Iron Deposition in Brain: Does Aging Matter?
Abstract
:1. Introduction
2. Determination of Iron Deposition in Brain
3. Iron Brain in Aging
Iron vs. Age Relationship: Quantitative Correlations
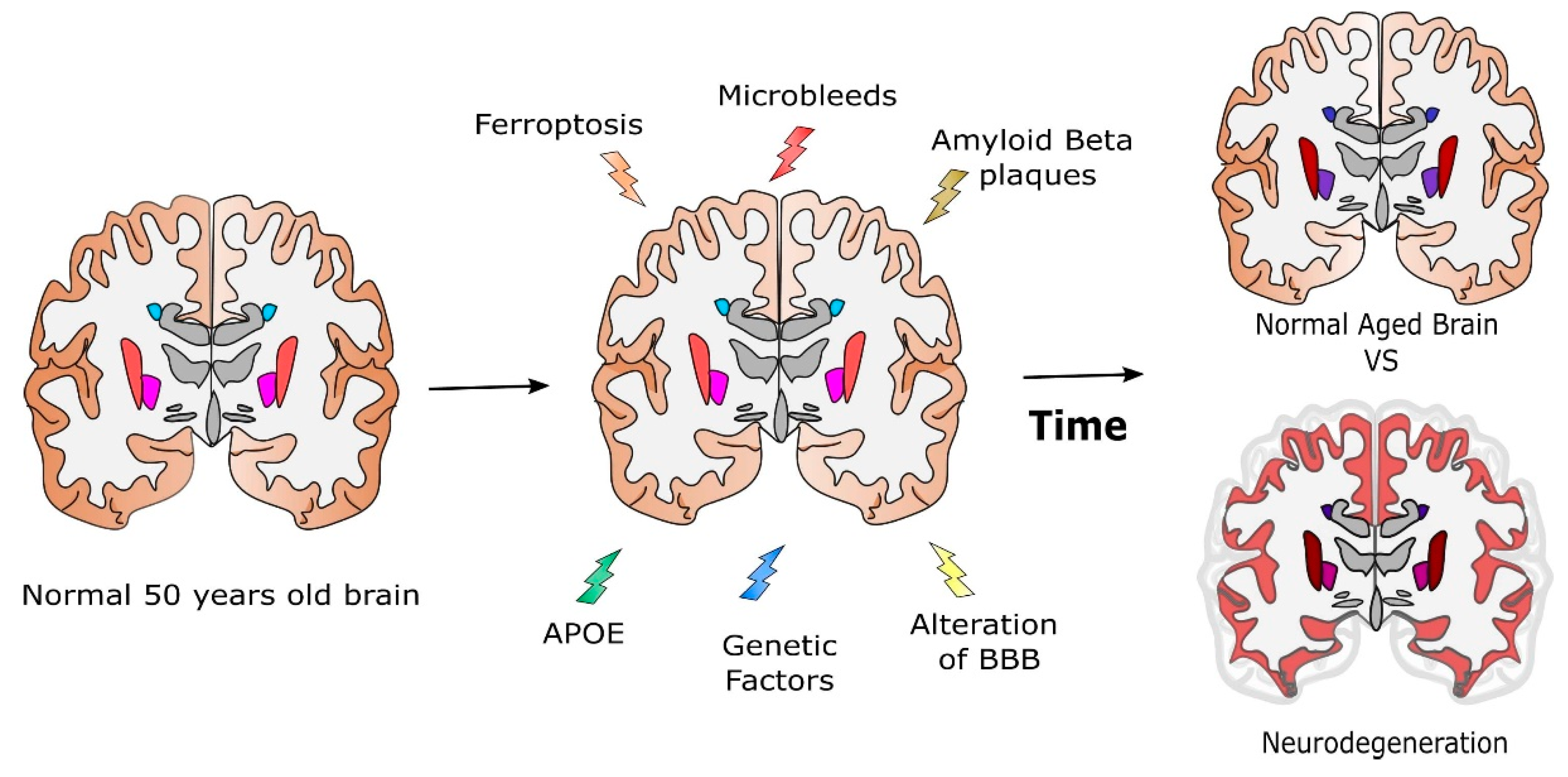
4. Where Does Brain Iron Excess Come from?
4.1. The Main Players: Transporters, Free Iron, and the Role of the Barriers
4.2. Blood–Brain Barrier and Age
4.3. The Interplay of Iron with Other Factors
4.3.1. Potential Iron Alteration Due to Microbleeds
4.3.2. Interaction of Iron with Amyloid Beta-APP and Tau
4.3.3. ApoE and the Genetic Origin of AD
5. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Magnetic Resonance Imaging | MRI |
| Transverse Relaxation Rate | R2* (1/T2*) |
| Quantitative Susceptibility Mapping | QSM |
| Susceptibility Weighted Imaging | SWI |
| Non-Transferrin Bound Iron | NTBI |
| Cerebro Spinal Fluid | CSF |
| Blood–brain barrier | BBB |
| Brain Microbleeds | BMB |
| Ferroportin | Fpn |
| Amyloid Beta Protein | Aβ |
| Amyloid Precursor Protein | APP |
| Pre Senilin | PS |
| Apolipoprotein E | ApoE |
| Hemojuvelin | HJV |
References
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to Tango: Regulation of Mammalian Iron Metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Villar, I.; García-Erce, J.A. An Update on Iron Physiology. World J. Gastroenterol. 2009, 15, 4617–4626. [Google Scholar] [CrossRef] [PubMed]
- Alle, H.; Roth, A.; Geiger, J.R.P. Energy-Efficient Action Potentials in Hippocampal Mossy Fibers. Science 2009, 325, 1405–1408. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. A Cellular Perspective on Brain Energy Metabolism and Functional Imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef] [PubMed]
- Madsen, E.; Gitlin, J.D. Copper and Iron Disorders of the Brain. Annu. Rev. Neurosci. 2007, 30, 317–337. [Google Scholar] [CrossRef] [PubMed]
- Salvador, G.A. Iron in neuronal function and dysfunction. BioFactors 2010, 36, 103–110. [Google Scholar] [CrossRef]
- Andersen, H.H.; Johnsen, K.B.; Moos, T. Iron Deposits in the Chronically Inflamed Central Nervous System and Contributes to Neurodegeneration. Cell. Mol. Life Sci. 2014, 71, 1607–1622. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef]
