Revealing the Therapeutic Targets and Mechanism of Ginsenoside Rg1 for Liver Damage Related to Anti-Oxidative Stress Using Proteomic Analysis
Abstract
:1. Introduction
2. Results
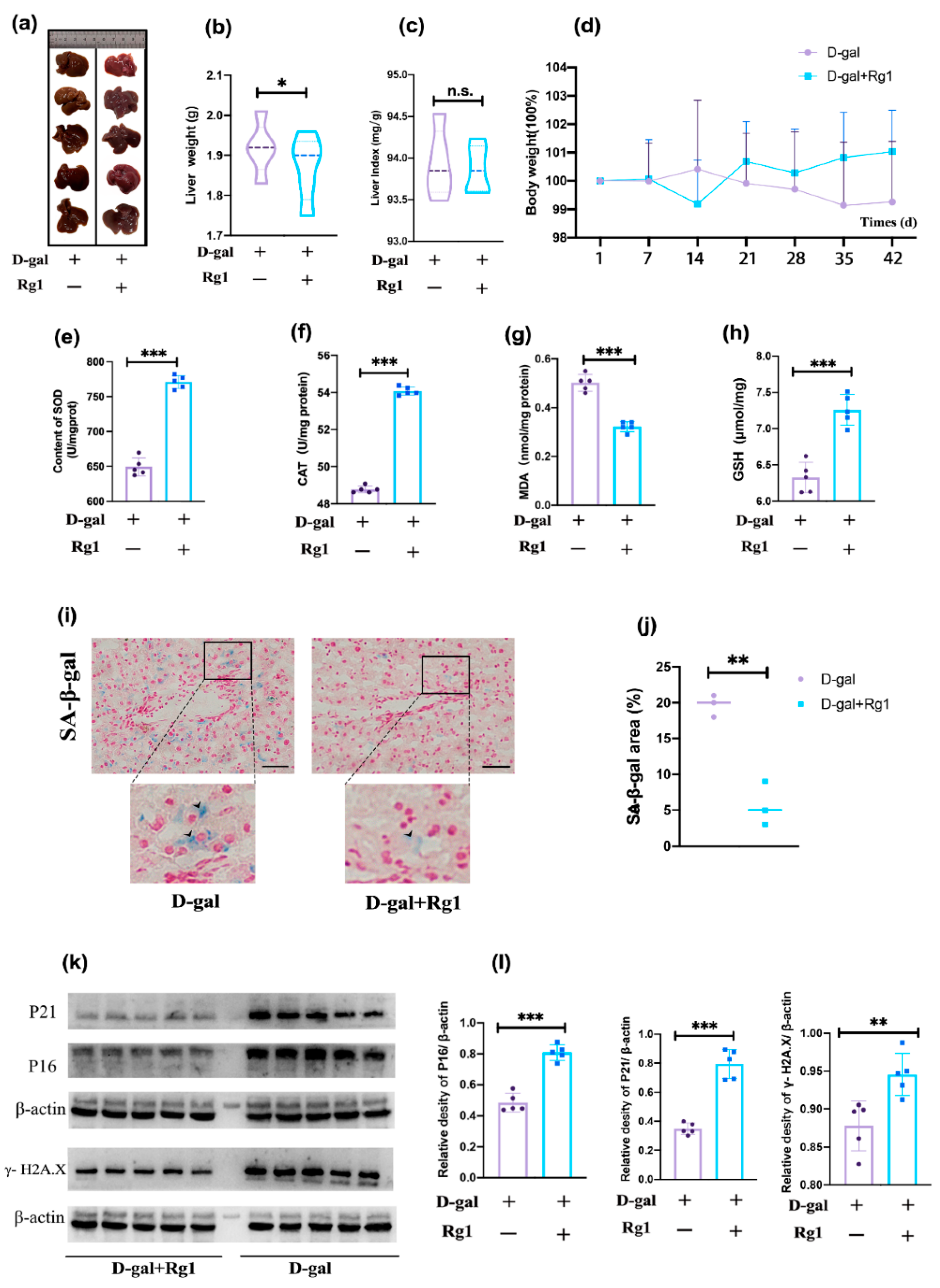
2.1. Validation of the D-Gal Damage Model and Observation of the Effect of Rg1 in Alleviating Oxidative Stress
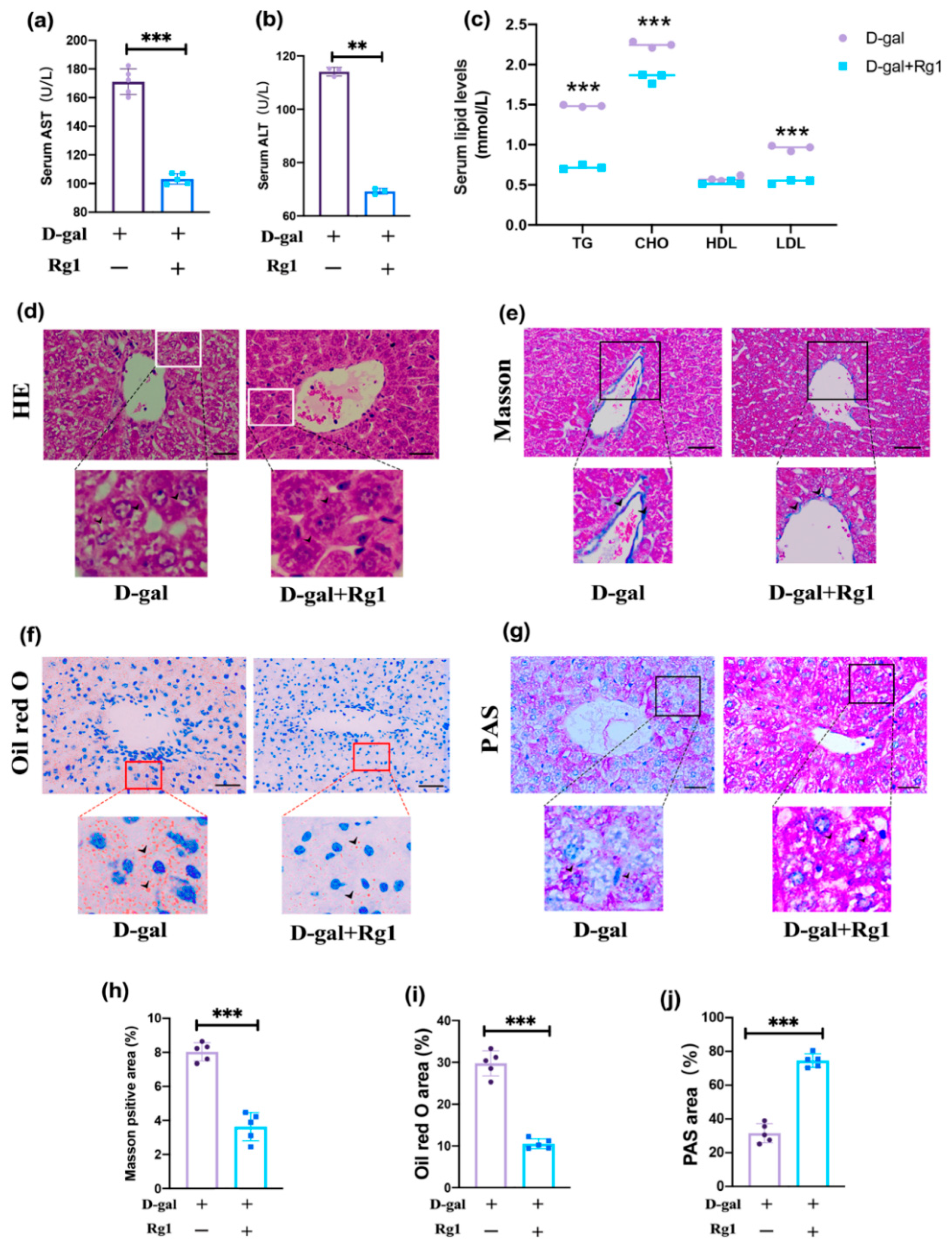
2.2. Rg1 Alleviated Liver Function Damage in D-Gal-Treated Mice
2.3. The Results of 4D-Label-Free Proteomic Analysis of Liver Tissue
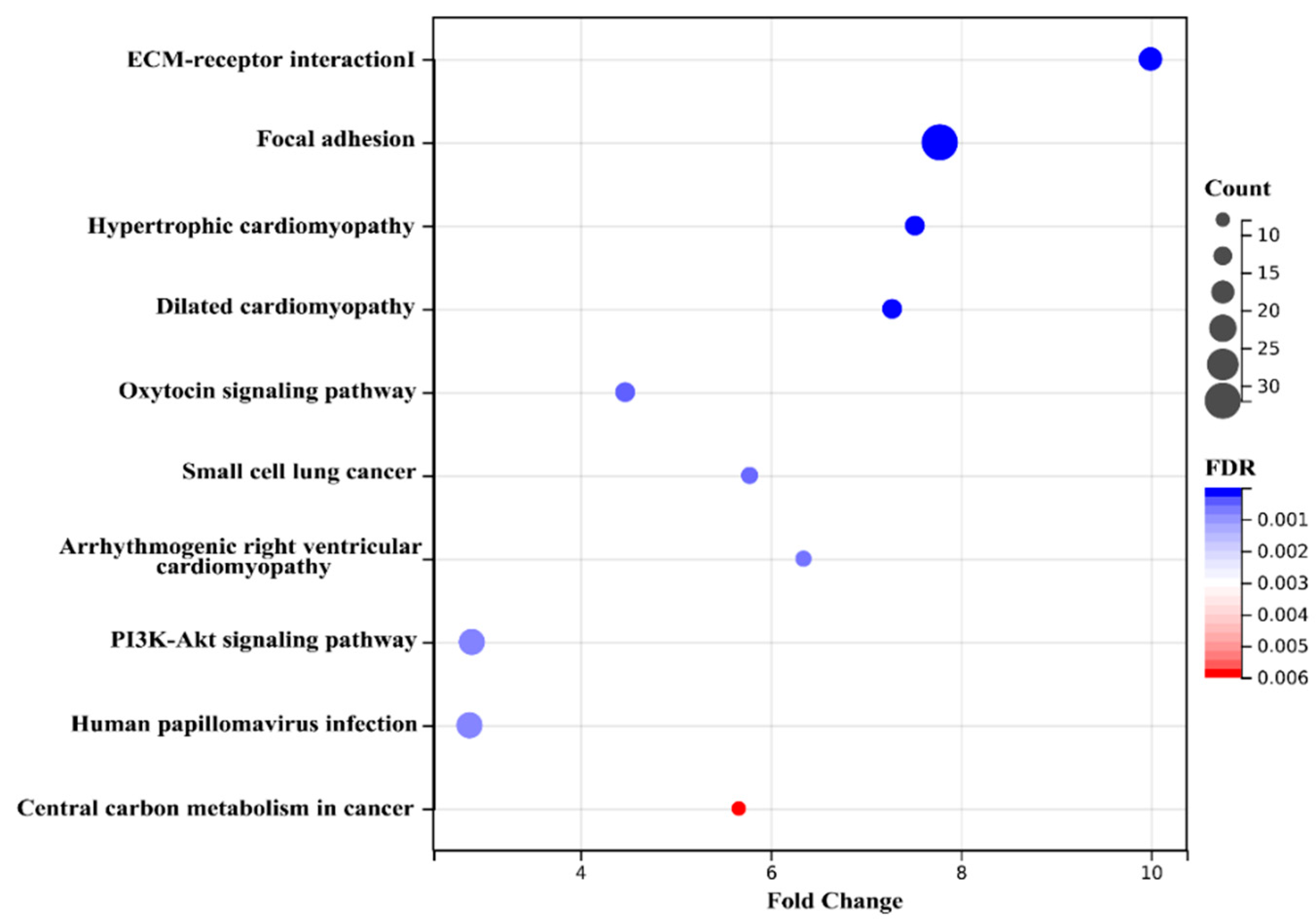
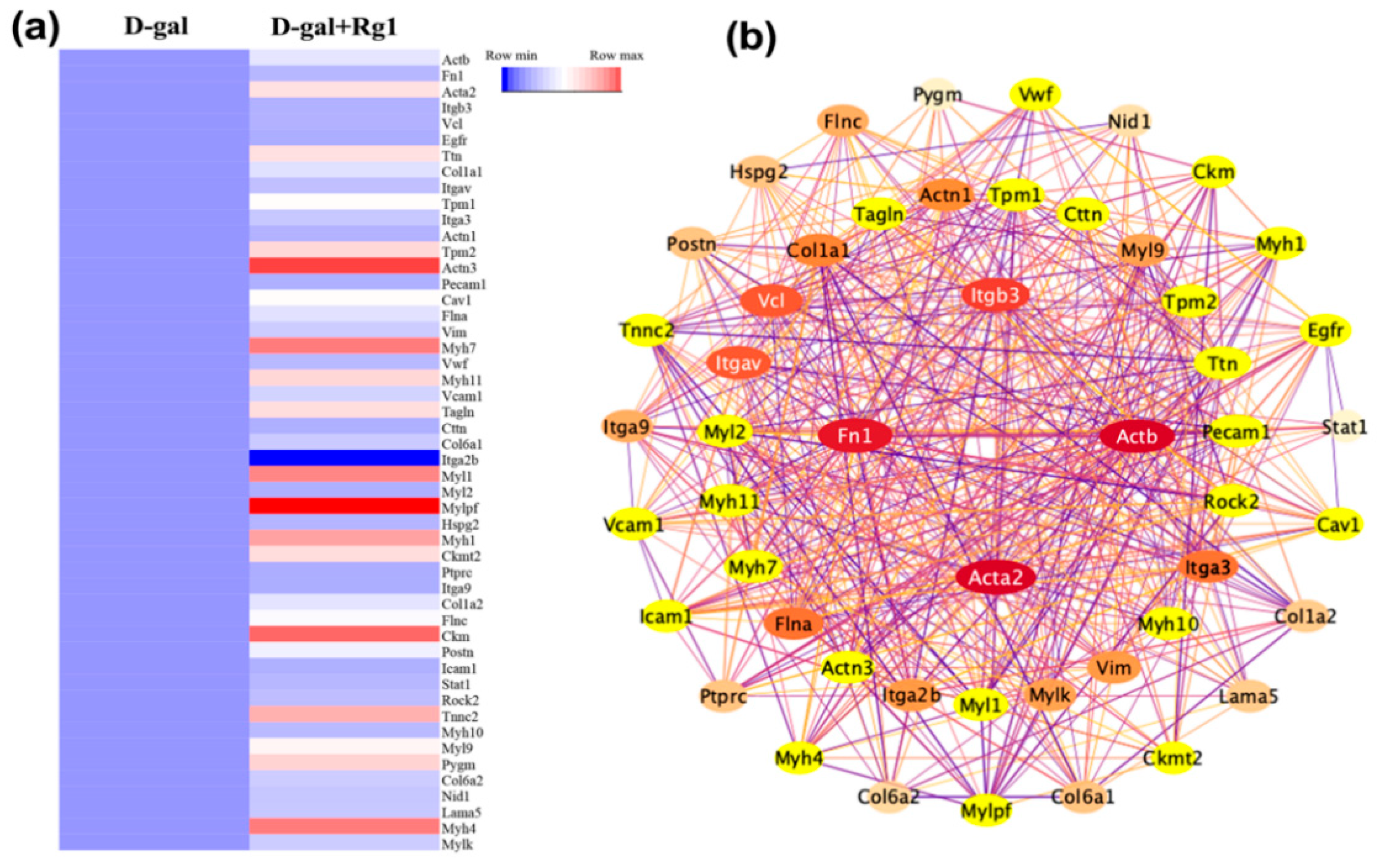
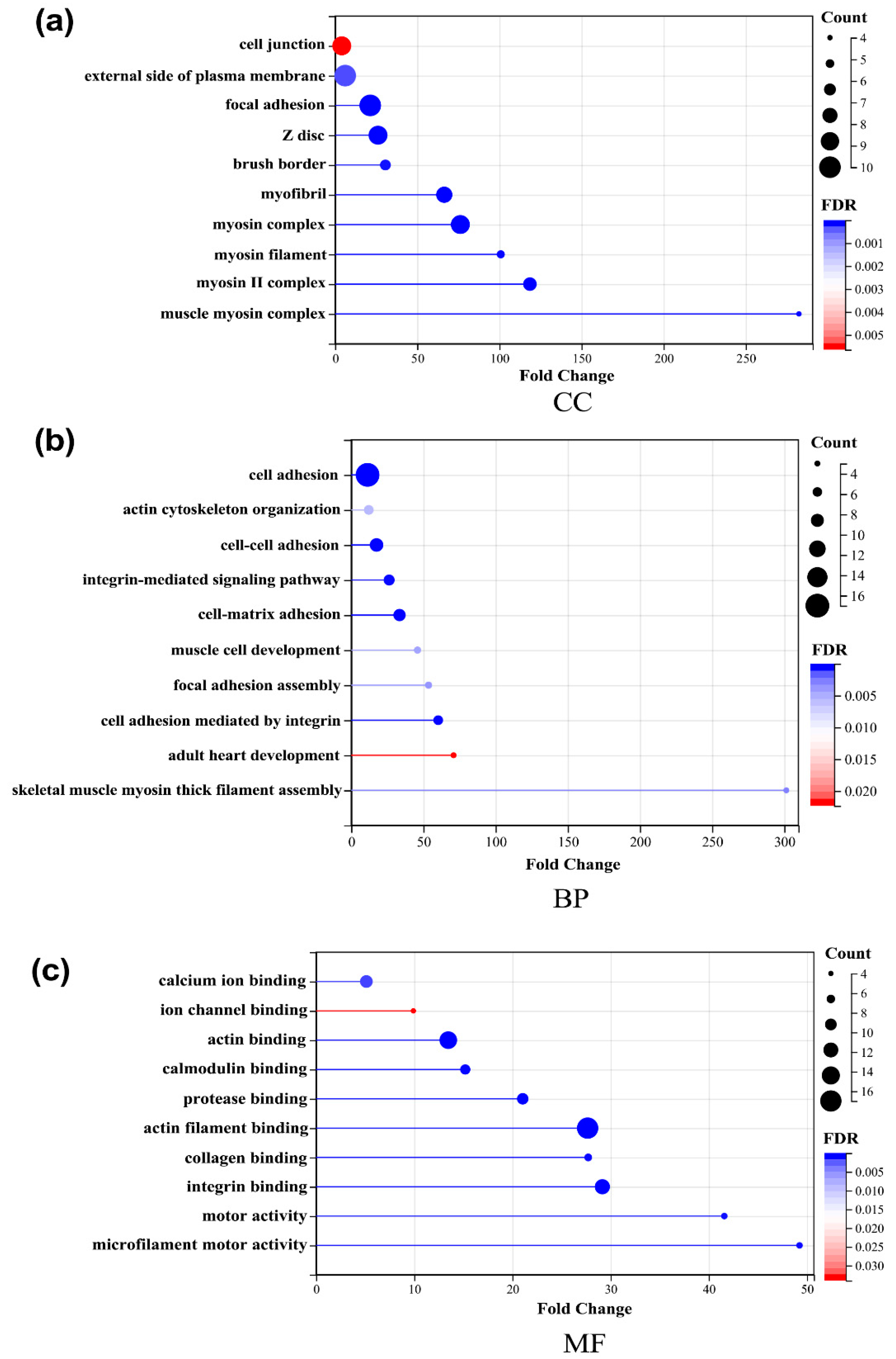
2.4. Bioinformatics Analysis of Key Proteins
2.5. Parallel Reaction Monitoring (PRM) Validates Proteomic Results
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Antioxidant Enzymes and Redox Products
4.2.1. Protein Concentration Measurement by BCA Protein Assay Reagent
4.2.2. Detection of SOD, CAT, and MDA
4.2.3. Detection of GSH
4.3. Senescence-Associated β-Galactosidase (SA-β-Gal) Staining and Oil Red O Staining
4.4. Immunoblot Assay
4.5. Paraffin Section
4.6. 4D-Label-Free Proteomic Analysis
4.6.1. Protein Extraction and Gel Separation
4.6.2. Filter Aided Sample Preparation (FASP) Enzymatic Digestion
4.6.3. NanoElute Chromatography
4.7. Mass Spectrometry Identification
4.8. Bioinformatics Analysis of Differentially Expressed Proteins
4.9. Parallel Reaction Monitoring (PRM)
4.9.1. Extraction of Peptides
4.9.2. Mass Spectrometry Detection
4.9.3. Mass Spectrometry Detection
