EVOO’s Effects on Incretin Production: Is There a Rationale for a Combination in T2DM Therapy?
Abstract
:1. Introduction
2. Glucagon-like Peptide-1 Receptor Agonists (GLP-1RAs)
GLP-1 RA Effects on Atherogenesis and Oxidative Stress
3. Extra Virgin Olive Oil (EVOO)
3.1. EVOO and Inflammatory Markers
3.2. EVOO and Bioactive Compounds
3.3. EVOO and Atherosclerosis
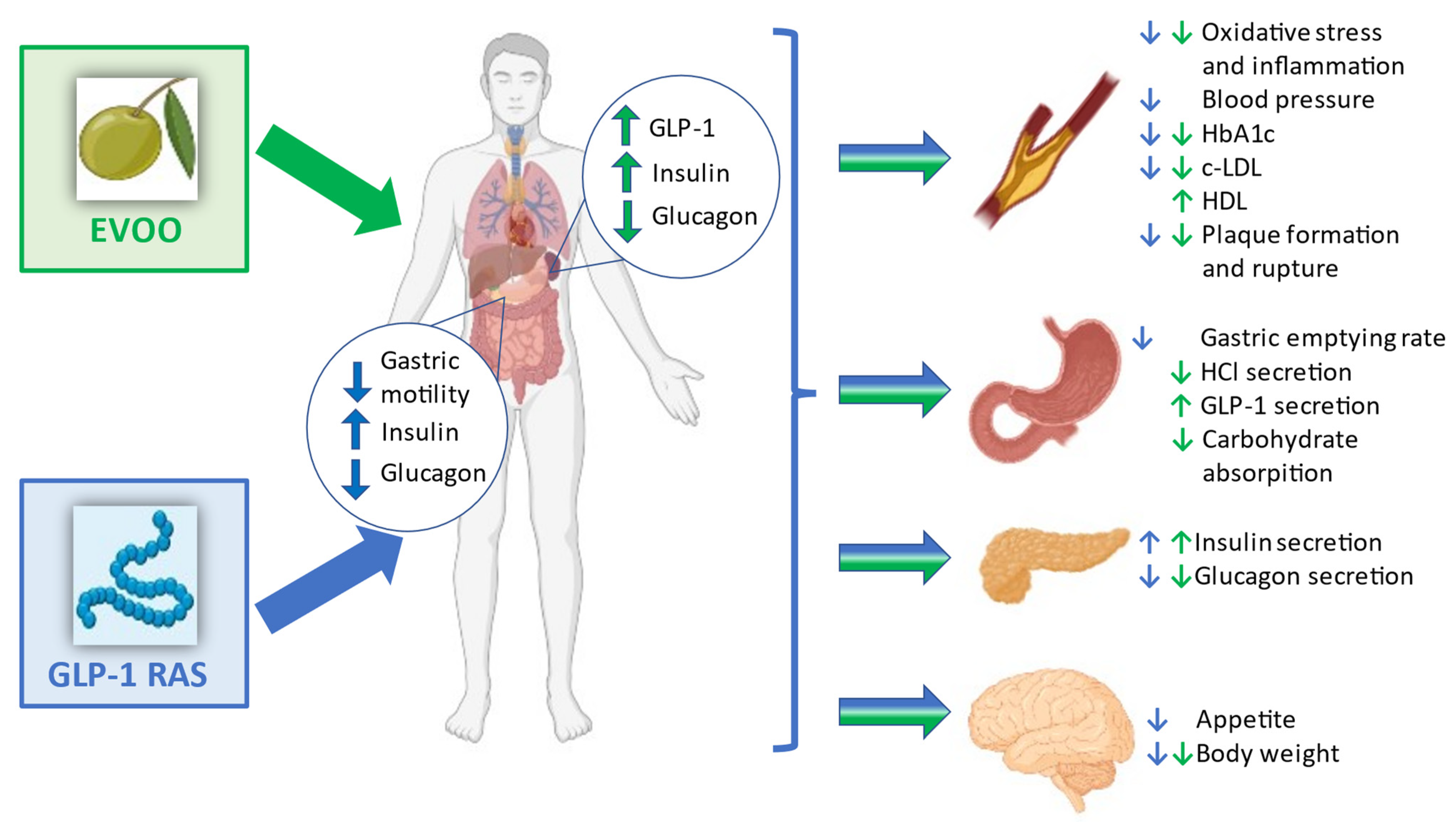
4. EVOO, GLP-1 and Glycaemic Control Improvement: Possible Underlying Mechanisms
| Author/Year | Design of the Study | Drug | Outcome(s) | Conclusion |
|---|---|---|---|---|
| Fuentes et al. [71], 2001 | Intervention dietary study Patients n°: 22 | Low-saturated fat diet vs. MedDiet | Determine endothelial function in hyper-cholesterolemic patients in the two different diets | Flow-mediated dilatation increased during the MedDiet levels of plasma cholesterol, LDL, ApoB and P-selectin decreased with both diets |
| Esposito et al. [53], 2004 | Randomized, single-blind trial Patients n°: 180 with metabolic syndrome (MetS) | MedDiet vs. Control diet | Nutrient intake endothelial function score lipid and glucose parameters, insulin sensitivity and circulating levels of hs-CRP and interleukins 6 (IL-6), 7 (IL-7) and 18 (IL-18). | Body weight and hs-CRP decreased Endothelial function score improved The number of patients with metabolic syndrome decreased |
| Mitjavila et al. [83], 2012 | Randomized, controlled, trial Patients n°: 110 with MetS | MedDiet + EVOO 1Lt/week or MedDiet + mixed nuts vs. Low-fat diet | Test the efficacy of MedDiet on the primary prevention of cardiovascular diseases | MedDiet reduces oxidative damage to lipids and DNA in MetS individuals |
| Loued et al. [57], 2013 | Interventional study Patients n°: 20 healthy subjects (divided in two groups: 10 young and 10 elderly) | EVOO 25 mL/day administered to the two groups | Investigate the effect of ageing and the role of PON1 on the anti-inflammatory activity of HDL Determine whether EVOO consumption could improve the atheroprotective activity of HDL | EVOO consumption increased the anti-inflammatory activities of both HDL and PON1 The anti-inflammatory activity of HDL was modulated by PON1 and was lower in the elderly volunteers EVOO consumption increased the anti-inflammatory effect of HDL and reduced the age-related decrease in anti-atherogenic activity |
| Carnevale et al. [82], 2014 | Randomized controlled trial Patients n°: 25 healthy subjects | MedDiet with EVOO 10 gr/day vs. MedDiet without EVOO | Investigate the role of EVOO in the atherosclerotic process | Addition of EVOO to a MedDiet protects against postprandial oxidative stress |
