Neuropathology of the Basal Ganglia in SNCA Transgenic Rat Model of Parkinson’s Disease: Involvement of Parvalbuminergic Interneurons and Glial-Derived Neurotropic Factor
Abstract
1. Introduction
2. Results
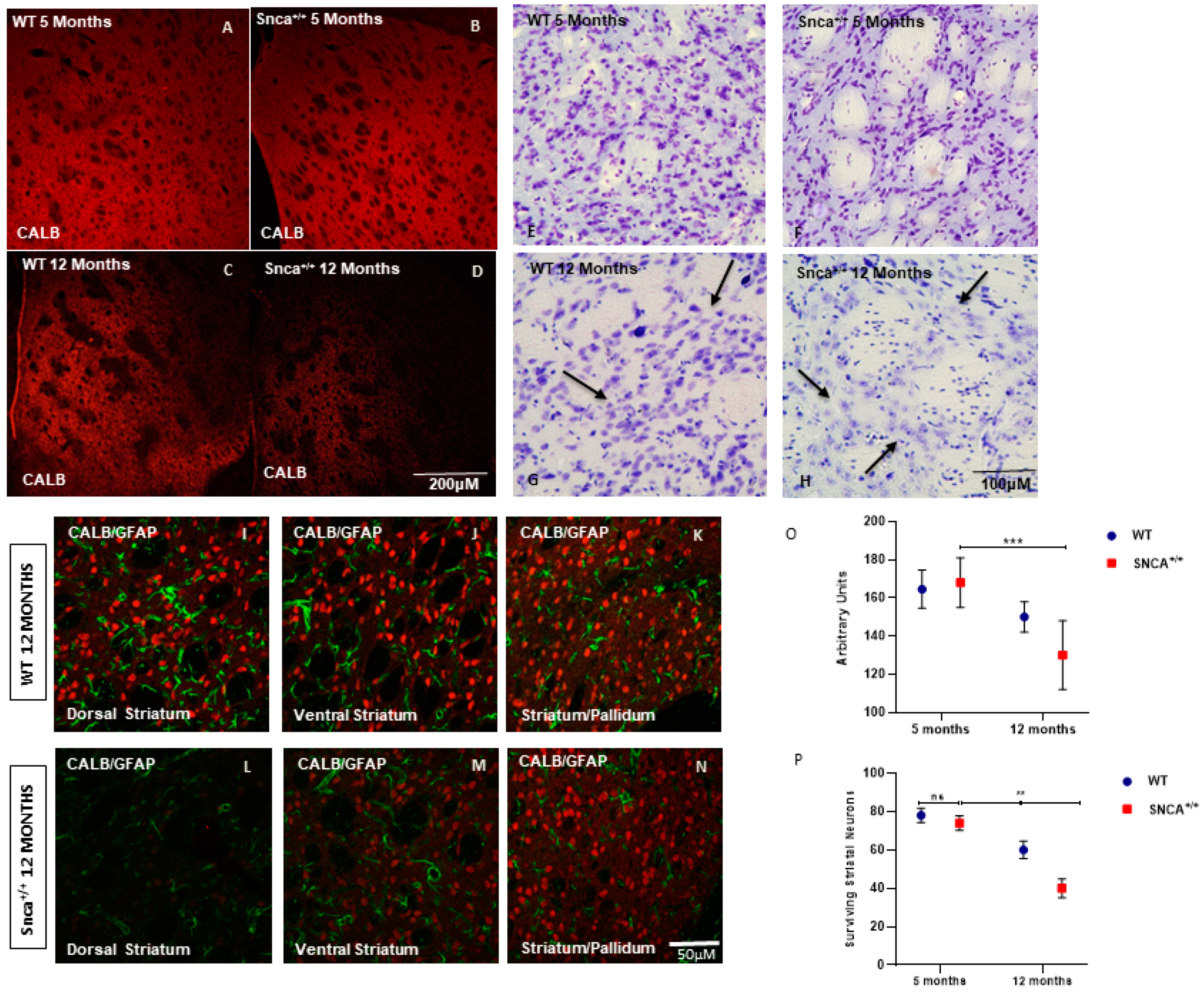
2.1. Striatal Neuronal Cell Counts
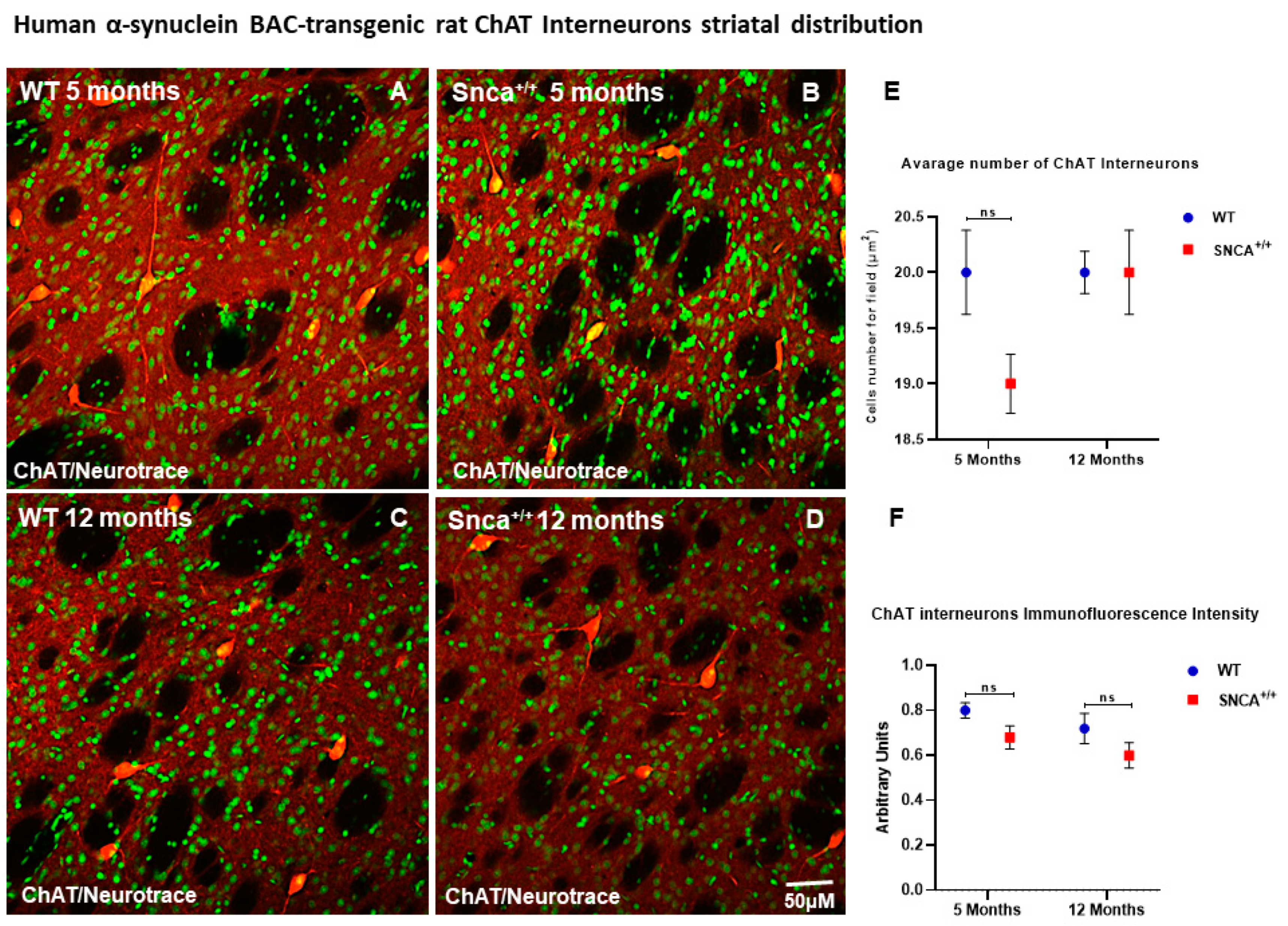
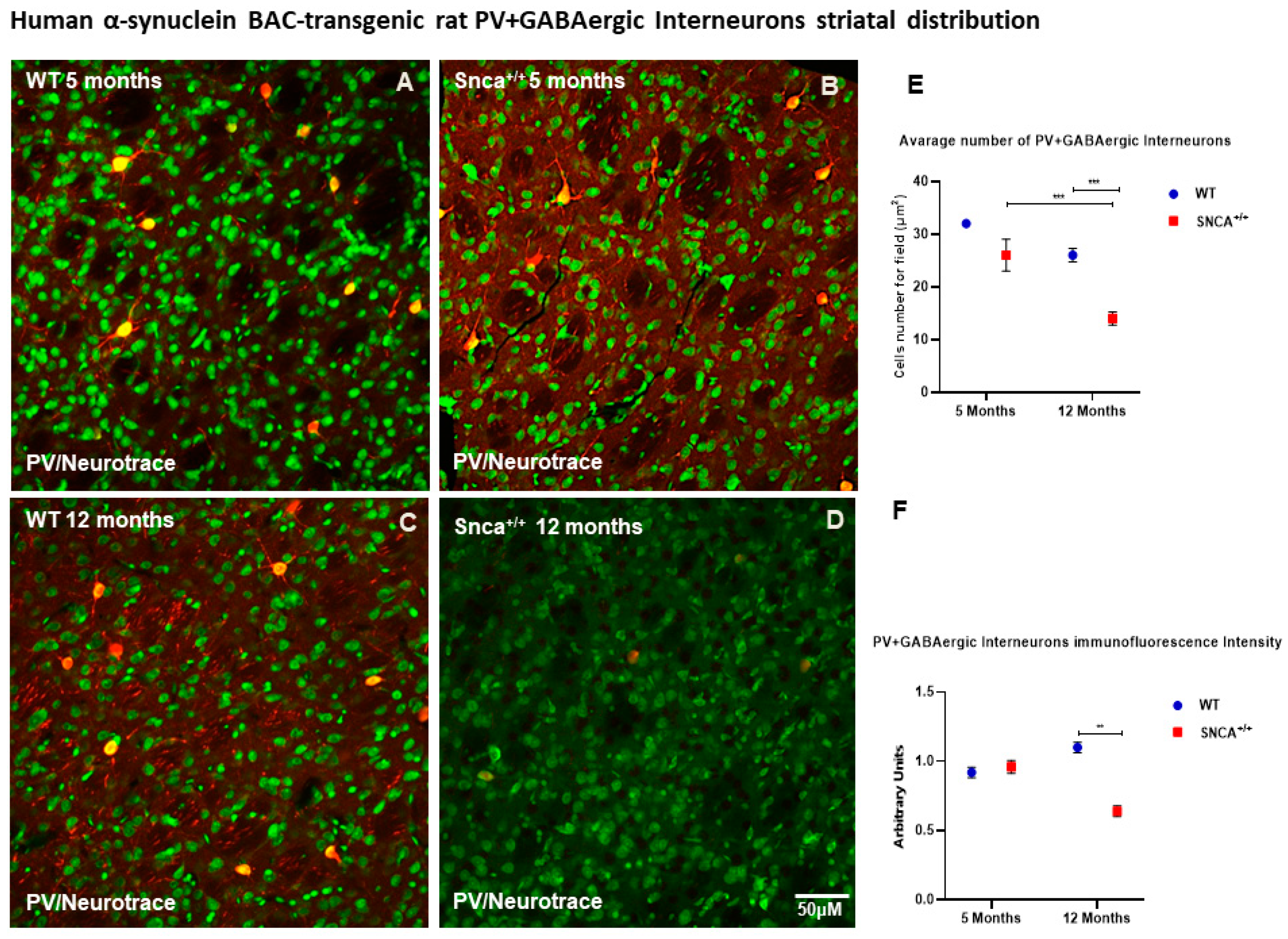
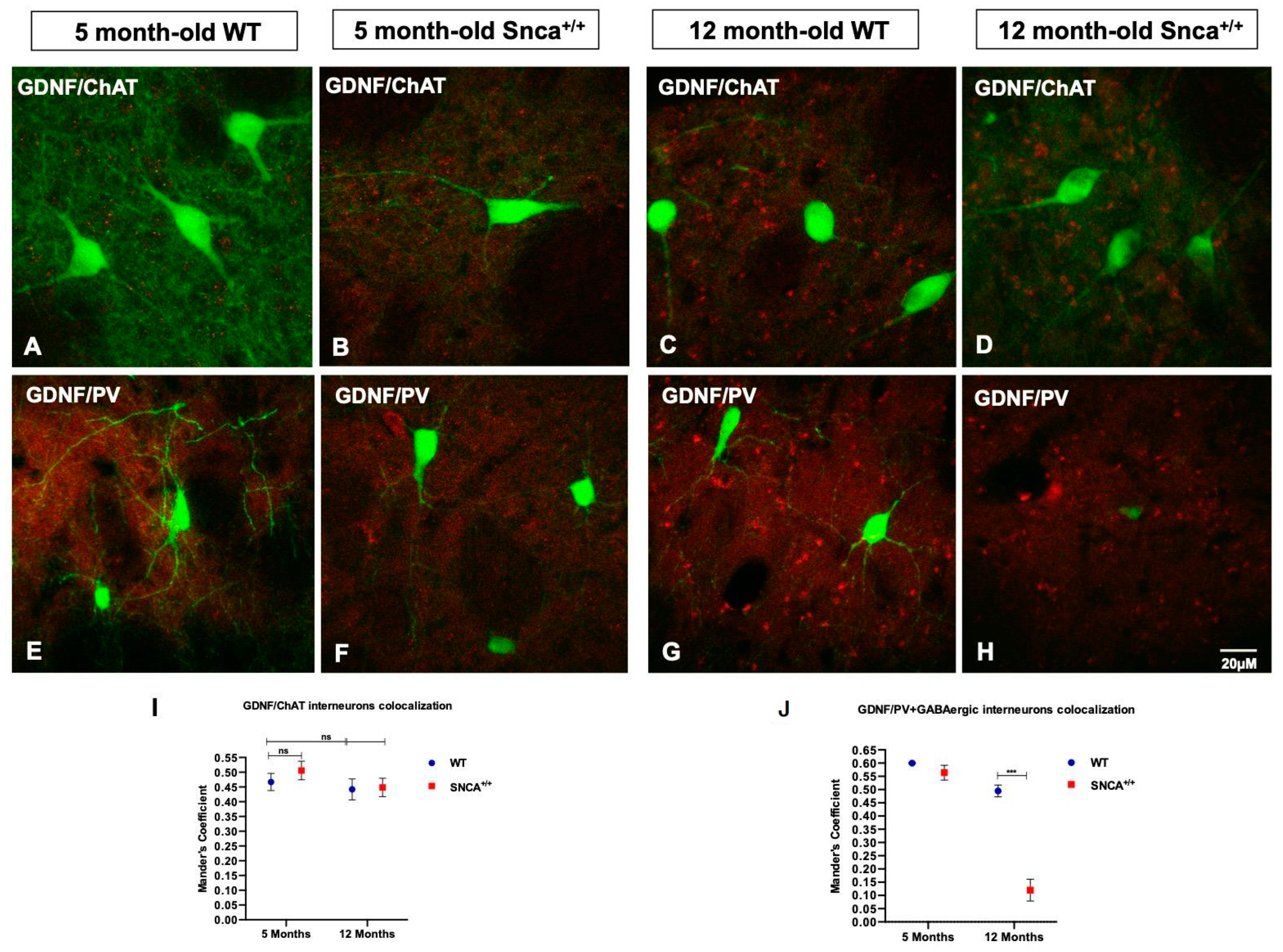
2.2. Interneuron Subtype Distribution
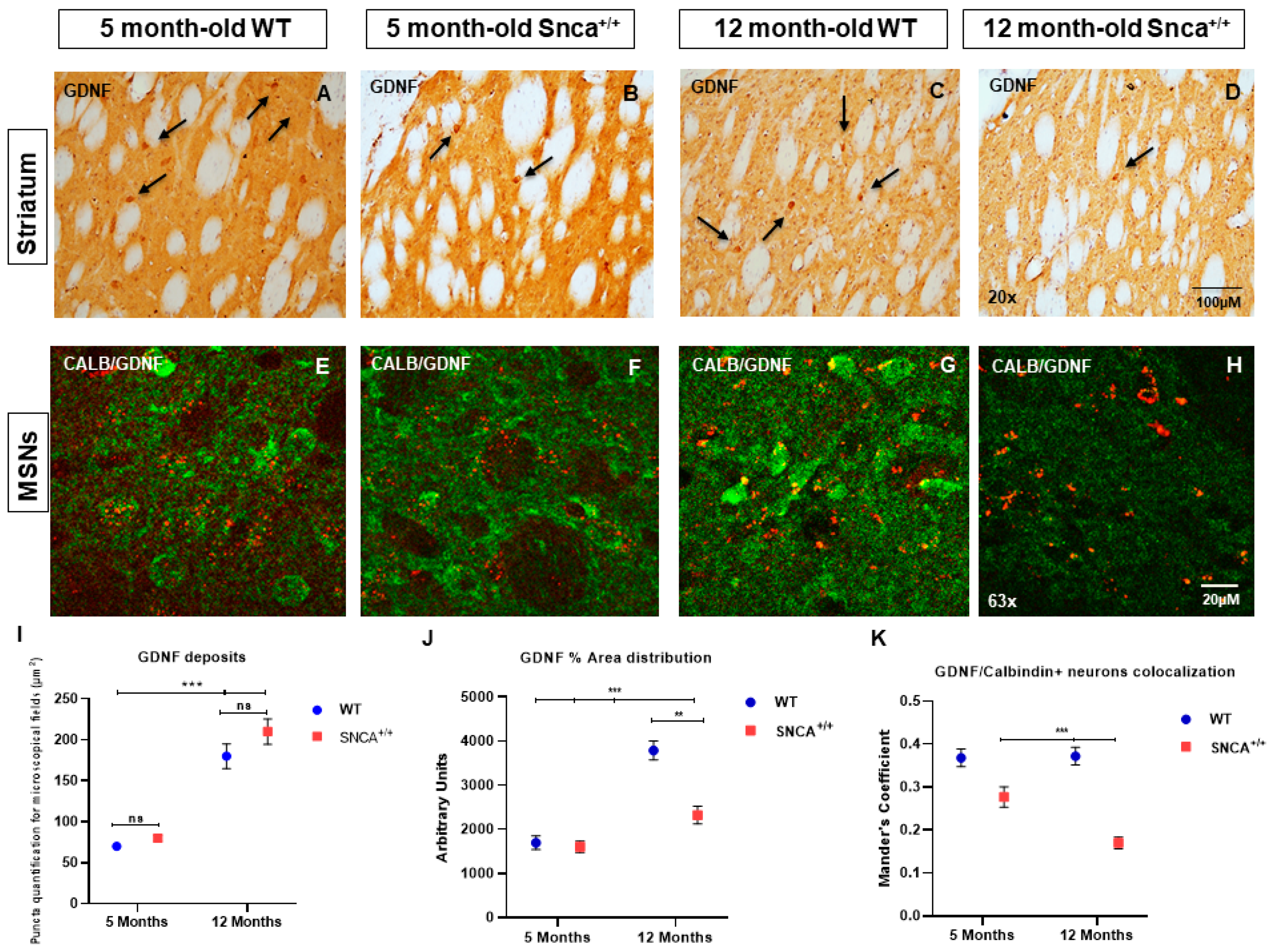
2.3. GDNF Expression in the Snca+/+ Rats’ Striatum
2.4. Altered GDNF Protein Expression in the Substantia Nigra
3. Discussion
4. Materials and Methods
4.1. Genetic Animal Model
4.2. Histological and Immunohistochemical Studies
- Tissue processing
- Histological and immunohistochemical studies
- Striatal interneuron subtype characterization
- Immunohistochemistry for GDNF
- Analysis of colocalization of GDNF in striatal projection neurons, interneurons, and dopaminergic neurons
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Monchi, O.; Petrides, M.; Mejia-Constain, B.; Strafella, A.P. Cortical activity in Parkinson’s disease during executive processing depends on striatal involvement. Brain 2007, 130, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Neuropathology of sporadic Parkinson’s disease evaluation and changes of concepts. Mov. Disord. 2012, 27, 8–30. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s diease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Owen, A.M. Cognitive dysfunction in Parkinson’s disease: The role of frontostriatal circuitry. Neuroscientist 2004, 10, 525–537. [Google Scholar] [CrossRef]
- Braak, H.; Ghebremedhin, E.; Rub, U.; Bratzke, H.; Del Tredici, K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004, 318, 121–134. [Google Scholar] [CrossRef]
- Tsuboi, Y.; Dickson, D.W. Dementia with Lewy bodies and Parkinson’s disease with dementia: Are they different? Parkinsonism Relat. Disord. 2005, 11 (Suppl. 1), S47–S51. [Google Scholar] [CrossRef]
- Steeves, T.D.; Miyasaki, J.; Zurowski, M.; Lang, A.E.; Pellecchia, G.; Van Eimeren, T.; Rusjan, P.; Houle, S.; Strafella, A.P. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: A [11C] raclopride PET study. Brain 2009, 132, 1376–1385. [Google Scholar] [CrossRef]
