Tear Biomarkers in Alzheimer’s and Parkinson’s Diseases, and Multiple Sclerosis: Implications for Diagnosis (Systematic Review)
Abstract
1. Introduction
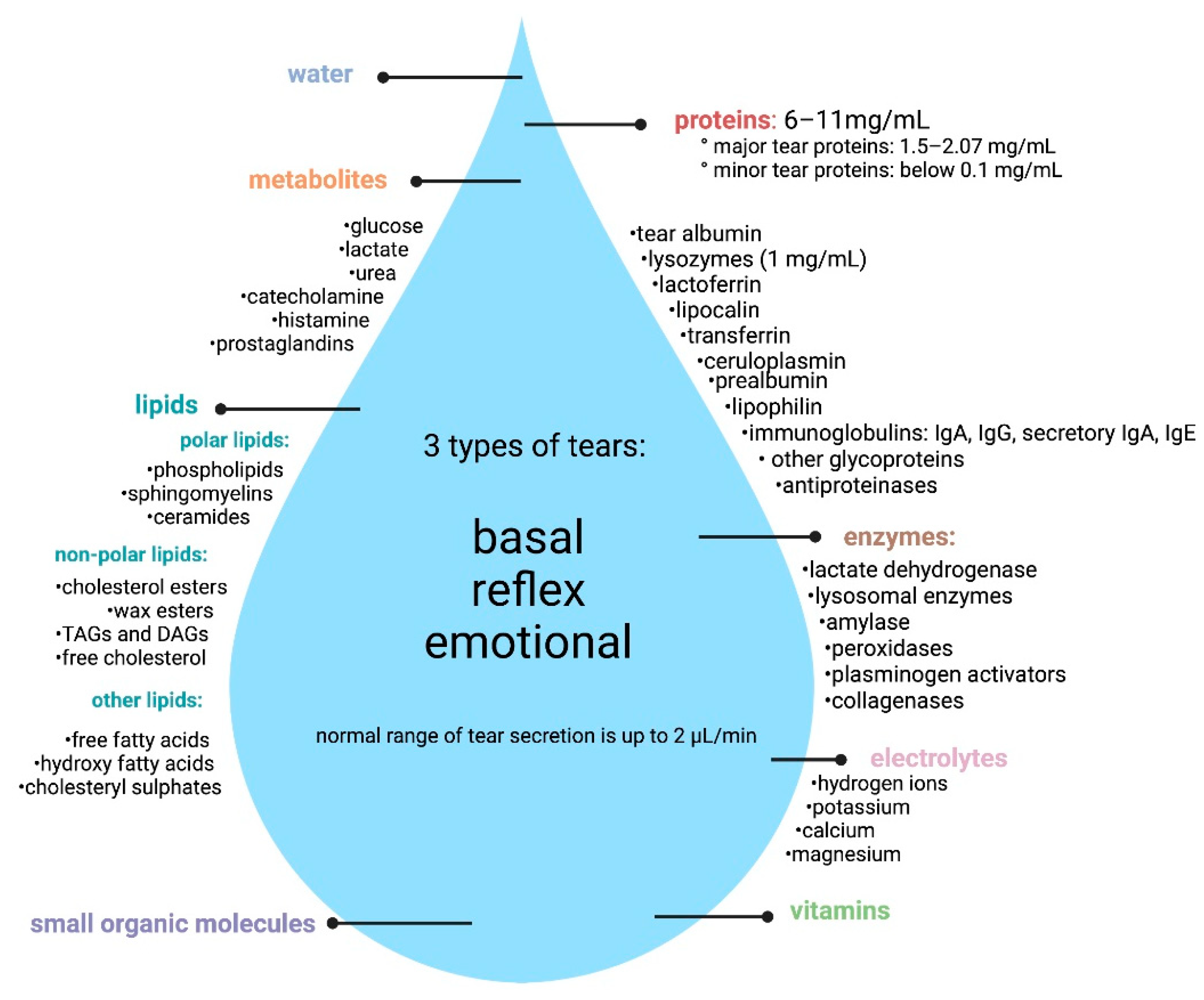
1.1. Composition of Tears
1.2. Methods of Tears Analysis
2. Methods
Literature Search Strategy
3. Results and Discussion
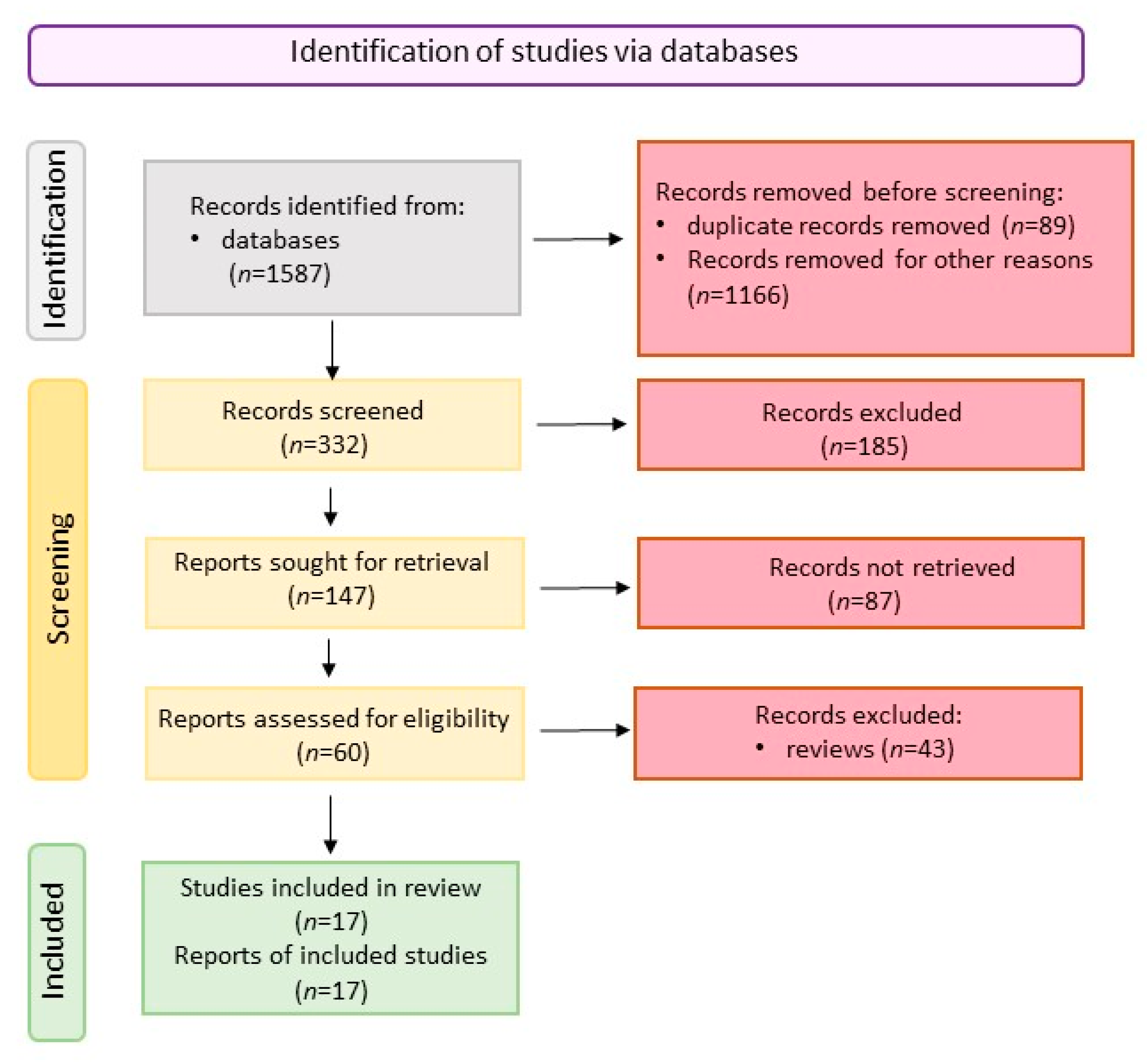
3.1. Literature Search
3.2. Parkinson’s Disease Tears Biomarkers
| Biomarker(s) | Number of Patients | Method of Tears Collection | Method of Identification/Analysis | Results | References |
|---|---|---|---|---|---|
| Oligomeric α-synunuklein (α-synOligo); total α-synuklein (α-synTotal); | CTR n = 84; PD n = 94 | Schirmer strip (basal tears) | ELISA | (1) α-synTotal decreased significantly in PD, compared to CTR (p = 0.004); (2) α-synOligo increased significantly in PD compared to CTR (p = 0.001); (3) the level of changes in analyzed parameters was associated with sex; (4) total protein, CCL2, DJ-1, and MMP9 were increased in PD but changes were not statistically significant. | [93] |
| Oligomeric α-synunuklein (α-synOligo); total α-synuklein (α-synTotal) CCL2; lactoferrin (LF) | CTR n = 82; PD n = 93 | Schirmer strip (relax tears) | ELISA | (1) α-synTotal decreased significantly in PD, compared to CTR (p-value = 0.05); (2) α-synOligo, lactoferrin, and CCL2 increased significantly in PD, compared to CTR (respectively: p = 0.005; p = 0.002; p = 0.003); (3) Level of changes analyzed parameters were associated with sex; (4) AUROC test for α-synOligo was 0.80 and for α-synOligo and CLL2 was 0.83. | [27] |
| Norepinephrine; adrenaline; α-2-macroglobulin | CTR n = 32, PD n = 31 | Schirmer strip | HPLC | (1) Noradrenaline increased in PD mainly on the ipsilateral side of pronounced motor symptoms (72%, p = 0.049); (2) a decrease in adrenaline level on both sides (ipsilateral—53%, p = 0.004; contralateral 42%, p = 0.02); (3) increased α-2-macroglobulin activity on both sides (ipsilateral 53%, p = 0.03; contralateral—56%, p = 0.037), compared to CTR; (4) adrenaline, noradrenaline, and the analysis of α-2-macroglobulin activity have the greatest potential as a biomarker (81.2%, 88.9% and 92%, respectively). | [82] |
| Epinephrine; norepinephrine; dopamine | CTR n = no data, PD n = 26 | Schirmer strip | HPLC | (1) In PD, the concentration of norepinephrine increased twice; (2) the dopamine content increased by approximately 50% (only the epinephrine ipsilateral site), and the concentration on both sides decreased twice. | [85] |
| Protein deglycase DJ-1 [PARK7]; S100 superfamily; peroxiredoxin-6 [PRDX6]; annexin-A5 [ANXA5]; glutathione S-transferase-A1 [GSTA1]; apolipoprotein superfamily | CRT n = 18, PD n = 36 | Schirmer strip | LC-MS/MS | (1) 571 proteins were identified in PD and CTR; 31 proteins were exclusively detected in the PD and only 7 in the CTR group; (2) 21 proteins were significantly increased and 19 decreased in the PD versus CTR; (3) Proteins involved in immune response, lipid metabolism, and oxidative stress were distinctly altered in PD; (4) Protein deglycase DJ-1 [PARK7], S100 superfamily (i.e., [S100A7], [S100A8] and [S100A11]), peroxiredoxin-6 [PRDX6], annexin-A5 [ANXA5], and glutathione S-transferase-A1 [GSTA1] were upregulated in the PD; (5) Several proteins from the apolipoprotein superfamily (i.e., [ApoD], [ApoA4] and [ApoA1]) were increased in tears of PD. | [87] |
| cathepsin D (CATD); acid ceramidase (ASAH1); cytoplasmic dynein-1 heavy chain (DYHC1) | CTR n = 27, PD n = 24, PD (with E46K-SNCA mutation) n = 3 | Capillaries (glass, 10 µL) | nLC-MS/MS | (1) 560 proteins have been identified in CRT and PD tears; (2) Proteins deregulated in the PD were mainly associated with immune response, apoptosis, collagen degradation, protein synthesis, lipid transport, and defense; (3) The group of 6 proteins that were up-regulated was distinguished: preamine AIC (LMNA), cathepsin D (CATD), acid ceramidase (ASAH1), transitional endoplasmic reticulum ATP-ase (TERA), and cytoplasmic dynein-1 heavy chain (DYHC1) and one down-regulated: tripeptidyl-peptidase 1 (TPP1) in PD; (4) tree proteins showed a high ability to classify patients with PD: CATD, ASAH1, and DYHC1. | [17] |
3.3. Alzheimer’s Disease Tears Biomarkers
| Biomarker/s | Number of Patent | Method of Tears Collection | Method of Identification/Analysis | Results | References |
|---|---|---|---|---|---|
| Lipocalin-1; dermcidin; lysozyme-C: lacritin; | CTR n = 9, AD n = 14 | Capillaries | LC-MS/MS; SRM-based targeted MS (with ROC analysis) | (1) Tear flow rates were significantly higher in AD (12 ± 2 μL/min) than in CTR (6 ± 2 μL/min); (2) Total protein concentration in tears was significantly higher in AD (8.8 ± 2.9 μg/μL) than in CTR (4.4 ± 1.4 μg/μL); (3) 10 proteins presented a significantly different intensity in AD group, compared to CTR; (4) The ROC analysis of the quantitative SRM-based proteomic results demonstrated the combination of 4 proteins that could be a potential biomarker of AD (with 81% sensitivity and 77% specificity); (5) Lacrimal gland dysfunction could be possible in AD as three of biomarker proteins: lipocalin-1, lysozyme-C, and lacritin (all typical of the lacrimal glands) were found to be downregulated in AD group. | [101] |
