Biochemical Neuroadaptations in the Rat Striatal Dopaminergic System after Prolonged Exposure to Methamphetamine Self-Administration
Abstract
:1. Introduction
2. Results
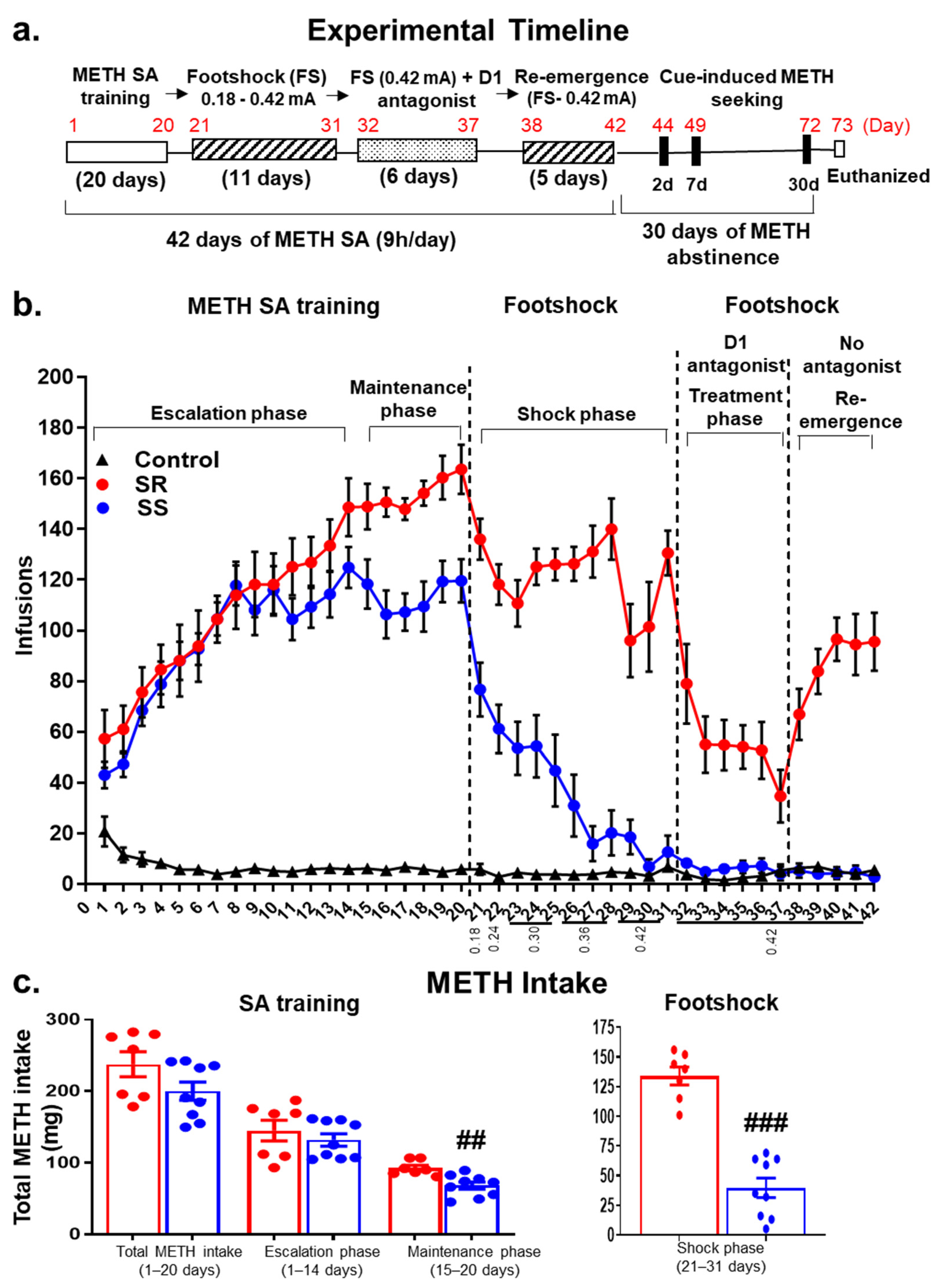
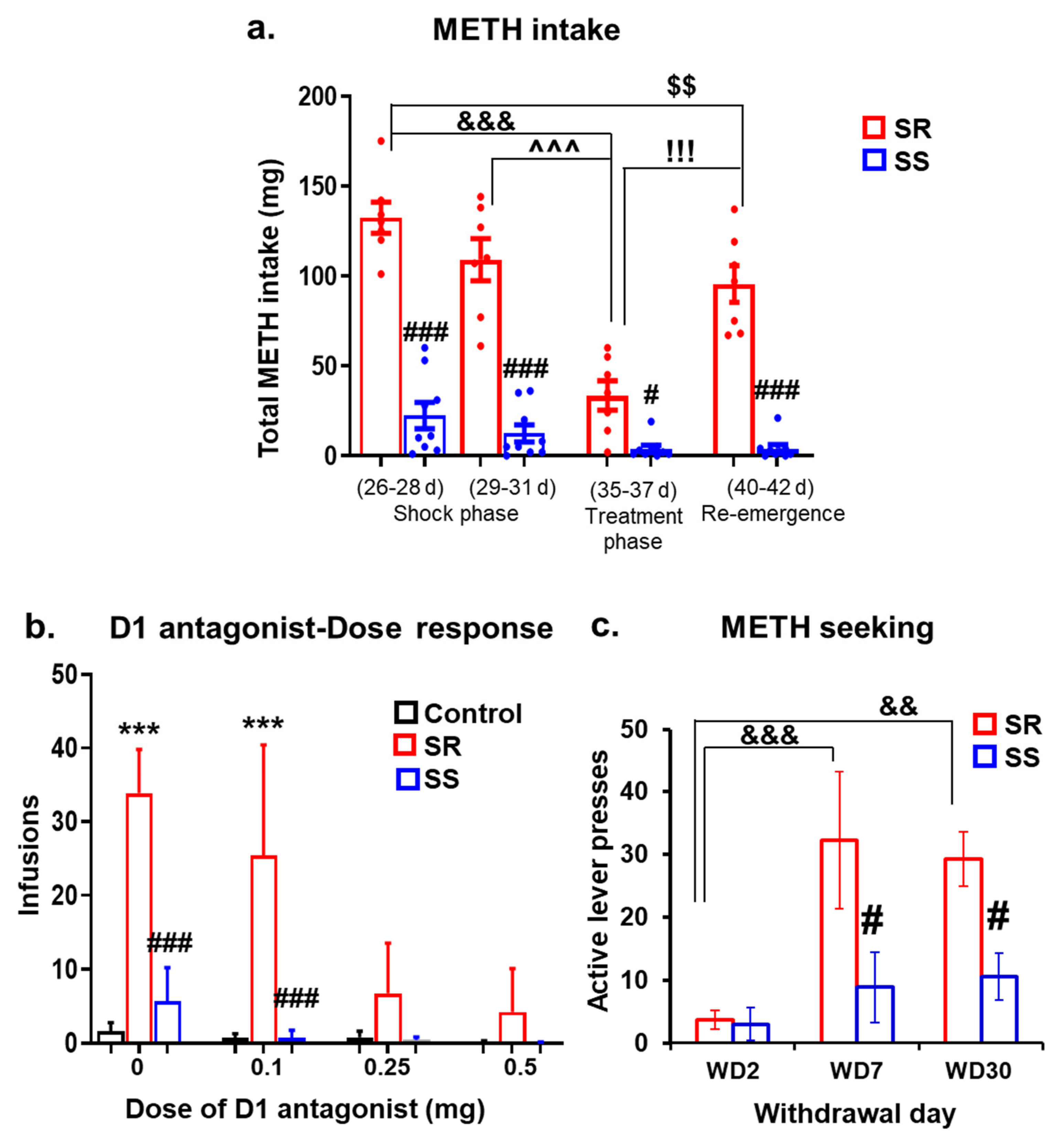
2.1. The DA D1 Antagonist, SCH23390, Suppresses Compulsive METH Taking in the Presence of Contingent Footshock Punishment
2.2. Shock-Resistant Rats Exhibit Greater Incubation of METH Seeking Than Shock-Sensitive Rats after Prolonged METH Withdrawal
2.3. Withdrawal from METH SA Elicits Increased Dopamine Metabolism in the Dorsal Striatum of Shock-Sensitive Rats
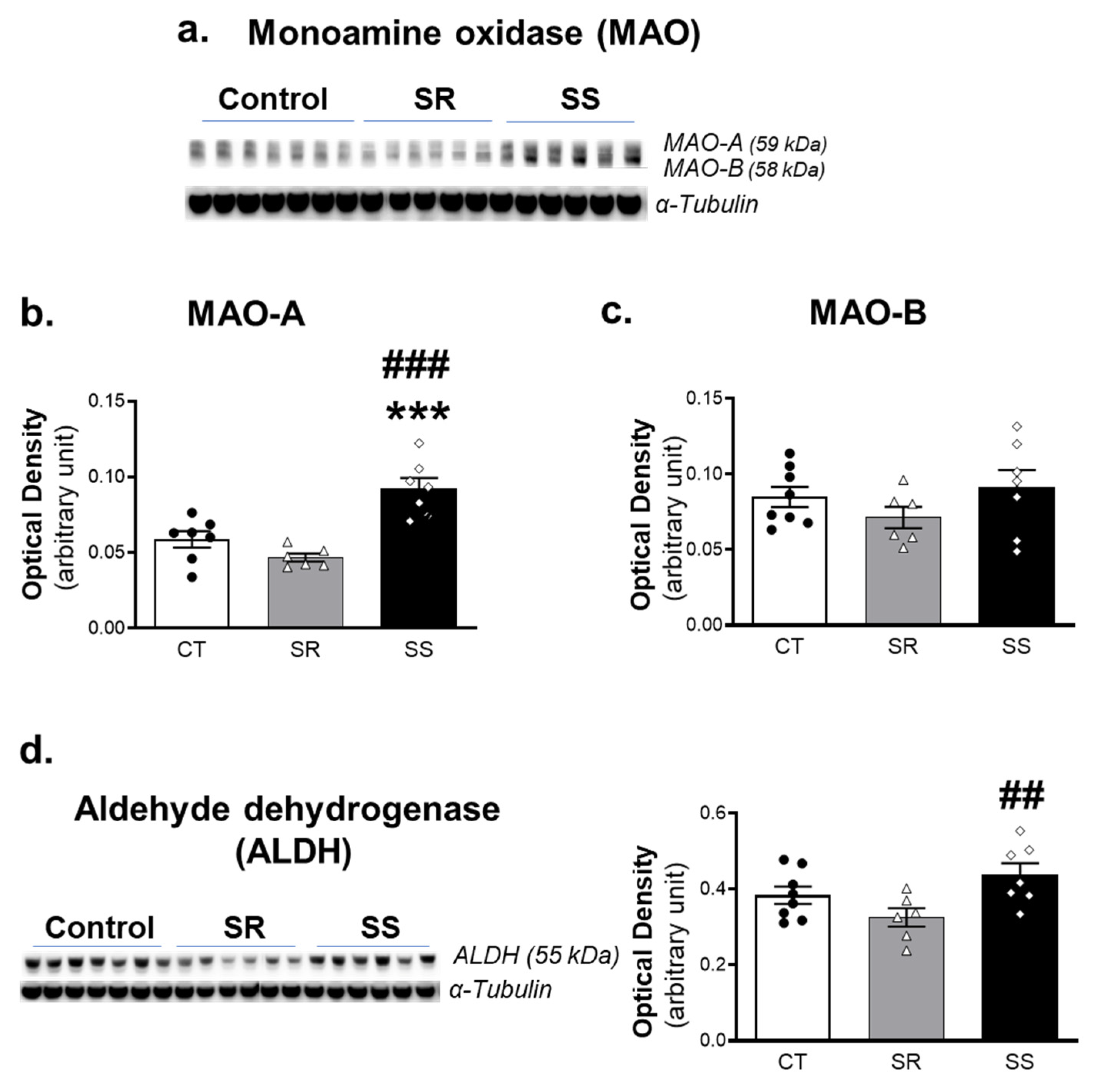
2.4. Shock-Sensitive Rats Have Higher MAO-A Protein Levels Than Shock-Resistant and Control Rats
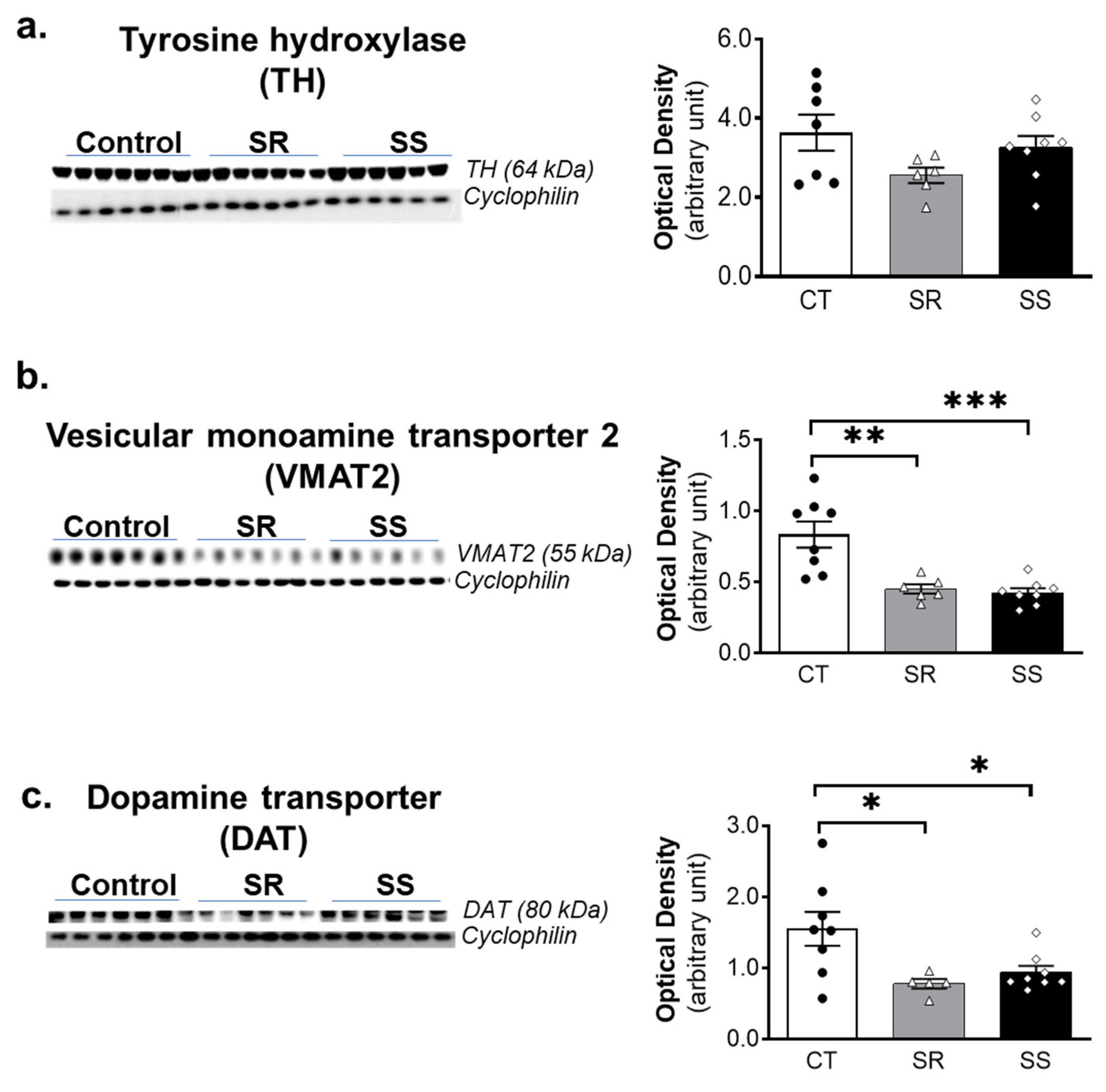
2.5. METH SA Caused Decreased VMAT2 and DAT Protein Levels in the Dorsal Striatum
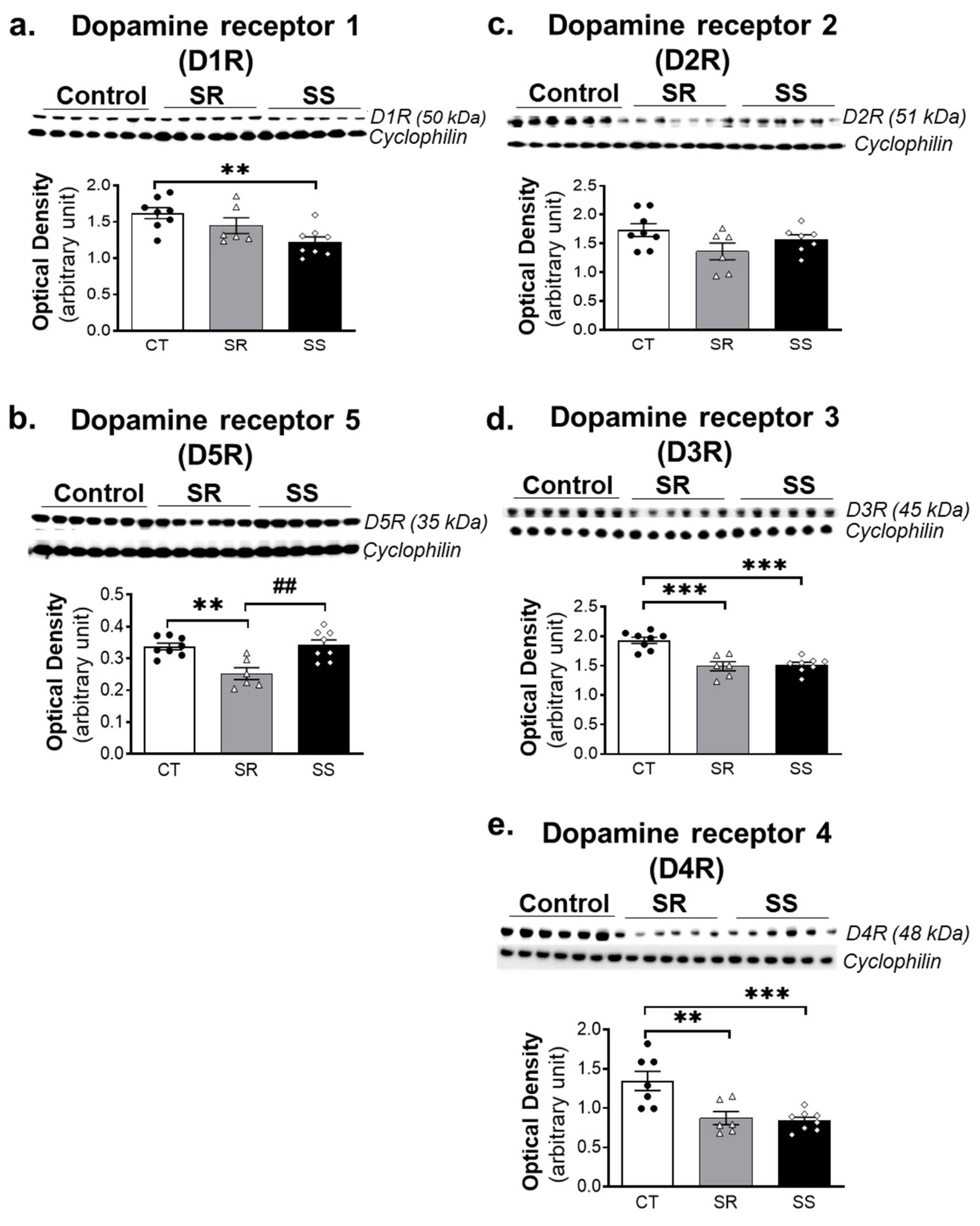
2.6. METH SA Caused Alterations in the Protein Expression of DA D1-like and D2-like Receptors in the Dorsal Striatum
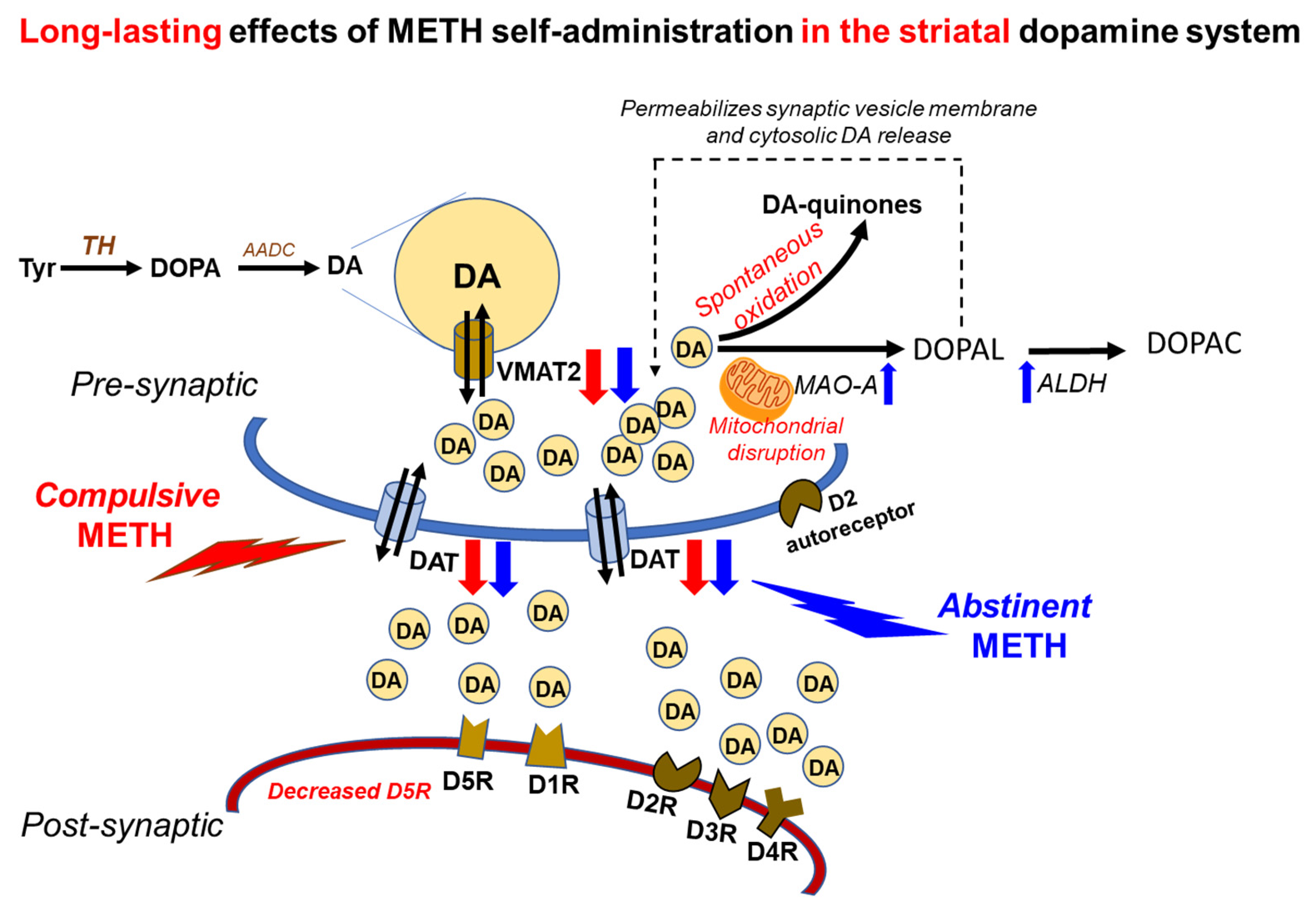
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Apparatus
4.4. Intravenous Catheter Implantation Surgery
4.5. METH Self-Administration and Effects of DA D1 Receptor Antagonist (SCH23390)
4.5.1. Self-Administration (SA) Training Phase (Days 1–20)
4.5.2. Footshock Phase (Days 21–31)
4.5.3. DA D1 Antagonist (SCH23390) Treatment Phase (Days 32–37)
4.5.4. Resurgence Phase (Training Days 38–42)
4.6. Withdrawal
4.7. Measurement of Dopamine and Metabolites
4.8. Western Blot
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chomchai, C.; Chomchai, S. Global patterns of methamphetamine use. Curr. Opin. Psychiatry 2015, 28, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.S.; Kasper, Z.A.; Cicero, T.J. Twin epidemics: The surging rise of methamphetamine use in chronic opioid users. Drug Alcohol Depend. 2018, 193, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.C.; Havens, J.R.; Stoops, W.W. A nationally representative analysis of “twin epidemics”: Rising rates of methamphetamine use among persons who use opioids. Drug Alcohol Depend. 2019, 204, 107592. [Google Scholar] [CrossRef] [PubMed]
- The Lancet. Opioids and methamphetamine: A tale of two crises. Lancet 2018, 391, 713. [Google Scholar] [CrossRef]
- UNODC. World Drug Report 2020: Global Drug Use Rising; While COVID-19 Has Far Reaching Impact on Global Drug Markets; (Sales No. E.20.XI.6); United Nations Publication: New York, NY, USA, 2020. [Google Scholar]
- Hedegaard, H.; Minino, A.M.; Warner, M. Drug Overdose Deaths in the United States, 1999–2017. NCHS Data Brief 2018, 329, 1–8. [Google Scholar]