| Salas-Salvadó et al. [54], 2014 | Randomized controlled trial Patients n°: 3541 without diabetes at high cardiovascular risk | MedDiet supplemented with EVOO vs. MedDiet supplemented with nuts vs. Control diet (advice on a low-fat diet) | Assess the efficacy of MedDiet for the primary prevention of diabetes | MedDiet supplemented with EVOO reduced diabetes risk among persons with high cardiovascular risk |
| Violi et al. [84], 2015 | Interventional cross-over study Patients n°: 25 healthy subjects | MedDiet with EVOO 10 gr/day vs. MedDiet without EVOO | Find the mechanisms that make EVOO effective in the prevention of cardiovascular disease | Decrease in blood glucose, DPP-4 protein and activity, LDL-C, oxLDL Increase in insulin, GLP-1, GIP |
| Santangelo et al. [55], 2016 | Interventional study Patients n°: 11 with T2DM, overweight but non-insulin treated | Abutal diet supplemented with EVOO 25 mL/day | Improvement in anthropometric parameters, fasting glycaemia, HbA1c, high-sensitive CRP, plasma lipid profile, liver function and serum levels of TNF-α, IL-6, adiponectin, visfatin. | EVOO significantly reduced fasting plasma glucose, HbA1c, BMI, and body weight, serum levels of AST and ALT and serum visfatin levels |
| Carnevale et al. [87], 2017 | Interventional cross-over study Patients n°: 30 with IFG | Meal with 10 gr of EVOO vs. Meal without EVOO | Improvement in postprandial glycaemia | EVOO reduces glycemia and DPP-4 activity Increases insulin and GLP-1 and decreases triglycerides and Apo B |
| Marrano et al. [68], 2021 | In vitro study | INS-1E cells were exposed to 10 μM of the main EVOO PCs for up to 24 h | To investigate the effects of several phenolic compounds (PCs) on beta-cell function and survival | EVOO may improve insulin secretion and promote glycaemic control in T2DM patients |
| Bartimoccia et al. [93], 2022 | Interventional study Patients n°: 20 with IFG and 20 healthy subjects | Mediterranean-type meal with 10 gr of EVOO vs. Mediterranean-type meal without EVOO | Improvement in postprandial glycaemia by reducing gut permeability-derived low-grade endotoxemia | IFG patients assuming EVOO showed a less significant increase in blood glucose, blood insulin and GLP1 and a significant reduction in LPS and zonulin compared to IFG patients not given EVOO |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 1 July 2022).
