007, 38, S3–S6. [Google Scholar] [CrossRef]
- Tiberio, F.; Cacciotti, I.; Frassanito, P.; Nocca, G.; Tamburrini, G.; Arcovito, A.; Lattanzi, W. Personalized Bone Reconstructionand Regeneration in the Treatment of Craniosynostosis. Appl. Sci. 2021, 11, 2649. [Google Scholar] [CrossRef]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90. [Google Scholar]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone Grafts: Which is the Ideal Biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- Palmieri, V.; Barba, M.; Di Pietro, L.; Gentilini, S.; Braidotti, M.C.; Ciancico, C.; Bugli, F.; Ciasca, G.; Larciprete, R.; Lattanzi, W.; et al. Reduction and Shaping of Graphene-Oxide by Laser-Printing for Controlled Bone Tissue Regeneration and Bacterial Killing. 2D Mater. 2018, 5, 15027. [Google Scholar] [CrossRef]
- Corona-Gomez, J.; Chen, X.; Yang, Q. Effect of Nanoparticle Incorporation and Surface Coating on Mechanical Properties of Bone Scaffolds: A Brief Review. J. Funct. Biomater. 2016, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Wang, W.; Bártolo, P. Investigating the Effect of Carbon Nanomaterials Reinforcing Poly (ε-Caprolactone) Printed Scaffolds for Bone Repair Applications. Int. J. Bioprint. 2020, 6, 266. [Google Scholar] [CrossRef]
- Su, J.; Du, Z.; Xiao, L.; Wei, F.; Yang, Y.; Li, M.; Qiu, Y.; Liu, J.; Chen, J.; Xiao, Y. Graphene Oxide Coated Titanium Surfaces with Osteoimmunomodulatory Role to Enhance Osteogenesis. Mater. Sci. Eng. C 2020, 113, 110983. [Google Scholar] [CrossRef]
- He, J.; You, D.; Li, Q.; Wang, J.; Ding, S.; He, X.; Zheng, H.; Ji, Z.; Wang, X.; Ye, X. Osteogenesis-Inducing Chemical Cues Enhance the Mechanosensitivity of Human Mesenchymal Stem Cells for Osteogenic Differentiation on a Microtopographically Patterned Surface. Adv. Sci. 2022, 9, 2200053. [Google Scholar] [CrossRef]
- Omidi, M.; Fatehinya, A.; Farahani, M.; Akbari, Z.; Shahmoradi, S.; Yazdian, F.; Tahriri, M.; Moharamzadeh, K.; Tayebi, L.; Vashaee, D. Characterization of Biomaterials. In Biomaterials for Oral and Dental Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 97–115. [Google Scholar]
- Reznikov, N.; Shahar, R.; Weiner, S. Bone Hierarchical Structure in Three Dimensions. Acta Biomater. 2014, 10, 3815–3826. [Google Scholar] [CrossRef]
- Burnett, T.L.; Withers, P.J. Completing the Picture through Correlative Characterization. Nat. Mater. 2019, 18, 1041–1049. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.D.; Wang, H.-L.; Bernard, J.-P.; Sammartino, G. New Biomaterials and Regenerative Medicine Strategies in Periodontology, Oral Surgery, Esthetic and Implant Dentistry. BioMed Res. Int. 2015, 2015, 210792. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable Materials for Bone Defect Repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Prakoso, A.T.; Syahrom, A.; Sulong, M.A.; Saad, A.P.M.; Yani, I.; Nasution, J.D.; Basri, H. A Comparison of Degradation Rate Bone Scaffold Morphology between Computer Simulation and Experimental Approach. Malays. J. Fundam. Appl. Sci. 2017, 13, 529–532. [Google Scholar] [CrossRef]
- Maroulakos, M.; Kamperos, G.; Tayebi, L.; Halazonetis, D.; Ren, Y. Applications of 3D Printing on Craniofacial Bone Repair: A Systematic Review. J. Dent. 2018, 80, 1–14. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B. 3D Printing of Bone Tissue Engineering Scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone Tissue Engineering Using 3D Printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Suga, M.; Asahina, S.; Sakuda, Y.; Kazumori, H.; Nishiyama, H.; Nokuo, T.; Alfredsson, V.; Kjellman, T.; Stevens, S.M.; Cho, H.S. Recent Progress in Scanning Electron Microscopy for the Characterization of Fine Structural Details of Nano Materials. Prog. Solid State Chem. 2014, 42, 1–21. [Google Scholar] [CrossRef]
- Shah, F.A.; Ruscsák, K.; Palmquist, A. 50 Years of Scanning Electron Microscopy of Bone—A Comprehensive Overview of the Important Discoveries Made and Insights Gained into Bone Material Properties in Health, Disease, and Taphonomy. Bone Res. 2019, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold Design for Bone Regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [Green Version]
- Palmroth, A.; Pitkänen, S.; Hannula, M.; Paakinaho, K.; Hyttinen, J.; Miettinen, S.; Kellomäki, M. Evaluation of Scaffold Microstructure and Comparison of Cell Seeding Methods Using Micro-Computed Tomography-Based Tools. J. R. Soc. Interface 2020, 17, 20200102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous Scaffolds for Bone Regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the Mechanical Strength of Biomaterials Used as a Bone Scaffold in Oral and Maxillofacial Defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Kang, J.-H. Mechanical Properties of Compact Bone Defined by the Stress-Strain Curve Measured Using Uniaxial Tensile Test: A Concise Review and Practical Guide. Materials 2021, 14, 4224. [Google Scholar] [CrossRef]
- Vazquez, O.R.; Avila, I.O.; Díaz, J.C.S.; Hernandez, E. An Overview of Mechanical Tests for Polymeric Biomaterial Scaffolds Used in Tissue Engineering. J. Res. Updates Polym. Sci. 2015, 4, 168–178. [Google Scholar]
- Oevreeide, I.H.; Szydlak, R.; Luty, M.; Ahmed, H.; Prot, V.; Skallerud, B.H.; Zemła, J.; Lekka, M.; Stokke, B.T. On the Determination of Mechanical Properties of Aqueous Microgels—Towards High-Throughput Characterization. Gels 2021, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hughes, R.; Mullin, N.; Hawkins, R.J.; Holen, I.; Brown, N.J.; Hobbs, J.K. Mechanical Heterogeneity in the Bone Microenvironment as Characterized by Atomic Force Microscopy. Biophys. J. 2020, 119, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Butt, H.-J.; Cappella, B.; Kappl, M. Force Measurements with the Atomic Force Microscope: Technique, Interpretation and Applications. Surf. Sci. Rep. 2005, 59, 1–152. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.P. Protein Adsorption on Biomaterial Surfaces: Subsequent Conformational and Biological Consequences—A Review. J. Surf. Sci Technol. 2020, 36, 7–38. [Google Scholar]
