The Diagnostic Value of MRI-Based Radiomic Analysis of Lacrimal Glands in Patients with Sjögren’s Syndrome
Abstract
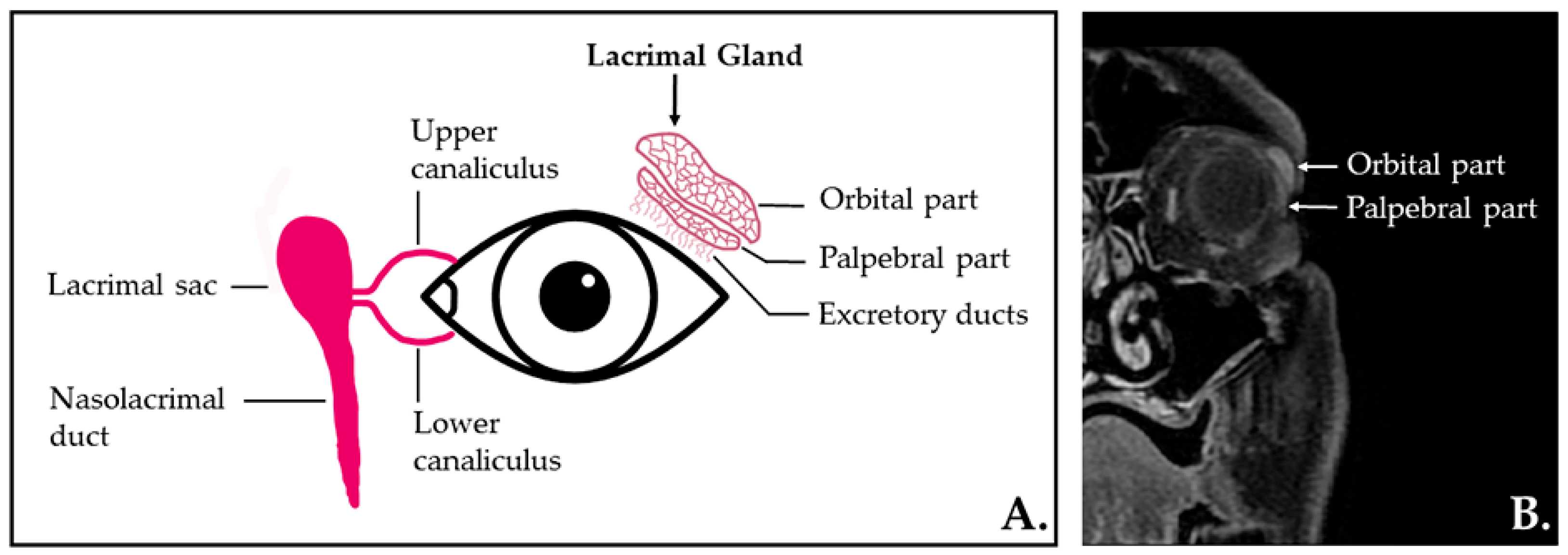
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Groups
4.2. MRI Protocol
4.3. Texture Analysis
4.3.1. Image Pre-Processing and Segmentation
4.3.2. Feature Extraction
4.3.3. Feature Selection, Class Prediction, and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jonsson, R.; Brokstad, K.A.; Jonsson, M.V.; Delaleu, N.; Skarstein, K. Current concepts on Sjögren’s syndrome-classification criteria and biomarkers. Eur. J. Oral Sci. 2018, 126 (Suppl. 1), 37–48. [Google Scholar] [CrossRef]
- Bjordal, O.; Norheim, K.B.; Rødahl, E.; Jonsson, R.; Omdal, R. Primary Sjögren’s syndrome and the eye. Surv. Ophthalmol. 2020, 65, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Skarlis, C.; Raftopoulou, S.; Mavragani, C.P. Sjogren’s Syndrome: Recent Updates. J. Clin. Med. 2022, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Vivino, F.B. Sjogren’s syndrome: Clinical aspects. Clin. Immunol. 2017, 182, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Fidelix, T.; Czapkowski, A.; Azjen, S.; Andriolo, A.; Trevisani, V. Salivary gland ultrasonography as a predictor of clinical activity in Sjögren’s syndrome. PLoS ONE 2017, 12, e0182287. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; International Sjögren’s Syndrome Criteria Working Group; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef]
- Bădărînză, M.; Serban, O.; Maghear, L.; Bocsa, C.; Micu, M.; Damian, L.; Felea, I.; Fodor, D. Shear wave elastography as a new method to identify parotid lymphoma in primary Sjögren Syndrome patients: An observational study. Rheumatol. Int. 2020, 40, 1275–1281. [Google Scholar] [CrossRef]
- Baldini, C.; Luciano, N.; Tarantini, G.; Pascale, R.; Sernissi, F.; Mosca, M.; Caramella, D.; Bombardieri, S. Salivary gland ultrasonography: A highly specific tool for the early diagnosis of primary Sjögren’s syndrome. Arthritis Res. Ther. 2015, 17, 146. [Google Scholar] [CrossRef]
- André, R.; Becker, M.; Lombardi, T.; Buchholzer, S.; Marchal, F.; Seebach, J.D. Comparison of Clinical Characteristics and Magnetic Resonance Imaging of Salivary Glands With Magnetic Resonance Sialography in Sjögren’s Syndrome. Laryngoscope 2021, 131, E83–E89. [Google Scholar] [CrossRef]
- Conrady, C.D.; Joos, Z.P.; Patel, B.C. Review: The Lacrimal Gland and Its Role in Dry Eye. J. Ophthalmol. 2016, 2016, 7542929. [Google Scholar] [CrossRef] [Green Version]
- Machiele, R.; Lopez, M.J.; Czyz, C.N. Anatomy, Head and Neck, Eye Lacrimal Gland. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532914/ (accessed on 3 May 2022).