- Marrano, N.; Biondi, G.; Cignarelli, A.; Perrini, S.; Laviola, L.; Giorgino, F.; Natalicchio, A. Functional loss of pancreatic islets in type 2 diabetes: How can we halt it? Metabolism 2020, 110, 154304. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.D.; Langenberg, C.; Rapsomaniki, E.; Denaxas, S.; Pujades-Rodriguez, M.; Gale, C.P.; Deanfield, J.; Smeeth, L.; Timmis, A.; Hemingway, H. Type 2 diabetes and incidence of cardiovascular diseases: A cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015, 3, 105–113. [Google Scholar] [PubMed]
- Gregg, E.W.; Li, Y.; Wang, J.; Rios Burrows, N.; Ali, M.K.; Rolka, D.; Williams, D.E.; Geiss, L. Changes in diabetes-related complications in the United States, 1990–2010. N. Engl. J. Med. 2014, 370, 1514–1523. [Google Scholar] [PubMed]
- Rawshani, A.; Rawshani, A.; Franzén, S.; Eliasson, B.; Svensson, A.-M.; Miftaraj, M.; McGuire, D.K.; Sattar, N.; Rosengren, A.; Gudbjörnsdottir, S. Mortality and cardiovascular disease in Type 1 and Type 2 diabetes. N. Engl. J. Med. 2017, 376, 1407–1418. [Google Scholar]
- Muilwijk, M.; Ho, F.; Waddell, H.; Sillars, A.; Welsh, P.; Iliodromiti, S.; Brown, R.; Ferguson, L.; Stronks, K.; van Valkengoed, I.; et al. Contribution of type 2 diabetes to all-cause mortality, cardiovascular disease incidence and cancer incidence in white Europeans and South Asians: Findings from the UK Biobank population-based cohort study. BMJ Open Diabetes Res. Care 2019, 7, e000765. [Google Scholar]
- Bengaluru Jayanna, M.; Robinson, J.G. The extent to which statins have improved cardiovascular outcomes: Lessons from randomized trials and observational studies of “real world” practice in people with diabetes. Diabetes Obes. Metab. 2019, 21, 17–27. [Google Scholar]
- Steg, P.G.; Bhatt, D.L.; Simon, T.; Fox, K.; Mehta, S.R.; Harrington, R.A.; Held, C.; Andersson, M.; Himmelmann, A.; Ridderstråle, W.; et al. Ticagrelor in patients with stable coronary disease and diabetes. N. Engl. J. Med. 2019, 381, 1309–1320. [Google Scholar]
- Bhatt, D.L.; Eikelboom, J.W.; Connolly, S.J.; Steg, P.G.; Anand, S.S.; Verma, S.; Branch, K.; Probstfield, J.; Bosch, J.; Shestakovska, O.; et al. The Role of Combination Antiplatelet and Anticoagulation Therapy in Diabetes and Cardiovascular Disease: Insights from the COMPASS Trial. Circulation 2020. [Google Scholar] [CrossRef]
- Thrasher, J. Pharmacologic management of type 2 diabetes mellitus: Available therapies. Am. J. Med. 2017, 130, S4–S17. [Google Scholar] [CrossRef]
- Karagiannis, T.; Liakos, A.; Bekiari, E.; Athanasiadou, E.; Paschos, P.; Vasilakou, D.; Mainou, M.; Rika, M.; Boura, P.; Matthews, D.R.; et al. Efficacy and safetyof once-weekly glucagon-like peptide 1 receptor agonists for themanagement of type 2 diabetes: A systematic review and metaanalysis of randomized controlled trials. Diabetes Obes. Metab. 2015, 17, 1065–1074. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, A Consensus Report di l’American Diabetes Association (ADA) e l’European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Missbach, B.; Konig, J.; Hoffmann, G. Adherence to a Mediterranean diet and risk of diabetes: A systematic review and meta-analysis. Public Health Nutr. 2015, 18, 1292–1299. [Google Scholar] [PubMed]
- Huo, R.; Du, T.; Xu, Y.; Xu, W.; Chen, X.; Sun, K.; Yu, X. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: A meta-analysis. Eur. J. Clin. Nutr. 2015, 69, 1200–1208. [Google Scholar] [PubMed]
- Lopez-Miranda, J.; Perez-Jimenez, F.; Ros, E.; De Caterina, R.; Badimon, L.; Covas, M.I.; Escrich, E.; Ordovás, J.; Soriguer, F.; Abiá, R.; et al. Olive oil and health: Summary of the II International Conference on Olive Oil and Health Consensus Report, Jaen and Cordoba (Spain) 2008. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 284–294. [Google Scholar] [PubMed]