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A Review of Biomaterials in Bone Defect Healing, Remaining Shortcomings and Future Opportunities for Bone Tissue Engineering: The Unsolved Challenge. Bone Jt. Res. 2018, 7, 232–243. [Google Scholar] [CrossRef]
- Strohbach, A.; Busch, R. Predicting the In Vivo Performance of Cardiovascular Biomaterials: Current Approaches In Vitro Evaluation of Blood-Biomaterial Interactions. Int. J. Mol. Sci. 2021, 22, 11390. [Google Scholar] [CrossRef]
- Palmieri, V.; De Spirito, M.; Papi, M. Nanofeatures of Orthopedic Implant Surfaces 2021. Nanomedicine 2021, 16, 1733–1736. [Google Scholar] [CrossRef]
- Migliorini, E.; Weidenhaupt, M.; Picart, C. Practical Guide to Characterize Biomolecule Adsorption on Solid Surfaces. Biointerphases 2018, 13, 06D303. [Google Scholar] [CrossRef] [Green Version]
- Kratz, F.; Grass, S.; Umanskaya, N.; Scheibe, C.; Müller-Renno, C.; Davoudi, N.; Hannig, M.; Ziegler, C. Cleaning of Biomaterial Surfaces: Protein Removal by Different Solvents. Colloids Surf. B Biointerfaces 2015, 128, 28–35. [Google Scholar] [CrossRef]
- Cordeiro, A.L.; Rückel, M.; Bartels, F.; Maitz, M.F.; Renner, L.D.; Werner, C. Protein Adsorption Dynamics to Polymer Surfaces Revisited—A Multisystems Approach. Biointerphases 2019, 14, 51005. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Carmagnola, I.; Ciardelli, G. Quartz Crystal Microbalance with Dissipation Monitoring: A Powerful Method to Predict the in Vivo Behavior of Bioengineered Surfaces. Front. Bioeng. Biotechnol. 2018, 6, 158. [Google Scholar] [CrossRef]
- Dabare, P.R.L.; Bachhuka, A.; Parkinson-Lawrence, E.; Vasilev, K. Surface Nanotopography Mediated Albumin Adsorption, Unfolding and Modulation of Early Innate Immune Responses. Mater. Today Adv. 2021, 12, 100187. [Google Scholar] [CrossRef]
- Ekdahl, K.N.; Huang, S.; Nilsson, B.; Teramura, Y. Complement Inhibition in Biomaterial-and Biosurface-Induced Thromboinflammation. Semin. Immunol. 2016, 28, 268–277. [Google Scholar] [CrossRef]
- Papi, M.; Palmieri, V.; Palchetti, S.; Pozzi, D.; Digiacomo, L.; Guadagno, E.; del Basso De Caro, M.; Di Domenico, M.; Ricci, S.; Pani, R. Exploitation of Nanoparticle-Protein Interactions for Early Disease Detection. Appl. Phys. Lett. 2019, 114, 163702. [Google Scholar] [CrossRef] [Green Version]
- Corbo, C.; Molinaro, R.; Tabatabaei, M.; Farokhzad, O.C.; Mahmoudi, M. Personalized Protein Corona on Nanoparticles and Its Clinical Implications. Biomater. Sci. 2017, 5, 378–387. [Google Scholar] [CrossRef]
- Brown, B.N.; Haschak, M.J.; Lopresti, S.T.; Stahl, E.C. Effects of Age-Related Shifts in Cellular Function and Local Microenvironment upon the Innate Immune Response to Implants. Semin. Immunol. 2017, 29, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Kapp, M.; Boccaccini, A.R. Protein Interactions with Bioactive Glass Surfaces: A Review. Appl. Mater. Today 2019, 15, 350–371. [Google Scholar] [CrossRef]
- Helmus, M.N.; Gibbons, D.F.; Cebon, D. Biocompatibility: Meeting a Key Functional Requirement of Next-Generation Medical Devices. Toxicol. Pathol. 2008, 36, 70–80. [Google Scholar] [CrossRef]
- Huzum, B.; Puha, B.; Necoara, R.; Gheorghevici, S.; Puha, G.; Filip, A.; Sirbu, P.; Alexa, O. Biocompatibility Assessment of Biomaterials Used in Orthopedic Devices: An Overview (Review). Exp. Ther. Med. 2021, 22, 1315. [Google Scholar] [CrossRef]
- Seweryn, A.; Pielok, A.; Lawniczak-Jablonska, K.; Pietruszka, R.; Marcinkowska, K.; Sikora, M.; Witkowski, B.S.; Godlewski, M.; Marycz, K.; Smieszek, A. Zirconium Oxide Thin Films Obtained by Atomic Layer Deposition Technology Abolish the Anti-Osteogenic Effect Resulting from MiR-21 Inhibition in the Pre-Osteoblastic MC3T3 Cell Line. Int. J. Nanomed. 2020, 15, 1595–1610. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Deng, H.; Fan, Y.; Zheng, L.; Che, J.; Li, X.; Aifantis, K.E. Conductive Nanostructured Si Biomaterials Enhance Osteogeneration through Electrical Stimulation. Mater. Sci. Eng. C 2019, 103, 109748. [Google Scholar] [CrossRef]
- Mielan, B.; Sousa, D.M.; Krok-Borkowicz, M.; Eloy, P.; Dupont, C.; Lamghari, M.; Pamuła, E. Polymeric Microspheres/Cells/Extracellular Matrix Constructs Produced by Auto-Assembly for Bone Modular Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 7897. [Google Scholar] [CrossRef]
- Li, M.; Bai, J.; Tao, H.; Hao, L.; Yin, W.; Ren, X.; Gao, A.; Li, N.; Wang, M.; Fang, S.; et al. Rational Integration of Defense and Repair Synergy on PEEK Osteoimplants via Biomimetic Peptide Clicking Strategy. Bioact. Mater. 2022, 8, 309–324. [Google Scholar] [CrossRef]
- Comini, S.; Sparti, R.; Coppola, B.; Mohammadi, M.; Scutera, S.; Menotti, F.; Banche, G.; Cuffini, A.M.; Palmero, P.; Allizond, V. Novel Silver-functionalized Poly(Ɛ-caprolactone)/Biphasic Calcium Phosphate Scaffolds Designed to Counteract Post-surgical Infections in Orthopedic Applications. Int. J. Mol. Sci. 2021, 22, 10176. [Google Scholar] [CrossRef]
- Hua, L.; Qian, H.; Lei, T.; Zhang, Y.; Lei, P.; Hu, Y. 3D-Printed Porous Tantalum Coated with Antitubercular Drugs Achieving Antibacterial Properties and Good Biocompatibility. Macromol. Biosci. 2022, 22, 2100338. [Google Scholar] [CrossRef]
- Luo, S.; Wang, P.; Ma, M.; Pan, Z.; Lu, L.; Yin, F.; Cai, J. Genistein Loaded into Microporous Surface of Nano Tantalum/PEEK Composite with Antibacterial Effect Regulating Cellular Response in Vitro, and Promoting Osseointegration in Vivo. J. Mech. Behav. Biomed. Mater. 2022, 125, 104972. [Google Scholar] [CrossRef]
- Chen, L.; Wang, D.; Qiu, J.; Zhang, X.; Liu, X.; Qiao, Y.; Liu, X. Synergistic Effects of Immunoregulation and Osteoinduction of Ds-Block Elements on Titanium Surface. Bioact. Mater. 2021, 6, 191–207. [Google Scholar] [CrossRef]