- Palamar, J.J.; Han, B.H.; Keyes, K.M. Trends in characteristics of individuals who use methamphetamine in the United States, 2015–2018. Drug Alcohol Depend. 2020, 213, 108089. [Google Scholar] [CrossRef]
- Mattson, C.L.; Tanz, L.J.; Quinn, K.; Kariisa, M.; Patel, P.; Davis, N.L. Trends and Geographic Patterns in Drug and Synthetic Opioid Overdose Deaths—United States, 2013–2019. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 202–207. [Google Scholar] [CrossRef]
- Brecht, M.L.; Herbeck, D. Time to relapse following treatment for methamphetamine use: A long-term perspective on patterns and predictors. Drug Alcohol Depend. 2014, 139, 18–25. [Google Scholar] [CrossRef]
- McKetin, R.; Kothe, A.; Baker, A.L.; Lee, N.K.; Ross, J.; Lubman, D.I. Predicting abstinence from methamphetamine use after residential rehabilitation: Findings from the Methamphetamine Treatment Evaluation Study. Drug Alcohol Rev. 2018, 37, 70–78. [Google Scholar] [CrossRef]
- Paulus, M.P.; Stewart, J.L. Neurobiology, Clinical Presentation, and Treatment of Methamphetamine Use Disorder: A Review. JAMA Psychiatry 2020, 77, 959–966. [Google Scholar] [CrossRef]
- Li, X.; Rubio, F.J.; Zeric, T.; Bossert, J.M.; Kambhampati, S.; Cates, H.M.; Kennedy, P.J.; Liu, Q.R.; Cimbro, R.; Hope, B.T.; et al. Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J. Neurosci. 2015, 35, 8232–8244. [Google Scholar] [CrossRef] [PubMed]
- Scheyer, A.F.; Loweth, J.A.; Christian, D.T.; Uejima, J.; Rabei, R.; Le, T.; Dolubizno, H.; Stefanik, M.T.; Murray, C.H.; Sakas, C.; et al. AMPA Receptor Plasticity in Accumbens Core Contributes to Incubation of Methamphetamine Craving. Biol. Psychiatry 2016, 80, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.D.; Anderson, S.M.; Famous, K.R.; Kumaresan, V.; Pierce, R.C. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur. J. Pharmacol. 2005, 526, 65–76. [Google Scholar] [PubMed]
- Cadet, J.L.; Patel, R.; Jayanthi, S. Compulsive methamphetamine taking and abstinence in the presence of adverse consequences: Epigenetic and transcriptional consequences in the rat brain. Pharmacol. Biochem. Behav. 2019, 179, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, S.; Peesapati, R.; McCoy, M.T.; Ladenheim, B.; Cadet, J.L. Footshock-Induced Abstinence from Compulsive Methamphetamine Self-administration in Rat Model Is Accompanied by Increased Hippocampal Expression of Cannabinoid Receptors (CB1 and CB2). Mol. Neurobiol. 2022, 59, 1238–1248. [Google Scholar] [CrossRef]
