The GEM-GECO Calcium Indicator Is Useable in Ogataea parapolymorpha Yeast, but Aggravates Effects of Increased Cytosolic Calcium Levels
Abstract
:1. Introduction
2. Results and Discussion
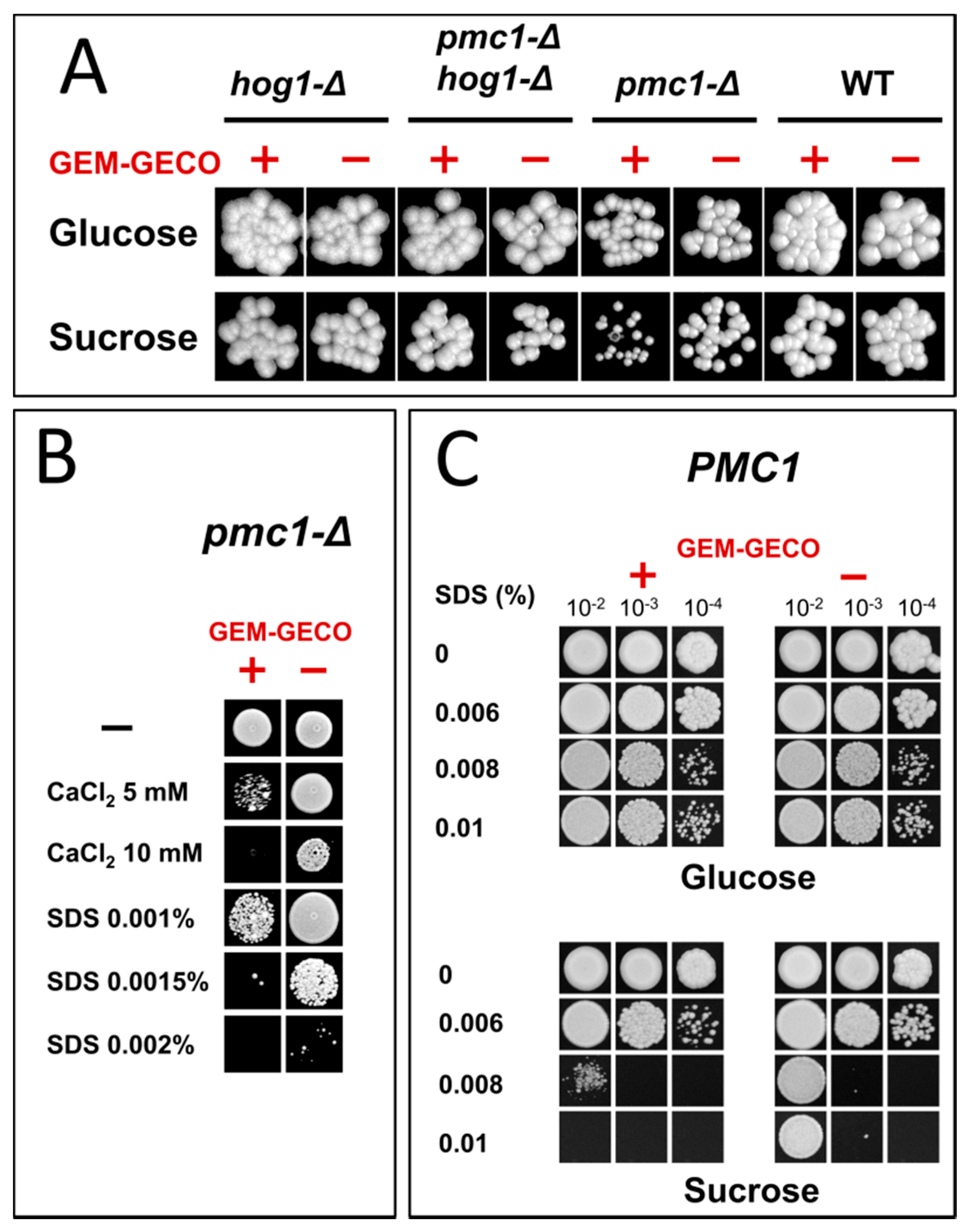
2.1. GEM-GECO Expression Affects Cell Growth in a Pmc1-Dependent Manner
2.2. GEM-GECO Does Not Complement the Loss of Endogenous CaM in Ogataea Yeast
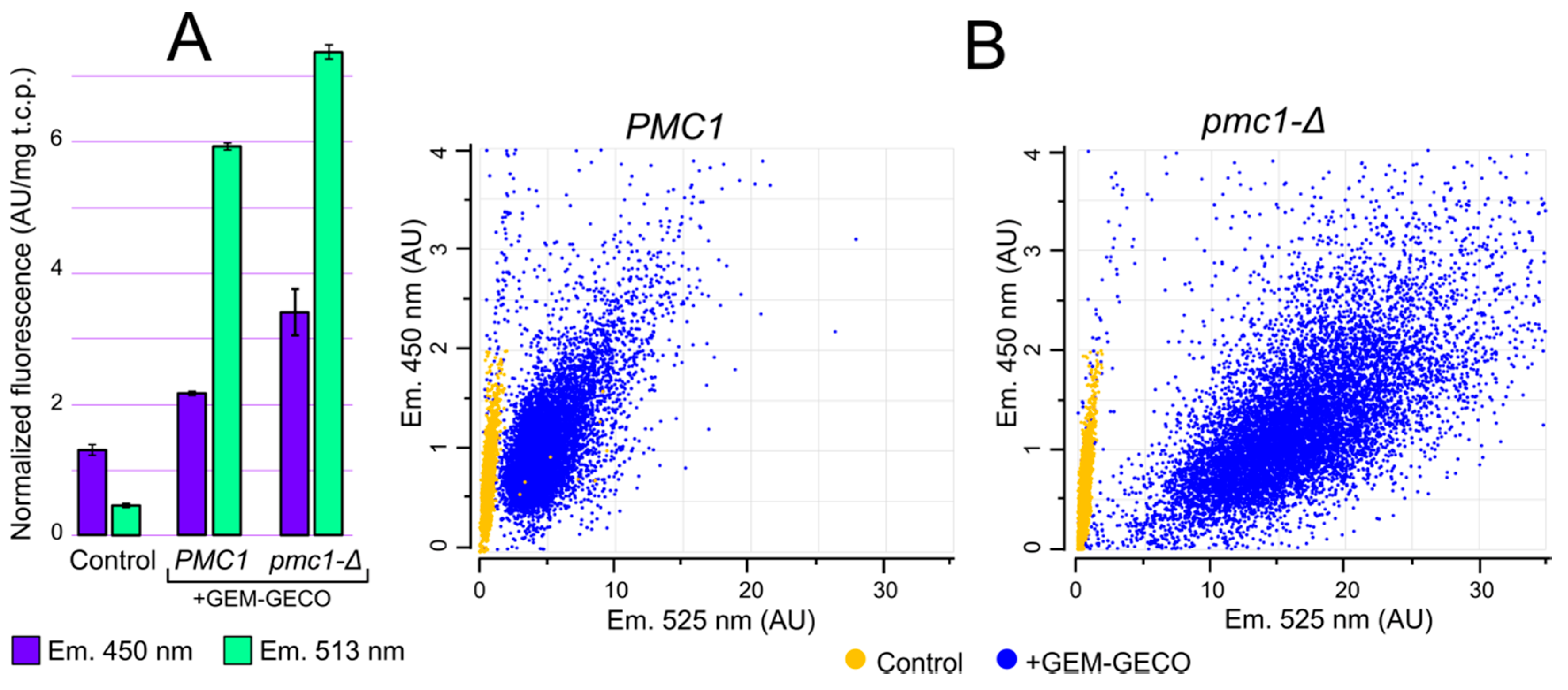
2.3. GEM-GECO Allows Monitoring Ca2+ Concentration in Cytosol in O. parapolymorpha
3. Materials and Methods
3.1. Yeast and Escherichia coli Strains
3.2. Culture Media and Transformation Procedures
3.3. Plasmid Construction
3.4. Analysis of GEM-GECO and Fluo-4 Fluorescence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef]
- Giorgi, C.; Danese, A.; Missiroli, S.; Patergnani, S.; Pinton, P. Calcium Dynamics as a Machine for Decoding Signals. Trends Cell Biol. 2018, 28, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Poddar, A.; Sidibe, O.; Ray, A.; Chen, Q. Calcium Spikes Accompany Cleavage Furrow Ingression and Cell Separation during Fission Yeast Cytokinesis. Mol. Biol. Cell 2021, 32, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O.; Johnson, F.H. Calcium Binding, Quantum Yield, and Emitting Molecule in Aequorin Bioluminescence. Nature 1970, 227, 1356–1357. [Google Scholar] [CrossRef]
- Nakajima-Shimada, J.; Iida, H.; Tsuji, F.I.; Anraku, Y. Monitoring of Intracellular Calcium in Saccharomyces Cerevisiae with an Apoaequorin CDNA Expression System. Proc. Natl. Acad. Sci. USA 1991, 88, 6878–6882. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Nasu, Y.; Shkolnikov, I.; Kim, A.; Campbell, R.E. Engineering Genetically Encoded Fluorescent Indicators for Imaging of Neuronal Activity: Progress and Prospects. Neurosci. Res. 2020, 152, 3–14. [Google Scholar] [CrossRef]