- Álvarez-Córdoba, M.; Fernández Khoury, A.; Villanueva-Paz, M.; Gómez-Navarro, C.; Villalón-García, I.; Suárez-Rivero, J.M.; Povea-Cabello, S.; de la Mata, M.; Cotán, D.; Talaverón-Rey, M.; et al. Pantothenate Rescues Iron Accumulation in Pantothenate Kinase-Associated Neurodegeneration Depending on the Type of Mutation. Mol. Neurobiol. 2019, 56, 3638–3656. [Google Scholar] [CrossRef]
- Kim, Y.; Connor, J.R. The roles of iron and HFE genotype in neurological diseases. Mol. Asp. Med. 2020, 75, 100867. [Google Scholar] [CrossRef]
- Tang, S.; Gao, P.; Chen, H.; Zhou, X.; Ou, Y.; He, Y. The Role of Iron, Its Metabolism and Ferroptosis in Traumatic Brain Injury. Front. Cell. Neurosci. 2020, 14, 590789. [Google Scholar] [CrossRef]
- Słomka, A.; Świtońska, M.; Zekanowska, E. Hepcidin Levels Are Increased in Patients with Acute Ischemic Stroke: Preliminary Report. J. Stroke Cerebrovasc. Dis. 2015, 24, 1570–1576. [Google Scholar] [CrossRef]
- Young, G.-H.; Tang, S.-C.; Wu, V.-C.; Wang, K.-C.; Nong, J.-Y.; Huang, P.-Y.; Hu, C.-J.; Chiou, H.-Y.; Jeng, J.-S.; Hsu, C.Y. The functional role of hemojuvelin in acute ischemic stroke. J. Cereb. Blood Flow Metab. 2020, 40, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Ghadery, C.; Pirpamer, L.; Hofer, E.; Langkammer, C.; Petrovic, K.; Loitfelder, M.; Schwingenschuh, P.; Seiler, S.; Duering, M.; Jouvent, E.; et al. R2* mapping for brain iron: Associations with cognition in normal aging. Neurobiol. Aging 2015, 36, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Kalpouzos, G.; Garzón, B.; Sitnikov, R.; Heiland, C.; Salami, A.; Persson, J.; Bäckman, L. Higher Striatal Iron Concentration is Linked to Frontostriatal Underactivation and Poorer Memory in Normal Aging. Cereb. Cortex 2017, 27, 3427–3436. [Google Scholar] [CrossRef]
- Ramos, P.; Santos, A.; Pinto, N.R.; Mendes, R.; Magalhães, T.; Almeida, A. Iron levels in the human brain: A post-mortem study of anatomical region differences and age-related changes. J. Trace Elem. Med. Biol. 2014, 28, 13–17. [Google Scholar] [CrossRef]
- McAllum, E.J.; Hare, D.J.; Volitakis, I.; McLean, C.A.; Bush, A.I.; Finkelstein, D.; Roberts, B.R. Regional iron distribution and soluble ferroprotein profiles in the healthy human brain. Prog. Neurobiol. 2020, 186, 101744. [Google Scholar] [CrossRef]
- Brain Map—Brain-Map.Org. Available online: https://portal.brain-map.org/ (accessed on 10 July 2022).