- Dozio, S.M.; Montesi, M.; Campodoni, E.; Sandri, M.; Piattelli, A.; Tampieri, A.; Panseri, S. Differences in Osteogenic Induction of Human Mesenchymal Stem Cells between a Tailored 3D Hybrid Scaffold and a 2D Standard Culture. J. Mater. Sci. Mater. Med. 2019, 30, 136. [Google Scholar] [CrossRef]
- Rodrigues, A.A.; Batista, N.A.; Malmonge, S.M.; Casarin, S.A.; Agnelli, J.A.M.; Santos, A.R.; Belangero, W.D. Osteogenic Differentiation of Rat Bone Mesenchymal Stem Cells Cultured on Poly (Hydroxybutyrate-Co-Hydroxyvalerate), Poly (ε-Caprolactone) Scaffolds. J. Mater. Sci. Mater. Med. 2021, 32, 138. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wu, P.; Xiao, L.; Zhao, Y.; Yan, F.; Liu, X.; Xie, Y.; Zhang, C.; Chen, Y.; Cai, L. Biomimetic Mineralization of Novel Hydroxyethyl Cellulose/Soy Protein Isolate Scaffolds Promote Bone Regeneration in Vitro and in Vivo. Int. J. Biol. Macromol. 2020, 162, 1627–1641. [Google Scholar] [CrossRef] [PubMed]
- Aslam Khan, M.U.; Raza, M.A.; Mehboob, H.; Kadir, M.R.A.; Razak, S.I.A.; Shah, S.A.; Iqbal, M.Z.; Amin, R. Development and: In Vitro Evaluation of κ-Carrageenan Based Polymeric Hybrid Nanocomposite Scaffolds for Bone Tissue Engineering. RSC Adv. 2020, 10, 40529–40542. [Google Scholar] [CrossRef]
- Careta, O.; Salicio-Paz, A.; Pellicer, E.; Ibáñez, E.; Fornell, J.; García-Lecina, E.; Sort, J.; Nogués, C. Electroless Palladium-Coated Polymer Scaffolds for Electrical Stimulation of Osteoblast-like Saos-2 Cells. Int. J. Mol. Sci. 2021, 22, 528. [Google Scholar] [CrossRef] [PubMed]
- Elashry, M.I.; Baulig, N.; Wagner, A.S.; Klymiuk, M.C.; Kruppke, B.; Hanke, T.; Wenisch, S.; Arnhold, S. Combined Macromolecule Biomaterials Together with Fluid Shear Stress Promote the Osteogenic Differentiation Capacity of Equine Adipose-Derived Mesenchymal Stem Cells. Stem Cell Res. Ther. 2021, 12, 116. [Google Scholar] [CrossRef]
- Lukaszewska-Kuska, M.; Wirstlein, P.; Majchrowski, R.; Dorocka-Bobkowska, B. The Effects of Titanium Topography and Chemical Composition on Human Osteoblast Cell. Physiol. Res. 2021, 70, 413–423. [Google Scholar] [CrossRef]
- da Silva Dias, C.; Rossi, M.C.; Apolonio, E.V.P.; dos Santos Rosa, G.; Pfeifer, J.P.H.; Hussni, C.A.; Watanabe, M.J.; Alves, A.L.G. Low Mg Content on Ti-Nb-Sn Alloy When in Contact with EBMMSCs Promotes Improvement of Its Biological Functions. J. Mater. Sci. Mater. Med. 2021, 32, 144. [Google Scholar] [CrossRef]
- El-Habashy, S.E.; Eltaher, H.M.; Gaballah, A.; Zaki, E.I.; Mehanna, R.A.; El-Kamel, A.H. Hybrid Bioactive Hydroxyapatite/Polycaprolactone Nanoparticles for Enhanced Osteogenesis. Mater. Sci. Eng. C 2021, 119, 111599. [Google Scholar] [CrossRef]
- Ignat, S.R.; Lazăr, A.D.; Şelaru, A.; Samoilă, I.; Vlăsceanu, G.M.; Ioniţă, M.; Radu, E.; Dinescu, S.; Costache, M. Versatile Biomaterial Platform Enriched with Graphene Oxide and Carbon Nanotubes for Multiple Tissue Engineering Applications. Int. J. Mol. Sci. 2019, 20, 3868. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.S.; Choi, S.H.; Choi, E.H.; Kim, K.M.; Chu, P.K. Enhanced Osteogenic Differentiation of Human Mesenchymal Stem Cells on Amine-Functionalized Titanium Using Humidified Ammonia Supplied Nonthermal Atmospheric Pressure Plasma. Int. J. Mol. Sci. 2020, 21, 6085. [Google Scholar] [CrossRef]
- Marques-Almeida, T.; Cardoso, V.F.; Gama, M.; Lanceros-Mendez, S.; Ribeiro, C. Patterned Piezoelectric Scaffolds for Osteogenic Differentiation. Int. J. Mol. Sci. 2020, 21, 8352. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Torreggiani, E.; Mazziotta, C.; Ruffini, A.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F.; Mazzoni, E. In Vitro Osteoinductivity Assay of Hydroxylapatite Scaffolds, Obtained with Biomorphic Transformation Processes, Assessed Using Human Adipose Stem Cell Cultures. Int. J. Mol. Sci. 2021, 22, 7092. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Huang, Y.; Jian, P.; Xie, Z.; Wu, Y.; Li, H.; Zeng, R.; Situ, F.; Tu, M. Enhanced Cell Affinity and Osteogenic Differentiation of Liquid Crystal-Based Substrate via Surface Bio-Functionalization. J. Biomed. Mater. Res. Part A 2021, 109, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chu, X.; Wang, D.; Jian, L.; Liu, L.; Yao, M.; Zhang, D.; Zheng, Y.; Liu, X.; Zhang, Y.; et al. Tuning the Surface Potential to Reprogram Immune Microenvironment for Bone Regeneration. Biomaterials 2022, 282, 121408. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, W.; Xiong, Z.; Hu, Y.; Xiao, J. Effects of Biomimetic Hydroxyapatite Coatings on Osteoimmunomodulation. Mater. Sci. Eng. C 2022, 5, 112640. [Google Scholar] [CrossRef]
- Huo, S.; Meng, X.; Zhang, S.; Yue, B.; Zhao, Y.; Long, T.; Nie, B.; Wang, Y. Hydrofluoric Acid and Nitric Acid Cotreatment for Biofunctionalization of Polyetheretherketone in M2 Macrophage Polarization and Osteogenesis. J. Biomed. Mater. Res. Part A 2021, 109, 879–892. [Google Scholar] [CrossRef]
- Liu, S.; Li, P.; Liu, X.; Wang, P.; Xue, W.; Ren, Y.; Yang, R.; Chi, B.; Ye, Z. Bioinspired Mineral-Polymeric Hybrid Hyaluronic Acid/Poly (γ-Glutamic Acid) Hydrogels as Tunable Scaffolds for Stem Cells Differentiation. Carbohydr. Polym. 2021, 264, 118048. [Google Scholar] [CrossRef]
- Lu, Y.C.; Chang, T.K.; Yeh, S.T.; Lin, T.C.; Lin, H.S.; Chen, C.H.; Huang, C.H.; Huang, C.H. Evaluation of Graphene-Derived Bone Scaffold Exposure to the Calvarial Bone_in-Vitro and in-Vivo Studies. Nanotoxicology 2022, 16, 1–15. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.; Kwok, T.; Yang, T.; Liu, C.; Li, W.; Zhang, X. A Bi-Layered Membrane with Micro-Nano Bioactive Glass for Guided Bone Regeneration. Colloids Surf. B Biointerfaces 2021, 205, 111886. [Google Scholar] [CrossRef]