- Mank, M.; Griesbeck, O. Genetically Encoded Calcium Indicators. Chem. Rev. 2008, 108, 1550–1564. [Google Scholar] [CrossRef]
- Zhao, Y.; Araki, S.; Wu, J.; Teramoto, T.; Chang, Y.-F.; Nakano, M.; Abdelfattah, A.S.; Fujiwara, M.; Ishihara, T.; Nagai, T.; et al. An Expanded Palette of Genetically Encoded Ca2+ Indicators. Science 2011, 333, 1888–1891. [Google Scholar] [CrossRef]
- Dana, H.; Mohar, B.; Sun, Y.; Narayan, S.; Gordus, A.; Hasseman, J.P.; Tsegaye, G.; Holt, G.T.; Hu, A.; Walpita, D.; et al. Sensitive Red Protein Calcium Indicators for Imaging Neural Activity. eLife 2016, 5, e12727. [Google Scholar] [CrossRef]
- Subach, O.M.; Barykina, N.V.; Chefanova, E.S.; Vlaskina, A.V.; Sotskov, V.P.; Ivashkina, O.I.; Anokhin, K.V.; Subach, F.V. FRCaMP, a Red Fluorescent Genetically Encoded Calcium Indicator Based on Calmodulin from Schizosaccharomyces Pombe Fungus. Int. J. Mol. Sci. 2020, 22, 111. [Google Scholar] [CrossRef]
- Gasterstädt, I.; Jack, A.; Stahlhut, T.; Rennau, L.-M.; Gonda, S.; Wahle, P. Genetically Encoded Calcium Indicators Can Impair Dendrite Growth of Cortical Neurons. Front. Cell. Neurosci. 2020, 14, 570596. [Google Scholar] [CrossRef]
- Liu, S.; Hou, Y.; Liu, W.; Lu, C.; Wang, W.; Sun, S. Components of the Calcium-Calcineurin Signaling Pathway in Fungal Cells and Their Potential as Antifungal Targets. Eukaryot. Cell 2015, 14, 324–334. [Google Scholar] [CrossRef]
- Cui, J.; Kaandorp, J.A.; Sloot, P.M.A.; Lloyd, C.M.; Filatov, M.V. Calcium Homeostasis and Signaling in Yeast Cells and Cardiac Myocytes. FEMS Yeast Res. 2009, 9, 1137–1147. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Saltsman, K.; Gasch, A.P.; Li, H.X.; Ogawa, N.; Botstein, D.; Brown, P.O.; Cyert, M.S. Genome-Wide Analysis of Gene Expression Regulated by the Calcineurin/Crz1p Signaling Pathway in Saccharomyces Cerevisiae. J. Biol. Chem. 2002, 277, 31079–31088. [Google Scholar] [CrossRef]