- Qian, F.; Korat, A.A.; Malik, V.; Hu, F.B. Metabolic effects of monounsaturated fatty acid–enriched diets compared with carbohydrate or polyunsaturated fatty acid–enriched diets in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2016, 39, 1448–1457. [Google Scholar] [PubMed]
- Schwingshackl, L.; Strasser, B. High-MUFA diets reduce fasting glucose in patients with type 2 diabetes. Ann. Nutr. Metab. 2012, 60, 33–34. [Google Scholar] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar]
- Zappas, M.P.; Gentes, M.; Walton-Moss, B. Use of Incretin Therapy in the Treatment of Type 2 Diabetes Mellitus. J. Nurse Pract. 2017, 13, 418–424. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes e state-of-the-art. Mol. Metab. 2020, 46, 101102. [Google Scholar]
- Suzuki, S.; Kawai, K.; Ohashi, S.; Mukai, H.; Murayama, Y.; Yamashita, K. Reduced insulinotropic effects of glucagonlike peptide 1-(7-36)-amide and gastric inhibitory polypeptide in isolated perfused diabetic rat pancreas. Diabetes 1990, 39, 1320–1325. [Google Scholar]
- Deacon, C.F.; Pridal, L.; Klarskov, L.; Olesen, M.; Holst, J.J. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am. J. Physiol. 1996, 271, E458–E464. [Google Scholar]
- Kendall, D.M.; Cuddihy, R.M.; Bergenstal, R.M. Clinical Application of Incretin-Based Therapy: Therapeutic Potential, Patient Selection and Clinical Use. Am. J. Med. 2009, 122, S37–S50. [Google Scholar] [CrossRef] [PubMed]
- Tasyurek, H.M.; Altunbas, H.A.; Balci, M.K.; Sanlioglu, S. Incretins: Their Physiology and Application in the Treatment of Diabetes Mellitus. Diabetes Metab. Res. Rev. 2014, 30, 354–371. [Google Scholar] [CrossRef]
- Nauck, M.A.; Heimesaat, M.M.; Ørskov, C.; Holst, J.J.; Ebert, R.; Creutzfeldt, W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Investig. 1993, 91, 301–307. [Google Scholar] [PubMed]
- Nauck, M.A.; Kleine, N.; Ørskov, C.; Holst, J.J.; Willms, B.; Creutzfeldt, W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993, 36, 741–744. [Google Scholar] [PubMed]
- Flint, A.; Raben, A.; Astrup, A.; Holst, J.J. Glucagon-like peptide-1 promotes satiety and suppresses energy intake in humans. J. Clin. Investig. 1998, 101, 515–520. [Google Scholar]
- Eng, J.; Kleinman, W.A.; Singh, L.; Singh, G.; Raufman, J.P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from Guinea pig pancreas. J. Biol. Chem. 1992, 267, 7402–7405. [Google Scholar]
- Göke, R.; Fehmann, H.C.; Linn, T.; Schmidt, H.; Krause, M.; Eng, J.; Göke, B. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J. Biol. Chem. 1993, 268, 19650–19655. [Google Scholar]
- Melo, M.; Gavina, C.; Silva-Nunes, J.; Andrade, L.; Carvalho, D. Heterogeneity amongst GLP-1 RA cardiovascular outcome trials results: Can definition of established cardiovascular disease be the missing link? Diabetol. Metab. Syndr. 2021, 13, 81. [Google Scholar] [CrossRef]
- Trujillo, J.M.; Nuffer, W.; Smith, B.A. GLP-1 receptor agonists: An updated review of head-to-head clinical studies. Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018821997320. [Google Scholar] [CrossRef]
- Dorecka, M.; Siemianowicz, K.; Francuz, T.; Garczorz, W.; Chyra, A.; Klych, A.; Romaniuk, W. Exendin-4 and GLP-1 decreases induced expression of ICAM-1, VCAM-1 and RAGE in human retinal pigment epithelial cells. Pharmacol. Rep. 2013, 65, 884–890. [Google Scholar] [CrossRef]
- Wei, R.; Ma, S.; Wang, C.; Ke, K.; Yang, J.; Li, W.; Liu, Y.; Hou, W.; Feng, X.; Wang, G.; et al. Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/ eNOS pathway in a GLP-1 receptor-dependent manner. Am. J. Physiol. 2016, 310, E947–E957. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Zhu, F.; Zheng, H.; Zhou, Z.; Miao, P.; Zhao, L.; Mao, Z. Glucagon-like peptide-1 receptor agonist dulaglutide prevents ox-LDL- induced adhesion of monocytes to human endothelial cells: An implication in the treatment of atherosclerosis. Mol. Immunol. 2019, 116, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Mehta, J.L.; Chen, M. Glucagon-like peptide-1 receptor agonist liraglutide inhibits endothelin-1 in endothelial cell by repressing nuclear Factor-kappa B activation. Cardiovasc. Drugs Ther. 2013, 27, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, M.; Mita, T.; Azuma, K.; Ebato, C.; Goto, H.; Nomiyama, T.; Fujitani, Y.; Hirose, T.; Kawamori, R.; Watada, H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin- 4. Diabetes 2010, 59, 1030–1037. [Google Scholar] [CrossRef]
