Ocular Lymphatic and Glymphatic Systems: Implications for Retinal Health and Disease
Abstract
1. Introduction
2. Aqueous Humour
2.1. Production and Secretion
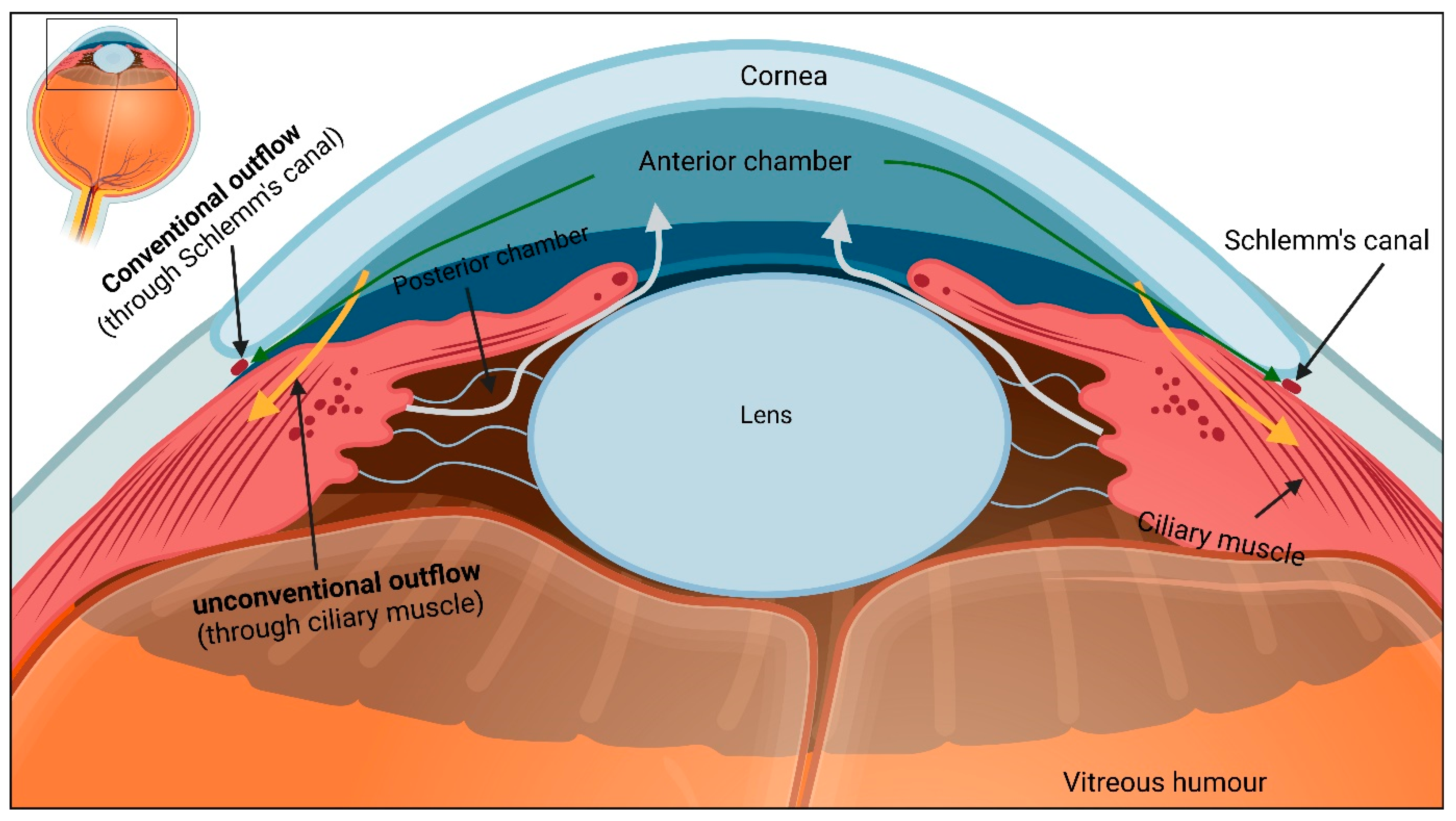
2.2. Aqueous Humour Outflow
3. Fluid Regulation in Retina
4. Lymphatic System
4.1. Structure–Function Relationship of Lymphatic Vessels
4.2. Functions of the Lymphatic System
4.3. Challenges in Identification of Lymphatic Vessels
4.4. Lymphatics in the Eye
5. Glymphatic System
5.1. Components of the Glymphatic System
5.1.1. Cerebrospinal Fluid
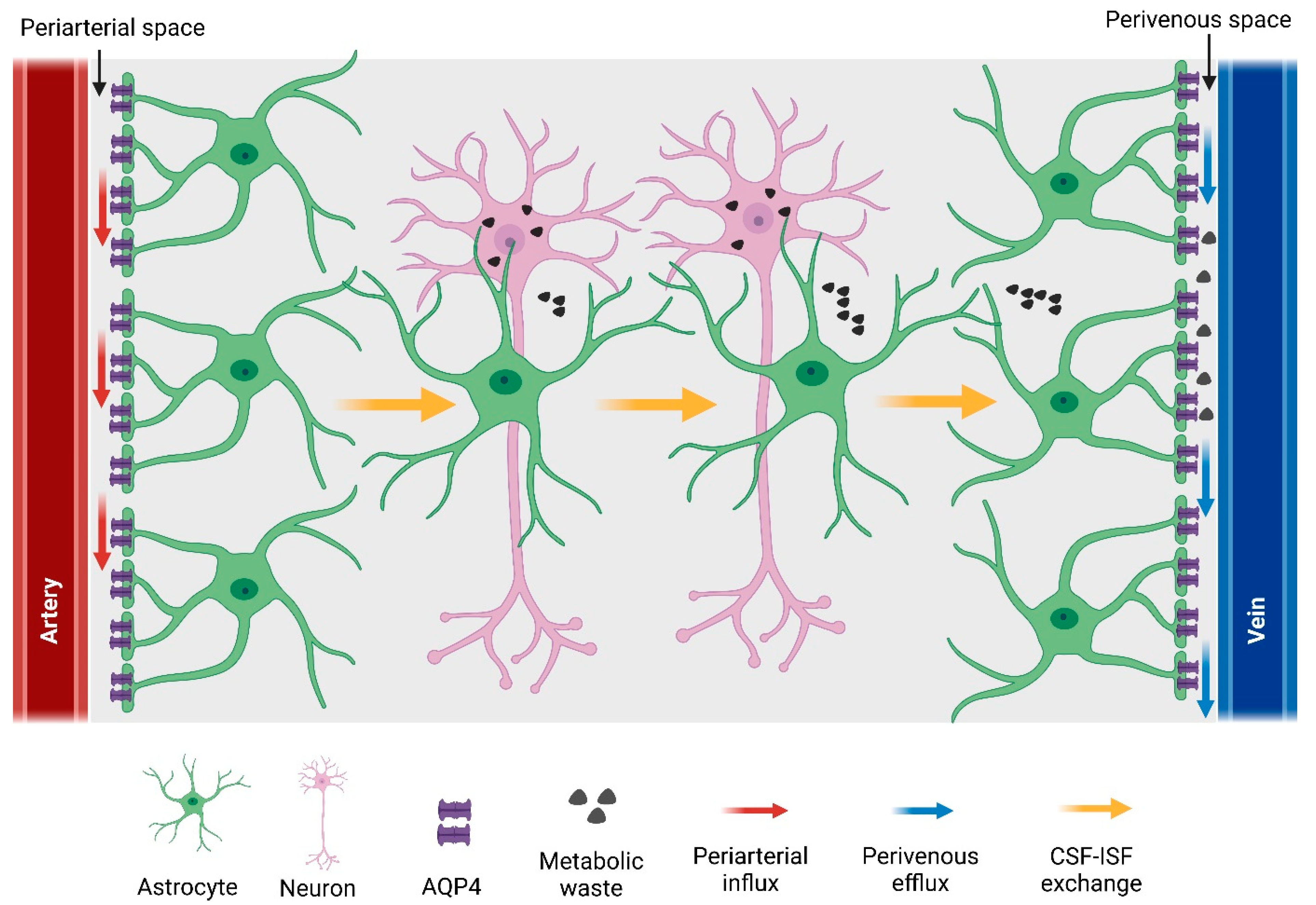
5.1.2. Cerebral Blood Vessels and Perivascular Space
5.1.3. Astrocytic Water Channel Aquaporin-4
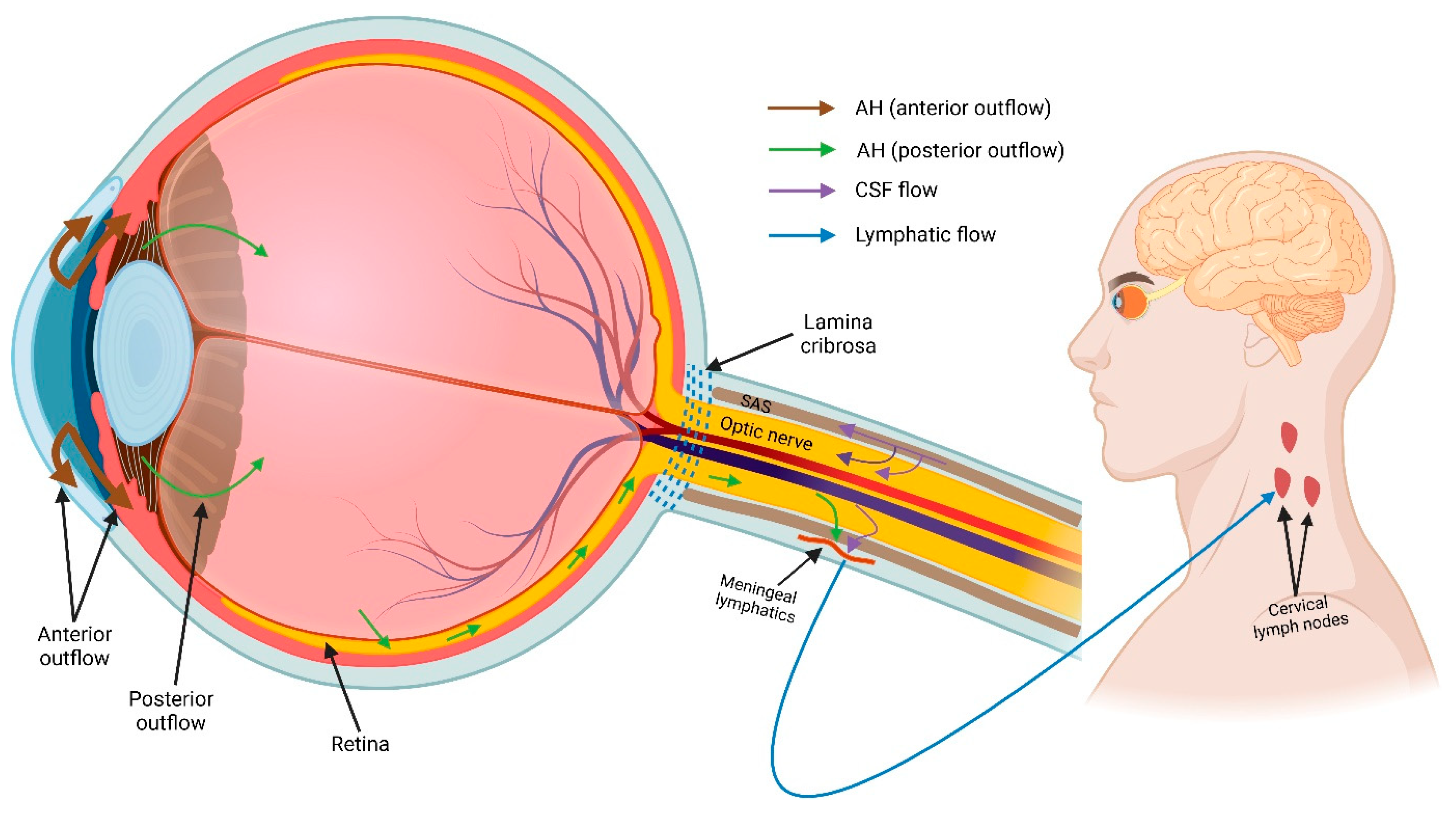
5.1.4. Ocular Glymphatic System
6. Lymphatics/Glymphatics in Retinal Degenerative Diseases
6.1. Glaucoma
6.2. Age-Related Macular Degerations
6.3. Other Retinal Diseases
7. Potential Avenues for Lymphatic/Glymphatic Modulation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open J. Ophthalmol. 2010, 4, 52–59. [Google Scholar] [CrossRef]
- Johnson, M.; McLaren, J.W.; Overby, D.R. Unconventional aqueous humor outflow: A review. Exp. Eye Res. 2017, 158, 94–111. [Google Scholar] [CrossRef]
- Cueni, L.N.; Detmar, M. The lymphatic system in health and disease. Lymphat Res. Biol. 2008, 6, 109–122. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Stamper, R.L.; Lieberman, M.F.; Drake, M.V. CHAPTER 2—Aqueous humor formation. In Becker-Shaffer’s Diagnosis and Therapy of the Glaucomas (Eighth Edition); Stamper, R.L., Lieberman, M.F., Drake, M.V., Eds.; Mosby: Edinburgh, UK, 2009; pp. 8–24. [Google Scholar]
- Civan, M.M. Chapter 1 Formation of the Aqueous Humor: Transport Components and Their Integration. In Current Topics in Membranes; Academic Press: Cambridge, MA, USA, 2008; Volume 62, pp. 1–45. [Google Scholar]
- Nesterova, A.P.; Klimov, E.A.; Zharkova, M.; Sozin, S.; Sobolev, V.; Ivanikova, N.V.; Shkrob, M.; Yuryev, A. Chapter 6—Diseases of the eye. In Disease Pathways; Nesterova, A.P., Klimov, E.A., Zharkova, M., Sozin, S., Sobolev, V., Ivanikova, N.V., Shkrob, M., Yuryev, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 259–296. [Google Scholar]