- Taylor, A.E.; Saint-Cyr, J.A.; Lang, A.E. Frontal lobe dysfunction in Parkinson’s disease. The cortical focus of neostriatal outflow. Brain 1986, 109, 845–883. [Google Scholar] [CrossRef]
- Zgaljardic, D.J.; Foldi, N.S.; Borod, J.C. Cognitive and behavioral dysfunction in Parkinson’s disease: Neurochemical and clinicopathological contributions. J. Neural Transm. 2004, 111, 1287–1301. [Google Scholar] [CrossRef]
- Alexander, G.E.; DeLong, M.R.; Strick, P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986, 9, 357–381. [Google Scholar] [CrossRef] [PubMed]
- Connolly, B.; Fox, S.H. Treatment of cognitive, psychiatric, and affective disorders associated with Parkinson’s disease. Neurother. J. Am. Soc. Exp. Neurother. 2014, 11, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.W.; Khoo, T.K.; Yarnall, A.J.; O’Brien, J.T.; Coleman, S.Y.; Brooks, D.J.; Barker, R.A.; Burn, D.J. Health-related quality of life in early Parkinson’s disease: The impact of nonmotor symptoms. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Mufti, S.; LaFaver, K. Mood Disorders in Parkinsons Disease. Psychiatr. Ann. 2020, 50, 95–99. [Google Scholar] [CrossRef]
- Lin, L.F.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef]
- Ibáñez, C.F. Catecholaminergic neuron survival: Getting hooked on GDNF. Nat. Neurosci. 2008, 11, 735–736. [Google Scholar] [CrossRef]
- Pascual, A.; Hidalgo-Figueroa, M.; Piruat, J.I.; Pintado, C.O.; Gómez-Díaz, R.; López-Barneo, J. Absolute requirement of GDNF for adult cat- echolaminergic neuron survival. Nat. Neurosci. 2008, 11, 755–761. [Google Scholar] [CrossRef]
- Beck, K.D.; Valverde, J.; Alexi, T.; Poulsen, K.; Moffat, B.; Vandlen, R.A.; Rosenthal, A.; Hefti, F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature 1995, 373, 339–341. [Google Scholar] [CrossRef]
- Hidalgo-Figueroa, M.; Bonilla, S.; Gutiérrez, F.; Pascual, A.; López-Barneo, J. GDNF Is Predominantly Expressed in the PV+ Neostriatal Interneuronal Ensemble in Normal Mouse and after Injury of the Nigrostriatal Pathway. J. Neurosci. 2012, 32, 864–872. [Google Scholar] [CrossRef]
- Björklund, A.; Dunnett, S.B. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef]