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LDL | Low-density lipoprotein |
| CHO | Cholesterol |
| TG | Triglycerides |
| HDL | High-density lipoprotein |
| EGFR | Epidermal growth factor receptor |
| STAT1 | Activator of transcription 1 |
| ROS | Reactive oxygen species |
| D-gal | D-galactose |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| SOD | Superoxide Dismutase |
| GSH | Glutathione |
| CAT | Catalase |
| MDA | Malondialdehyde |
| SA-β-gal | Senescence-associated β-galactosidase |
| NAFLD | Nonalcoholic fatty liver disease |
| γ-H2A.X | phospho-histone H2A.X |
| ALT | Alanine transaminase |
| AST | Aspartate aminotransferase |
| HE | Hematoxylin and eosin |
| PAS | Periodic acid–Schiff |
| DEPs | Differential express protein |
| FDR | False discovery rate |
| GO | Gene ontology |
| BP | Biological processes |
| MF | Molecular functions |
| CC | Cellular components |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PPI | Protein-protein interaction |
| PRM | Parallel Reaction Monitoring |
| LC-PRM/MS | Liquid chromatography tandem mass spectrometry |
| AGEs | Advanced glycosylation end products |
| NKCC1 | Na+/K+/2Cl− cotransporter |
| ItgaV | Alpha v integrins |
| FASP | Filter aided sample preparation |
| IAA | iodoacetamide |
| DTT | Dithiothreitol |
| HCD | Higher-energy collisional dissociation |
| SD | Standard deviation |
References