- Chowdhury, U.R.; Madden, B.J.; Charlesworth, M.C.; Fautsch, M.P. Proteome Analysis of Human Aqueous Humor. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4921–4931. [Google Scholar] [CrossRef]
- To, C.H.; Kong, C.W.; Chan, C.Y.; Shahidullah, M.; Do, C.W. The mechanism of aqueous humour formation. Clin. Exp. Optom. 2002, 85, 335–349. [Google Scholar]
- Thygesen, J. 88—Late Hypotony. In Glaucoma (Second Edition); Shaarawy, T.M., Sherwood, M.B., Hitchings, R.A., Crowston, J.G., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2015; pp. 863–881. [Google Scholar]
- Brubaker, R.F. Flow of aqueous humor in humans [The Friedenwald Lecture]. Investig. Ophthalmol. Vis. Sci. 1991, 32, 3145–3166. [Google Scholar]
- Yamaguchi, Y.; Watanabe, T.; Hirakata, A.; Hida, T. Localization and ontogeny of aquaporin-1 and -4 expression in iris and ciliary epithelial cells in rats. Cell Tissue Res. 2006, 325, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Tamm, E.R. The trabecular meshwork outflow pathways: Structural and functional aspects. Exp. Eye Res. 2009, 88, 648–655. [Google Scholar] [CrossRef]
- Braunger, B.M.; Fuchshofer, R.; Tamm, E.R. The aqueous humor outflow pathways in glaucoma: A unifying concept of disease mechanisms and causative treatment. Eur. J. Pharm. Biopharm. 2015, 95, 173–181. [Google Scholar] [CrossRef]
- Toris, C.B. Chapter 7 Aqueous Humor Dynamics I: Measurement Methods and Animal Studies. In Current Topics in Membranes; Academic Press: Cambridge, MA, USA, 2008; Volume 62, pp. 193–229. [Google Scholar]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Bill, A. The Aqueous Humor Drainage Mechanism in the Cynomolgus Monkey (Macaca irus) with Evidence for Unconventional Routes. Investig. Ophthalmol. Vis. Sci. 1965, 4, 911–919. [Google Scholar]
- Pederson, J.E.; Gaasterland, D.E.; MacLellan, H.M. Uveoscleral aqueous outflow in the rhesus monkey: Importance of uveal reabsorption. Investig. Ophthalmol. Vis. Sci. 1977, 16, 1008–1017. [Google Scholar]