- Tepper, J.M.; Koós, T.; Ibanez-Sandoval, O.; Tecuapetla, F.; Faust, T.W.; Assous, M. Heterogeneity and Diversity of Striatal GABAergic Interneurons: Update 2018. Front. Neuroanat. 2018, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Bizon, J.L.; Lauterborn, J.C.; Gall, C.M. Subpopulations of striatal interneurons can be distinguished on the basis of neurotrophic factor expression. J. Comp. Neurol. 1999, 408, 283–298. [Google Scholar] [CrossRef]
- Tanimura, A.; Pancani, T.; Lim, S.A.O.; Tubert, C.; Melendez, A.E.; Shen, W.; Surmeier, D.J. Striatal cholinergic interneurons and Parkinson’s disease. Eur. J. Neurosci. 2018, 47, 1148–1158. [Google Scholar] [CrossRef]
- Fusco, F.R.; Zuccato, C.; Tartari, M.; Martorana, A.; de March, Z.; Giampà, C.; Cattaneo, E.; Bernardi, G. Co-localization of brain-derived neurotrophic factor (BDNF) and wild-type huntingtin in normal and quinolinic acid-lesioned rat brain. Eur. J. Neurosci. 2003, 18, 1093–1102. [Google Scholar] [CrossRef]
- Graybiel, A.M.; Hirsch, E.C.; Agid, Y. The nigrostriatal system in Parkinson’s disease. Adv. Neurol. 1990, 53, 17–29. [Google Scholar]
- Billingsley, K.J.; Bandres-Ciga, S.; Saez-Atienzar, S.; Singleton, A.B. Genetic risk factors in Parkinson’s disease. Cell Tissue Res. 2018, 373, 9–20. [Google Scholar] [CrossRef]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef]
- Valente, E.M.; Abou-Sleiman, P.M.; Caputo, V.; Muqit, M.M.; Harvey, K.; Gispert, S.; Ali, Z.; Del Turco, D.; Bentivoglio, A.R.; Healy, D.G.; et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 2004, 304, 1158–1160. [Google Scholar] [CrossRef]
- Zimprich, A.; Biskup, S.; Leitner, P.; Lichtner, P.; Farrer, M.; Lincoln, S.; Kachergus, J.; Hulihan, M.; Uitti, R.J.; Calne, D.B.; et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004, 44, 601–607. [Google Scholar] [CrossRef]
- Nuber, S.; Harmuth, F.; Kohl, Z.; Adame, A.; Trejo, M.; Schönig, K.; Zimmermann, F.; Bauer, C.; Casadei, N.; Giel, C.; et al. A progressive dopaminergic phenotype associated with neurotoxic conversion of α-synuclein in BAC-transgenic rats. Brain J. Neurol. 2013, 136, 412–432. [Google Scholar] [CrossRef] [PubMed]
- Brimblecombe, K.R.; Cragg, S.J. The Striosome and Matrix Compartments of the Striatum: A Path through the Labyrinth from Neurochemistry toward Function. ACS Chem Neurosci. 2017, 8, 235–242. [Google Scholar] [CrossRef]