- Zhan, Y.; Sun, H.L.; Chen, H.; Zhang, H.; Sun, J.; Zhang, Z.; Cai, D.H. Glucagon-like peptide-1 (GLP-1) protects vascular endothelial cells against advanced glycation end products (AGEs)-induced apoptosis. Med. Sci. Monit. 2012, 18, BR286-91. [Google Scholar] [CrossRef]
- Lambadiari, V.; Thymis, J.; Kouretas, D.; Skaperda, Z.; Tekos, F.; Kousathana, F.; Kountouri, A.; Balampanis, K.; Parissis, J.; Andreadou, I.; et al. Effects of a 12-Month Treatment with Glucagon-like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Oxidant and Antioxidant Biomarkers in Patients with Type 2 Diabetes. Antioxidants 2021, 10, 1379. [Google Scholar] [CrossRef]
- Hirata, Y.; Kurobe, H.; Nishio, C.; Tanaka, K.; Fukuda, D.; Uematsu, E.; Nishimoto, S.; Soeki, T.; Harada, N.; Sakaue, H.; et al. Exendin-4, a glucagon-like peptide-1 receptor agonist, attenuates neointimal hyperplasia after vascular injury. Eur. J. Pharmacol. 2013, 699, 106–111. [Google Scholar] [CrossRef]
- Jojima, T.; Uchida, K.; Akimoto, K.; Tomotsune, T.; Yanagi, K.; Iijima, T.; Suzuki, K.; Kasai, K.; Aso, Y. Liraglutide, a GLP-1 receptor agonist, inhibits vascular smooth muscle cell proliferation by enhancing AMP-activated protein kinase and cell cycle regulation, and delays atherosclerosis in ApoE deficient mice. Atherosclerosis 2017, 261, 44–51. [Google Scholar] [CrossRef]
- Tang, S.T.; Tang, H.Q.; Su, H.; Wang, Y.; Zhou, Q.; Zhang, Q.; Wang, Y.; Zhu, H.-Q. Glucagon-like peptide-1 attenuates endothelial barrier injury in diabetes via cAMP/PKA mediated down-regulation of MLC phosphorylation. Biomed. Pharmacother. 2019, 113, 108667. [Google Scholar] [CrossRef]
- Krasner, N.M.; Ido, Y.; Ruderman, N.B.; Cacicedo, J.M. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS ONE 2014, 9, era97554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regulation (EU) No 1308/2013. 2014, 189. Available online: http://eur-lex.europa.eu/legal-content/IT/TXT/?uri=celex:32013R1308 (accessed on 20 October 2017).
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. The Mediterranean diet: Science and practice. Public Health Nutr. 2006, 9, 105–110. [Google Scholar] [CrossRef]
- Turner, R.; Etienne, N.; Alonso, M.G.; de Pascual-Teresa, S.; Minihane, A.M.; Weinberg, P.D.; Rimbach, G. Antioxidant and anti-atherogenic activities of olive oil phenolics. Int. J. Vitam. Nutr. Res. 2005, 75, 61–70. [Google Scholar] [CrossRef]
- Lucas, L.; Russell, A.; Keast, R. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2019, 72 (Suppl. 1), 8–17. [Google Scholar] [CrossRef]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas-Salvadó, J.; Bulló, M.; Estruch, R.; Ros, E.; Covas, M.-I.; Ibarrola-Jurado, N.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; et al. La prevenzione del diabete con le diete mediterranee. Anna. Stagista. Med. 2014, 160, 1–10. [Google Scholar] [CrossRef]
- Santangelo, C.; Filesi, C.; Varì, R.; Scazzocchio, B.; Filardi, T.; Fogliano, V.; D’Archivio, M.; Giovannini, C.; Lenzi, A.; Morano, S.; et al. Consumption of extra-virgin olive oil rich in phenolic compounds improves metabolic control in patients with type 2 diabetes mellitus: A possible involvement of reduced levels of circulating visfatin. J. Endocrinol. Investig. 2016, 39, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Massaro, M.; Carluccio, M.A.; Pellegrino, M.; Wabitsch, M.; Calabriso, N.; Storelli, C.; de Caterina, R. Additive regulation of adiponectin expression by the mediterranean diet olive oil components oleic Acid and hydroxytyrosol in human adipocytes. PLoS ONE 2015, 10, e0128218. [Google Scholar] [CrossRef] [PubMed]