- Yücel, Y.H.; Johnston, M.G.; Ly, T.; Patel, M.; Drake, B.; Gümüş, E.; Fraenkl, S.A.; Moore, S.; Tobbia, D.; Armstrong, D.; et al. Identification of lymphatics in the ciliary body of the human eye: A novel “uveolymphatic” outflow pathway. Exp. Eye Res. 2009, 89, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.W.; Lee, C.-J.; Gardiner, B.S. No flow through the vitreous humor: How strong is the evidence? Prog. Retin. Eye Res. 2020, 78, 100845. [Google Scholar] [CrossRef]
- Marmor, M.F. Mechanisms of fluid accumulation in retinal edema. Doc. Ophthalmol. 1999, 97, 239–249. [Google Scholar] [CrossRef]
- Bringmann, A.; Reichenbach, A.; Wiedemann, P. Pathomechanisms of cystoid macular edema. Ophthalmic Res. 2004, 36, 241–249. [Google Scholar] [CrossRef]
- Bringmann, A.; Uckermann, O.; Pannicke, T.; Iandiev, I.; Reichenbach, A.; Wiedemann, P. Neuronal versus glial cell swelling in the ischaemic retina. Acta Ophthalmol. Scand. 2005, 83, 528–538. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Reichenbach, A.; Wurm, A.; Pannicke, T.; Iandiev, I.; Wiedemann, P.; Bringmann, A. Müller cells as players in retinal degeneration and edema. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 627–636. [Google Scholar] [CrossRef]
- Goodyear, M.J.; Crewther, S.G.; Junghans, B.M. A role for aquaporin-4 in fluid regulation in the inner retina. Vis. Neurosci. 2009, 26, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Nagelhus, E.A.; Veruki, M.L.; Torp, R.; Haug, F.M.; Laake, J.H.; Nielsen, S.; Agre, P.; Ottersen, O.P. Aquaporin-4 water channel protein in the rat retina and optic nerve: Polarized expression in Müller cells and fibrous astrocytes. J. Neurosci. 1998, 18, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Goodyear, M.J.; Junghans, B.M.; Giummarra, L.; Murphy, M.J.; Crewther, D.P.; Crewther, S.G. A role for aquaporin-4 during induction of form deprivation myopia in chick. Mol. Vis. 2008, 14, 298–307. [Google Scholar] [PubMed]
- Dibas, A.; Yang, M.-H.; He, S.; Bobich, J.; Yorio, T. Changes in ocular aquaporin-4 (AQP4) expression following retinal injury. Mol. Vis. 2008, 14, 1770–1783. [Google Scholar] [PubMed]