- Prensa, L.; Giménez-Amaya, J.M.; Parent, A. Chemical heterogeneity of the striosomal compartment in the human striatum. J. Comp. Neurol. 1999, 413, 603–618. [Google Scholar] [CrossRef]
- Johnston, J.G.; Gerfen, C.R.; Haber, S.N.; van der Kooy, D. Mechanisms of striatal pattern formation: Conservation of mammalian compartmentalization. Dev. Brain Res. 1990, 57, 93–102. [Google Scholar] [CrossRef]
- Ferrante, R.J.; Kowall, N.W. Tyrosine hydroxylase-like immunoreactivity is distributed in the matrix compartment of normal human and Huntington’s disease striatum. Brain Res. 1987, 416, 141–146. [Google Scholar] [CrossRef]
- Prensa, L.; Cossette, M.; Parent, A. Dopaminergic innervation of human basal ganglia. J. Chem. Neuroanat. 2000, 20, 207–213. [Google Scholar] [CrossRef]
- Huot, P.; Lévesque, M.; Parent, A. The fate of striatal dopaminergic neurons in Parkinson’s disease and Huntington’s chorea. Brain 2007, 130, 222–232. [Google Scholar] [CrossRef][Green Version]
- Graybiel, A.M. The basal ganglia and chunking of action repertoires. Neurobiol. Learn. Mem. 1998, 70, 119–136. [Google Scholar] [CrossRef]
- Schultz, W. Getting formal with dopamine and reward. Neuron 2002, 36, 241–263. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Bolam, J.P. Chapter 1-The Neuroanatomical Organization of the Basal Ganglia. Handb. Behav. Neurosci. 2010, 20, 3–28. [Google Scholar]
- Petryszyn, S.; Parent, A.; Parent, M. The calretinin interneurons of the striatum: Comparisons between rodents and primates under normal and pathological conditions. J. Neural. Transm. 2018, 128, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.A.; Ding, J.B.; Surmeier, D.J. Muscarinic Modulation of Striatal Function and Circuitry. In Muscarinic Receptors; Springer: Berlin/Heidelberg, Germany, 2012; pp. 223–241. [Google Scholar]
- Pisani, A.; Bernardi, G.; Ding, J.; Surmeier, D.J. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007, 30, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Won, L.; Britt, J.P.; Lim, S.A.O.; McGehee, D.S.; Kang, U.J. Enhanced striatal cholinergic neuronal activity mediates l-DOPA–induced dyskinesia in parkinsonian mice. Proc. Natl. Acad. Sci. USA 2011, 108, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Gerfen, C.R.; Herkenham, M.; Thibault, J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J. Neurosci. 1987, 7, 3915–3934. [Google Scholar] [CrossRef]