- Krasnova, I.N.; Gerra, M.C.; Walther, D.; Jayanthi, S.; Ladenheim, B.; McCoy, M.T.; Brannock, C.; Cadet, J.L. Compulsive methamphetamine taking in the presence of punishment is associated with increased oxytocin expression in the nucleus accumbens of rats. Sci. Rep. 2017, 7, 8331. [Google Scholar] [CrossRef]
- Subu, R.; Jayanthi, S.; Cadet, J.L. Compulsive methamphetamine taking induces autophagic and apoptotic markers in the rat dorsal striatum. Arch. Toxicol. 2020, 94, 3515–3526. [Google Scholar] [CrossRef]
- Torres, O.V.; Jayanthi, S.; Ladenheim, B.; McCoy, M.T.; Krasnova, I.N.; Cadet, J.L. Compulsive methamphetamine taking under punishment is associated with greater cue-induced drug seeking in rats. Behav. Brain Res. 2017, 326, 265–271. [Google Scholar] [CrossRef]
- Cadet, J.L.; Brannock, C.; Krasnova, I.N.; Jayanthi, S.; Ladenheim, B.; McCoy, M.T.; Walther, D.; Godino, A.; Pirooznia, M.; Lee, R.S. Genome-wide DNA hydroxymethylation identifies potassium channels in the nucleus accumbens as discriminators of methamphetamine addiction and abstinence. Mol. Psychiatry 2017, 22, 1196–1204. [Google Scholar] [CrossRef]
- Cadet, J.L.; Krasnova, I.N.; Walther, D.; Brannock, C.; Ladenheim, B.; McCoy, M.T.; Collector, D.; Torres, O.V.; Terry, N.; Jayanthi, S. Increased expression of proenkephalin and prodynorphin mRNAs in the nucleus accumbens of compulsive methamphetamine taking rats. Sci. Rep. 2016, 6, 37002. [Google Scholar] [CrossRef]
- Lee, B.; London, E.D.; Poldrack, R.A.; Farahi, J.; Nacca, A.; Monterosso, J.R.; Mumford, J.A.; Bokarius, A.V.; Dahlbom, M.; Mukherjee, J.; et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J. Neurosci. 2009, 29, 14734–14740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Chang, L.; Wang, G.J.; Fowler, J.S.; Ding, Y.S.; Sedler, M.; Logan, J.; Franceschi, D.; Gatley, J.; Hitzemann, R.; et al. Low level of brain dopamine D-2 receptors in methamphetamine abusers: Association with metabolism in the orbitofrontal cortex. Am. J. Psychiatry 2001, 158, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Chang, L.; Wang, G.J.; Fowler, J.S.; Franceschi, D.; Sedler, M.; Gatley, S.J.; Miller, E.; Hitzemann, R.; Ding, Y.S.; et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J. Neurosci. 2001, 21, 9414–9418. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Kalasinsky, K.S.; Levey, A.I.; Bergeron, C.; Reiber, G.; Anthony, R.M.; Schmunk, G.A.; Shannak, K.; Haycock, J.W.; Kish, S.J. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat. Med. 1996, 2, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.J.; Smith, L.; Volkow, N.D.; Telang, F.; Logan, J.; Tomasi, D.; Wong, C.T.; Hoffman, W.; Jayne, M.; Alia-Klein, N.; et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol. Psychiatry 2012, 17, 918–925. [Google Scholar] [CrossRef]