- Amann, B.; Kleinwort, K.J.H.; Hirmer, S.; Sekundo, W.; Kremmer, E.; Hauck, S.M.; Deeg, C.A. Expression and Distribution Pattern of Aquaporin 4, 5 and 11 in Retinas of 15 Different Species. Int. J. Mol. Sci. 2016, 17, 1145. [Google Scholar] [CrossRef]
- Brinker, T.; Stopa, E.; Morrison, J.; Klinge, P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 2014, 11, 10. [Google Scholar] [CrossRef]
- Bringmann, A.; Wiedemann, P. Müller glial cells in retinal disease. Ophthalmologica 2012, 227, 1–19. [Google Scholar] [CrossRef]
- Nagelhus, E.A.; Horio, Y.; Inanobe, A.; Fujita, A.; Haug, F.M.; Nielsen, S.; Kurachi, Y.; Ottersen, O.P. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Müller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia 1999, 26, 47–54. [Google Scholar] [CrossRef]
- Hamann, S. Molecular mechanisms of water transport in the eye. Int. Rev. Cytol. 2002, 215, 395–431. [Google Scholar]
- Frishman, L.J.; Yamamoto, F.; Bogucka, J.; Steinberg, R.H. Light-evoked changes in [K+]o in proximal portion of light-adapted cat retina. J. Neurophysiol. 1992, 67, 1201–1212. [Google Scholar] [CrossRef]
- Uckermann, O.; Vargová, L.; Ulbricht, E.; Klaus, C.; Weick, M.; Rillich, K.; Wiedemann, P.; Reichenbach, A.; Syková, E.; Bringmann, A. Glutamate-Evoked Alterations of Glial and Neuronal Cell Morphology in the Guinea Pig Retina. J. Neurosci. 2004, 24, 10149. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Q.; Kobayashi, H.; Jin, Z.-B.; Wada, A.; Nao-I, N. Differential expression of Kir4.1 and aquaporin 4 in the retina from endotoxin-induced uveitis rat. Mol. Vis. 2007, 13, 309–317. [Google Scholar]
- Newman, E.A.; Frambach, D.A.; Odette, L.L. Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science 1984, 225, 1174–1175. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Horio, Y.; Tada, Y.; Hibino, H.; Inanobe, A.; Ito, M.; Yamada, M.; Gotow, T.; Uchiyama, Y.; Kurachi, Y. Expression and clustered distribution of an inwardly rectifying potassium channel, KAB-2/Kir4.1, on mammalian retinal Müller cell membrane: Their regulation by insulin and laminin signals. J. Neurosci. 1997, 17, 7725–7735. [Google Scholar] [CrossRef] [PubMed]