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy Metabolism in the Liver. In Comprehensive Physiology; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Hunt, N.J.; Sun, W.; Lockwood, G.P.; Couteur, D.; Cogger, V.C. Hallmarks of Aging in the Liver. Comput. Struct. Biotechnol. J. 2019, 17, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Tu, Y.; He, W.; Liu, Y.; Wu, W.; Fang, Q.; Tang, H.; Tang, R.; Wan, Z.; Sun, W.; et al. Hyperoside attenuates renal aging and injury induced by D-galactose via inhibiting AMPK-ULK1 signaling-mediated autophagy. Aging 2018, 10, 4197–4212. [Google Scholar] [CrossRef]
- Fan, J.; Yang, X.; Li, J.; Shu, Z.; Dai, J.; Liu, X.; Li, B.; Jia, S.; Kou, X.; Yang, Y. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget 2017, 8, 17475–17490. [Google Scholar] [CrossRef]
- Chen, P.; Lei, J.; Chen, F.; Zhou, B. Ameliorative effect of urolithin A on D-gal-induced liver and kidney damage in aging mice via its antioxidative, anti-inflammatory and antiapoptotic properties. R. Soc. Chem. 2020, 10, 8027–8038. [Google Scholar]
- Wang, Y.; Zhang, H.; Chen, Q.; Jiao, F.; Gong, Z. TNF-α/HMGB1 inflammation signalling pathway regulates pyroptosis during liver failure and acute kidney injury. Cell Prolif. 2020, 53, e12829. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. d -Galactose-induced accelerated aging model: An overview. Biogerontology 2019, 20, 763–782. [Google Scholar] [CrossRef]
- Hu, S.Y. A contribution to our knowledge of ginseng. Am. J. Chin. Med. 1977, 5, 1–23. [Google Scholar] [CrossRef]