- Bucher, M.L.; Barrett, C.W.; Moon, C.J.; Mortimer, A.D.; Burton, E.A.; Greenamyre, J.T.; Hastings, T.G. Acquired dysregulation of dopamine homeostasis reproduces features of Parkinson’s disease. NPJ Parkinsons Dis. 2020, 6, 34. [Google Scholar] [CrossRef]
- Masato, A.; Plotegher, N.; Boassa, D.; Bubacco, L. Impaired dopamine metabolism in Parkinson’s disease pathogenesis. Mol. Neurodegener. 2019, 14, 35. [Google Scholar] [CrossRef]
- Callaghan, R.C.; Cunningham, J.K.; Sajeev, G.; Kish, S. Incidence of Parkinson’s disease among hospital patients with methamphetamine-use disorders. Mov. Disord. 2010, 25, 2333–2339. [Google Scholar] [CrossRef]
- Callaghan, R.C.; Cunningham, J.K.; Sykes, J.; Kish, S.J. Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2012, 120, 35–40. [Google Scholar] [CrossRef]
- D’Arcy, C.; Luevano, J.E.; Miranda-Arango, M.; Pipkin, J.A.; Jackson, J.A.; Castaneda, E.; Gosselink, K.L.; O’Dell, L.E. Extended access to methamphetamine self-administration up-regulates dopamine transporter levels 72 hours after withdrawal in rats. Behav. Brain Res. 2016, 296, 125–128. [Google Scholar] [CrossRef]
- Krasnova, I.N.; Justinova, Z.; Ladenheim, B.; Jayanthi, S.; McCoy, M.T.; Barnes, C.; Warner, J.E.; Goldberg, S.R.; Cadet, J.L. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS ONE 2010, 5, e8790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacan, G.; Hadamitzky, M.; Kuczenski, R.; Melega, W.P. Alterations in the striatal dopamine system during intravenous methamphetamine exposure: Effects of contingent and noncontingent administration. Synapse 2013, 67, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Schweppe, C.A.; Burzynski, C.; Jayanthi, S.; Ladenheim, B.; Cadet, J.L.; Gardner, E.L.; Xi, Z.X.; van Praag, H.; Newman, A.H.; Keck, T.M. Neurochemical and behavioral comparisons of contingent and non-contingent methamphetamine exposure following binge or yoked long-access self-administration paradigms. Psychopharmacology 2020, 237, 1989–2005. [Google Scholar] [CrossRef]
- Alleweireldt, A.T.; Weber, S.M.; Kirschner, K.F.; Bullock, B.L.; Neisewander, J.L. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology 2002, 159, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Crombag, H.S.; Grimm, J.W.; Shaham, Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology 2002, 27, 1006–1015. [Google Scholar] [CrossRef]