- Zhongxing, L.; Shaohong, W.; Jinlong, L.; Limin, Z.; Yuanzheng, W.; Haipeng, G.; Jian, C. Three-Dimensional Printed Hydroxyapatite Bone Tissue Engineering Scaffold with Antibacterial and Osteogenic Ability. J. Biol. Eng. 2021, 15, 21. [Google Scholar] [CrossRef]
- Sun, H.; Zheng, K.; Zhou, T.; Boccaccini, A.R. Incorporation of Zinc into Binary SiO2-CaO Mesoporous Bioactive Glass Nanoparticles Enhances Anti-Inflammatory and Osteogenic Activities. Pharmaceutics 2021, 13, 2124. [Google Scholar] [CrossRef]
- Przekora, A.; Audemar, M.; Pawlat, J.; Canal, C.; Thomann, J.S.; Labay, C.; Wojcik, M.; Kwiatkowski, M.; Terebun, P.; Ginalska, G.; et al. Positive Effect of Cold Atmospheric Nitrogen Plasma on the Behavior of Mesenchymal Stem Cells Cultured on a Bone Scaffold Containing Iron Oxide-Loaded Silica Nanoparticles Catalyst. Int. J. Mol. Sci. 2020, 21, 4738. [Google Scholar] [CrossRef]
- Du, Z.; Zhao, Z.; Liu, H.; Liu, X.; Zhang, X.; Huang, Y.; Leng, H.; Cai, Q.; Yang, X. Macroporous Scaffolds Developed from CaSiO3 Nanofibers Regulating Bone Regeneration via Controlled Calcination. Mater. Sci. Eng. C 2020, 113, 111005. [Google Scholar] [CrossRef]
- Goto, R.; Nishida, E.; Kobayashi, S.; Aino, M.; Ohno, T.; Iwamura, Y.; Kikuchi, T.; Hayashi, J.I.; Yamamoto, G.; Asakura, M.; et al. Gelatin Methacryloyl–riboflavin (Gelma–rf) Hydrogels for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 1635. [Google Scholar] [CrossRef]
- Guo, C.; Lu, R.; Wang, X.; Chen, S. Antibacterial Activity, Bio-Compatibility and Osteogenic Differentiation of Graphene Oxide Coating on 3D-Network Poly-Ether-Ether-Ketone for Orthopaedic Implants. J. Mater. Sci. Mater. Med. 2021, 32, 135. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, D.; Li, M.; Liu, X.; Zhang, Y.; Qian, S.; Peng, F. Osteogenesis, Angiogenesis and Immune Response of Mg-Al Layered Double Hydroxide Coating on Pure Mg. Bioact. Mater. 2021, 6, 91–105. [Google Scholar] [CrossRef]
- Yao, Y.-T.; Yang, Y.; Ye, Q.; Cao, S.-S.; Zhang, X.-P.; Zhao, K.; Jian, Y. Effects of Pore Size and Porosity on Cytocompatibility and Osteogenic Differentiation of Porous Titanium. J. Mater. Sci. Mater. Med. 2021, 32, 72. [Google Scholar] [CrossRef]
- Li, X.; Huang, Q.; Hu, X.; Wu, D.; Li, N.; Liu, Y.; Li, Q.; Wu, H. Evaluating the Osteoimmunomodulatory Properties of Micro-Arc Oxidized Titanium Surface at Two Different Biological Stages Using an Optimized in Vitro Cell Culture Strategy. Mater. Sci. Eng. C 2020, 110, 110722. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, N.; Zhang, Y.; Zhao, B.; Zhang, Z.; Zhou, X.; Zhou, Y.; Liu, H.; Zhang, Y.; Liu, J. Enhanced Osteogenic Proliferation and Differentiation of Human Adipose-Derived Stem Cells on a Porous n-HA/PGS-M Composite Scaffold. Sci. Rep. 2019, 9, 7960. [Google Scholar] [CrossRef]
- Suvarnapathaki, S.; Wu, X.; Lantigua, D.; Nguyen, M.A.; Camci-Unal, G. Hydroxyapatite-Incorporated Composite Gels Improve Mechanical Properties and Bioactivity of Bone Scaffolds. Macromol. Biosci. 2020, 20, e2000176. [Google Scholar] [CrossRef]
- López-González, I.; Zamora-ledezma, C.; Sanchez-lorencio, M.I.; Barrenechea, E.T.; Gabaldón-hernández, J.A.; Meseguer-Olmo, L. Modifications in Gene Expression in the Process of Osteoblastic Differentiation of Multipotent Bone Marrow-derived Human Mesenchymal Stem Cells Induced by a Novel Osteoinductive Porous Medical-grade 3d-printed Poly(Ε-caprolactone)/Β-tricalcium Phosphate Composite. Int. J. Mol. Sci. 2021, 22, 11216. [Google Scholar] [CrossRef]
- Spreda, M.; Hauptmann, N.; Lehner, V.; Biehl, C.; Liefeith, K.; Lips, K.S. Porous 3D Scaffolds Enhance Msc Vitality and Reduce Osteoclast Activity. Molecules 2021, 26, 6258. [Google Scholar] [CrossRef]
- Marx, D.; Yazdi, A.R.; Papini, M.; Towler, M. In Vitro Osteogenic Performance of Two Novel Strontium and Zinc-Containing Glass Polyalkenoate Cements. J. Biomed. Mater. Res. Part A 2021, 109, 1366–1378. [Google Scholar] [CrossRef]
- El-Habashy, S.; Eltaher, H.; Gaballah, A.; Mehanna, R.; El-Kamel, A.H. Biomaterial-Based Nanocomposite for Osteogenic Repurposing of Doxycycline. Int. J. Nanomed. 2021, 16, 1103–1126. [Google Scholar] [CrossRef]
- Keikhosravani, P.; Maleki-Ghaleh, H.; Khosrowshahi, A.K.; Bodaghi, M.; Dargahi, Z.; Kavanlouei, M.; Khademi-Azandehi, P.; Fallah, A.; Beygi-Khosrowshahi, Y.; Siadati, M.H. Bioactivity and Antibacterial Behaviors of Nanostructured Lithium-Doped Hydroxyapatite for Bone Scaffold Application. Int. J. Mol. Sci. 2021, 22, 9214. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, W.; Liu, X.; Liang, C.; Zheng, Y.; Li, Z.; Liang, Y.; Zheng, D.; Zhu, S.; Cui, Z.; et al. Self-Activating Anti-Infection Implant. Nat. Commun. 2021, 12, 6907. [Google Scholar] [CrossRef]
- Spirandeli, B.R.; Ribas, R.G.; Amaral, S.S.; Martins, E.F.; Esposito, E.; Vasconcellos, L.M.R.; Campos, T.M.B.; Thim, G.P.; Trichês, E.S. Incorporation of 45S5 Bioglass via Sol-Gel in β-TCP Scaffolds: Bioactivity and Antimicrobial Activity Evaluation. Mater. Sci. Eng. C 2021, 131, 112453. [Google Scholar] [CrossRef]
- Hashemi, N.; Vaezi, Z.; Khanmohammadi, S.; Sohi, A.N.; Masoumi, S.; Hruschka, V.; Wolbank, S.; Redl, H.; Presen, D.M.; Naderi-Manesh, H. A Novel Fluorescent Hydroxyapatite Based on Iron Quantum Cluster Template to Enhance Osteogenic Differentiation. Mater. Sci. Eng. C 2020, 111, 110775. [Google Scholar] [CrossRef]
- Da Fonseca, G.F.; Avelino, S.D.O.M.; Mello, D.D.C.R.; Prado, R.F.D.; Campos, T.M.B.; De Vasconcellos, L.M.R.; Trichês, E.D.S.; Borges, A.L.S. Scaffolds of PCL Combined to Bioglass: Synthesis, Characterization and Biological Performance. J. Mater. Sci. Mater. Med. 2020, 31, 41. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Pereira, A.R.; Hermann, M.; Hansmann, J.; Delgado-López, J.M.; Sprio, S.; Tampieri, A.; Sandri, M. Biomimetic Mineralization Promotes Viability and Differentiation of Human Mesenchymal Stem Cells in a Perfusion Bioreactor. Int. J. Mol. Sci. 2021, 22, 1447. [Google Scholar] [CrossRef]