- Gao, X.Q.; Du, Z.R.; Yuan, L.J.; Zhang, W.D.; Chen, W.F. Ginsenoside Rg1 Exerts Anti-inflammatory Effects via G Protein-Coupled Estrogen Receptor in Lipopolysaccharide-Induced Microglia Activation. Front. Neurosci. 2019, 13, 1168. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alolga, R.N.; Nuer-Allornuvor, G.F.; Kuugbee, E.D.; Yin, X.; Ma, G. Ginsenoside Rg1 and the control of inflammation implications for the therapy of type 2 diabetes: A review of scientific findings and call for further research. Pharmacol. Res. 2020, 152, 104630. [Google Scholar] [CrossRef] [PubMed]
- Im, D.-S. Pro-Resolving Effect of Ginsenosides as an Anti-Inflammatory Mechanism of Panax ginseng. Biomolecules 2020, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Litao, B.; Jialiang, G.; Fan, W.; Jing, Z.; Danwei, W.; Junping, W. Therapeutic Potential of Ginsenosides as an Adjuvant Treatment for Diabetes. Front Pharm. 2018, 9, 423. [Google Scholar]
- Esra, S.; Sievenpiper, J.L.; Vladimir, D.; Cozma, A.I.; Vanessa, H.; Jayalath, V.H.; Jenkins, D.; Blanco, M.S.; De, S.; Elena, J. The Effect of Ginseng (The Genus Panax) on Glycemic Control: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. PLoS ONE 2014, 9, e107391. [Google Scholar]
- Liu, Q.; Zhang, F.G.; Zhang, W.S.; An, P.; Yang, Y.L.; Liu, J.F.; Ping, L.; Liu, B.L.; Qi, L.W. Ginsenoside Rg1 Inhibits Glucagon-Induced Hepatic Gluconeogenesis through Akt-FoxO1 Interaction. Theranostics 2017, 7, 4001–4012. [Google Scholar] [CrossRef]
- Qi, R.; Jiang, R.; Xiao, H.; Wang, Z.; He, S.; Wang, L.; Wang, Y. Ginsenoside Rg1 protects against d -galactose induced fatty liver disease in a mouse model via FOXO1 transcriptional factor. Life Sci. 2020, 254, 117776. [Google Scholar] [CrossRef]