- Fokina, A.V.; Sokolov, S.S.; Kang, H.A.; Kalebina, T.S.; Ter-Avanesyan, M.D.; Agaphonov, M.O. Inactivation of Pmc1 Vacuolar Ca2+ ATPase Causes G2 Cell Cycle Delay in Hansenula Polymorpha. Cell Cycle 2012, 11, 778–784. [Google Scholar] [CrossRef]
- Suppi, S.; Michelson, T.; Viigand, K.; Alamäe, T. Repression vs. Activation of MOX, FMD, MPP1 and MAL1 Promoters by Sugars in Hansenula Polymorpha: The Outcome Depends on Cell’s Ability to Phosphorylate Sugar. FEMS Yeast Res. 2013, 13, 219–232. [Google Scholar] [CrossRef]
- Davis, T.N.; Urdea, M.S.; Masiarz, F.R.; Thorner, J. Isolation of the Yeast Calmodulin Gene: Calmodulin Is an Essential Protein. Cell 1986, 47, 423–431. [Google Scholar] [CrossRef]
- Kurtzman, C.P. A New Methanol Assimilating Yeast, Ogataea Parapolymorpha, the Ascosporic State of Candida Parapolymorpha. Antonie van Leeuwenhoek 2011, 100, 455–462. [Google Scholar] [CrossRef]
- Gee, K.R.; Brown, K.A.; Chen, W.-N.U.; Bishop-Stewart, J.; Gray, D.; Johnson, I. Chemical and Physiological Characterization of Fluo-4 Ca2+-Indicator Dyes. Cell Calcium 2000, 27, 97–106. [Google Scholar] [CrossRef]
- Cunningham, K.W.; Fink, G.R. Calcineurin-Dependent Growth Control in Saccharomyces Cerevisiae Mutants Lacking PMC1, a Homolog of Plasma Membrane Ca2+ ATPases. J. Cell Biol. 1994, 124, 351–363. [Google Scholar] [CrossRef]
- Sohn, J.H.; Choi, E.S.; Kim, C.H.; Agaphonov, M.O.; Ter-Avanesyan, M.D.; Rhee, J.S.; Rhee, S.K. A Novel Autonomously Replicating Sequence (ARS) for Multiple Integration in the Yeast Hansenula Polymorpha DL-1. J. Bacteriol. 1996, 178, 4420–4428. [Google Scholar] [CrossRef]
- Karginov, A.V.; Fokina, A.V.; Kang, H.A.; Kalebina, T.S.; Sabirzyanova, T.A.; Ter-Avanesyan, M.D.; Agaphonov, M.O. Dissection of Differential Vanadate Sensitivity in Two Ogataea Species Links Protein Glycosylation and Phosphate Transport Regulation. Sci. Rep. 2018, 8, 16428. [Google Scholar] [CrossRef]