- Motulsky, E.; Koch, P.; Janssens, S.; Liénart, M.; Vanbellinghen, A.-M.; Bolaky, N.; Chan, C.-C.; Caspers, L.; Martin-Martinez, M.-D.; Xu, H.; et al. Aquaporin expression in blood-retinal barrier cells during experimental autoimmune uveitis. Mol. Vis. 2010, 16, 602–610. [Google Scholar] [CrossRef]
- Kofuji, P.; Newman, E.A. Potassium buffering in the central nervous system. Neuroscience 2004, 129, 1045–1056. [Google Scholar] [CrossRef]
- Stamer, W.D.; Bok, D.; Hu, J.; Jaffe, G.J.; McKay, B.S. Aquaporin-1 channels in human retinal pigment epithelium: Role in transepithelial water movement. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2803–2808. [Google Scholar] [CrossRef]
- Willermain, F.; Janssens, S.; Arsenijevic, T.; Piens, I.; Bolaky, N.; Caspers, L.; Perret, J.; Delporte, C. Osmotic stress decreases aquaporin-4 expression in the human retinal pigment epithelial cell line, ARPE-19. Int. J. Mol. Med. 2014, 34, 533–538. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Moore, J.E., Jr.; Bertram, C.D. Lymphatic System Flows. Annu. Rev. Fluid Mech. 2018, 50, 459–482. [Google Scholar] [CrossRef]
- Pepper, M.S.; Skobe, M. Lymphatic endothelium: Morphological, molecular and functional properties. J. Cell Biol. 2003, 163, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.R.; Choi, H.; Lee, E. Blood and Lymphatic Vasculatures On-Chip Platforms and Their Applications for Organ-Specific In Vitro Modeling. Micromachines 2020, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Skobe, M.; Detmar, M. Structure, function, and molecular control of the skin lymphatic system. J. Investig. Dermatol. Symp. Proc. 2000, 5, 14–19. [Google Scholar] [CrossRef]
- Shang, T.; Liang, J.; Kapron, C.M.; Liu, J. Pathophysiology of aged lymphatic vessels. Aging (Albany NY) 2019, 11, 6602–6613. [Google Scholar] [CrossRef]
- Koina, M.E.; Baxter, L.; Adamson, S.J.; Arfuso, F.; Hu, P.; Madigan, M.C.; Chan-Ling, T. Evidence for lymphatics in the developing and adult human choroid. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1310–1327. [Google Scholar] [CrossRef]