- Avchalumov, Y.; Trenet, W.; Pina-Crespo, J.; Mandyam, C. SCH23390 Reduces Methamphetamine Self-Administration and Prevents Methamphetamine-Induced Striatal LTD. Int. J. Mol. Sci. 2020, 21, 6491. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, C.J.; Gopalakrishnan, S.; Cogan, E.S.; Yager, L.M.; Meyer, P.J.; Lovic, V.; Saunders, B.T.; Parker, C.C.; Gonzales, N.M.; Aryee, E.; et al. Variation in the form of Pavlovian conditioned approach behavior among outbred male Sprague-Dawley rats from different vendors and colonies: Sign-tracking vs. goal-tracking. PLoS ONE 2013, 8, e75042. [Google Scholar] [CrossRef]
- Soares, E.; Pereira, F.C. Pharmacotherapeutic strategies for methamphetamine use disorder: Mind the subgroups. Expert Opin. Pharmacother. 2019, 20, 2273–2293. [Google Scholar] [CrossRef]
- Wachtel, S.R.; Abercrombie, E.D. L-3,4-dihydroxyphenylalanine-induced dopamine release in the striatum of intact and 6-hydroxydopamine-treated rats: Differential effects of monoamine oxidase A and B inhibitors. J. Neurochem. 1994, 63, 108–117. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Kopin, I.J.; Sharabi, Y. Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders. Pharmacol. Ther. 2014, 144, 268–282. [Google Scholar] [CrossRef]
- Jayanthi, S.; Daiwile, A.P.; Cadet, J.L. Neurotoxicity of methamphetamine: Main effects and mechanisms. Exp. Neurol. 2021, 344, 113795. [Google Scholar] [PubMed]
- Beaulieu, J.M.; Espinoza, S.; Gainetdinov, R.R. Dopamine receptors—IUPHAR Review 13. Br. J. Pharmacol. 2015, 172, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Centonze, D.; Grande, C.; Saulle, E.; Martin, A.B.; Gubellini, P.; Pavon, N.; Pisani, A.; Bernardi, G.; Moratalla, R.; Calabresi, P. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J. Neurosci. 2003, 23, 8506–8512. [Google Scholar] [CrossRef]
- Gagnon, D.; Petryszyn, S.; Sanchez, M.G.; Bories, C.; Beaulieu, J.M.; De Koninck, Y.; Parent, A.; Parent, M. Striatal Neurons Expressing D1 and D2 Receptors are Morphologically Distinct and Differently Affected by Dopamine Denervation in Mice. Sci. Rep. 2017, 7, 41432. [Google Scholar] [CrossRef] [PubMed]
- Mateu, C.; Rodriguez-Arias, M.; Gil-Miravet, I.; Benito, A.; Tomas, J.M.; Haro, G. The Association between a MAOB Variable Number Tandem Repeat Polymorphism and Cocaine and Opiate Addictions in Polyconsumers. Brain Sci. 2021, 11, 1265. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.C.; Lin, C.H.; Chang, Y.Y.; Lung, F.W. Association of VNTR polymorphisms in the MAOA promoter and DRD4 exon 3 with heroin dependence in male Chinese addicts. World J. Biol. Psychiatry 2010, 11, 409–416. [Google Scholar] [CrossRef] [PubMed]