- Xiao, M.-H.; Xia, J.-Y.; Wang, Z.-L.; Hu, W.-X.; Fan, Y.-L. Ginsenoside Rg1 attenuates liver injury induced by D-galactose in mice. Exp. Ther. Med. 2018, 16, 4100–4106. [Google Scholar] [CrossRef]
- Xu, S.; Bao, M.; Li, D.; Yong, M.L. Advanced glycation in d-galactose induced mouse aging model. Mech. Ageing Dev. 1999, 108, 239–251. [Google Scholar]
- Du, Z.; Yang, Q.; Liu, L.; Li, S.; Zhao, J.; Hu, J.; Liu, C.; Qian, D.; Gao, C. NADPH oxidase 2-dependent oxidative stress, mitochondrial damage and apoptosis in the ventral cochlear nucleus of D-galactose-induced aging rats. Neuroscience 2015, 286, 281–292. [Google Scholar] [CrossRef]
- Tian, W.; Chen, L.; Zhang, L.; Wang, B.; Li, Y. Effects of ginsenoside Rg1 on glucose metabolism and liver injury in streptozotocin-induced type 2 diabetic rats. Genet. Mol. Res. GMR 2017, 16, gmr16019463. [Google Scholar] [CrossRef]
- Schlessinger, J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 2002, 110, 669–672. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2013, 2, 127–137. [Google Scholar] [CrossRef]
- Karin, K.; Maria, S. EGFR Signaling in Liver Diseases. Int. J. Mol. Sci. 2015, 17, 30. [Google Scholar]
- Bonus, M.; Häussinger, D.; Gohlke, H. Liver cell hydration and integrin signaling. Biol. Chem. 2021, 402, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.C.; Ma, P.Y.; Blanchard, A.; Urner, T.M.; Mattheyses, A.L. EGFR activation attenuates the mechanical threshold for integrin tension and focal adhesion formation. J. Cell Sci. 2020, 133, jcs-238840. [Google Scholar] [CrossRef]