- Zeng, Y.; DiGiacomo, P.S.; Madsen, S.J.; Zeineh, M.M.; Sinclair, R. Exploring valence states of abnormal mineral deposits in biological tissues using correlative microscopy and spectroscopy techniques: A case study on ferritin and iron deposits from Alzheimer’s disease patients. Ultramicroscopy 2021, 231, 113254. [Google Scholar] [CrossRef]
- Langkammer, C.; Krebs, N.; Goessler, W.; Scheurer, E.; Ebner, F.; Yen, K.; Fazekas, F.; Ropele, S. Quantitative MR Imaging of Brain Iron: A Postmortem Validation Study. Radiology 2010, 257, 455–462. [Google Scholar] [CrossRef]
- Langkammer, C.; Schweser, F.; Krebs, N.; Deistung, A.; Goessler, W.; Scheurer, E.; Sommer, K.; Reishofer, G.; Yen, K.; Fazekas, F.; et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. NeuroImage 2012, 62, 1593–1599. [Google Scholar] [CrossRef] [Green Version]
- Ordidge, R.J.; Gorell, J.M.; Deniau, J.C.; Knight, R.A.; Helpern, J.A. Assessment of relative brain iron concentrations usingT2-weighted andT2*-weighted MRI at 3 Tesla. Magn. Reson. Med. 1994, 32, 335–341. [Google Scholar] [CrossRef]
- Acosta-Cabronero, J.; Betts, M.J.; Cardenas-Blanco, A.; Yang, S.; Nestor, P.J. In Vivo MRI Mapping of Brain Iron Deposition across the Adult Lifespan. J. Neurosci. 2016, 36, 364–374. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T. Quantitative susceptibility mapping (QSM): DecodingMRIdata for a tissue magnetic biomarker. Magn. Reson. Med. 2014, 73, 82–101. [Google Scholar] [CrossRef]
- Langkammer, C.; Bredies, K.; Poser, B.A.; Barth, M.; Reishofer, G.; Fan, A.P.; Bilgic, B.; Fazekas, F.; Mainero, C.; Ropele, S. Fast quantitative susceptibility mapping using 3D EPI and total generalized variation. NeuroImage 2015, 111, 622–630. [Google Scholar] [CrossRef]
- Treit, S.; Naji, N.; Seres, P.; Rickard, J.; Stolz, E.; Wilman, A.H.; Beaulieu, C. R2 * and quantitative susceptibility mapping in deep gray matter of 498 healthy controls from 5 to 90 years. Hum. Brain Mapp. 2021, 42, 4597–4610. [Google Scholar] [CrossRef]
- Schenker, C.; Meier, D.; Wichmann, W.; Boesiger, P.; Valavanis, A. Age distribution and iron dependency of the T2 relaxation time in the globus pallidus and putamen. Neuroradiology 1993, 35, 119–124. [Google Scholar] [CrossRef]
- Yao, B.; Li, T.; Van Gelderen, P.; Shmueli, K.; De Zwart, J.A.; Duyn, J.H. Susceptibility contrast in high field MRI of human brain as a function of tissue iron content. NeuroImage 2009, 44, 1259–1266. [Google Scholar] [CrossRef]
- Péran, P.; Cherubini, A.; Luccichenti, G.; Hagberg, G.; Demonet, J.-F.; Rascol, O.; Celsis, P.; Caltagirone, C.; Spalletta, G.; Sabatini, U. Volume and iron content in basal ganglia and thalamus. Hum. Brain Mapp. 2009, 30, 2667–2675. [Google Scholar] [CrossRef]
- Khan, S.; Cohen, D. Using the magnetoencephalogram to noninvasively measure magnetite in the living human brain. Hum. Brain Mapp. 2018, 40, 1654–1665. [Google Scholar] [CrossRef]
- Cullen, K.M.; Kócsi, Z.; Stone, J. Pericapillary Haem-Rich Deposits: Evidence for Microhaemorrhages in Aging Human Cerebral Cortex. J. Cereb. Blood Flow Metab. 2005, 25, 1656–1667. [Google Scholar] [CrossRef] [Green Version]