| eIF4E (and others 11 proteins); 38 miRNAs (mainly miRNA-200b-5) | CTR n = 15, MCI n = 8, AD n = 9 | Schirmer strips | high throughput RP-LC-MS/MS; genome-wide high-throughput qPCR-based microRNA platform (OpenArray) | (1) Tears flow rates were not significantly different between groups (Control (12 ± 9); MCI (9.25 ± 6.3) and AD (8.5 ± 2.9) estimated in mm/5min); (2) Total proteins concentration in tears was similar between all groups; (3) Profiling of proteins in a complete tears fluid proteome analysis did not show the presence of classical markers for AD; (4) GO analysis did not show a clear pattern in AD patients, but typical AD protein categories were found to contribute to the regulation of endopeptidases activity, proteins folding, cellular amino acid metabolic processes, and mRNA stability; (5) A unique proteomic and microRNA composition may be present in tears fluid of AD patients—12 AD-specific proteins were identified (with eIF4E presented only in AD group) and 38 distinct microRNAs (with miRNA-200b-5p detected in AD samples only). | [113] |
| Aβ38, Aβ40, Aβ42, t-Tau, p-Tau | CTR n = 9, SCD n = 23, MCI n = 22, AD n = 11 | Schirmer strips | Multiplex immunoassays with electrochemiluminescence | (1) Tear fluid classical AD biomarkers detectability: Aβ40 and t-Tau were detectable in more than 94% of samples, while Aβ38, Aβ42, and p-Tau were detectable in less than 23% of samples; p-Tau was not detectable in the TR; Aβ42 was better detectable in CTR (78%), as compared to all patients (<18%); (2) Levels of classic AD biomarkers levels: detection level for amyloid peptides was pg/mL and for Tau protein forms was ng/mL (10 times higher than in CSF); Aβ38, Aβ40, Aβ42, and t-Tau were higher in patients, compared to CTR (but not significantly); p-Tau did not differ between patient groups; (3) Levels of biomarkers in tears of patients classified according to the ATN criteria: t-Tau levels were significantly elevated in patients with neurodegeneration (N positive), compared to patients without neurodegeneration (N negative). | [112] |
| Aβ42 | CTR n = 1 (healthy, no family risk), AD n = 2 (healthy, with family risk), | No data | Immunocytochemistry assay (and fundus camera examination for retinal plaques) | (1) Numerous plaques on the retina were found in patients with a family risk of AD only; (2) Aβ-42 peptide was present in tears of patients with a family risk of AD only; (3) retinal plaques were directly linked with Aβ-42 in tears and Aβ-42 was not linked to the expression of dementia symptoms; (4) negative test for Aβ-42 in “no AD risk” patient just after eye viral infection suggests that inflammatory conditions do not cause false-positive findings; (5) Aβ-42 in tears could have a predictive value in the diagnosis of AD | [110] |
| Aβ40, Aβ42 | CTR n = 50 healthy donors (wide age spectrum 20 to 79) | Schirmer strips | Electrochemical immunosensor | (1) Testing of a new biosensor: relatively high sensitivity and a low detection limit (detection range 1–100 pg/mL); (2) Testing of AD classical biomarkers: tears of healthy people were found to contain 10 times more Aβ peptides than their blood (10 pg/mL in tears; 1 pg/mL in blood); Aβ content in healthy subjects was inversely proportional to the age; the youngest age group (ages 20–39) and the oldest age group (ages 60–79) differed significantly in their Aβ ratio (p < 0.01). | [111] |
3.4. Multiple Sclerosis (MS) Disease Tears Biomarkers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chojnowska, S.; Baran, T.; Wilińska, I.; Sienicka, P.; Cabaj-Wiater, I.; Knaś, M. Human Saliva as a Diagnostic Material. Adv. Med. Sci. 2018, 63, 185–191. [Google Scholar] [CrossRef]
- Woźniak, M.; Paluszkiewicz, C.; Kwiatek, W.M. Saliva as a Non-Invasive Material for Early Diagnosis. Acta Biochim. Pol. 2019, 66, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Pieragostino, D.; Lanuti, P.; Cicalini, I.; Cufaro, M.C.; Ciccocioppo, F.; Ronci, M.; Simeone, P.; Onofrj, M.; van der Pol, E.; Fontana, A.; et al. Proteomics Characterization of Extracellular Vesicles Sorted by Flow Cytometry Reveals a Disease-Specific Molecular Cross-Talk from Cerebrospinal Fluid and Tears in Multiple Sclerosis. J. Proteom. 2019, 204, 103403. [Google Scholar] [CrossRef] [PubMed]
- Barmada, A.; Shippy, S.A. Tear Analysis as the next Routine Body Fluid Test. Eye 2020, 34, 1731–1733. [Google Scholar] [CrossRef] [PubMed]
- Cicalini, I.; Rossi, C.; Pieragostino, D.; Agnifili, L.; Mastropasqua, L.; di Ioia, M.; De Luca, G.; Onofrj, M.; Federici, L.; Del Boccio, P. Integrated Lipidomics and Metabolomics Analysis of Tears in Multiple Sclerosis: An Insight into Diagnostic Potential of Lacrimal Fluid. Int. J. Mol. Sci. 2019, 20, 1265. [Google Scholar] [CrossRef]
- Börger, M.; Funke, S.; Bähr, M.; Grus, F.; Lingor, P. Biomarker Sources for Parkinson’s Disease: Time to Shed Tears? Basal Ganglia 2015, 5, 63–69. [Google Scholar] [CrossRef]
- Örnek, N.; Dağ, E.; Örnek, K. Corneal Sensitivity and Tear Function in Neurodegenerative Diseases. Curr. Eye Res. 2015, 40, 423–428. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Boccardi, M.; Barkhof, F.; Blennow, K.; Cappa, S.; Chiotis, K.; Démonet, J.-F.; Garibotto, V.; Giannakopoulos, P.; Gietl, A.; et al. Strategic Roadmap for an Early Diagnosis of Alzheimer’s Disease Based on Biomarkers. Lancet Neurol. 2017, 16, 661–676. [Google Scholar] [CrossRef]