- Hussinger, D.; Lang, F. Cell volume and hormone action. Trends Pharmacol. Sci. 1992, 13, 371–373. [Google Scholar] [CrossRef]
- Boini, K.M.; Graf, D.; Hennige, A.M.; Koka, S.; Kempe, D.S.; Wang, K.; Ackermann, T.F.; Foller, M.; Vallon, V.; Pfeifer, K. Enhanced insulin sensitivity of gene-targeted mice lacking functional KCNQ1. Am. J. Physiol. Regul Integr. Comp. Physiol. 2009, 296, R1695–R1701. [Google Scholar] [CrossRef]
- Reinehr, R.; Sommerfeld, A.; Haussinger, D. Insulin Induces Swelling-dependent Activation of the Epidermal Growth Factor Receptor in Rat Liver. J. Biol. Chem. 2010, 285, 25904. [Google Scholar] [CrossRef]
- Yasuda, K.; Miyake, K.; Horikawa, Y.; Hara, K.; Kasuga, M. Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat. Genet. 2008, 40, 1092. [Google Scholar] [CrossRef]
- Jeong, W.I.; Park, O.; Radaeva, S.; Gao, B. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology 2010, 44, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Albanis, E.; Friedman, S.L. Antifibrotic Agents for Liver Disease. Am. J. Transplant. 2010, 6, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Pinzani, M. PDGF and signal transduction in hepatic stellate cells. Front. Biosci. 2002, 7, d1720. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Takikawa, Y.; Tabuchi, T.; Satoh, T.; Kosaka, K.; Suzuki, K. Carnosic acid (CA) prevents lipid accumulation in hepatocytes through the EGFR/MAPK pathway. J. Gastroenterol. 