- Janaway, B.M.; Simpson, J.E.; Hoggard, N.; Highley, R.; Forster, G.; Drew, D.; Gebril, O.; Matthews, F.; Brayne, C.; Wharton, S.B.; et al. Brain haemosiderin in older people: Pathological evidence for an ischaemic origin of magnetic resonance imaging (MRI) microbleeds. Neuropathol. Appl. Neurobiol. 2014, 40, 258–269. [Google Scholar] [CrossRef]
- Hasiloglu, Z.; Asik, M.; Ure, E.; Ertem, F.; Apak, H.; Albayram, S. The utility of susceptibility-weighted imaging to evaluate the extent of iron accumulation in the choroid plexus of patients with β-thalassaemia major. Clin. Radiol. 2017, 72, 903.e1–903.e7. [Google Scholar] [CrossRef]
- Bartzokis, G.; Beckson, M.; Hance, D.B.; Marx, P.; Foster, J.A.; Marder, S.R. MR evaluation of age-related increase of brain iron in young adult and older normal males. Magn. Reson. Imaging 1997, 15, 29–35. [Google Scholar] [CrossRef]
- Wayne Martin, W.R.; Ye, F.Q.; Allen, P.S. Increasing Striatal Iron Content Associated with Normal Aging. Mov. Disord. 1998, 13, 281–286. [Google Scholar] [CrossRef]
- Pfefferbaum, A.; Adalsteinsson, E.; Rohlfing, T.; Sullivan, E.V. MRI estimates of brain iron concentration in normal aging: Comparison of field-dependent (FDRI) and phase (SWI) methods. NeuroImage 2009, 47, 493–500. [Google Scholar] [CrossRef]
- Cherubini, A.; Péran, P.; Caltagirone, C.; Sabatini, U.; Spalletta, G. Aging of subcortical nuclei: Microstructural, mineralization and atrophy modifications measured in vivo using MRI. NeuroImage 2009, 48, 29–36. [Google Scholar] [CrossRef]
- Gracien, R.-M.; Nürnberger, L.; Hok, P.; Hof, S.-M.; Reitz, S.C.; Rüb, U.; Steinmetz, H.; Hilker-Roggendorf, R.; Klein, J.C.; Deichmann, R.; et al. Evaluation of brain ageing: A quantitative longitudinal MRI study over 7 years. Eur. Radiol. 2017, 27, 1568–1576. [Google Scholar] [CrossRef]
- Burgetova, R.; Dusek, P.; Burgetova, A.; Pudlac, A.; Vaneckova, M.; Horakova, D.; Krasensky, J.; Varga, Z.; Lambert, L. Age-related magnetic susceptibility changes in deep grey matter and cerebral cortex of normal young and middle-aged adults depicted by whole brain analysis. Quant. Imaging Med. Surg. 2021, 11, 3906–3919. [Google Scholar] [CrossRef]
- Penke, L.; Valdés Hernandéz, M.C.; Maniega, S.M.; Gow, A.J.; Murray, C.; Starr, J.M.; Bastin, M.E.; Deary, I.J.; Wardlaw, J.M. Brain Iron Deposits Are Associated with General Cognitive Ability and Cognitive Aging. Neurobiol. Aging 2012, 33, 510–517.e2. [Google Scholar] [CrossRef]
- Rodrigue, K.M.; Daugherty, A.; Haacke, E.M.; Raz, N. The Role of Hippocampal Iron Concentration and Hippocampal Volume in Age-Related Differences in Memory. Cereb. Cortex 2013, 23, 1533–1541. [Google Scholar] [CrossRef] [Green Version]
- Bastian, T.W.; Rao, R.; Tran, P.V.; Georgieff, M.K. The Effects of Early-Life Iron Deficiency on Brain Energy Metabolism. J. Exp. Neurosci. 2020, 15, 2633105520935104. [Google Scholar] [CrossRef] [PubMed]
- Hect, J.L.; Daugherty, A.M.; Hermez, K.M.; Thomason, M. Developmental variation in regional brain iron and its relation to cognitive functions in childhood. Dev. Cogn. Neurosci. 2018, 34, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, A.; Daugherty, A.M.; Bennett, I.J. Neuroimaging measures of iron and gliosis explain memory performance in aging. Hum. Brain Mapp. 2021, 42, 5761–5770. [Google Scholar] [CrossRef]