- De Lucia, O.; Zandonella Callegher, S.; De Souza, M.V.; Battafarano, N.; Del Papa, N.; Gerosa, M.; Giovannini, I.; Tullio, A.; Valent, F.; Zabotti, A.; et al. Ultrasound assessment of lacrimal glands: A cross-sectional study in healthy subjects and a preliminary study in primary Sjögren’s syndrome patients. Clin. Exp. Rheumatol. 2020, 38 (Suppl. 126), 203–209. [Google Scholar] [PubMed]
- Izumi, M.; Eguchi, K.; Uetani, M.; Nakamura, H.; Takagi, Y.; Hayashi, K.; Nakamura, T. MR features of the lacrimal gland in Sjögren’s syndrome. Am. J. Roentgenol. 1998, 170, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Sumi, M.; Kitamori, H.; Takagi, Y.; Nakamura, T. Diffusion-weighted MR microimaging of the lacrimal glands in patients with Sjogren’s syndrome. Am. J. Roentgenol. 2005, 184, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging-“how-to” guide and critical reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef]
- Ștefan, R.A.; Ștefan, P.A.; Mihu, C.M.; Csutak, C.; Melincovici, C.S.; Crivii, C.B.; Maluțan, A.M.; Hîțu, L.; Lebovici, A. Ultrasonography in the Differentiation of Endometriomas from Hemorrhagic Ovarian Cysts: The Role of Texture Analysis. J. Pers. Med. 2021, 11, 611. [Google Scholar] [CrossRef]
- Mărginean, L.; Ștefan, P.A.; Lebovici, A.; Opincariu, I.; Csutak, C.; Lupean, R.A.; Coroian, P.A.; Suciu, B.A. CT in the Differentiation of Gliomas from Brain Metastases: The Radiomics Analysis of the Peritumoral Zone. Brain Sci. 2022, 12, 109. [Google Scholar] [CrossRef]
- Stefan, P.A.; Puscas, M.E.; Csuak, C.; Lebovici, A.; Petresc, B.; Lupean, R.; Mihu, C.M. The utility of texture-based classification of different types of ascites on magnetic resonance. J. Buon. 2020, 25, 1237–1244. [Google Scholar]
- Boca, I.; Ciurea, A.I.; Ciortea, C.A.; Ștefan, P.A.; Lisencu, L.A.; Dudea, S.M. Differentiating Breast Tumors from Background Parenchymal Enhancement at Contrast-Enhanced Mammography: The Role of Radiomics—A Pilot Reader Study. Diagnostics 2021, 11, 1248. [Google Scholar] [CrossRef]
- Lecler, A.; Duron, L.; Balvay, D.; Savatovsky, J.; Bergès, O.; Zmuda, M.; Farah, E.; Galatoire, O.; Bouchouicha, A.; Fournier, L.S. Combining Multiple Magnetic Resonance Imaging Sequences Provides Independent Reproducible Radiomics Features. Sci. Rep. 2019, 9, 2068. [Google Scholar] [CrossRef]
- Guo, J.; Li, Z.; Qu, X.; Xian, J. Value of MRI-based radiomics analysis for differentiation of benign and malignant epithelial neoplasms in the lacrimal gland: A retrospective study. Acta Radiol. 2021, 62, 743–751. [Google Scholar] [CrossRef]