- Ziemssen, T.; Akgün, K.; Brück, W. Molecular Biomarkers in Multiple Sclerosis. J. Neuroinflamm. 2019, 16, 272. [Google Scholar] [CrossRef]
- Paul, A.; Comabella, M.; Gandhi, R. Biomarkers in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a029058. [Google Scholar] [CrossRef]
- Nandi, S.K.; Singh, D.; Upadhay, J.; Gupta, N.; Dhiman, N.; Mittal, S.K.; Mahindroo, N. Identification of Tear-Based Protein and Non-Protein Biomarkers: Its Application in Diagnosis of Human Diseases Using Biosensors. Int. J. Biol. Macromol. 2021, 193, 838–846. [Google Scholar] [CrossRef]
- Masoudi, S. Biochemistry of Human Tear Film: A Review. Exp. Eye Res. 2022, 220, 109101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Beuerman, R.W. The Power of Tears: How Tear Proteomics Research Could Revolutionize the Clinic. Expert Rev. Proteom. 2017, 14, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Aparna, R.; Shanti Iyer, R. Tears and Eyewear in Forensic Investigation-A Review. Forensic. Sci. Int. 2020, 306, 110055. [Google Scholar] [CrossRef] [PubMed]
- Dor, M.; Eperon, S.; Lalive, P.H.; Guex-Crosier, Y.; Hamedani, M.; Salvisberg, C.; Turck, N. Investigation of the Global Protein Content from Healthy Human Tears. Exp. Eye Res. 2019, 179, 64–74. [Google Scholar] [CrossRef]
- Anitua, E.; Muruzabal, F.; Tayebba, A.; Riestra, A.; Perez, V.L.; Merayo-Lloves, J.; Orive, G. Autologous Serum and Plasma Rich in Growth Factors in Ophthalmology: Preclinical and Clinical Studies. Acta Ophthalmol. 2015, 93, e605–e614. [Google Scholar] [CrossRef]
- Acera, A.; Abad, B.; Pereiro, X.; Rodríguez, F.D.; Ruzafa, N.; Duran, J.A.; Vecino, E. Comparative Study of the Lipid Profile of Tears and Plasma Enriched in Growth Factors. Exp. Eye Res. 2022, 219, 109061. [Google Scholar] [CrossRef]
- Gao, F.; Hong, X.; Ding, F.; Huang, S.; Lian, W.; Wang, H.; Zheng, W.; Ni, J.; Chen, M.; Liu, Q. High Level of Inflammatory Cytokines in the Tears: A Bridge of Patients with Concomitant Exotropia and Dry Eye. Oxid. Med. Cell Longev. 2021, 2021, e5662550. [Google Scholar] [CrossRef]
- Sahay, P.; Rao, A.; Padhy, D.; Sarangi, S.; Das, G.; Reddy, M.M.; Modak, R. Functional Activity of Matrix Metalloproteinases 2 and 9 in Tears of Patients With Glaucoma. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO106–BIO113. [Google Scholar] [CrossRef]
- Caselli, E.; Soffritti, I.; Lamberti, G.; D’Accolti, M.; Franco, F.; Demaria, D.; Contoli, M.; Passaro, A.; Contini, C.; Perri, P. Anti-SARS-Cov-2 IgA Response in Tears of COVID-19 Patients. Biology 2020, 9, 374. [Google Scholar] [CrossRef]
- Kwon, J.; Surenkhuu, B.; Raju, I.; Atassi, N.; Mun, J.; Chen, Y.-F.; Sarwar, M.A.; Rosenblatt, M.; Pradeep, A.; An, S.; et al. Pathological Consequences of Anti-Citrullinated Protein Antibodies in Tear Fluid and Therapeutic Potential of Pooled Human Immune Globulin-Eye Drops in Dry Eye Disease. Ocul. Surf. 2020, 18, 80–97. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Serjersen, H.; Hjortdal, J.; Zieske, J.D.; Karamichos, D. Characterization of Tear Immunoglobulins in a Small-Cohort of Keratoconus Patients. Sci. Rep. 2020, 10, 9426. [Google Scholar] [CrossRef]
- Muyldermans, A.; Bjerke, M.; Demuyser, T.; De Geyter, D.; Wybo, I.; Soetens, O.; Weets, I.; Kuijpers, R.; Allard, S.D.; Piérard, D.; et al. SARS-CoV-2 RNA and Antibodies in Tear Fluid. BMJ Open Ophthalmol. 2021, 6, e000733. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Ren, C.; Cai, W.; Wei, Q.; Song, Y.; Yu, J. Proteomic Analysis of Tears Following Acupuncture Treatment for Menopausal Dry Eye Disease by Two-Dimensional Nano-Liquid Chromatography Coupled with Tandem Mass Spectrometry. IJN 2017, 12, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.G.-J.; Rahimi-Oztan, M.; Proschogo, N.; Khoo, P.; Watson, S.L. Evaluation of Sex Hormone and Cholesterol Sterols in Human Tears by Mass Spectroscopy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3276. [Google Scholar]
- Gibson, E.J.; Bucknall, M.P.; Golebiowski, B.; Stapleton, F. Comparative Limitations and Benefits of Liquid Chromatography-Mass Spectrometry Techniques for Analysis of Sex Steroids in Tears. Exp. Eye Res. 2019, 179, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Hamm-Alvarez, S.F.; Okamoto, C.T.; Janga, S.R.; Feigenbaum, D.; Edman, M.C.; Freire, D.; Shah, M.; Ghanshani, R.; Mack, W.J.; Lew, M.F. Oligomeric α-Synuclein Is Increased in Basal Tears of Parkinson’s Patients. Biomark. Med. 2019, 13, 941–952. [Google Scholar] [CrossRef]
- Lekhanont, K.; Sathianvichitr, K.; Pisitpayat, P.; Anothaisintawee, T.; Soontrapa, K.; Udomsubpayakul, U. Association between the Levels of Prostaglandin E2 in Tears and Severity of Dry Eye. Int. J. Ophthalmol. 2019, 12, 1127–1133. [Google Scholar] [CrossRef]
- Dikovskaya, M.; Korolenko, T.; Trunov, A. P43: Endogenous Inhibitors of Cysteine Proteases Cystatin C and Cystatin SN in Biological Fluids of Patients with Intraocular Melanoma as Possible Biomarkers and Therapy Targets. Eur. J. Cancer Suppl. 2015, 13, 13–14. [Google Scholar] [CrossRef]