- Raz, N.; Rodrigue, K.M.; Haacke, E.M. Brain Aging and Its Modifiers. Ann. N. Y. Acad. Sci. 2007, 1097, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Siemonsen, S.; Finsterbusch, J.; Matschke, J.; Lorenzen, A.; Ding, X.-Q.; Fiehler, J. Age-Dependent Normal Values of T2* and T2′ in Brain Parenchyma. Am. J. Neuroradiol. 2008, 29, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Ficiarà, E.; Munir, Z.; Boschi, S.; Caligiuri, M.; Guiot, C. Alteration of Iron Concentration in Alzheimer’s Disease as a Possible Diagnostic Biomarker Unveiling Ferroptosis. Int. J. Mol. Sci. 2021, 22, 4479. [Google Scholar] [CrossRef]
- Singh, N.; Haldar, S.; Tripathi, A.K.; Horback, K.; Wong, J.; Sharma, D.; Beserra, A.; Suda, S.; Anbalagan, C.; Dev, S.; et al. Brain Iron Homeostasis: From Molecular Mechanisms To Clinical Significance and Therapeutic Opportunities. Antioxid. Redox Signal. 2014, 20, 1324–1363. [Google Scholar] [CrossRef]
- Hare, D.J.; Ayton, S.; Bush, A.I.; Lei, P. A delicate balance: Iron metabolism and diseases of the brain. Front. Aging Neurosci. 2013, 5, 34. [Google Scholar] [CrossRef]
- Connor, J.R.; Benkovic, S.A. Iron Regulation in the Brain: Histochemical, Biochemical, and Molecular Considerations. Ann. Neurol. 1992, 32, S51–S61. [Google Scholar] [CrossRef]
- Benkovic, S.A.; Connor, J.R. Ferritin, transferrin, and iron in selected regions of the adult and aged rat brain. J. Comp. Neurol. 1993, 338, 97–113. [Google Scholar] [CrossRef]
- Connor, J.R.; Snyder, B.S.; Beard, J.L.; Fine, R.E.; Mufson, E.J. Regional distribution of iron and iron-regulatory proteins in the brain in aging and Alzheimer’s disease. J. Neurosci. Res. 1992, 31, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Bartzokis, G.; Tishler, T.A.; Lu, P.H.; Villablanca, P.; Altshuler, L.L.; Carter, M.; Huang, D.; Edwards, N.; Mintz, J. Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol. Aging 2007, 28, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Raha, A.A.; Vaishnav, R.A.; Friedland, R.P.; Bomford, A.; Raha-Chowdhury, R. The systemic iron-regulatory proteins hepcidin and ferroportin are reduced in the brain in Alzheimer’s disease. Acta Neuropathol. Commun. 2013, 1, 55. [Google Scholar] [CrossRef]
- Bao, W.-D.; Pang, P.; Zhou, X.-T.; Hu, F.; Xiong, W.; Chen, K.; Wang, J.; Wang, F.; Xie, D.; Hu, Y.-Z.; et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021, 28, 1548–1562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.-S. Control of Systemic Iron Homeostasis by the Hemojuvelin-Hepcidin Axis. Adv. Nutr. 2010, 1, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Erdő, F.; Denes, L.; de Lange, E. Age-Associated Physiological and Pathological Changes at the Blood–Brain Barrier: A Review. J. Cereb. Blood Flow Metab. 2017, 37, 4–24. [Google Scholar] [CrossRef]
- Banks, W.A.; Reed, M.J.; Logsdon, A.F.; Rhea, E.M.; Erickson, M.A. Healthy aging and the blood–brain barrier. Nat. Aging 2021, 1, 243–254. [Google Scholar] [CrossRef]
- Bernier, M.; Cunnane, S.C.; Whittingstall, K. The morphology of the human cerebrovascular system. Hum. Brain Mapp. 2018, 39, 4962–4975. [Google Scholar] [CrossRef]