- Gerli, R.; Ibba, L.; Fruschelli, C. A fibrillar elastic apparatus around human lymph capillaries. Anat. Embryol. 1990, 181, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Schönbein, G.W. Mechanisms causing initial lymphatics to expand and compress to promote lymph flow. Arch. Histol. Cytol. 1990, 53, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, T.; Norrmén, C.; Petrova, T.V. Molecular mechanisms of lymphatic vascular development. Cell Mol. Life Sci. 2007, 64, 1915–1929. [Google Scholar] [CrossRef]
- Suami, H.; Scaglioni, M.F. Anatomy of the Lymphatic System and the Lymphosome Concept with Reference to Lymphedema. Semin. Plast. Surg. 2018, 32, 5–11. [Google Scholar]
- Baluk, P.; Fuxe, J.; Hashizume, H.; Romano, T.; Lashnits, E.; Butz, S.; Vestweber, D.; Corada, M.; Molendini, C.; Dejana, E.; et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007, 204, 2349–2362. [Google Scholar] [CrossRef]
- Aspelund, A.; Robciuc, M.R.; Karaman, S.; Makinen, T.; Alitalo, K. Lymphatic System in Cardiovascular Medicine. Circ. Res. 2016, 118, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G.; Isogai, S.; Weinstein, B.M. Lymphatic development. Birth Defects Res. C Embryo Today 2009, 87, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Randolph, G.J.; Angeli, V.; Swartz, M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 2005, 5, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-C. Lymph Nodes and Cancer Metastasis: New Perspectives on the Role of Intranodal Lymphatic Sinuses. Int J. Mol. Sci 2016, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Schwager, S.; Detmar, M. Inflammation and Lymphatic Function. Front. Immunol. 2019, 10, 308. [Google Scholar] [CrossRef]
- Liu, X.; De la Cruz, E.; Gu, X.; Balint, L.; Oxendine-Burns, M.; Terrones, T.; Ma, W.; Kuo, H.H.; Lantz, C.; Bansal, T.; et al. Lymphoangiocrine signals promote cardiac growth and repair. Nature 2020, 588, 705–711. [Google Scholar] [CrossRef]