- McFadden, L.M.; Hadlock, G.C.; Allen, S.C.; Vieira-Brock, P.L.; Stout, K.A.; Ellis, J.D.; Hoonakker, A.J.; Andrenyak, D.M.; Nielsen, S.M.; Wilkins, D.G.; et al. Methamphetamine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by a subsequent methamphetamine exposure. J. Pharmacol. Exp. Ther. 2012, 340, 295–303. [Google Scholar] [CrossRef] [PubMed]
- McFadden, L.M.; Stout, K.A.; Vieira-Brock, P.L.; Allen, S.C.; Nielsen, S.M.; Wilkins, D.G.; Hanson, G.R.; Fleckenstein, A.E. Methamphetamine self-administration acutely decreases monoaminergic transporter function. Synapse 2012, 66, 240–245. [Google Scholar] [CrossRef]
- Schwendt, M.; Rocha, A.; See, R.E.; Pacchioni, A.M.; McGinty, J.F.; Kalivas, P.W. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J. Pharmacol. Exp. Ther. 2009, 331, 555–562. [Google Scholar] [CrossRef]
- Chang, L.; Alicata, D.; Ernst, T.; Volkow, N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction 2007, 102.S1, 16–32. [Google Scholar] [CrossRef]
- Johanson, C.E.; Frey, K.A.; Lundahl, L.H.; Keenan, P.; Lockhart, N.; Roll, J.; Galloway, G.P.; Koeppe, R.A.; Kilbourn, M.R.; Robbins, T.; et al. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology 2006, 185, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, O.; Tokunaga, I.; Gotohda, T.; Kubo, S. Immunohistochemical investigation of dopaminergic terminal markers and caspase-3 activation in the striatum of human methamphetamine users. Int. J. Leg. Med. 2007, 121, 163–168. [Google Scholar] [CrossRef] [PubMed]
- German, C.L.; Baladi, M.G.; McFadden, L.M.; Hanson, G.R.; Fleckenstein, A.E. Regulation of the Dopamine and Vesicular Monoamine Transporters: Pharmacological Targets and Implications for Disease. Pharmacol. Rev. 2015, 67, 1005–1024. [Google Scholar] [CrossRef] [PubMed]
- Lohr, K.M.; Stout, K.A.; Dunn, A.R.; Wang, M.; Salahpour, A.; Guillot, T.S.; Miller, G.W. Increased Vesicular Monoamine Transporter 2 (VMAT2; Slc18a2) Protects against Methamphetamine Toxicity. ACS Chem. Neurosci. 2015, 6, 790–799. [Google Scholar] [CrossRef]
- Lohr, K.M.; Bernstein, A.I.; Stout, K.A.; Dunn, A.R.; Lazo, C.R.; Alter, S.P.; Wang, M.; Li, Y.; Fan, X.; Hess, E.J.; et al. Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 9977–9982. [Google Scholar] [CrossRef]
- Guillot, T.S.; Shepherd, K.R.; Richardson, J.R.; Wang, M.Z.; Li, Y.; Emson, P.C.; Miller, G.W. Reduced vesicular storage of dopamine exacerbates methamphetamine-induced neurodegeneration and astrogliosis. J. Neurochem. 2008, 106, 2205–2217. [Google Scholar] [CrossRef]
- Gurevich, E.V.; Gainetdinov, R.R.; Gurevich, V.V. G protein-coupled receptor kinases as regulators of dopamine receptor functions. Pharmacol. Res. 2016, 111, 1–16. [Google Scholar] [CrossRef]