- Loued, S.; Berrougui, H.; Componova, P.; Ikhlef, S.; Helal, O.; Khalil, A. Extra-virgin olive oil consumption reduces the age-related decrease in HDL and paraoxonase 1 anti-inflammatory activities. Br. J. Nutr. 2013, 110, 1272–1284. [Google Scholar] [CrossRef]
- Llorente-Cortés, V.; Estruch, R.; Mena, M.P.; Ros, E.; Martínez González, M.A.; Fitó, M.; Lamuela-Raventós, R.M.; Badimon, L. Effect of Mediterranean diet on the expression of pro-atherogenic genes in a population at high cardiovascular risk. Atherosclerosis 2010, 208, 442–450. [Google Scholar] [CrossRef]
- Pedan, V.; Popp, M.; Rohn, S.; Nyfeler, M.; Bongartz, A. Characterization of Phenolic Compounds and Their Contribution to Sensory Properties of Olive Oil. Molecules 2019, 24, 2041. [Google Scholar] [CrossRef]
- Mirarchi, L.; Amodeo, S.; Citarrella, R.; Licata, A.; Soresi, M.; Giannitrapani, L. SGLT2 Inhibitors as the Most Promising Influencers on the Outcome of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 3668. [Google Scholar] [CrossRef]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; di Maio, I.; Selvaggini, R.; Taticchi, A. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants 2013, 3, 1–23. [Google Scholar] [CrossRef]
- Bermudez, B.; Lopez, S.; Ortega, A.; Varela, L.M.; Pacheco, Y.M.; Abia, R.; Muriana, J.G.M. Oleic acid in olive oil: From a metabolic framework toward a clinical perspective. Curr. Pharm. Des. 2011, 17, 831–843. [Google Scholar] [CrossRef]
- Galli, F.; Azzi, A.; Birringer, M.; Cook-Mills, J.M.; Eggersdorfer, M.; Frank, J.; Cruciani, G.; Lorkowski, S.; Özer, N.K. Vitamin E: Emerging aspects and new directions. Free Radic. Biol. Med. 2016, 102, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—A review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef] [PubMed]
- Tsimidou, M.Z. Virgin Olive Oil (VOO) and Other Olive Tree Products as Sources of a-Tocopherol. Updating and Perspective. In Tocopherol: Sources, Uses and Health Benefits; Catala, A., Ed.; Nova Science Publisher: New York, NY, USA, 2012; pp. 1–21. [Google Scholar]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed]
- European Union. Commission Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, L136, 1–40. [Google Scholar]
- Marrano, N.; Spagnuolo, R.; Biondi, G.; Cignarelli, A.; Perrini, S.; Vincenti, L.; Laviola, L.; Giorgino, F.; Natalicchio, A. Effects of Extra Virgin Olive Oil Polyphenols on Beta-Cell Function and Survival. Plants 2021, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, D.P.; Gotto, A.M. Biological Relevance of Inflammation and Oxidative Stress in the Pathogenesis of Arterial Diseases. Am. J. Pathol. 2013, 182, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Mengi, S.A.; Xu, Y.-J.; Arneja, A.S.; Dhalla, N.S. Pathogenesis of Atherosclerosis: A Multifactorial Process. Exp. Clin. Cardiol. 2002, 7, 40–53. [Google Scholar] [PubMed]
- Fuentes, F.; Lopez-Miranda, J.; Sánchez, E.; Sánchez, F.; Paez, J.; Paz-Rojas, E.; Marín, C.; Gómez, P.; Jimenez, F.F.; Ordovás, J.M.; et al. Mediterranean and low-fat diets improve endothelial function in hypercholesterolemic men. Ann. Intern. Med. 2001, 134, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Ramirez, R.; Delgado-Lista, J.; Yubero-Serrano, E.M.; Martínez, P.P.; Carracedo, J.; Garcia-Rios, A.; Rodriguez, F.; Mariscal, F.M.G.; Gomez, P.; et al. Mediterranean diet reduces endothelial damage and improves the regenerative capacity of endothelium. Am. J. Clin. Nutr. 2011, 93, 267–274. [Google Scholar] [CrossRef]