- Matsuda, W.; Furuta, T.; Nakamura, K.C.; Hioki, H.; Fujiyama, F.; Arai, R.; Kaneko, T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J. Neurosci. 2009, 29, 444–453. [Google Scholar] [CrossRef]
- Stefanova, N.; Puschban, Z.; Fernagut, P.O.; Brouillet, E.; Tison, F.; Reindl, M.; Jellinger, K.A.; Poewe, W.; Wenning, G.K. Neuropathological and behavioral changes induced by various treatment paradigms with MPTP and 3-nitropropionic acid in mice: Towards a model of striatonigral degeneration (multiple system atrophy). Acta. Neuropathol. 2003, 106, 157–166. [Google Scholar] [CrossRef]
- Simioni, A.C.; Dagher, A.; Fellows, L.K. Compensatory striatal-cerebel- lar connectivity in mild-moderate Parkinson’s disease. Neuroimage Clin. 2016, 10, 54–62. [Google Scholar] [CrossRef]
- Horne, M.K.; Butler, E.G. The role of the cerebello-thalamo-cortical pathway in skilled movement. Prog. Neurogibol. 1995, 46, 199–213. [Google Scholar] [CrossRef]
- Shen, B.; Pan, Y.; Jiang, X.; Wu, Z.; Zhu, J.; Dong, J.; Zhang, W.; Xu, P.; Dai, Y.; Gao, Y.; et al. Altered putamen and cerebellum connectivity among different subtypes of Parkinson’s disease. CNS Neurosci. Ther. 2020, 26, 207–214. [Google Scholar] [CrossRef]
- Cheshire, P.; Ayton, S.; Bertram, K.L.; Ling, H.; Li, A.; McLean, C.; Halliday, G.M.; O’Sullivan, S.S.; Revesz, T.; Finkelstein, D.I.; et al. Serotonergic markers in Parkinson’s disease and levodopa-induced dyskinesias. Mov. Disord. 2015, 30, 796–804. [Google Scholar] [CrossRef]
- Sian-Hulsmann, J.; Mandel, S.; Youdim, M.B.; Riederer, P. The relevance of iron in the pathogenesis of Parkinson’s disease. J. Neurochem. 2011, 118, 939–957. [Google Scholar] [CrossRef] [PubMed]
- Michel, P.P.; Alvarez-Fischer, D.; Guerreiro, S.; Hild, A.; Hartmann, A.; Hirsch, E.C. Role of activity-dependent mechanisms in the control of dopaminergic neuron survival. J. Neurochem. 2007, 101, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Bugiani, O.; Perdelli, F.; Salvarani, S.; Leonardi, A.; Mancardi, G.L. Loss of striatal neurons in Parkinson’s disease: A cytometric study. Eur. Neurol. 