- Ren, K.; Guo, B.; Dai, C.; Yao, H.; Sun, T.; Liu, X.; Bai, Z.; Wang, W.; Wu, S. Striatal Distribution and Cytoarchitecture of Dopamine Receptor Subtype 1 and 2: Evidence from Double-Labeling Transgenic Mice. Front. Neural Circuits 2017, 11, 57. [Google Scholar] [CrossRef]
- Xin, J.; Fan, T.; Guo, P.; Wang, J. Identification of functional divergence sites in dopamine receptors of vertebrates. Comput. Biol. Chem. 2019, 83, 107140. [Google Scholar] [CrossRef]
- Charuchinda, C.; Supavilai, P.; Karobath, M.; Palacios, J.M. Dopamine D2 receptors in the rat brain: Autoradiographic visualization using a high-affinity selective agonist ligand. J. Neurosci. 1987, 7, 1352–1360. [Google Scholar] [CrossRef]
- Savasta, M.; Dubois, A.; Scatton, B. Autoradiographic localization of D1 dopamine receptors in the rat brain with [3H] SCH 23390. Brain Res. 1986, 375, 291–301. [Google Scholar] [CrossRef]
- Ledonne, A.; Mercuri, N.B. Current Concepts on the Physiopathological Relevance of Dopaminergic Receptors. Front. Cell. Neurosci. 2017, 11, 27. [Google Scholar] [CrossRef]
- Sarinana, J.; Kitamura, T.; Kunzler, P.; Sultzman, L.; Tonegawa, S. Differential roles of the dopamine 1-class receptors, D1R and D5R, in hippocampal dependent memory. Proc. Natl. Acad. Sci. USA 2014, 111, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, R.M.; Hefner, K.R.; Sibley, D.R.; Holmes, A. Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology 2008, 200, 117–127. [Google Scholar] [CrossRef]
- Hayashizaki, S.; Hirai, S.; Ito, Y.; Honda, Y.; Arime, Y.; Sora, I.; Okado, H.; Kodama, T.; Takada, M. Methamphetamine increases locomotion and dopamine transporter activity in dopamine d5 receptor-deficient mice. PLoS ONE 2013, 8, e75975. [Google Scholar] [CrossRef] [PubMed]
- Kish, S.J.; Boileau, I.; Callaghan, R.C.; Tong, J. Brain dopamine neurone ‘damage’: Methamphetamine users vs. Parkinson’s disease—a critical assessment of the evidence. Eur. J. Neurosci. 2017, 45, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.; Przedborski, S.; Cadet, J.L. Retrograde degeneration of nigrostriatal neurons induced by intrastriatal 6-hydroxydopamine injection in rats. Brain Res. Bull. 1991, 26, 301–307. [Google Scholar] [CrossRef]
- Cadet, J.L.; Brannock, C. Free radicals and the pathobiology of brain dopamine systems. Neurochem. Int. 1998, 32, 117–131. [Google Scholar] [CrossRef]
- Curtin, K.; Fleckenstein, A.E.; Robison, R.J.; Crookston, M.J.; Smith, K.R.; Hanson, G.R. Methamphetamine/amphetamine abuse and risk of Parkinson’s disease in Utah: A population-based assessment. Drug Alcohol Depend. 2015, 146, 30–38. [Google Scholar] [CrossRef]