- Edman, M.C.; Janga, S.R.; Meng, Z.; Bechtold, M.; Chen, A.F.; Kim, C.; Naman, L.; Sarma, A.; Teekappanavar, N.; Kim, A.Y.; et al. Increased Cathepsin S Activity Associated with Decreased Protease Inhibitory Capacity Contributes to Altered Tear Proteins in Sjögren’s Syndrome Patients. Sci. Rep. 2018, 8, 11044. [Google Scholar] [CrossRef]
- Magalhães, B.; Trindade, F.; Barros, A.S.; Klein, J.; Amado, F.; Ferreira, R.; Vitorino, R. Reviewing Mechanistic Peptidomics in Body Fluids Focusing on Proteases. Proteomics 2018, 18, e1800187. [Google Scholar] [CrossRef]
- Fu, R.; Klinngam, W.; Heur, M.; Edman, M.C.; Hamm-Alvarez, S.F. Tear Proteases and Protease Inhibitors: Potential Biomarkers and Disease Drivers in Ocular Surface Disease. Eye Contact Lens. 2020, 46 (Suppl. S2), S70–S83. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Menon, N.G.; de Almeida, L.G.N.; Woods, P.S.; Heynen, M.L.; Jay, G.D.; Caffery, B.; Jones, L.; Krawetz, R.; Schmidt, T.A.; et al. Proteomics Analysis of Tears and Saliva From Sjogren’s Syndrome Patients. Front. Pharmacol. 2021, 12, 3299. [Google Scholar] [CrossRef] [PubMed]
- Chng, C.-L.; Seah, L.L.; Yang, M.; Shen, S.Y.; Koh, S.K.; Gao, Y.; Deng, L.; Tong, L.; Beuerman, R.W.; Zhou, L. Tear Proteins Calcium Binding Protein A4 (S100A4) and Prolactin Induced Protein (PIP) Are Potential Biomarkers for Thyroid Eye Disease. Sci. Rep. 2018, 8, 1–10. Available online: https://www.nature.com/articles/s41598-018-35096-x (accessed on 14 July 2022). [CrossRef] [PubMed]
- Zong, R.-R.; Zhu, F.-F.; Han, W.; Wang, Y.-X.; Wang, G.-L.; Wang, Y.-Z.; Mao, Y.-B.; Guan, T.-J.; Liu, Z.-G.; Xue, Y.-H.; et al. Tear Dynamics Testing and Quantitative Proteomics Analysis in Patients with Chronic Renal Failure. J. Proteom. 2021, 248, 104351. [Google Scholar] [CrossRef]
- Nguyen-Khuong, T.; Everest-Dass, A.V.; Kautto, L.; Zhao, Z.; Willcox, M.D.P.; Packer, N.H. Glycomic Characterization of Basal Tears and Changes with Diabetes and Diabetic Retinopathy. Glycobiology 2015, 25, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Schmelter, C.; Perumal, N.; Funke, S.; Pfeiffer, N.; Grus, F.H. Identification and Characterization of Human Tear Film Glycoproteins. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2489. [Google Scholar]
- Tambe, M.; Ng, B.; Freeze, H. Beyond ERAD: N-Glycanase Will Bring You to Tears. FASEB J. 2018, 32, 673–674. [Google Scholar] [CrossRef]
- Pieragostino, D.; D’Alessandro, M.; di Ioia, M.; Di Ilio, C.; Sacchetta, P.; Del Boccio, P. Unraveling the Molecular Repertoire of Tears as a Source of Biomarkers: Beyond Ocular Diseases. Proteom. Clin. Appl. 2015, 9, 169–186. [Google Scholar] [CrossRef]
- Ngo, W.; Chen, J.; Panthi, S.; Nichols, K.K.; Nichols, J.J. Comparison of Collection Methods for the Measure of Human Meibum and Tear Film-Derived Lipids Using Mass Spectrometry. Curr. Eye Res. 2018, 43, 1244–1252. [Google Scholar] [CrossRef]
- Chen, J.; Nichols, K.K.; Wilson, L.; Barnes, S.; Nichols, J.J. Untargeted Lipidomic Analysis of Human Tears: A New Approach for Quantification of O-Acyl-Omega Hydroxy Fatty Acids. Ocul. Surf. 2019, 17, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, B.J. Evidence for Phospholipids on the Surface of Human Tears. Investig. Ophthalmol. Vis. Sci. 2020, 61, 19. [Google Scholar] [CrossRef] [PubMed]
- Fineide, F.; Chen, X.; Bjellaas, T.; Vitelli, V.; Utheim, T.P.; Jensen, J.L.; Galtung, H.K. Characterization of Lipids in Saliva, Tears and Minor Salivary Glands of Sjögren’s Syndrome Patients Using an HPLC/MS-Based Approach. Int. J. Mol. Sci. 2021, 22, 8997. [Google Scholar] [CrossRef]
- Masoudi, S.; Mitchell, T.W.; Willcox, M.D. Profiling of Non-Polar Lipids in Tears of Contact Lens Wearers during the Day. Exp. Eye Res. 2021, 207, 108567. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P. Antimicrobial Tear Lipids in the Ocular Surface Defense. Front. Cell Infect. Microbiol. 2022, 12, 866900. [Google Scholar]
- van den Berg, M.M.J.; Krauskopf, J.; Ramaekers, J.G.; Kleinjans, J.C.S.; Prickaerts, J.; Briedé, J.J. Circulating MicroRNAs as Potential Biomarkers for Psychiatric and Neurodegenerative Disorders. Prog. Neurobiol. 2020, 185, 101732. [Google Scholar] [CrossRef] [PubMed]
- Quah, J.H.M.; Tong, L.; Barbier, S. Patient Acceptability of Tear Collection in the Primary Healthcare Setting. Optom. Vis. Sci. 2014, 91, 452–458. [Google Scholar] [CrossRef]
- Soria, J.; Durán, J.A.; Etxebarria, J.; Merayo, J.; González, N.; Reigada, R.; García, I.; Acera, A.; Suárez, T. Tear Proteome and Protein Network Analyses Reveal a Novel Pentamarker Panel for Tear Film Characterization in Dry Eye and Meibomian Gland Dysfunction. J. Proteom. 2013, 78, 94–112. [Google Scholar] [CrossRef]
- Versura, P.; Nanni, P.; Bavelloni, A.; Blalock, W.L.; Piazzi, M.; Roda, A.; Campos, E.C. Tear Proteomics in Evaporative Dry Eye Disease. Eye 2010, 24, 1396–1402. [Google Scholar] [CrossRef]
- Nichols, J.J.; Green-Church, K.B. Mass Spectrometry-Based Proteomic Analyses in Contact Lens-Related Dry Eye. Cornea 2009, 28, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Acera, A.; Rocha, G.; Vecino, E.; Lema, I.; Durán, J.A. Inflammatory Markers in the Tears of Patients with Ocular Surface Disease. Ophthalmic Res. 2008, 40, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Seifert, K.; Gandia, N.C.; Wilburn, J.K.; Bower, K.S.; Sia, R.K.; Ryan, D.S.; Deaton, M.L.; Still, K.M.; Vassilev, V.C.; Laurie, G.W.; et al. Tear Lacritin Levels by Age, Sex, and Time of Day in Healthy Adults. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6610–6616. [Google Scholar] [CrossRef]