- Gøransson, L.G.; Haldorsen, K.; Brun, J.G.; Harboe, E.; Jonsson, M.V.; Skarstein, K.; Time, K.; Omdal, R. The point prevalence of clinically relevant primary Sjögren’s syndrome in two Norwegian counties. Scand. J. Rheumatol. 2011, 40, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Shen, L.; Malyavantham, K.; Pankewycz, O.; Ambrus, J.L., Jr.; Suresh, L. Temporal histological changes in lacrimal and major salivary glands in mouse models of Sjogren’s syndrome. BMC Oral Health 2013, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Jousse-Joulin, S.; D’Agostino, M.A.; Nicolas, C.; Naredo, E.; Ohrndorf, S.; Backhaus, M.; Tamborrini, G.; Chary-Valckenaere, I.; Terslev, L.; Iagnocco, A.; et al. Video clip assessment of a salivary gland ultrasound scoring system in Sjögren’s syndrome using consensual definitions: An OMERACT ultrasound working group reliability exercise. Ann. Rheum. Dis. 2019, 78, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Jousse-Joulin, S.; Coiffier, G. Current status of imaging of Sjogren’s syndrome. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101592. [Google Scholar] [CrossRef]
- Mafee, M.F.; Yang, G. Clinical Magnetic Resonance Imaging; W.B. Saunders: Philadelphia, PA, USA, 2006. [Google Scholar]
- Jansen, J.; Parra, C.; Lu, Y.; Shukla-Dave, A. Evaluation of Head and Neck Tumors with Functional MR Imaging. Magn. Reson. Imaging Clin. N. Am. 2016, 24, 123–133. [Google Scholar] [CrossRef]
- Expert Panel on MR Safety; Kanal, E.; Barkovich, A.J.; Bell, C.; Borgstede, J.P.; Bradley, W.G., Jr.; Froelich, J.W.; Gimbel, J.R.; Gosbee, J.W.; Kuhni-Kaminski, E.; et al. ACR guidance document on MR safe practices: 2013. J. Magn. Reson. Imaging JMRI 2013, 37, 501–530. [Google Scholar] [CrossRef]
- Jabehdar Maralani, P.; Schieda, N.; Hecht, E.M.; Litt, H.; Hindman, N.; Heyn, C.; Davenport, M.S.; Zaharchuk, G.; Hess, C.P.; Weinreb, J. MRI safety and devices: An update and expert consensus. J. Magn. Reson. Imaging JMRI 2020, 51, 657–674. [Google Scholar] [CrossRef]
- Available online: https://pyradiomics.readthedocs.io/en/latest/features.html (accessed on 1 August 2021).
- van Ginkel, M.S.; Glaudemans, A.; van der Vegt, B.; Mossel, E.; Kroese, F.; Bootsma, H.; Vissink, A. Imaging in Primary Sjögren’s Syndrome. J. Clin. Med. 2020, 9, 2492. [Google Scholar] [CrossRef]
- Qin, B.; Wang, J.; Yang, Z.; Yang, M.; Ma, N.; Huang, F.; Zhong, R. Epidemiology of primary Sjögren’s syndrome: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 1983–1989. [Google Scholar] [CrossRef]
- Seror, R.; Bowman, S.J.; Brito-Zeron, P.; Theander, E.; Bootsma, H.; Tzioufas, A.; Gottenberg, J.E.; Ramos-Casals, M.; Dörner, T.; Ravaud, P.; et al. EULAR Sjögren’s syndrome disease activity index (ESSDAI): A user guide. RMD Open 2015, 1, e000022. [Google Scholar] [CrossRef]
- Ștefan, P.A.; Lupean, R.A.; Mihu, C.M.; Lebovici, A.; Oancea, M.D.; Hîțu, L.; Duma, D.; Csutak, C. Ultrasonography in the Diagnosis of Adnexal Lesions: The Role of Texture Analysis. Diagnostics 2021, 11, 812. [Google Scholar] [CrossRef] [PubMed]


| Variable (n = 23) | |
|---|---|
| Age (years) | 58.79 ± 12.64 |
| Female:male | 21:2 |
| BMI (kg/m2) | 26.15 ± 4.78 |
| Disease duration (months) | 29 [15.5–60] |