1980, 19, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Burguière, E.; Monteiro, P.; Feng, G.; Graybiel, A.M. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science 2013, 340, 1243–1246. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, S.; Wang, H.L.; Barker, D.J.; Miranda-Barrientos, J.; Morales, M. VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat. Neurosci. 2016, 19, 725–733. [Google Scholar] [CrossRef]
- Soós, J.; Engelhardt, J.I.; Siklós, L.; Havas, L.; Majtényi, K. The expression of PARP, NF-κB and parvalbumin is increased in Parkinson disease. Neuroreport 2004, 15, 1715–1718. [Google Scholar] [CrossRef]
- d’anglemont de Tassigny, X.; Pascual, A.; López-Barneo, J. GDNF-based therapies, GDNF-producing interneurons, and trophic support of the dopaminergic nigrostriatal pathway. Implications for Parkinson’s disease. Front. Neuroanat. 2015, 9, 10. [Google Scholar]
- Duarte Azevedo, M.; Sander, S.; Tenenbaum, L. GDNF, A Neuron-Derived Factor Upregulated in Glial Cells during Disease. J. Clin. Med. 2020, 9, 456. [Google Scholar] [CrossRef]
- Merienne, N.; Meunier, C.; Schneider, A.; Seguin, J.; Nair, S.S.; Rocher, A.B.; Le Gras, S.; Keime, C.; Faull, R.; Pellerin, L.; et al. Cell-type-specific gene expression profiling in adult mouse brain reveals normal and disease-state signatures. Cell Rep. 2019, 26, 2477–2493. [Google Scholar] [CrossRef]
- Marco, S.; Canudas, A.M.; Canals, J.M.; Gavalda, N.; Perez-Navarro, E.; Alberch, J. Excitatory amino acids differentially regulate the expression of GDNF, neurturin, and their receptors in the adult rat striatum. Exp. Neurol. 2002, 174, 243–252. [Google Scholar] [CrossRef]
- Arenas, E.; Trupp, M.; Akerud, P.; Ibáñez, C.F. GDNF prevents degeneration and promotes the phenotype of brain noradrenergic neurons in vivo. Neuron 1995, 15, 1465–1473. [Google Scholar] [CrossRef]
- Sauer, H.; Rosenblad, C.; Bjorklund, A. Glial cell line-derived neurotrophic factor but not transforming growth factor beta 3 prevents delayed degeneration of nigral dopaminergic neurons following striatal 6-hydroxydopamine lesion. Proc. Natl. Acad. Sci. USA 1995, 92, 8935–8939. [Google Scholar] [CrossRef] [PubMed]