- Enríquez-de-Salamanca, A.; Castellanos, E.; Stern, M.E.; Fernández, I.; Carreño, E.; García-Vázquez, C.; Herreras, J.M.; Calonge, M. Tear Cytokine and Chemokine Analysis and Clinical Correlations in Evaporative-Type Dry Eye Disease. Mol. Vis. 2010, 16, 862–873. [Google Scholar]
- Sonoda, S.; Uchino, E.; Nakao, K.; Sakamoto, T. Inflammatory Cytokine of Basal and Reflex Tears Analysed by Multicytokine Assay. Br. J. Ophthalmol. 2006, 90, 120–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pankratov, D.; González-Arribas, E.; Blum, Z.; Shleev, S. Tear Based Bioelectronics. Electroanalysis 2016, 28, 1250–1266. [Google Scholar] [CrossRef]
- Kukkar, D.; Zhang, D.; Jeon, B.H.; Kim, K.-H. Recent Advances in Wearable Biosensors for Non-Invasive Monitoring of Specific Metabolites and Electrolytes Associated with Chronic Kidney Disease: Performance Evaluation and Future Challenges. TrAC Trends Anal. Chem. 2022, 150, 116570. [Google Scholar] [CrossRef]
- Yang, X.; Yao, H.; Zhao, G.; Ameer, G.A.; Sun, W.; Yang, J.; Mi, S. Flexible, Wearable Microfluidic Contact Lens with Capillary Networks for Tear Diagnostics. J. Mater. Sci. 2020, 55, 9551–9561. [Google Scholar] [CrossRef]
- Lin, C.-E.; Hiraka, K.; Matloff, D.; Johns, J.; Deng, A.; Sode, K.; La Belle, J. Development toward a Novel Integrated Tear Lactate Sensor Using Schirmer Test Strip and Engineered Lactate Oxidase. Sens. Actuators B Chem. 2018, 270, 525–529. [Google Scholar] [CrossRef]
- Lee, S.H.; Cho, Y.C.; Bin Choy, Y. Noninvasive Self-Diagnostic Device for Tear Collection and Glucose Measurement. Sci. Rep. 2019, 9, 4747. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jeon, H.M.; Choi, K.C.; Sung, G.Y. Testing the Effectiveness of Curcuma Longa Leaf Extract on a Skin Equivalent Using a Pumpless Skin-on-a-Chip Model. Int. J. Mol. Sci. 2020, 21, 3898. [Google Scholar] [CrossRef]
- Kownacka, A.E.; Vegelyte, D.; Joosse, M.; Anton, N.; Toebes, B.J.; Lauko, J.; Buzzacchera, I.; Lipinska, K.; Wilson, D.A.; Geelhoed-Duijvestijn, N. Clinical Evidence for Use of a Noninvasive Biosensor for Tear Glucose as an Alternative to Painful Finger-Prick for Diabetes Management Utilizing a Biopolymer Coating. Biomacromolecules 2018, 19, 4504–4511. [Google Scholar] [CrossRef] [PubMed]
- Moreddu, R.; Wolffsohn, J.S.; Vigolo, D.; Yetisen, A.K. Laser-Inscribed Contact Lens Sensors for the Detection of Analytes in the Tear Fluid. Sens. Actuators B Chem. 2020, 317, 128183. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Brazaca, L.C.; García-Carmona, L.; Bolat, G.; Campbell, A.S.; Martin, A.; Tang, G.; Shah, R.; Mishra, R.K.; Kim, J.; et al. Eyeglasses-Based Tear Biosensing System: Non-Invasive Detection of Alcohol, Vitamins and Glucose. Biosens. Bioelectron. 2019, 137, 161–170. [Google Scholar] [CrossRef]
- Solomon, A.; Dursun, D.; Liu, Z.; Xie, Y.; Macri, A.; Pflugfelder, S.C. Pro- and Anti-Inflammatory Forms of Interleukin-1 in the Tear Fluid and Conjunctiva of Patients with Dry-Eye Disease. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2283–2292. [Google Scholar]
- de Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s Disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Love, S. Neuropathological Investigation of Dementia: A Guide for Neurologists. J. Neurol. Neurosurg. Psychiatry 2005, 76 (Suppl. S5), v8–v14. Available online: https://jnnp.bmj.com/content/76/suppl_5/v8 (accessed on 15 July 2022). [PubMed]
- Okun, M.; Malaty, I.A.; Deeb, W. Living with Parkinson’s Disease: A Complete Guide for Patients and Caregivers; Robert Rose Incorporated: Toronto, ON, Canada, 2020; ISBN 978-0-7788-0672-1. [Google Scholar]
- Sharma, S.; Moon, C.S.; Khogali, A.; Haidous, A.; Chabenne, A.; Ojo, C.; Jelebinkov, M.; Kurdi, Y.; Ebadi, M. Biomarkers in Parkinson’s Disease (Recent Update). Neurochem. Int. 2013, 63, 201–229. [Google Scholar] [CrossRef]
- Delenclos, M.; Jones, D.R.; McLean, P.J.; Uitti, R.J. Biomarkers in Parkinson’s Disease: Advances and Strategies. Parkinsonism Relat. Disord. 2016, 22 (Suppl. S1), S106–S110. [Google Scholar] [CrossRef] [PubMed]
- Ugrumov, M. Development of Early Diagnosis of Parkinson’s Disease: Illusion or Reality? CNS Neurosci. Ther. 2020, 26, 997–1009. [Google Scholar] [CrossRef]
- Chahine, L.M.; Stern, M.B. Parkinson’s Disease Biomarkers: Where Are We and Where Do We Go Next? Mov. Disord. Clin. Pract. 2017, 4, 796–805. [Google Scholar] [CrossRef]
- Datta, I.; Ganapathy, K.; Razdan, R.; Bhonde, R. Location and Number of Astrocytes Determine Dopaminergic Neuron Survival and Function Under 6-OHDA Stress Mediated Through Differential BDNF Release. Mol. Neurobiol. 2018, 55, 5505–5525. [Google Scholar] [CrossRef] [PubMed]
- Dehay, B.; Bourdenx, M.; Gorry, P.; Przedborski, S.; Vila, M.; Hunot, S.; Singleton, A.; Olanow, C.W.; Merchant, K.M.; Bezard, E.; et al. Targeting α-Synuclein for Treatment of Parkinson’s Disease: Mechanistic and Therapeutic Considerations. Lancet Neurol. 2015, 14, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Nagano, I.; Shiote, M.; Narai, H.; Murakami, T.; Hayashi, T.; Shoji, M.; Abe, K. Elevation of MCP-1 and MCP-1/VEGF Ratio in Cerebrospinal Fluid of Amyotrophic Lateral Sclerosis Patients. Neurol. Res. 2007, 29, 772–776. [Google Scholar] [CrossRef]