- Palikuqi, B.; Rispal, J.; Reyes, E.A.; Vaka, D.; Boffelli, D.; Klein, O. Lymphangiocrine signals are required for proper intestinal repair after cytotoxic injury. Cell Stem Cell 2022, 29, 1262–1272.e1265. [Google Scholar] [CrossRef]
- Sleeman, J.P.; Krishnan, J.; Kirkin, V.; Baumann, P. Markers for the lymphatic endothelium: In search of the holy grail? Microsc. Res. Tech. 2001, 55, 61–69. [Google Scholar] [CrossRef]
- Kong, L.L.; Yang, N.Z.; Shi, L.H.; Zhao, G.H.; Zhou, W.; Ding, Q.; Wang, M.H.; Zhang, Y.S. The optimum marker for the detection of lymphatic vessels. Mol. Clin. Oncol. 2017, 7, 515–520. [Google Scholar] [CrossRef]
- Schroedl, F.; Kaser-Eichberger, A.; Schlereth, S.L.; Bock, F.; Regenfuss, B.; Reitsamer, H.A.; Lutty, G.A.; Maruyama, K.; Chen, L.; Lütjen-Drecoll, E.; et al. Consensus Statement on the Immunohistochemical Detection of Ocular Lymphatic Vessels. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6440–6442. [Google Scholar] [CrossRef] [PubMed]
- Nakao, S.; Hafezi-Moghadam, A.; Ishibashi, T. Lymphatics and Lymphangiogenesis in the Eye. J. Ophthalmol. 2012, 2012, 783163. [Google Scholar] [CrossRef] [PubMed]
- Collin, H.B. Endothelial cell lined lymphatics in the vascularized rabbit cornea. Investig. Ophthalmol 1966, 5, 337–354. [Google Scholar]
- Collin, H.B. The ultrastructure of conjunctival lymphatic anchoring filaments. Exp. Eye Res. 1969, 8, 102–105. [Google Scholar] [CrossRef]
- Chen, L.; Hamrah, P.; Cursiefen, C.; Zhang, Q.; Pytowski, B.; Streilein, J.W.; Dana, M.R. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat. Med. 2004, 10, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Chen, L. Ocular lymphatics: State-of-the-art review. Lymphology 2009, 42, 66–76. [Google Scholar]
- Wu, Y.; Seong, Y.J.; Li, K.; Choi, D.; Park, E.; Daghlian, G.H.; Jung, E.; Bui, K.; Zhao, L.; Madhavan, S.; et al. Organogenesis and distribution of the ocular lymphatic vessels in the anterior eye. JCI Insight 2020, 5, e135121. [Google Scholar] [CrossRef]
- Birke, K.; Lütjen-Drecoll, E.; Kerjaschki, D.; Birke, M.T. Expression of Podoplanin and Other Lymphatic Markers in the Human Anterior Eye Segment. Investig. Ophthalmol. Vis. Sci. 2010, 51, 344–354. [Google Scholar] [CrossRef]