- Varatharaj, A.; Liljeroth, M.; Darekar, A.; Larsson, H.B.; Galea, I.; Cramer, S.P. Blood-brain barrier permeability measured using dynamic contrast-enhanced magnetic resonance imaging: A validation study. J. Physiol. 2019, 597, 699–709. [Google Scholar] [CrossRef]
- Schipper, H. Heme oxygenase-1: Role in brain aging and neurodegeneration. Exp. Gerontol. 2000, 35, 821–830. [Google Scholar] [CrossRef]
- Schipper, H.M. Heme oxygenase expression in human central nervous system disorders. Free Radic. Biol. Med. 2004, 37, 1995–2011. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, W.; McAuley, G.; Holshouser, B.; Petersen, F.; Ayaz, M.; Vinters, H.V.; Dickson, C.; Haacke, E.M.; Britt III, W.; Larsen, J.; et al. Serial Susceptibility Weighted MRI Measures Brain Iron and Microbleeds in Dementia. J. Alzheimer’s Dis. 2009, 17, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Vernooij, M.W.; Cordonnier, C.; Viswanathan, A.; Salman, R.A.-S.; Warach, S.; Launer, L.J.; Van Buchem, M.A.; Breteler, M.M. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009, 8, 165–174. [Google Scholar] [CrossRef]
- Ayaz, M.; Boikov, A.S.; Haacke, E.M.; Kido, D.K.; Kirsch, W.M. Imaging Cerebral Microbleeds Using Susceptibility Weighted Imaging: One Step toward Detecting Vascular Dementia. J. Magn. Reson. Imaging 2010, 31, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.Y.; Guo-Ross, S.X.; Bondy, S.C. The Stabilization of Ferrous Iron by a Toxic β-Amyloid Fragment and by an Aluminum Salt. Brain Res. 1999, 839, 221–226. [Google Scholar] [CrossRef]
- Leskovjan, A.C.; Kretlow, A.; Lanzirotti, A.; Barrea, R.; Vogt, S.; Miller, L.M. Increased brain iron coincides with early plaque formation in a mouse model of Alzheimer’s disease. NeuroImage 2011, 55, 32–38. [Google Scholar] [CrossRef]
- Sanmartín, C.D.; Paula-Lima, A.C.; Hidalgo, C.; Nunez, M.T. Sub-lethal levels of amyloid β-peptide oligomers decrease non-transferrin-bound iron uptake and do not potentiate iron toxicity in primary hippocampal neurons. BioMetals 2012, 25, 805–813. [Google Scholar] [CrossRef]
- Chuang, J.-Y.; Lee, C.-W.; Shih, Y.-H.; Yang, T.; Yu, L.; Kuo, Y.-M. Interactions between Amyloid-β and Hemoglobin: Implications for Amyloid Plaque Formation in Alzheimer’s Disease. PLoS ONE 2012, 7, e33120. [Google Scholar] [CrossRef]
- Ghosh, C.; Seal, M.; Mukherjee, S.; Ghosh Dey, S. Alzheimer’s Disease: A Heme–Aβ Perspective. Acc. Chem. Res. 2015, 48, 2556–2564. [Google Scholar] [CrossRef]
- Gong, N.-J.; Dibb, R.; Bulk, M.; van der Weerd, L.; Liu, C. Imaging beta amyloid aggregation and iron accumulation in Alzheimer’s disease using quantitative susceptibility mapping MRI. NeuroImage 2019, 191, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Spotorno, N.; Acosta-Cabronero, J.; Stomrud, E.; Lampinen, B.; Strandberg, O.T.; Van Westen, D.; Hansson, O. Relationship between cortical iron and tau aggregation in Alzheimer’s disease. Brain 2020, 143, 1341–1349. [Google Scholar] [CrossRef]