- Lev, N.; Roncevic, D.; Roncevich, D.; Ickowicz, D.; Melamed, E.; Offen, D. Role of DJ-1 in Parkinson’s Disease. J. Mol. Neurosci. 2006, 29, 215–225. [Google Scholar] [CrossRef]
- Danjo, Y.; Lee, M.; Horimoto, K.; Hamano, T. Ocular Surface Damage and Tear Lactoferrin in Dry Eye Syndrome. Acta Ophthalmol. 1994, 72, 433–437. [Google Scholar] [CrossRef]
- Manicone, A.M.; McGuire, J.K. Matrix Metalloproteinases as Modulators of Inflammation. Semin. Cell Dev. Biol. 2008, 19, 34–41. [Google Scholar] [CrossRef]
- Bonini, S.; Rama, P.; Olzi, D.; Lambiase, A. Neurotrophic Keratitis. Eye 2003, 17, 989–995. [Google Scholar] [CrossRef]
- Scott, B.; Borgman, A.; Engler, H.; Johnels, B.; Aquilonius, S.M. Gender Differences in Parkinson’s Disease Symptom Profile. Acta Neurol. Scand. 2000, 102, 37–43. [Google Scholar] [CrossRef]
- Kim, A.R.; Pavlenko, T.A.; Katargina, L.A.; Chesnokova, N.B.; Ugrumov, M.V. Biochemical and Functional Changes in the Eye As a Manifestation of Systemic Degeneration of the Nervous System in Parkinsonism. Acta Nat. 2018, 10, 62–67. [Google Scholar] [CrossRef]
- Armstrong, R.A. Visual Symptoms in Parkinson’s Disease. Parkinson's Dis. 2011, 2011, 908306. [Google Scholar] [CrossRef]
- Bogdanov, V.; Kim, A.; Nodel, M.; Pavlenko, T.; Pavlova, E.; Blokhin, V.; Chesnokova, N.; Ugrumov, M. A Pilot Study of Changes in the Level of Catecholamines and the Activity of α-2-Macroglobulin in the Tear Fluid of Patients with Parkinson’s Disease and Parkinsonian Mice. Int. J. Mol. Sci. 2021, 22, 4736. [Google Scholar] [CrossRef]
- Cater, J.H.; Wilson, M.R.; Wyatt, A.R. Alpha-2-Macroglobulin, a Hypochlorite-Regulated Chaperone and Immune System Modulator. Oxid. Med. Cell Longev. 2019, 2019, 5410657. [Google Scholar] [CrossRef] [PubMed]
- Barcelona, P.F.; Saragovi, H.U. A Pro-Nerve Growth Factor (ProNGF) and NGF Binding Protein, A2-Macroglobulin, Differentially Regulates P75 and TrkA Receptors and Is Relevant to Neurodegeneration Ex Vivo and In Vivo. Mol. Cell Biol. 2015, 35, 3396–3408. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Nodel, M.R.; Pavlenko, T.A.; Chesnokova, N.B.; Yakhno, N.N.; Ugrumov, M.V. Tear Fluid Catecholamines As Biomarkers of the Parkinson’s Disease: A Clinical and Experimental Study. Acta Nat. 2019, 11, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Nigmatullina, R.; Zalyalova, Z.; Soshnikova, N.; Krasnov, A.; Vorobyeva, N.; Georgieva, S.; Kudrin, V.; Narkevich, V.; Ugrumov, M. Upgraded Methodology for the Development of Early Diagnosis of Parkinson’s Disease Based on Searching Blood Markers in Patients and Experimental Models. Mol. Neurobiol. 2019, 56, 3437–3450. [Google Scholar] [CrossRef] [PubMed]
- Boerger, M.; Funke, S.; Leha, A.; Roser, A.-E.; Wuestemann, A.-K.; Maass, F.; Bähr, M.; Grus, F.; Lingor, P. Proteomic Analysis of Tear Fluid Reveals Disease-Specific Patterns in Patients with Parkinson’s Disease—A Pilot Study. Parkinsonism Relat. Disord. 2019, 63, 3–9. [Google Scholar] [CrossRef]
- Ordoñez, C.; Navarro, A.; Perez, C.; Astudillo, A.; Martínez, E.; Tolivia, J. Apolipoprotein D Expression in Substantia Nigra of Parkinson Disease. Histol. Histopathol. 2006, 21, 361–366. [Google Scholar] [CrossRef]
- Zintzaras, E.; Hadjigeorgiou, G.M. Association of Paraoxonase 1 Gene Polymorphisms with Risk of Parkinson’s Disease: A Meta-Analysis. J. Hum. Genet. 2004, 49, 474–481. [Google Scholar] [CrossRef]
- Zarranz, J.J.; Alegre, J.; Gómez-Esteban, J.C.; Lezcano, E.; Ros, R.; Ampuero, I.; Vidal, L.; Hoenicka, J.; Rodriguez, O.; Atarés, B.; et al. The New Mutation, E46K, of Alpha-Synuclein Causes Parkinson and Lewy Body Dementia. Ann. Neurol. 2004, 55, 164–173. [Google Scholar] [CrossRef]
- Partanen, S.; Haapanen, A.; Kielar, C.; Pontikis, C.; Alexander, N.; Inkinen, T.; Saftig, P.; Gillingwater, T.H.; Cooper, J.D.; Tyynelä, J. Synaptic Changes in the Thalamocortical System of Cathepsin D-Deficient Mice: A Model of Human Congenital Neuronal Ceroid-Lipofuscinosis. J. Neuropathol. Exp. Neurol. 2008, 67, 16–29. [Google Scholar] [CrossRef]
- Chu, J.; Thomas, L.M.; Watkins, S.C.; Franchi, L.; Núñez, G.; Salter, R.D. Cholesterol-Dependent Cytolysins Induce Rapid Release of Mature IL-1beta from Murine Macrophages in a NLRP3 Inflammasome and Cathepsin B-Dependent Manner. J. Leukoc. Biol. 2009, 86, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Hamm-Alvarez, S.F.; Janga, S.R.; Edman, M.C.; Feigenbaum, D.; Freire, D.; Mack, W.J.; Okamoto, C.T.; Lew, M.F. Levels of Oligomeric α-Synuclein in Reflex Tears Distinguish Parkinson’s Disease Patients from Healthy Controls. Biomark. Med. 2019, 13, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, S.; Didier, M.-A.; Vinjamuri, S. The Who, When, Why, and How of PET Amyloid Imaging in Management of Alzheimer’s Disease—Review of Literature and Interesting Images. Diagnostics 2019, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- World Alzheimer Report 2021: Journey through the Diagnosis of Dementia. Available online: https://www.alzint.org/u/World-Alzheimer-Report-2021.pdf (accessed on 26 July 2022).