- Shen, C.C.; Hsu, S.H.; Chang, K.B.; Yeh, C.A.; Chang, H.C.; Tang, C.M.; Yang, Y.C.; Hsieh, H.H.; Hung, H.S. Physical Gold Nanoparticle-Decorated Polyethylene Glycol-Hydroxyapatite Composites Guide Osteogenesis and Angiogenesis of Mesenchymal Stem Cells. Biomedicines 2021, 9, 1632. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Komasa, S.; Morimoto, Y.; Sekino, T.; Kawazoe, T.; Okazaki, J. UV/Ozone Irradiation Manipulates Immune Response for Antibacterial Activity and Bone Regeneration on Titanium. Mater. Sci. Eng. C 2021, 129, 112377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, M.; Zhang, W.; Zhang, X. Construction of Tellurium-Doped Mesoporous Bioactive Glass Nanoparticles for Bone Cancer Therapy by Promoting ROS-Mediated Apoptosis and Antibacterial Activity. J. Colloid Interface Sci. 2022, 610, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Litvinova, L.; Yurova, K.; Shupletsova, V.; Khaziakhmatova, O.; Malashchenko, V.; Shunkin, E.; Melashchenko, E.; Todosenko, N.; Khlusova, M.; Sharkeev, Y.; et al. Gene Expression Regulation and Secretory Activity of Mesenchymal Stem Cells upon in Vitro Contact with Microarc Calcium Phosphate Coating. Int. J. Mol. Sci. 2020, 21, 7682. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xie, G.; Lu, Y.; Wang, J.; Feng, B.; Wang, Q.; Xu, K.; Bao, J. An Improved Osseointegration of Metal Implants by Pitavastatin Loaded Multilayer Films with Osteogenic and Angiogenic Properties. Biomaterials 2022, 280, 121260. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, C.; Liang, W.; Kang, F.; Bai, Y.; Ma, B.; Wu, C.; Dong, S. Mn-Containing Bioceramics Inhibit Osteoclastogenesis and Promote Osteoporotic Bone Regeneration via Scavenging ROS. Bioact. Mater. 2021, 6, 3839–3850. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, E.J.; Lemoine, M.; Clarke, D.; Vazquez, A.G.; O’Brien, F.J. The Incorporation of Marine Coral Microparticles into Collagen-Based Scaffolds Promotes Osteogenesis of Human Mesenchymal Stromal Cells via Calcium Ion Signalling. Mar. Drugs 2020, 18, 74. [Google Scholar] [CrossRef] [Green Version]
- Kazimierczak, P.; Koziol, M.; Przekora, A. The Chitosan/Agarose/Nanoha Bone Scaffold-Induced M2 Macrophage Polarization and Its Effect on Osteogenic Differentiation in Vitro. Int. J. Mol. Sci. 2021, 22, 1109. [Google Scholar] [CrossRef]
- Gieroba, B.; Przekora, A.; Kalisz, G.; Kazimierczak, P.; Song, C.L.; Wojcik, M.; Ginalska, G.; Kazarian, S.G.; Sroka-Bartnicka, A. Collagen Maturity and Mineralization in Mesenchymal Stem Cells Cultured on the Hydroxyapatite-Based Bone Scaffold Analyzed by ATR-FTIR Spectroscopic Imaging. Mater. Sci. Eng. C 2021, 119, 111634. [Google Scholar] [CrossRef]
- Parrilla, C.; Almadori, A.; Longobardi, Y.; Lattanzi, W.; Salgarello, M.; Almadori, G. Regenerative Strategy for Persistent Periprosthetic Leakage around Tracheoesophageal Puncture: Is It an Effective Long-Term Solution? Cells 2021, 10, 1695. [Google Scholar] [CrossRef]
- Di Taranto, G.; Cicione, C.; Visconti, G.; Isgrò, M.A.; Barba, M.; Di Stasio, E.; Stigliano, E.; Bernardini, C.; Michetti, F.; Salgarello, M.; et al. Qualitative and Quantitative Differences of Adipose-Derived Stromal Cells from Superficial and Deep Subcutaneous Lipoaspirates: A Matter of Fat. Cytotherapy 2015, 17, 1076–1089. [Google Scholar] [CrossRef]
- Barba, M.; Di Taranto, G.; Lattanzi, W. Adipose-Derived Stem Cell Therapies for Bone Regeneration. Expert Opin. Biol. Ther. 2017, 17, 677–689. [Google Scholar] [CrossRef]
- El-Ghannam, A.; Nakamura, M.; Muguruza, L.B.; Sarwar, U.; Hassan, M.; Al Fotawi, R.; Horowitz, R. Inhibition of Osteoclast Activities by SCPC Bioceramic Promotes Osteoblast-Mediated Graft Resorption and Osteogenic Differentiation. J. Biomed. Mater. Res. Part A 2021, 109, 1714–1725. [Google Scholar] [CrossRef]
- Latour, M.L.; Pelling, A.E. Mechanosensitive Osteogenesis on Native Cellulose Scaffolds for Bone Tissue Engineering. J. Biomech. 2022, 135, 111030. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Ismail, S.O.; Bowoto, O.K.; Omigbodun, F.T.; Olawumi, M.A.; Muhammad, M.A. Lattice Design and 3D-Printing of PEEK with Ca10(OH)(PO4)3 and in-Vitro Bio-Composite for Bone Implant. Int. J. Biol. Macromol. 2020, 165, 50–62. [Google Scholar] [CrossRef]
- Palmieri, V.; Barba, M.; Di Pietro, L.; Conti, C.; De Spirito, M.; Lattanzi, W.; Papi, M. Graphene Oxide Induced Osteogenesis Quantification by In-Situ 2D-Fluorescence Spectroscopy. Int. J. Mol. Sci. 2018, 19, 3336. [Google Scholar] [CrossRef] [Green Version]
- Bellucci, D.; Salvatori, R.; Anesi, A.; Chiarini, L.; Cannillo, V. SBF Assays, Direct and Indirect Cell Culture Tests to Evaluate the Biological Performance of Bioglasses and Bioglass-Based Composites: Three Paradigmatic Cases. Mater. Sci. Eng. C 2019, 96, 757–764. [Google Scholar] [CrossRef]
- Zhao, R.; Shi, L.; Gu, L.; Qin, X.; Song, Z.; Fan, X.; Zhao, P.; Li, C.; Zheng, H.; Li, Z.; et al. Evaluation of Bioactive Glass Scaffolds Incorporating SrO or ZnO for Bone Repair: In Vitro Bioactivity and Antibacterial Activity. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211040910. [Google Scholar] [CrossRef]
- Unalan, I.; Fuggerer, T.; Slavik, B.; Buettner, A.; Boccaccini, A.R. Antibacterial and Antioxidant Activity of Cinnamon Essential Oil-Laden 45S5 Bioactive Glass/Soy Protein Composite Scaffolds for the Treatment of Bone Infections and Oxidative Stress. Mater. Sci. Eng. C 2021, 128, 112320. [Google Scholar] [CrossRef]
- Rajendran, A.; Pattanayak, D.K. Bioactive and Antimicrobial Macro-/Micro-Nanoporous Selective Laser Melted Ti–6Al–4V Alloy for Biomedical Applications. Heliyon 2022, 8, e09122. [Google Scholar] [CrossRef]
- Kandel, R.; Jang, S.R.; Shrestha, S.; Ghimire, U.; Shrestha, B.K.; Park, C.H.; Kim, C.S. A Bimetallic Load-Bearing Bioceramics of TiO2 @ ZrO2 Integrated Polycaprolactone Fibrous Tissue Construct Exhibits Anti Bactericidal Effect and Induces Osteogenesis in MC3T3-E1 Cells. Mater. Sci. Eng. C 2021, 131, 112501. [Google Scholar] [CrossRef]