- Streit, W.J.; Rotter, J.; Winter, K.; Müller, W.; Khoshbouei, H.; Bechmann, I. Droplet Degeneration of Hippocampal and Cortical Neurons Signifies the Beginning of Neuritic Plaque Formation. J. Alzheimer’s Dis. 2022, 85, 1701–1720. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Finkelstein, D.I.; Adlard, P.A. Interactions of Metals and Apolipoprotein E in Alzheimer’s Disease. Front. Aging Neurosci. 2014, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Belaidi, A.A.; Masaldan, S.; Southon, A.; Kalinowski, P.; Acevedo, K.; Appukuttan, A.T.; Portbury, S.; Lei, P.; Agarwal, P.; Leurgans, S.E.; et al. Apolipoprotein E potently inhibits ferroptosis by blocking ferritinophagy. Mol. Psychiatry 2022, 1–10. [Google Scholar] [CrossRef]
- Küpper, C.; Levin, J.; Klopstock, T. Eisen im alternden Gehirn. Aktuelle Neurol. 2016, 47, 32–40. [Google Scholar] [CrossRef]
- Timmers, P.R.H.J.; Wilson, J.F.; Joshi, P.K.; Deelen, J. Multivariate Genomic Scan Implicates Novel Loci and Haem Metabolism in Human Ageing. Nat. Commun. 2020, 11, 3570. [Google Scholar] [CrossRef]
- Ayton, S.; Portbury, S.; Kalinowski, P.; Agarwal, P.; Diouf, I.; Schneider, J.A.; Morris, M.C.; Bush, A.I. Regional Brain Iron Associated with Deterioration in Alzheimer’s Disease: A Large Cohort Study and Theoretical Significance. Alzheimer’s Dement. 2021, 17, 1244–1256. [Google Scholar] [CrossRef]
- IIslam, S.; Sun, Y.; Gao, Y.; Nakamura, T.; Noorani, A.A.; Li, T.; Wong, P.C.; Kimura, N.; Matsubara, E.; Kasuga, K.; et al. Presenilin Is Essential for ApoE Secretion, a Novel Role of Presenilin Involved in Alzheimer’s Disease Pathogenesis. J. Neurosci. 2022, 42, 1574–1586. [Google Scholar] [CrossRef]
- Sun, H.; Walsh, A.J.; Lebel, R.M.; Blevins, G.; Catz, I.; Lu, J.-Q.; Johnson, E.S.; Emery, D.J.; Warren, K.G.; Wilman, A.H. Validation of quantitative susceptibility mapping with Perls’ iron staining for subcortical gray matter. NeuroImage 2015, 105, 486–492. [Google Scholar] [CrossRef]
- Hametner, S.; Endmayr, V.; Deistung, A.; Palmrich, P.; Prihoda, M.; Haimburger, E.; Menard, C.; Feng, X.; Haider, T.; Leisser, M.; et al. The influence of brain iron and myelin on magnetic susceptibility and effective transverse relaxation—A biochemical and histological validation study. NeuroImage 2018, 179, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Baek, S.-Y.; Chun, S.Y.; Lee, J.-H.; Cho, H. Specific visualization of neuromelanin-iron complex and ferric iron in the human post-mortem substantia nigra using MR relaxometry at 7T. NeuroImage 2018, 172, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Ravanfar, P.; Loi, S.M.; Syeda, W.T.; Van Rheenen, T.E.; Bush, A.I.; Desmond, P.; Cropley, V.L.; Lane, D.J.R.; Opazo, C.M.; Moffat, B.A.; et al. Systematic Review: Quantitative Susceptibility Mapping (QSM) of Brain Iron Profile in Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 618435. [Google Scholar] [CrossRef] [PubMed]
- The Brain Vascular System. Annals of Internal Medicine. Available online: https://www.acpjournals.org/doi/10.7326/0003-4819-65-5-1158_2 (accessed on 10 July 2022).
- Viviani, R. A Digital Atlas of Middle to Large Brain Vessels and Their Relation to Cortical and Subcortical Structures. Front. Neuroanat. 2016, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Ingrassia, R.; Garavaglia, B.; Memo, M. DMT1 Expression and Iron Levels at the Crossroads Between Aging and Neurodegeneration. Front. Neurosci. 2019, 13, 575. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Ni, W.; Gu, Y. Effects of deferoxamine on blood-brain barrier disruption after subarachnoid hemorrhage. PLoS ONE 2017, 12, e0172784. [Google Scholar] [CrossRef]