- Agaphonov, M.; Alexandrov, A. Self-Excising Integrative Yeast Plasmid Vectors Containing an Intronated Recombinase Gene. FEMS Yeast Res. 2014, 14, 1048–1054. [Google Scholar] [CrossRef]
- Bogdanova, A.I.; Agaphonov, M.O.; Ter-Avanesyan, M.D. Plasmid Reorganization during Integrative Transformation InHansenula Polymorpha. Yeast 1995, 11, 343–353. [Google Scholar] [CrossRef]
- Agaphonov, M.O.; Beburov, M.Y.; Ter-Avanesyan, M.D.; Smirnov, V.N. A Disruption-Replacement Approach for the Targeted Integration of Foreign Genes in Hansenula Polymorpha. Yeast 1995, 11, 1241–1247. [Google Scholar] [CrossRef]
- Inoue, H.; Nojima, H.; Okayama, H. High Efficiency Transformation of Escherichia Coli with Plasmids. Gene 1990, 96, 23–28. [Google Scholar] [CrossRef]
- Gietz, R.D.; Schiestl, R.H. Quick and Easy Yeast Transformation Using the LiAc/SS Carrier DNA/PEG Method. Nat. Protoc. 2007, 2, 35–37. [Google Scholar] [CrossRef]
- Karginov, A.V.; Alexandrov, A.I.; Kushnirov, V.V.; Agaphonov, M.O. Perturbations in the Heme and Siroheme Biosynthesis Pathways Causing Accumulation of Fluorescent Free Base Porphyrins and Auxotrophy in Ogataea Yeasts. J. Fungi 2021, 7, 884. [Google Scholar] [CrossRef]
- Agaphonov, M.; Romanova, N.; Choi, E.-S.; Ter-Avanesyan, M. A Novel Kanamycin/G418 Resistance Marker for Direct Selection of Transformants in Escherichia Coli and Different Yeast Species. Yeast 2010, 27, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Herbert, D.; Phipps, P.J.; Strange, R.E. Chapter III Chemical Analysis of Microbial Cells. In Methods in Microbiology; Academic Press: Cambridge, MA, USA, 1971; pp. 209–344. [Google Scholar]