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments in Alzheimer Disease: An Update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K.; Honea, R.A.; Vidoni, E.D.; Swerdlow, R.H.; Burns, J.M. Is Alzheimer’s Disease a Systemic Disease? Biochim. Biophys. Acta 2014, 1842, 1340–1349. [Google Scholar] [CrossRef]
- Romaus-Sanjurjo, D.; Regueiro, U.; López-López, M.; Vázquez-Vázquez, L.; Ouro, A.; Lema, I.; Sobrino, T. Alzheimer’s Disease Seen through the Eye: Ocular Alterations and Neurodegeneration. Int. J. Mol. Sci. 2022, 23, 2486. [Google Scholar] [CrossRef]
- Roda, M.; Ciavarella, C.; Giannaccare, G.; Versura, P. Biomarkers in Tears and Ocular Surface: A Window for Neurodegenerative Diseases. Eye Contact Lens. 2020, 46 (Suppl. S2), S129–S134. [Google Scholar] [CrossRef]
- Ausó, E.; Gómez-Vicente, V.; Esquiva, G. Biomarkers for Alzheimer’s Disease Early Diagnosis. J. Pers. Med. 2020, 10, 114. [Google Scholar] [CrossRef]
- Kalló, G.; Emri, M.; Varga, Z.; Ujhelyi, B.; Tőzsér, J.; Csutak, A.; Csősz, É. Changes in the Chemical Barrier Composition of Tears in Alzheimer’s Disease Reveal Potential Tear Diagnostic Biomarkers. PLoS ONE 2016, 11, e0158000. [Google Scholar] [CrossRef]
- Wu, S.; Fitzpatrick, J.; Cronin, K.; Miao, S. Effects of Calcium Chelation on the Neutralization of Milk Protein Isolate and Casein Micelle Reassembling. Food Chem. 2020, 332, 127440. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Dekosky, S.T.; Barberger-Gateau, P.; Cummings, J.; Delacourte, A.; Galasko, D.; Gauthier, S.; Jicha, G.; et al. Research Criteria for the Diagnosis of Alzheimer’s Disease: Revising the NINCDS-ADRDA Criteria. Lancet Neurol. 2007, 6, 734–746. [Google Scholar] [CrossRef]
- Jack, C.R.; Albert, M.S.; Knopman, D.S.; McKhann, G.M.; Sperling, R.A.; Carrillo, M.C.; Thies, B.; Phelps, C.H. Introduction to the Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer's Dement. 2011, 7, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimer's Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.C.; Karlawish, J. The Problem of Aducanumab for the Treatment of Alzheimer Disease. Ann. Intern. Med. 2021, 174, 1303–1304. [Google Scholar] [CrossRef] [PubMed]
- Hornung, S.; Dutta, S.; Bitan, G. CNS-Derived Blood Exosomes as a Promising Source of Biomarkers: Opportunities and Challenges. Front. Mol. Neurosci. 2020, 13, 38. [Google Scholar] [CrossRef]
- Qiu, T.; Liu, Q.; Chen, Y.-X.; Zhao, Y.-F.; Li, Y.-M. Aβ42 and Aβ40: Similarities and Differences. J. Pept. Sci. 2015, 21, 522–529. [Google Scholar] [CrossRef]
- Del Prete, S.; Marasco, D.; Sabetta, R.; Del Prete, A.; Marino, F.Z.; Franco, R.; Troisi, S.; Troisi, M.; Cennamo, G. Tear Liquid for Predictive Diagnosis of Alzheimer’s Disease. Reports 2021, 4, 26. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Z.; Zhao, X.; Han, R.; Huang, D.; Yang, Y.; Cheng, G. Comparative Proteomic Characterization of Bovine Milk Containing β-Casein Variants A1A1 and A2A2, and Their Heterozygote A1A2. J. Sci. Food Agric. 2021, 101, 718–725. [Google Scholar] [CrossRef]
- Gijs, M.; Ramakers, I.H.G.B.; Visser, P.J.; Verhey, F.R.J.; van de Waarenburg, M.P.H.; Schalkwijk, C.G.; Nuijts, R.M.M.A.; Webers, C.A.B. Association of Tear Fluid Amyloid and Tau Levels with Disease Severity and Neurodegeneration. Sci. Rep. 2021, 11, 22675. [Google Scholar] [CrossRef]
- Kenny, A.; Jiménez-Mateos, E.M.; Zea-Sevilla, M.A.; Rábano, A.; Gili-Manzanaro, P.; Prehn, J.H.; Henshall, D.C.; Ávila, J.; Engel, T.; Hernández, F. Proteins and MicroRNAs Are Differentially Expressed in Tear Fluid from Patients with Alzheimer’s Disease. Sci. Rep. 2019, 9, 1–14. Available online: https://www.nature.com/articles/s41598-019-51837-y (accessed on 14 July 2022). [CrossRef] [PubMed]
- Swarbrick, S.; Wragg, N.; Ghosh, S.; Stolzing, A. Systematic Review of MiRNA as Biomarkers in Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 6156–6167. [Google Scholar] [CrossRef] [PubMed]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple Sclerosis—A Review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Pastare, D.; Bennour, M.R.; Polunosika, E.; Karelis, G. Biomarkers of Multiple Sclerosis. Open Immunol. J. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Lebrun, C.; Forzy, G.; Collongues, N.; Cohen, M.; de Seze, J.; Hautecoeur, P. Tear Analysis as a Tool to Detect Oligoclonal Bands in Radiologically Isolated Syndrome. Rev. Neurol. 2015, 171, 390–393. [Google Scholar] [CrossRef]
- Marchisio, M.; Lanuti, P.; Pierdomenico, L.; Bologna, G.; Simeone, P.; Ercolino, E.; Pieragostino, L.; Cicalini, I.; Del Boccio, P.; Grifone, G.; et al. Proteomic Insights in Extracellular Microvesicles from Multiple Sclerosis Patients. Ital. J. Anat. Embryol. 2017, 122, 135. [Google Scholar]
- Bachhuber, F. Investigating Oligoclonal IgG Bands in the Tear Fluid of Multiple Sclerosis Patients. Ph.D. Thesis, Universität Ulm, Ulm, Germany, 2021. [Google Scholar]
- Hümmert, M.W.; Wurster, U.; Bönig, L.; Schwenkenbecher, P.; Sühs, K.-W.; Alvermann, S.; Gingele, S.; Skripuletz, T.; Stangel, M. Investigation of Oligoclonal IgG Bands in Tear Fluid of Multiple Sclerosis Patients. Front. Immunol. 2019, 10, 1110. [Google Scholar] [CrossRef]