| ESSDAI | |
| Inactive | 17 (73.9) |
| Moderately active | 4 (17.3) |
| Severely active | 2 (8.69) |
| ESSDAI score | 3.71 ± 5.94 |
| Positive Schirmer’s test | 20 (86.9) |
| Schirmer’s test (mm) | 1 [1.5, 3.75] |
| Xerophthalmia | |
| Absent | 1 (4.3) |
| Mild under treatment | 18 (78.3) |
| Severe under treatment | 4 (17.4) |
| Anti-Ro/La autoantibodies | 20 (86.9) |
| Rheumatoid factor | 18 (78.2) |
| Parameter | p-Value | pSS Group (n = 23) | Control Group (n = 23) | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| RunPercentage | 0.000025 | 0.89 | 0.85–0.90 | 0.85 | 0.82–0.86 |
| InverseVariance | 0.000027 | 0.30 | 0.27–0.35 | 0.36 | 0.34–0.4 |
| ZoneVariance | 0.000028 | 1.61 | 0.80–2.44 | 3.26 | 2.09–4.27 |
| LargeDependenceEmphasis | 0.000029 | 4.56 | 3.90–5.96 | 6.29 | 5.35–7.92 |
| RunLengthNonUniformityNormalized | 0.000031 | 0.80 | 0.75–0.84 | 0.75 | 0.70–0.77 |
| ShortRunEmphasis | 0.000032 | 0.91 | 0.89–0.93 | 0.88 | 0.86–0.90 |
| GrayLevelNonUniformity | 0.000033 | 8.39 | 7.04–10.01 | 11.63 | 8.88–15.70 |
| SmallDependenceEmphasis | 0.000035 | 0.56 | 0.47–0.60 | 0.45 | 0.41–0.49 |
| DependenceNonUniformityNormalized | 0.000047 | 0.34 | 0.27–0.37 | 0.27 | 0.24–0.30 |
| LongRunEmphasis | 0.000050 | 1.42 | 1.35–1.57 | 1.62 | 1.51–1.82 |
| DependenceVariance | 0.000141 | 1.05 | 0.79–1.47 | 1.49 | 1.28–1.85 |
| SizeZoneNonUniformityNormalized | 0.000149 | 0.53 | 0.48–0.60 | 0.47 | 0.40–0.50 |
| RunVariance | 0.000154 | 0.16 | 0.12–0.20 | 0.23 | 0.18–0.30 |
| SmallAreaEmphasis | 0.000189 | 0.75 | 0.72–0.80 | 0.71 | 0.65–0.74 |
| LargeAreaHighGrayLevelEmphasis | 0.000200 | 1304.26 | 922.01–2309.91 | 2835.71 | 1510.48–4198.58 |
| Kurtosis | 0.000335 | 3.29 | 2.85–3.84 | 4.97 | 3.22–6.73 |
| RobustMeanAbsoluteDeviation | 0.000480 | 71.89 | 54.54–86.86 | 55.64 | 42.45–67.38 |
| Maximum2DDiameterColumn | 0.000550 | 5.03 | 4.65–5.73 | 5.77 | 5.35–6.47 |
| Skewness | 0.000550 | -0.59 | −0.96–−0.11 | −1.10 | −1.46–−0.44 |
| Independent Variables | Coefficient | Std. Error | t | p | r partial | r semipartial | VIF |
|---|---|---|---|---|---|---|---|
| DependenceNonUniformityNormalized | −8.77 | 6.47 | −1.35 | 0.179 | −0.15 | 0.11 | 141.98 |
| DependenceVariance | −0.60 | 1.00 | −0.60 | 0.548 | −0.06 | 0.04 | 220.03 |
| GrayLevelNonUniformity | 0.00 | 0.01 | 0.48 | 0.626 | 0.05 | 0.04 | 3.10 |
| InverseVariance | −3.05 | 1.89 | −1.61 | 0.109 | −0.18 | 0.13 | 9.69 |
| Kurtosis | −0.02 | 0.05 | −0.39 | 0.696 | −0.04 | 0.03 | 6.54 |
| LargeAreaHighGrayLevelEmphasis | −0.00 | 5.06 | −1.03 | 0.304 | −0.11 | 0.08 | 5.82 |
| LargeDependenceEmphasis | −1.04 | 1.31 | −0.79 | 0.431 | −0.09 | 0.06 | 6242.02 |
| LongRunEmphasis | −5.83 | 4.41 | −1.32 | 0.189 | −0.15 | 0.10 | 1440.47 |
| Maximum2DDiameterColumn | −0.09 | 0.04 | −2.12 | 0.037 | −0.23 | 0.17 | 1.30 |