2012, 47, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Berasain, C.; Avila, M.A. The EGFR signalling system in the liver: From hepatoprotection to hepatocarcinogenesis. J. Gastroenterol. 2014, 49, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Aravalli, R.N.; Cressman, E.N.K.; Steer, C.J. Cellular and molecular mechanisms of hepatocellular carcinoma: An update. Arch. Toxicol. 2013, 87, 227–247. [Google Scholar]
- Chinwalla, A.T.; Cook, L.L.; Delehaunty, K.D.; Fewell, G.A.; Fulton, L.A.; Fulton, R.S.; Graves, T.A.; Hillier, L.W.; Mardis, E.R.; McPherson, J.D.; et al. Initial sequencing and comparative analysis of the mouse genome. Nature 2002, 420, 520–562. [Google Scholar] [CrossRef]
- He, L.; Ling, L.; Wei, T.; Wang, Y.; Xiong, Z. Ginsenoside Rg1 Improves Fertility and Reduces Ovarian Pathological Damages in Premature Ovarian Failure Model of Mice. Exp. Biol. Med. 2017, 242, 1535370217693323. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Jiang, R.; Li, C.; Wang, Y. Ginsenoside Rg1 prevents bone marrow mesenchymal stem cell senescence via NRF2 and PI3K/Akt signaling. Free Radic. Biol. Med. 2021, 174, 182–194. [Google Scholar] [CrossRef]
- Jing, L.; Dachuan, C.; Xin, Y.; Yanyan, Z.; Linbo, C.; Pengwei, J.; Lu, W.; Yaping, W. Protective Effect of Ginsenoside Rg1 on Hematopoietic Stem/Progenitor Cells through Attenuating Oxidative Stress and the Wnt/β-Catenin Signaling Pathway in a Mouse Model of d-Galactose-induced Aging. Int. J. Mol. Sci. 2016, 17, 849. [Google Scholar]