- Gash, D.M.; Zhang, Z.; Ovadia, A.; Cass, W.A.; Yi, A.; Simmerman, L.; Russell, D.; Martin, D.; Lapchak, P.A.; Collins, F.; et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature 1996, 380, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Whone, A.; Luz, M.; Boca, M.; Woolley, M.; Mooney, L.; Dharia, S.; Broadfoot, J.; Cronin, D.; Schroers, C.; Barua, N.U.; et al. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain 2019, 142, 512–525. [Google Scholar] [CrossRef]
- Tomac, A.; Lindqvist, E.; Lin, L.F.; Ogren, S.O.; Young, D.; Hoffer, B.J.; Olson, L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 1995, 373, 335–339. [Google Scholar] [CrossRef]
- Oppenheim, R.W.; Houenou, L.J.; Johnson, J.E.; Lin, L.F.; Li, L.; Lo, A.C.; Newsome, A.L.; Prevette, D.M.; Wang, S. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature 1995, 373, 344–346. [Google Scholar] [CrossRef]
- Guatteo, E.; Rizzo, F.R.; Federici, M.; Cordella, A.; Ledonne, A.; Latini, L.; Nobili, A.; Viscomi, M.T.; Biamonte, F.; Landrock, K.K.; et al. Functional alterations of the dopaminergic and glutamatergic systems in spontaneous α-synuclein overexpressing rats. Exp. Neurol. 2017, 287, 21–33. [Google Scholar] [CrossRef]
- Yan, Q.; Matheson, C.; Lopez, O.T. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature 1995, 373, 341–344. [Google Scholar] [CrossRef]
- Tai, M.H.; Cheng, H.; Wu, J.P.; Liu, Y.L.; Lin, P.R.; Kuo, J.S.; Tseng, C.J.; Tzeng, S.F. Gene transfer of glial cell line-derived neurotrophic factor promotes functional recovery following spinal cord contusion. Exp. Neurol. 2003, 183, 508–515. [Google Scholar] [CrossRef]
- Sala, G.; Bocci, T.; Borzì, V.; Parazzini, M.; Priori, A.; Ferrarese, C. Direct current stimulation enhances neuronal alpha-synuclein degradation in vitro. Sci. Rep. 2021, 11, 2197. [Google Scholar] [CrossRef]
- Winkler, C.; Reis, J.; Hoffmann, N.; Gellner, A.K.; Münkel, C.; Curado, M.R.; Furlanetti, L.; Garcia, J.; Döbrössy, M.D.; Fritsch, B. Anodal Transcranial Direct Current Stimulation Enhances Survival and Integration of Dopaminergic Cell Transplants in a Rat Parkinson Model. eNeuro 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Machado-Vieira, R.; Zarate, C.A., Jr.; Vieira, E.L.; Valiengo, L.; Benseñor, I.M.; Lotufo, P.A.; Gattaz, W.F.; Teixeira, A.L. Assessment of non-BDNF neurotrophins and GDNF levels after depression treatment with sertraline