- Varela, J.N.; Yadav, V.G. A Pichia Biosensor for High-throughput Analyses of Compounds That Can Influence Mosquito Behavior. Microbiologyopen 2021, 10, e1139. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, A.I.; Dergalev, A.A. Increasing Throughput of Manual Microscopy of Cell Suspensions Using Solid Medium Pads. MethodsX 2019, 6, 329–332. [Google Scholar] [CrossRef] [PubMed]


| Strain | Genotype |
|---|---|
| DL1-L | leu2 |
| DLdaduA | leu2 ade2-Δura3::ADE2 |
| DL1-LC | leu2 {LEU2} |
| DL-pmc1-LC | leu2 pmc1::loxP {LEU2} |
| DL5 | leu2 {PMAL1-GEM-GECO} |
| DL5-LC | leu2 {PMAL1-GEM-GECO} {LEU2} |
| DL5-pmc1-LC | leu2 pmc1::loxP {PMAL1-GEM-GECO} {LEU2} |
| DL5-pmc1-hog1 | leu2 pmc1::loxP hog1::LEU2 {PMAL1-GEM-GECO} |
| DL5-hog1 | leu2 hog1::LEU2{PMAL1-GEM-GECO} |
| DLdaduAU-LC | leu2 ade2-Δura3::ADE2 {LEU2} {URA3} |
| DLdaduA-pmc1-LC | leu2 ade2-Δura3::ADE2 pmc1::URA3 {LEU2} |
| DLdaduA-hog1-UC | leu2 ade2-Δura3::ADE2 hog1::LEU2 {URA3} |
| DLdaduA-pmc1-hog1 | leu2 ade2-Δura3::ADE2 pmc1::URA3 hog1::LEU2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulakova, M.V.; Karginov, A.V.; Alexandrov, A.I.; Agaphonov, M.O. The GEM-GECO Calcium Indicator Is Useable in Ogataea parapolymorpha Yeast, but Aggravates Effects of Increased Cytosolic Calcium Levels. Int. J. Mol. Sci. 2022, 23, 10004. https://doi.org/10.3390/ijms231710004
Kulakova MV, Karginov AV, Alexandrov AI, Agaphonov MO. The GEM-GECO Calcium Indicator Is Useable in Ogataea parapolymorpha Yeast, but Aggravates Effects of Increased Cytosolic Calcium Levels. International Journal of Molecular Sciences. 2022; 23(17):10004. https://doi.org/10.3390/ijms231710004
Chicago/Turabian StyleKulakova, Maria V., Azamat V. Karginov, Alexander I. Alexandrov, and Michael O. Agaphonov. 2022. "The GEM-GECO Calcium Indicator Is Useable in Ogataea parapolymorpha Yeast, but Aggravates Effects of Increased Cytosolic Calcium Levels" International Journal of Molecular Sciences 23, no. 17: 10004. https://doi.org/10.3390/ijms231710004