| RobustMeanAbsoluteDeviation | 0.00 | 0.00 | 0.93 | 0.352 | 0.10 | 0.07 | 4.79 |
| RunLengthNonUniformityNormalized | 42.15 | 21.14 | 1.99 | 0.049 | 0.22 | 0.16 | 1386.23 |
| RunPercentage | −87.08 | 75.41 | −1.15 | 0.251 | −0.13 | 0.09 | 8747.97 |
| RunVariance | 9.60 | 7.76 | 1.23 | 0.219 | 0.14 | 0.10 | 710.30 |
| ShortRunEmphasis | −82.77 | 43.88 | −1.88 | 0.063 | −0.21 | 0.15 | 1750.14 |
| SizeZoneNonUniformityNormalized | −8.85 | 8.91 | −0.99 | 0.323 | −0.11 | 0.08 | 473.78 |
| Skewness | 0.08 | 0.12 | 0.70 | 0.480 | 0.08 | 0.05 | 4.32 |
| SmallAreaEmphasis | 4.35 | 11.61 | 0.37 | 0.709 | 0.04 | 0.03 | 511.95 |
| SmallDependenceEmphasis | 21.37 | 14.73 | 1.45 | 0.151 | 0.16 | 0.11 | 1570.01 |
| ZoneVariance | 0.06 | 0.08 | 0.76 | 0.449 | 0.08 | 0.06 | 75.30 |
| Texture Parameter | AUC | p-Value | J | Cut-Off | Se | Sp |
|---|---|---|---|---|---|---|
| Maximum2DDiameterColumn | 0.713 (0.611–0.8) | 0.0001 | 0.41 | ≤5.35 | 70.83 (55.9–83) | 70.83 (55.9–83) |
| RunLengthNonUniformityNormalized | 0.747 (0.648–0.83) | <0.0001 | 0.52 | >0.77 | 72.92 (58.2–84.7) | 79.17 (65.0–89.5) |
| Prediction model | 0.905 (0.828–0.956) | <0.0001 | 0.75 | >0.44 | 91.67 (80.0–97.7) | 83.33 (69.8–92.5) |
| Radiomic Feature | ESSDAI Score | Schirmer’s Test (mm) |
|---|---|---|
| Max2DDC | r = 0.315, p = 0.134 | r = −0.312, p = 0.138 |
| RLNonUN | r = −0.191, p = 0.372 | r = 0.334, p = 0.111 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muntean, D.D.; Bădărînză, M.; Ștefan, P.A.; Lenghel, M.L.; Rusu, G.M.; Csutak, C.; Coroian, P.A.; Lupean, R.A.; Fodor, D. The Diagnostic Value of MRI-Based Radiomic Analysis of Lacrimal Glands in Patients with Sjögren’s Syndrome. Int. J. Mol. Sci. 2022, 23, 10051. https://doi.org/10.3390/ijms231710051
Muntean DD, Bădărînză M, Ștefan PA, Lenghel ML, Rusu GM, Csutak C, Coroian PA, Lupean RA, Fodor D. The Diagnostic Value of MRI-Based Radiomic Analysis of Lacrimal Glands in Patients with Sjögren’s Syndrome. International Journal of Molecular Sciences. 2022; 23(17):10051. https://doi.org/10.3390/ijms231710051
Chicago/Turabian StyleMuntean, Delia Doris, Maria Bădărînză, Paul Andrei Ștefan, Manuela Lavinia Lenghel, Georgeta Mihaela Rusu, Csaba Csutak, Paul Alexandru Coroian, Roxana Adelina Lupean, and Daniela Fodor. 2022. "The Diagnostic Value of MRI-Based Radiomic Analysis of Lacrimal Glands in Patients with Sjögren’s Syndrome" International Journal of Molecular Sciences 23, no. 17: 10051. https://doi.org/10.3390/ijms231710051
APA StyleMuntean, D. D., Bădărînză, M., Ștefan, P. A., Lenghel, M. L., Rusu, G. M., Csutak, C., Coroian, P. A., Lupean, R. A., & Fodor, D. (2022). The Diagnostic Value of MRI-Based Radiomic Analysis of Lacrimal Glands in Patients with Sjögren’s Syndrome. International Journal of Molecular Sciences, 23(17), 10051. https://doi.org/10.3390/ijms231710051