and transcranial direct current stimulation in a factorial, randomized, sham-controlled trial (SELECT-TDCS): An exploratory analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 56, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Surguchov, A. Biomarkers in Parkinson’s Disease. In Neurodegenerative Diseases Biomarkers; Peplow, P.V., Martinez, B., Gennarelli, T.A., Eds.; Neuromethods; Humana: New York, NY, USA, 2022; Volume 173, pp. 155–180. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paldino, E.; D’angelo, V.; Massaro Cenere, M.; Guatteo, E.; Barattucci, S.; Migliorato, G.; Berretta, N.; Riess, O.; Sancesario, G.; Mercuri, N.B.; et al. Neuropathology of the Basal Ganglia in SNCA Transgenic Rat Model of Parkinson’s Disease: Involvement of Parvalbuminergic Interneurons and Glial-Derived Neurotropic Factor. Int. J. Mol. Sci. 2022, 23, 10126. https://doi.org/10.3390/ijms231710126
Paldino E, D’angelo V, Massaro Cenere M, Guatteo E, Barattucci S, Migliorato G, Berretta N, Riess O, Sancesario G, Mercuri NB, et al. Neuropathology of the Basal Ganglia in SNCA Transgenic Rat Model of Parkinson’s Disease: Involvement of Parvalbuminergic Interneurons and Glial-Derived Neurotropic Factor. International Journal of Molecular Sciences. 2022; 23(17):10126. https://doi.org/10.3390/ijms231710126
Chicago/Turabian StylePaldino, Emanuela, Vincenza D’angelo, Mariangela Massaro Cenere, Ezia Guatteo, Simone Barattucci, Giorgia Migliorato, Nicola Berretta, Olaf Riess, Giuseppe Sancesario, Nicola Biagio Mercuri, and et al. 2022. "Neuropathology of the Basal Ganglia in SNCA Transgenic Rat Model of Parkinson’s Disease: Involvement of Parvalbuminergic Interneurons and Glial-Derived Neurotropic Factor" International Journal of Molecular Sciences 23, no. 17: 10126. https://doi.org/10.3390/ijms231710126
APA StylePaldino, E., D’angelo, V., Massaro Cenere, M., Guatteo, E., Barattucci, S., Migliorato, G., Berretta, N., Riess, O., Sancesario, G., Mercuri, N. B., & Fusco, F. R. (2022). Neuropathology of the Basal Ganglia in SNCA Transgenic Rat Model of Parkinson’s Disease: Involvement of Parvalbuminergic Interneurons and Glial-Derived Neurotropic Factor. International Journal of Molecular Sciences, 23(17), 10126. https://doi.org/10.3390/ijms231710126