- Calabriso, N.; Massaro, M.; Scoditti, E.; D’Amore, S.; Gnoni, A.; Pellegrino, M.; Storelli, C.; De Caterina, R.; Palasciano, G.; Carluccio, M.A. Extra virgin olive oil rich in polyphenols modulates VEGF-induced angiogenic responses by preventing NADPH oxidase activity and expression. J. Nutr. Biochem. 2016, 28, 19–29. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.B.; Högger, P. Dietary polyphenols and type 2 diabetes: Current insights and future perspectives. Curr. Med. Chem. 2015, 22, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Soto-Alarcon, S.A.; Valenzuela, R.; Valenzuela, A.; Videla, L.A. Liver protective effects of extra virgin olive oil: Interaction between its chemical composition and the cell-signaling pathways involved in protection. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Cerezo, A.B.; de Pablos, R.M.; Krisa, S.; Richard, T.; García-Parrilla, M.C.; Troncoso, A.M. Phenolic compounds characteristic of the Mediterranean diet in mitigating microglia-mediated neuroinflammation. Front. Cell. Neurosci. 2018, 12, 373. [Google Scholar] [CrossRef]
- Singh, U.; Jialal, I. Oxidative Stress and Atherosclerosis. Pathophysiology 2006, 13, 129–142. [Google Scholar] [CrossRef]
- Carnevale, R.; Bartimoccia, S.; Nocella, C.; Di Santo, S.; Loffredo, L.; Illuminati, G.; Lombardi, E.; Boz, V.; Del Ben, M.; De Marco, L.; et al. LDL Oxidation by Platelets Propagates Platelet Activation via an Oxidative Stress-Mediated Mechanism. Atherosclerosis 2014, 237, 108–116. [Google Scholar] [CrossRef]
- Nocella, C.; Cammisotto, V.; Fianchini, L.; D’Amico, A.; Novo, M.; Castellani, V.; Stefanini, L.; Violi, F.; Carnevale, R. Extra Virgin Olive Oil and Cardiovascular Diseases: Benefits for Human Health. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 4–13. [Google Scholar] [CrossRef]
- Carnevale, R.; Pignatelli, P.; Nocella, C.; Loffredo, L.; Pastori, D.; Vicario, T.; Petruccioli, A.; Bartimoccia, S.; Violi, F. Extra Virgin Olive Oil Blunt Post-Prandial Oxidative Stress via NOX2 down- Regulation. Atherosclerosis 2014, 235, 649–658. [Google Scholar] [CrossRef]
- Mitjavila, M.T.; Fandos, M.; Salas-Salvadó, J.; Covas, M.-I.; Borrego, S.; Estruch, R.; Lamuela-Raventós, R.; Corella, D.; Martínez-Gonzalez, M.Á.; Sánchez, J.M.; et al. The mediterranean diet improves the systemic lipid and dna oxidative damage in metabolic syndrome individuals. A randomized, controlled, trial. Clin. Nutr. 2013, 32, 172–178. [Google Scholar] [CrossRef]
- Violi, F.; Loffredo, L.; Pignatelli, P.; Angelico, F.; Bartimoccia, S.; Nocella, C.; Cangemi, R.; Petruccioli, A.; Monticolo, R.; Pastori, D.; et al. Extra Virgin Olive Oil Use Is Associated with Improved Post-Prandial Blood Glucose and LDL Cholesterol in Healthy Subjects. Nutr. Diabetes 2015, 5, e172. [Google Scholar] [CrossRef] [PubMed]
- Reiner, Ž.; Catapano, A.L.; De Backer, G.; Graham, I.; Taskinen, M.R.; Wiklund, O.; Agewall, S.; Alegria, E.; Chapman, M.J.; Durrington, P.; et al. ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 2011, 32, 1769–1818. [Google Scholar] [PubMed] [Green Version]
- Covas, M.-I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.-J.F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. The Effect of Polyphenols in Olive Oil on Heart Disease Risk Factors: A Randomized Trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Loffredo, L.; Del Ben, M.; Angelico, F.; Nocella, C.; Petruccioli, A.; Bartimoccia, S.; Monticolo, R.; Cava, E.; Violi, F. Extra Virgin Olive Oil Improves Post-Prandial Glycemic and Lipid Profile in Patients with Impaired Fasting Glucose. Clin. Nutr. 2017, 36, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Serrano, S.; Ho-Plagaro, A.; Santiago-Fernandez, C.; Rodríguez-Díaz, C.; Martín-Reyes, F.; Valdes, S.; Moreno-Ruiz, F.J.; Lopez-Gómez, C.; García-Fuentes, E.; Rodríguez-Pacheco, F. An Isolated Dose of Extra-Virgin Olive Oil Produces a Better Postprandial Gut Hormone Response, Lipidic, and Anti-Inflammatory Profile that Sunflower Oil: Effect of Morbid Obesity. Mol. Nutr. Food Res. 2021, 65, 2100071. [Google Scholar] [CrossRef]
- Gomes, J.M.G.; Costa, J.A.; Alfenas, R.C.G. Metabolic endotoxemia and diabetes mellitus: A systematic review. Metabolism 2017, 68, 133–144. [Google Scholar] [CrossRef]