- Belviranli, S.; Oltulu, P.; Uca, A.U.; Gundogan, A.O.; Mirza, E.; Altas, M.; Turk, N.; Oltulu, R. Conjunctival Impression Cytology and Tear Film Parameters in Patients with Multiple Sclerosis. Int. Ophthalmol. 2022, 42, 593–600. [Google Scholar] [CrossRef]
| Method of Tears Collection | Method of Identification/ Analysis | References |
|---|---|---|
| Schirmer strips | Mass spectrometry | [13,24] |
| Absorbent materials/sponge | ELISA 2D-electrophoresis | [48,51] |
| Microcapillary tubes | ELISA Multiplex bead analysis | [53,54,64] |
| Mass spectrometry Microarray | [48,50] | |
| Micropipette | SDS-PAGE | [49] |
| Contact lens/Biosensors | Bioelectrochemical field/electrochemical techniques | [55,60,61,62] |
| ELISA | [52] |
| Biomarker/s | Number of Patients | Method of Tears Collection | Method of Identification/Analysis | Results | References |
|---|---|---|---|---|---|
| Corneal sensitivity/tear function | AD n = 20, MS n = 20, PD n = 30, Friedreich ataxia (FA) n = 10, epilepsy (EP) n = 21 | Schirmer test | (1) Central corneal sensitivity was measured using a Cochet–Bonnet esthesiometer. (2) Schirmer’s test score. (3) Tear function tests included tear break-up time (TBUT) | (1) Mean corneal sensitivity was significantly reduced in AD, MS, PD, and EP patients, in comparison to CTR; (2) mean TBUT level was significantly shorter in patients with AD and MS; (3) Mean Schirmer’s 1 test score was significantly lower in EP patients; (4) The reduction in mean corneal sensitivity in the AD and PD groups was significantly more than in FA and MS groups. Mean TBUT levels in AD, MS, and PD groups were significantly shorter than in FA and EP groups; (5) Mean Schirmer’s test scores in AD and PD groups were significantly lower than in MS, FA, and EP groups. | [7] |
| Conjunctival impression cytology (CIC) grades; tear break-up time (TBUT), Schirmer 1 test results; ocular surface disease index (OSDI) scores | CTR n = 33, MS n = 33 | Schirmer test | (1) TBUT and Schirmer 1 tests were performed; (2) CIC samples were collected | (1) Mean CIC grade was higher in the MS group than in the CTR (1.48 ± 0.71 and 0.39 ± 0.56, respectively; p = 0.001). In the MS group, the CIC of the 14 participants (42.4%) was grade 2–3. In CTR, CIC of the only one participant (3.3%) was grade 2, and none of them were grade 3. (2) TBUT (8.12 ± 3.16, 13.06 ± 4.23 s in MS and CTR, respectively; p = 0.001); (3) Schirmer 1 test results (8.45 ± 5.75, 17.36 ± 10.89 mm in MS and CTR, respectively; p = 0.001) were lower; (4) OSDI score (36.36 ± 19.19, 13.70 ± 15.36 in MS and CTR, respectively; p = 0.001) was higher in the MS group. | [122] |
| Oligoclonal bands (OCBs) | No data | No data | Flow cytometry, nLC-ESI-QTOF-MS/MS | (1) MVs form neuronal and microglial origin are detectable in the CSF and tears from MS patients | [119] |
| Oligoclonal bands (OCBs) | MS n = 59 | Schirmer strips; flush procedure and plastic capillary tubes | Isoelectric focusing in polyacrylamide gels; Immunoblotting | (1) The collection of IgG in tears was most reliable by using Schirmer strips; (2) The concordance of OCB in tears and CSF of all investigated MS patients was 39% with a high rate of only marginal pattern in tears; (3) Not recommended for tear OCB detection as replacement for CSF OCB detection in MS patients. | [121] |
| Oligoclonal bands (OCBs) | CTR n = 44, MS n = 22 | Capillary tubes or Schirmer strips | ELISA | (1) OCB in tear fluid was not specific for MS; (2) The presence of OCB in the tear fluid could not be related to the laboratory and clinical parameters. | [120] |
| Lipids containing choline; free carnitine; acylcarnitines and amino acids | CTR n = 21, MS n = 12 | LC-MS/MS | (1) Tear lipidomics showed 30 phospholipids significantly modulated and many sphingomyelins resulted lower in MS; (2) The metabolomics approach carried out in both tears and serum highlighted the diagnostic potential of specific amino acids and acylcarnitines. | [5] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król-Grzymała, A.; Sienkiewicz-Szłapka, E.; Fiedorowicz, E.; Rozmus, D.; Cieślińska, A.; Grzybowski, A. Tear Biomarkers in Alzheimer’s and Parkinson’s Diseases, and Multiple Sclerosis: Implications for Diagnosis (Systematic Review). Int. J. Mol. Sci. 2022, 23, 10123. https://doi.org/10.3390/ijms231710123
Król-Grzymała A, Sienkiewicz-Szłapka E, Fiedorowicz E, Rozmus D, Cieślińska A, Grzybowski A. Tear Biomarkers in Alzheimer’s and Parkinson’s Diseases, and Multiple Sclerosis: Implications for Diagnosis (Systematic Review). International Journal of Molecular Sciences. 2022; 23(17):10123. https://doi.org/10.3390/ijms231710123
Chicago/Turabian StyleKról-Grzymała, Angelika, Edyta Sienkiewicz-Szłapka, Ewa Fiedorowicz, Dominika Rozmus, Anna Cieślińska, and Andrzej Grzybowski. 2022. "Tear Biomarkers in Alzheimer’s and Parkinson’s Diseases, and Multiple Sclerosis: Implications for Diagnosis (Systematic Review)" International Journal of Molecular Sciences 23, no. 17: 10123. https://doi.org/10.3390/ijms231710123
APA StyleKról-Grzymała, A., Sienkiewicz-Szłapka, E., Fiedorowicz, E., Rozmus, D., Cieślińska, A., & Grzybowski, A. (2022). Tear Biomarkers in Alzheimer’s and Parkinson’s Diseases, and Multiple Sclerosis: Implications for Diagnosis (Systematic Review). International Journal of Molecular Sciences, 23(17), 10123. https://doi.org/10.3390/ijms231710123