- Daiwile, A.P.; Jayanthi, S.; Ladenheim, B.; McCoy, M.T.; Brannock, C.; Schroeder, J.; Cadet, J.L. Sex Differences in Escalated Methamphetamine Self-Administration and Altered Gene Expression Associated with Incubation of Methamphetamine Seeking. Int. J. Neuropsychopharmacol. 2019, 22, 710–723. [Google Scholar] [CrossRef]
- Casida, J.E.; Ford, B.; Jinsmaa, Y.; Sullivan, P.; Cooney, A.; Goldstein, D.S. Benomyl, aldehyde dehydrogenase, DOPAL, and the catecholaldehyde hypothesis for the pathogenesis of Parkinson’s disease. Chem. Res. Toxicol. 2014, 27, 1359–1361. [Google Scholar] [CrossRef] [PubMed]
- Landrock, K.K.; Sullivan, P.; Martini-Stoica, H.; Goldstein, D.S.; Graham, B.H.; Yamamoto, S.; Bellen, H.J.; Gibbs, R.A.; Chen, R.; D’Amelio, M.; et al. Pleiotropic neuropathological and biochemical alterations associated with Myo5a mutation in a rat Model. Brain Res. 2018, 1679, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Wey, M.C.; Fernandez, E.; Martinez, P.A.; Sullivan, P.; Goldstein, D.S.; Strong, R. Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: Implications for Parkinson’s disease. PLoS ONE 2012, 7, e31522. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayanthi, S.; Ladenheim, B.; Sullivan, P.; McCoy, M.T.; Krasnova, I.N.; Goldstein, D.S.; Cadet, J.L. Biochemical Neuroadaptations in the Rat Striatal Dopaminergic System after Prolonged Exposure to Methamphetamine Self-Administration. Int. J. Mol. Sci. 2022, 23, 10092. https://doi.org/10.3390/ijms231710092
Jayanthi S, Ladenheim B, Sullivan P, McCoy MT, Krasnova IN, Goldstein DS, Cadet JL. Biochemical Neuroadaptations in the Rat Striatal Dopaminergic System after Prolonged Exposure to Methamphetamine Self-Administration. International Journal of Molecular Sciences. 2022; 23(17):10092. https://doi.org/10.3390/ijms231710092
Chicago/Turabian StyleJayanthi, Subramaniam, Bruce Ladenheim, Patricia Sullivan, Michael T. McCoy, Irina N. Krasnova, David S. Goldstein, and Jean Lud Cadet. 2022. "Biochemical Neuroadaptations in the Rat Striatal Dopaminergic System after Prolonged Exposure to Methamphetamine Self-Administration" International Journal of Molecular Sciences 23, no. 17: 10092. https://doi.org/10.3390/ijms231710092
APA StyleJayanthi, S., Ladenheim, B., Sullivan, P., McCoy, M. T., Krasnova, I. N., Goldstein, D. S., & Cadet, J. L. (2022). Biochemical Neuroadaptations in the Rat Striatal Dopaminergic System after Prolonged Exposure to Methamphetamine Self-Administration. International Journal of Molecular Sciences, 23(17), 10092. https://doi.org/10.3390/ijms231710092