- Xiang, Y.; Wang, S.-H.; Wang, L.; ZWang, Z.-L.; Yao, H.; Chen, L.-B.; Wang, Y.-P. Effects of Ginsenoside Rg1 Regulating Wnt/β-Catenin Signaling on Neural Stem Cells to Delay Brain Senescence. Stem Cells Int. 2019, 2019, 5010184. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xia, J.; Jia, D.; Zhang, M.; Zhang, Y.; Huang, G.; Wang, Y. Mechanism of ginsenoside Rg1 renal protection in a mouse model of D-galactose-induced subacute damage. Pharm. Biol. 2016, 54, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, W.; Hebestedt, A.; Aschenbrenner, G.; Pichotka, B.; Blümel, G. A comparative study with various anesthetics in mice (pentobarbitone, ketamine-xylazine, carfentanyl-etomidate). Res. Exp. Med. 1984, 184, 159–169. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Ma, R.; Zhu, S.; Wang, Y. Revealing the Therapeutic Targets and Mechanism of Ginsenoside Rg1 for Liver Damage Related to Anti-Oxidative Stress Using Proteomic Analysis. Int. J. Mol. Sci. 2022, 23, 10045. https://doi.org/10.3390/ijms231710045
Hou J, Ma R, Zhu S, Wang Y. Revealing the Therapeutic Targets and Mechanism of Ginsenoside Rg1 for Liver Damage Related to Anti-Oxidative Stress Using Proteomic Analysis. International Journal of Molecular Sciences. 2022; 23(17):10045. https://doi.org/10.3390/ijms231710045
Chicago/Turabian StyleHou, Jiying, Ruoxiang Ma, Shisheng Zhu, and Yaping Wang. 2022. "Revealing the Therapeutic Targets and Mechanism of Ginsenoside Rg1 for Liver Damage Related to Anti-Oxidative Stress Using Proteomic Analysis" International Journal of Molecular Sciences 23, no. 17: 10045. https://doi.org/10.3390/ijms231710045