- Carnevale, R.; Sciarretta, S.; Valenti, V.; di Nonno, F.; Calvieri, C.; Nocella, C.; Frati, G.; Forte, M.; d’Amati, G.; Pignataro, M.G.; et al. Low-grade endotoxaemia enhances artery thrombus growth via Toll-like receptor 4: Implication for myocardial infarction. Eur. Heart J. 2020, 41, 3156–3165. [Google Scholar] [CrossRef]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Bartimoccia, S.; Cammisotto, V.; Nocella, C.; Del Ben, M.; D’Amico, A.; Castellani, V.; Baratta, F.; Pignatelli, P.; Loffredo, L.; Violi, F.; et al. Extra Virgin Olive Oil Reduces Gut Permeability and Metabolic Endotoxemia in Diabetic Patients. Nutrients 2022, 14, 2153. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenco-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Grob, K.; Lanfranchi, M.; Mariani, C. Evaluation of Olive Oils through the Fatty Alcohols, the Sterols and Their Esters by Coupled Lc-Gc. J. Am. Oil Chem. Soc. 1990, 67, 626–634. [Google Scholar] [CrossRef]
- Hodaifa, G.; Martinez Nieto, L.; Lozano, J.L.; Sanchez, S. Changes of the wax contents in mixtures of olive oils as determined by gas chromatography with a flame ionization detector. J. AOAC Int. 2012, 95, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Mariotti, R.; Stanzione, V.; Pandolfi, S.; Mastio, V.; Baldoni, L.; Cultrera, N.G.M. Evolution of Extra Virgin Olive Oil Quality under Different Storage Conditions. Foods 2021, 10, 1945. [Google Scholar] [CrossRef]
- Carr, R.D.; Larsen, M.O.; Winzell, M.S.; Jelic, K.; Lindgren, O.; Deacon, C.F.; Ahren, B. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E779–E784. [Google Scholar] [CrossRef]
- Feinle, C.; Chapman, I.M.; Wishart, J.; Horowitz, M. Plasma glucagon-like peptide-1 (GLP-1) responses to duodenal fat and glucose infusions in lean and obese men. Peptides 2002, 23, 1491–1495. [Google Scholar] [CrossRef]
- Mu, H.; Hoy, C.E. The digestion of dietary triacylglycerols. Prog. Lipid Res. 2004, 43, 105–133. [Google Scholar] [CrossRef]
- Pilichiewicz, A.; O’Donovan, D.; Feinle, C.; Lei, Y.; Wishart, J.M.; Bryant, L.; Meyer, J.H.; Horowitz, M.; Jones, K.L. Effect of lipase inhibition on gastric emptying of, and the glycemic and incretin responses to, an oil/aqueous drink in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2003, 88, 3829–3834. [Google Scholar] [CrossRef]
- Mandøe, M.J.; Hansen, K.B.; Hartmann, B.; Rehfeld, J.F.; Holst, J.J.; Hansen, H.S. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans. Am. J. Clin. Nutr. 2015, 102, 548–555. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amodeo, S.; Mirarchi, L.; Seidita, A.; Citarrella, R.; Licata, A.; Soresi, M.; Iovanna, J.L.; Giannitrapani, L. EVOO’s Effects on Incretin Production: Is There a Rationale for a Combination in T2DM Therapy? Int. J. Mol. Sci. 2022, 23, 10120. https://doi.org/10.3390/ijms231710120
Amodeo S, Mirarchi L, Seidita A, Citarrella R, Licata A, Soresi M, Iovanna JL, Giannitrapani L. EVOO’s Effects on Incretin Production: Is There a Rationale for a Combination in T2DM Therapy? International Journal of Molecular Sciences. 2022; 23(17):10120. https://doi.org/10.3390/ijms231710120
Chicago/Turabian StyleAmodeo, Simona, Luigi Mirarchi, Aurelio Seidita, Roberto Citarrella, Anna Licata, Maurizio Soresi, Juan Lucio Iovanna, and Lydia Giannitrapani. 2022. "EVOO’s Effects on Incretin Production: Is There a Rationale for a Combination in T2DM Therapy?" International Journal of Molecular Sciences 23, no. 17: 10120. https://doi.org/10.3390/ijms231710120
APA StyleAmodeo, S., Mirarchi, L., Seidita, A., Citarrella, R., Licata, A., Soresi, M., Iovanna, J. L., & Giannitrapani, L. (2022). EVOO’s Effects on Incretin Production: Is There a Rationale for a Combination in T2DM Therapy? International Journal of Molecular Sciences, 23(17), 10120. https://doi.org/10.3390/ijms231710120