- Nayak, V.V.; Tovar, N.; Hacquebord, J.H.; Duarte, S.; Panariello, B.H.D.; Tonon, C.; Atria, P.J.; Coelho, P.G.; Witek, L. Physiochemical and Bactericidal Activity Evaluation: Silver-Augmented 3D-Printed Scaffolds—An in Vitro Study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 195–209. [Google Scholar] [CrossRef]
- Meng, F.; Yang, Z.; Long, D.; Gu, M.; Shang, M.; Zeng, A.; Wen, X.; Xue, Y.; Zhao, X.; He, A. Hyaluronan Size Alters Chondrogenesis of Mesenchymal Stem Cells Cultured on Tricalcium Phosphate-Collagen-Hyaluronan Scaffolds. J. Biomed. Mater. Res. Part A 2022, 110, 838–850. [Google Scholar] [CrossRef]
- Nosoudi, N.; Hart, C.; McKnight, I.; Esmaeilpour, M.; Ghomian, T.; Zadeh, A.; Raines, R.; Ramirez Vick, J.E. Differentiation of Adipose-Derived Stem Cells to Chondrocytes Using Electrospraying. Sci. Rep. 2021, 11, 24301. [Google Scholar] [CrossRef]
- Wang, H.C.; Lin, T.H.; Hsu, C.C.; Yeh, M.L. Restoring Osteochondral Defects through the Differentiation Potential of Cartilage Stem/Progenitor Cells Cultivated on Porous Scaffolds. Cells 2021, 10, 3536. [Google Scholar] [CrossRef]
- Browe, D.C.; Díaz-Payno, P.J.; Freeman, F.E.; Schipani, R.; Burdis, R.; Ahern, D.P.; Nulty, J.M.; Guler, S.; Randall, L.D.; Buckley, C.T.; et al. Bilayered Extracellular Matrix Derived Scaffolds with Anisotropic Pore Architecture Guide Tissue Organization during Osteochondral Defect Repair. Acta Biomater. 2022, 143, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Levingstone, T.J.; Moran, C.; Almeida, H.V.; Kelly, D.J.; O’Brien, F.J. Layer-Specific Stem Cell Differentiation in Tri-Layered Tissue Engineering Biomaterials: Towards Development of a Single-Stage Cell-Based Approach for Osteochondral Defect Repair. Mater. Today Bio 2021, 12, 100173. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, P.; Chen, M.; Peng, L.; Luo, X.; Tian, G.; Wang, H.; Wu, L.; Tian, Q.; Li, H.; et al. Hydrogel Composite Scaffolds Achieve Recruitment and Chondrogenesis in Cartilage Tissue Engineering Applications. J. Nanobiotechnol. 2022, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M. Resorbable Biomaterials as Bone Graft Substitutes. Mater. Today 2010, 13, 24–30. [Google Scholar] [CrossRef]
- Heinemann, C.; Adam, J.; Kruppke, B.; Hintze, V.; Wiesmann, H.P.; Hanke, T. How to Get Them off?—Assessment of Innovative Techniques for Generation and Detachment of Mature Osteoclasts for Biomaterial Resorption Studies. Int. J. Mol. Sci. 2021, 22, 1329. [Google Scholar] [CrossRef]
- Eugen, G.; Claus, M.; Anna-Maria, S.; Niklas, D.; Philipp, S.; Andrea, E.; Andrea, M.; Elke, V. Bioactive Materials Degradation of 3D-Printed Magnesium Phosphate Ceramics in Vitro and a Prognosis on Their Bone Regeneration Potential. Bioact. Mater. 2022, 19, 376–391. [Google Scholar] [CrossRef]
- Bergara-Muguruza, L.; Mäkelä, K.; Yrjälä, T.; Salonen, J.; Yamashita, K.; Nakamura, M. Surface Electric Fields Increase Human Osteoclast Resorption through Improved Wettability on Carbonate-Incorporated Apatite. ACS Appl. Mater. Interfaces 2021, 13, 58270–58278. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y.; Hua, Z.; Li, S.; Yang, Z.; Liu, Q.; Fu, G.; Ji, P.; Wu, Q. BMP2 Immune Complexes Promote New Bone Formation by Facilitating the Direct Contact between Osteoclasts and Osteoblasts. Biomaterials 2021, 275, 120890. [Google Scholar] [CrossRef]
- Wu, H.; Yang, S.; Xiao, J.; Ouyang, Z.; Yang, M.; Zhang, M.; Zhao, D.; Huang, Q. Facile Synthesis of Multi-Functional Nano-Composites by Precise Loading of Cu2+ onto MgO Nano-Particles for Enhanced Osteoblast Differentiation, Inhibited Osteoclast Formation and Effective Bacterial Killing. Mater. Sci. Eng. C 2021, 130, 112442. [Google Scholar] [CrossRef]
- Vitale, M.; Ligorio, C.; McAvan, B.; Hodson, N.W.; Allan, C.; Richardson, S.M.; Hoyland, J.A.; Bella, J. Hydroxyapatite-Decorated Fmoc-Hydrogel as a Bone-Mimicking Substrate for Osteoclast Differentiation and Culture. Acta Biomater. 2022, 138, 144–154. [Google Scholar] [CrossRef]
- Ponzetti, M.; Rucci, N. Updates on Osteoimmunology: What’s New on the Cross-Talk between Bone and Immune System. Front. Endocrinol. 2019, 10, 236. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Komasa, S.; Kusumoto, T.; Kuwamoto, S.; Okunishi, T.; Kobayashi, Y.; Hashimoto, Y.; Sekino, T.; Okazaki, J. Immunomodulatory Properties and Osteogenic Activity of Polyetheretherketone Coated with Titanate Nanonetwork Structures. Int. J. Mol. Sci. 2022, 23, 612. [Google Scholar] [CrossRef]
- Mao, L.; Yin, Y.; Zhang, L.; Chen, X.; Wang, X.; Chen, F.; Liu, C. Regulation of Inflammatory Response and Osteogenesis to Citrate-Based Biomaterials through Incorporation of Alkaline Fragments. Adv. Healthc. Mater. 2022, 11, 2101590. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Gui, L.; Deng, Y.; Wang, L.; Jiao, J.; Hu, Y.; Lan, X.; Hou, J.; Li, Y.; et al. Sequential Gastrodin Release PU/n-HA Composite Scaffolds Reprogram Macrophages for Improved Osteogenesis and Angiogenesis. Bioact. Mater. 2023, 19, 24–37. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Q.; Fu, L.; Zheng, S.; Wang, C.; Han, L.; Gong, Z.; Wang, Z.; Tang, H.; Zhang, Y. CD301b+ Macrophages Mediate Angiogenesis of Calcium Phosphate Bioceramics by CaN/NFATc1/VEGF Axis. Bioact. Mater. 2022, 15, 446–455. [Google Scholar] [CrossRef]