- Puy, L.; Pasi, M.; Rodrigues, M.; van Veluw, S.J.; Tsivgoulis, G.; Shoamanesh, A.; Cordonnier, C. Cerebral microbleeds: From depiction to interpretation. J. Neurol. Neurosurg. Psychiatry 2021, 92, 598–607. [Google Scholar] [CrossRef]
- Cullen, K.M.; Kócsi, Z.; Stone, J. Microvascular pathology in the aging human brain: Evidence that senile plaques are sites of microhaemorrhages. Neurobiol. Aging 2006, 27, 1786–1796. [Google Scholar] [CrossRef]
- Ficiarà, E.; D’Agata, F.; Cattaldo, S.; Priano, L.; Mauro, A.; Guiot, C. A Compartmental Model for the Iron Trafficking Across the Blood-Brain Barriers in Neurodegenerative Diseases. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC), Mexico City, Mexico, 1–5 November 2021; pp. 4200–4203. [Google Scholar]
- Ficiarà, E.; D’Agata, F.; Ansari, S.; Boschi, S.; Rainero, I.; Priano, L.; Cattaldo, S.; Abollino, O.; Cavalli, R.; Guiot, C. A Mathematical Model for the Evaluation of Iron Transport across the Blood-Cerebrospinal Fluid Barrier in Neurodegenerative Diseases. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 2270–2273. [Google Scholar]
- Cherubini, A.; Caligiuri, M.E.; Péran, P.; Sabatini, U.; Cosentino, C.; Amato, F. Importance of Multimodal MRI in Characterizing Brain Tissue and Its Potential Application for Individual Age Prediction. IEEE J. Biomed. Health Inform. 2016, 20, 1232–1239. [Google Scholar] [CrossRef]
- Howard, C.M.; Jain, S.; Cook, A.D.; Packard, L.E.; Mullin, H.A.; Chen, N.; Liu, C.; Song, A.W.; Madden, D.J. Cortical Iron Mediates Age-Related Decline in Fluid Cognition. Hum. Brain Mapp. 2022, 43, 1047–1060. [Google Scholar] [CrossRef]

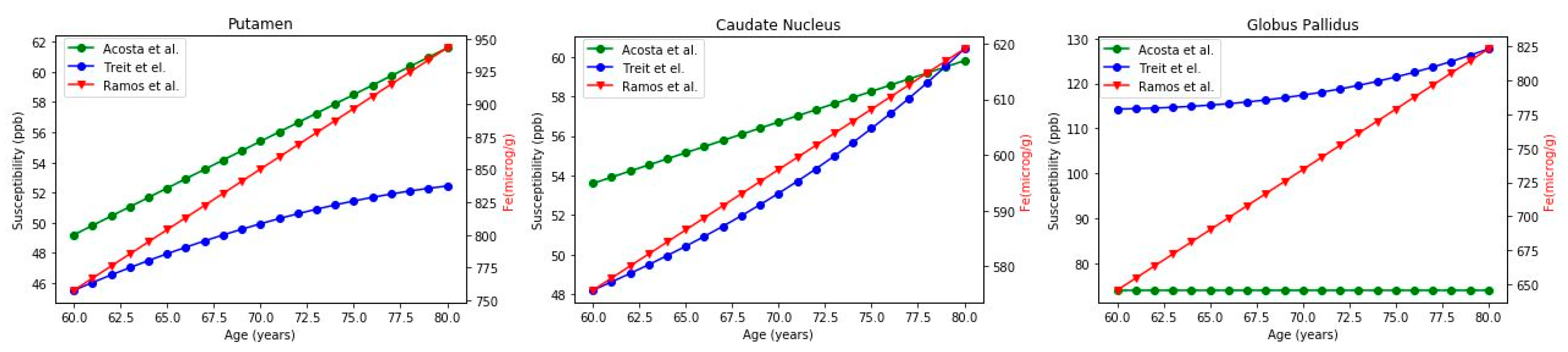
| Brain Region | Ref | Mean Iron Concentration (60–80 Years) | Correlation Coefficient | Proportion |
|---|---|---|---|---|
| Putamen | Treit et al. [26] Acosta et al. [23] Ramos et al. [16] | 59.15 ± 5.34 ppb 55.40 ± 3.75 ppb 850.37 ± 56.17 μg/g | r = 0.99, p = 2.6 × 10−17 1 | (17.1 ± 0.4) (15.4 ± 0.0) |
| Caudate Nucleus | Treit et al. [26] Acosta et al. [23] Ramos et al. [16] | 53.54 ± 3.67 ppb 56.7 ± 1.88 ppb 597.37 ± 13.14 μg/g | r= 0.99, p = 6.8 × 10−20 0.99 | (11.2 ± 0.5) (10.5 ± 0.1) |
| Globus Pallidus | Treit et al. [26] Acosta et al. [23] Ramos et al. [16] | 118.72 ± 4.11 ppb 74 ppb 734.42 ± 53.90 μg/g | r= 0.96, p = 9.8 × 10−12 | (6.2 ± 0.3) (9.9± 0.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ficiarà, E.; Stura, I.; Guiot, C. Iron Deposition in Brain: Does Aging Matter? Int. J. Mol. Sci. 2022, 23, 10018. https://doi.org/10.3390/ijms231710018
Ficiarà E, Stura I, Guiot C. Iron Deposition in Brain: Does Aging Matter? International Journal of Molecular Sciences. 2022; 23(17):10018. https://doi.org/10.3390/ijms231710018
Chicago/Turabian StyleFiciarà, Eleonora, Ilaria Stura, and Caterina Guiot. 2022. "Iron Deposition in Brain: Does Aging Matter?" International Journal of Molecular Sciences 23, no. 17: 10018. https://doi.org/10.3390/ijms231710018
APA StyleFiciarà, E., Stura, I., & Guiot, C. (2022). Iron Deposition in Brain: Does Aging Matter? International Journal of Molecular Sciences, 23(17), 10018. https://doi.org/10.3390/ijms231710018