- Guo, F.; Yuan, C.; Huang, H.; Deng, X.; Bian, Z.; Wang, D.; Dou, K.; Mei, L.; Zhou, Q. Regulation of T Cell Responses by Nano-Hydroxyapatite to Mediate the Osteogenesis. Front. Bioeng. Biotechnol. 2022, 10, 884291. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, C.; He, L.; Zhou, J.; Chen, T.; Ouyang, L.; Guo, X.; Qu, Y.; Luo, Z.; Duan, D. Stratified-Structural Hydrogel Incorporated with Magnesium-Ion-Modified Black Phosphorus Nanosheets for Promoting Neuro-Vascularized Bone Regeneration. Bioact. Mater. 2022, 16, 271–284. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Yang, J.; Ha, Y.; Zhang, Q.; Zhou, X.; He, C. 3D Bioprinted Gelatin/Gellan Gum-Based Scaffold with Double-Crosslinking Network for Vascularized Bone Regeneration. Carbohydr. Polym. 2022, 290, 119469. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Zhang, Y.; Wang, T.; Lin, K.; Liu, J. A Polydopamine-Assisted Strontium-Substituted Apatite Coating for Titanium Promotes Osteogenesis and Angiogenesis via FAK/MAPK and PI3K/AKT Signaling Pathways. Mater. Sci. Eng. C 2021, 131, 112482. [Google Scholar] [CrossRef]
- Pinto, T.S.; Martins, B.R.; Ferreira, M.R.; Bezerra, F.; Zambuzzi, W.F. Nanohydroxyapatite-Blasted Bioactive Surface Drives Shear-Stressed Endothelial Cell Growth and Angiogenesis. Biomed. Res. Int. 2022, 2022, 1433221. [Google Scholar] [CrossRef]
- Wang, P.; Meng, X.; Wang, R.; Yang, W.; Yang, L.; Wang, J.; Wang, D.; Fan, C. Biomaterial Scaffolds Made of Chemically Cross-Linked Gelatin Microsphere Aggregates (C-GMSs) Promote Vascularized Bone Regeneration. Adv. Healthc. Mater. 2022, 11, 2102818. [Google Scholar] [CrossRef]
- Hayashi, K.; Shimabukuro, M.; Ishikawa, K. Antibacterial Honeycomb Scaffolds for Achieving Infection Prevention and Bone Regeneration. ACS Appl. Mater. Interfaces 2022, 14, 3762–3772. [Google Scholar] [CrossRef]
- Lu, H.T.; Huang, G.Y.; Chang, W.J.; Lu, T.W.; Huang, T.W.; Ho, M.H.; Mi, F.L. Modification of Chitosan Nanofibers with CuS and Fucoidan for Antibacterial and Bone Tissue Engineering Applications. Carbohydr. Polym. 2022, 281, 119035. [Google Scholar] [CrossRef] [PubMed]
- Meshkini, A.; Sistanipour, E.; Izadi, A. Mg. ATP-Decorated Ultrafine Magnetic Nanofibers: A Bone Scaffold with High Osteogenic and Antibacterial Properties in the Presence of an Electromagnetic Field. Colloids Surf. B Biointerfaces 2022, 210, 112256. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Franco, D.; Petralia, S.; Monforte, F.; Condorelli, G.G.; Squarzoni, S.; Traina, F.; Conoci, S. Dual-Functional Nano-Functionalized Titanium Scaffolds to Inhibit Bacterial Growth and Enhance Osteointegration. Nanomaterials 2021, 11, 2634. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Salas, B.; Beltrán-Partida, E. Feasibility of Using H3PO4/H2O2 in the Synthesis of Antimicrobial TiO2 Nanoporous Surfaces. Bioinorg. Chem. Appl. 2021, 2021, 6209094. [Google Scholar] [CrossRef]
- Qiu, X.; Li, S.; Li, X.; Xiao, Y.; Li, S.; Fen, Q.; Kang, X.; Zhen, P. Experimental Study of β-TCP Scaffold Loaded with VAN/PLGA Microspheres in the Treatment of Infectious Bone Defects. Colloids Surf. B Biointerfaces 2022, 213, 112424. [Google Scholar] [CrossRef]
- Chodara, A.; Higuchi, J.; Klimek, K.; Wojnarowicz, J.; Opali, A. Electrospun Membrane Surface Modification by Sonocoating with HA and ZnO:Ag Nanoparticles—Characterization and Evaluation of Osteoblasts and Bacterial Cell Behavior In Vitro. Cells 2022, 11, 1582. [Google Scholar]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal Models for Bone Tissue Engineering and Modelling Disease. DMM Dis. Model. Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Ziadlou, R.; Grad, S.; Alini, M.; Wen, C.; Lai, Y.; Qin, L.; Zhao, Y.; Wang, X. Animal Models of Osteochondral Defect for Testing Biomaterials. Biochem. Res. Int. 2020, 2020, 9659412. [Google Scholar] [CrossRef] [Green Version]
- Klemmer, V.A.; Khera, N.; Siegenthaler, B.M.; Bhattacharya, I.; Weber, F.E.; Ghayor, C. Effect of N-Vinyl-2-Pyrrolidone (NVP), a Bromodomain-Binding Small Chemical, on Osteoblast and Osteoclast Differentiation and Its Potential Application for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 11052. [Google Scholar] [CrossRef]
- Browe, D.C.; Burdis, R.; Díaz-Payno, P.J.; Freeman, F.E.; Nulty, J.M.; Buckley, C.T.; Brama, P.A.J.; Kelly, D.J. Promoting Endogenous Articular Cartilage Regeneration Using Extracellular Matrix Scaffolds. Mater. Today Bio 2022, 16, 100343. [Google Scholar] [CrossRef]
- Jeuken, R.M.; Roth, A.K.; Peters, M.J.M.; Welting, T.J.M.; van Rhijn, L.W.; Koenen, J.; Peters, R.J.R.W.; Thies, J.C.; Emans, P.J. In Vitro and in Vivo Study on the Osseointegration of BCP-Coated versus Uncoated Nondegradable Thermoplastic Polyurethane Focal Knee Resurfacing Implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 3370–3382. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Z.; Yang, Y.; Wang, S.; Zhao, Y.; Xiong, Y.; Yang, S.; Qiu, Z.; Song, T.; Zhang, C.; et al. Biphasic Mineralized Collagen-Based Composite Scaffold for Cranial Bone Regeneration in Developing Sheep. Regen. Biomater. 2022, 9, rbac004. [Google Scholar] [CrossRef]
- Shah, F.A.; Jolic, M.; Micheletti, C.; Omar, O.; Norlindh, B.; Emanuelsson, L.; Engqvist, H.; Engstrand, T.; Palmquist, A.; Thomsen, P. Bone without Borders—Monetite-Based Calcium Phosphate Guides Bone Formation beyond the Skeletal Envelope. Bioact. Mater. 2023, 19, 103–114. [Google Scholar] [CrossRef]

| Bone Graft | Advantages | Disadvantages |
|---|---|---|
| Autologous |
| need of additional surgery |
| Xenografts |
|
|
| Natural biomaterials | Similarity to native extracellular matrix | Mechanical properties poor -biodegradability less controllable |
| Synthetic polymers |
|
|
| Synthetic bioceramics |
|
|
| Composite xenohybrid substitutes |
|
|
| Name of the Mechanical Property Parameter | Bones with Osteoporosis | Bones without Osteoporosis | |
|---|---|---|---|
| 1 | Range of the elastic region (in strain) (m/m) | 0–0.0063 | 0–0.0043 |
| 2 | Range of the plastic region (in strain) (m/m) | 0.0063–0.0089 | 0.0043–0.0129 |
| 3 | Proportional limit (in stress) (MPa) | 77.0934 | 80.3718 |
| 4 | Elastic limit (in stress) (MPa) | 88.3528 | 98.6828 |
| 5 | Failure strength (in stress) (MPa) | 94.9280 | 116.9657 |
| 6 | Brittleness coefficient (Dimensionless) | 0.7079 | 0.3333 |
| 7 | Modulus of resilience (MJ/m3) | 0.3394 | 0.2450 |
| 8 | Modulus of toughness (MJ/m3) | 0.5778 | 1.1751 |
| 9 | Modulus of elasticity (MPa) | 18283.2314 | 27544.2425 |
| 10 | Tangent modulus (MPa) | 2490.2230 | 2118.0671 |
| 11 | Strain hardening parameter (MPa) | 2882.8784 | 2294.5076 |
| Gene Name | Gene Symbol | Function |
|---|---|---|
| RUNX family transcription factor 2 | RUNX2 | Member of the RUNX family of transcription factors characterized by a Runt DNA-binding domain. It is fundamental for osteogenesis and skeletal morphogenesis. It acts as a scaffold for other regulatory factors involved in osteoblast maturation. Its expression increases forthwith, starting from the first steps of osteogenesis. |
| bone gamma-carboxyglutamate protein (osteocalcin) | BGLAP | Bone protein is extensively secreted by osteoblasts that regulates bone remodeling and energy metabolism by binding to calcium and hydroxyapatite rich in the mineral matrix. |
| secreted protein acidic and cysteine rich (osteonectin) | SPARC | Cysteine-rich acidic matrix-associated protein is involved in extracellular matrix synthesis and cell shape changes. |
| secreted phosphoprotein 1 (osteopontin) | SPP1 | Secreted bone protein that binds to hydroxyapatite with high affinity, thus representing an integral part of the mineralized matrix. It is probably important for cell-matrix interaction that is involved in the attachment of osteoclasts to the mineralized bone matrix.It also plays a key role in the activation of type I immunity, acting as a cytokine, enhancing the production of interferon-gamma and interleukin-12 and reducing the production of interleukin-10. |
| integrin binding sialoprotein | IBSP | One of the major structural proteins of the bone matrix, synthesized by skeletal-associated cell types, including hypertrophic chondrocytes, osteoblasts, osteocytes, and osteoclasts. It constitutes approximately 12% of the non-collagenous proteins in human bone. It binds to calcium and hydroxyapatite and mediates cell attachment. |
| alkaline phosphatase, biomineralization associated | ALPL | A membrane bound glycosylated enzyme that is a member of the alkaline phosphatase family of proteins. It plays an essential role in bone mineralization by acting at different levels of osteogenesis. |
| Sp7 transcription factor | SP7 (OSX) | Bone specific transcription factor required for osteoblast differentiation and bone formation. |
| bone morphogenetic protein 2 | BMP2 | Secreted ligand of the TGF-beta (transforming growth factor-beta) superfamily of proteins. The downstream activated signal cascade leads to the recruitment and activation of SMAD family transcription factors that regulate gene expression for bone and cartilage development. |
| bone morphogenetic protein 4 | BMP4 | Another secreted ligand of the TGF-beta superfamily of proteins that activates the SMAD pathway. This protein regulates heart development and adipogenesis. |
| bone morphogenetic protein 6 | BMP6 | Like BMP2 and BMP4, this secreted protein activates SMAD signaling and regulates a wide range of biological processes, including fat and bone development. |
| bone morphogenetic protein 7 | BMP7 | Secreted ligand of the TGF-beta superfamily, which plays a role in bone, kidney, and brown adipose tissue development. This protein is also involved in ectopic bone formation and may promote fracture healing in human patients. |
| collagen type I alpha 1 chain | COL1A1 | Pro-alpha1 chains of type I collagen are present in most connective tissues and particularly abundant in bone. |
| SMAD family member 1 | SMAD1 | Proteins belonging to the SMAD family mediate multiple signaling pathways. Specifically, SMAD1 mediates the signals of BMPs, and the activated phosphorylated form of this protein forms a complex with SMAD4, which is important for its function in transcription regulation. |
| SMAD family member 3 | SMAD3 | One of the principal master regulators of the osteogenic lineage during mesenchymal stem cell commitment. This protein forms a complex with other SMAD proteins and binds DNA, functioning as a transcription factor. For example, SMAD3 has been shown to bind to the SSP1 promoter as a sequence-specific activator. |
| SMAD family member 5 | SMAD5 | Protein activated by bone morphogenetic proteins type 1 receptor kinase and is involved in the transforming growth factor beta signaling pathway. |
| SMAD family member 9 | SMAD9 | Protein activated by bone morphogenetic proteins that interact with SMAD4. |
| Transforming growth factor beta 3 | TGFB3 | Secreted ligand of the TGF-beta superfamily of proteins that can form heterodimers with other TGF-beta family members. It is involved in embryogenesis and cell differentiation and may play a role in wound healing. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pietro, L.; Palmieri, V.; Papi, M.; Lattanzi, W. Translating Material Science into Bone Regenerative Medicine Applications: State-of-The Art Methods and Protocols. Int. J. Mol. Sci. 2022, 23, 9493. https://doi.org/10.3390/ijms23169493
Di Pietro L, Palmieri V, Papi M, Lattanzi W. Translating Material Science into Bone Regenerative Medicine Applications: State-of-The Art Methods and Protocols. International Journal of Molecular Sciences. 2022; 23(16):9493. https://doi.org/10.3390/ijms23169493
Chicago/Turabian StyleDi Pietro, Lorena, Valentina Palmieri, Massimiliano Papi, and Wanda Lattanzi. 2022. "Translating Material Science into Bone Regenerative Medicine Applications: State-of-The Art Methods and Protocols" International Journal of Molecular Sciences 23, no. 16: 9493. https://doi.org/10.3390/ijms23169493
APA StyleDi Pietro, L., Palmieri, V., Papi, M., & Lattanzi, W. (2022). Translating Material Science into Bone Regenerative Medicine Applications: State-of-The Art Methods and Protocols. International Journal of Molecular Sciences, 23(16), 9493. https://doi.org/10.3390/ijms23169493