- Kaser-Eichberger, A.; Schrödl, F.; Trost, A.; Strohmaier, C.; Bogner, B.; Runge, C.; Motloch, K.; Bruckner, D.; Laimer, M.; Schlereth, S.L.; et al. Topography of Lymphatic Markers in Human Iris and Ciliary Body. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4943–4953. [Google Scholar] [CrossRef]
- Reekie, I.R.; Sharma, S.; Foers, A.; Sherlock, J.; Coles, M.C.; Dick, A.D.; Denniston, A.K.; Buckley, C.D. The Cellular Composition of the Uveal Immune Environment. Front. Med. 2021, 8, 721953. [Google Scholar] [CrossRef] [PubMed]
- Subileau, M.; Vittet, D. Lymphatics in Eye Fluid Homeostasis: Minor Contributors or Significant Actors? Biology 2021, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Tammela, T.; Antila, S.; Nurmi, H.; Leppänen, V.M.; Zarkada, G.; Stanczuk, L.; Francois, M.; Mäkinen, T.; Saharinen, P.; et al. The Schlemm’s canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J. Clin. Investig. 2014, 124, 3975–3986. [Google Scholar] [CrossRef]
- Schrödl, F.; Kaser-Eichberger, A.; Trost, A.; Strohmaier, C.; Bogner, B.; Runge, C.; Motloch, K.; Bruckner, D.; Laimer, M.; Heindl, L.M.; et al. Lymphatic Markers in the Adult Human Choroid. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7406–7416. [Google Scholar] [CrossRef]
- Heindl, L.M.; Kaser-Eichberger, A.; Schlereth, S.L.; Bock, F.; Regenfuss, B.; Reitsamer, H.A.; McMenamin, P.; Lutty, G.A.; Maruyama, K.; Chen, L.; et al. Sufficient Evidence for Lymphatics in the Developing and Adult Human Choroid? Investig. Ophthalmol. Vis. Sci. 2015, 56, 6709–6710. [Google Scholar] [CrossRef] [PubMed]
- Chan-Ling, T.; Koina, M.E.; Arfuso, F.; Adamson, S.J.; Baxter, L.C.; Hu, P.; Madigan, M.C. Author Response: Sufficient Evidence for Lymphatics in the Developing and Adult Human Choroid? Investig. Ophthalmol. Vis. Sci. 2015, 56, 6711–6713. [Google Scholar] [CrossRef] [PubMed]
- Schroedl, F.; Brehmer, A.; Neuhuber, W.L.; Kruse, F.E.; May, C.A.; Cursiefen, C. The Normal Human Choroid Is Endowed with a Significant Number of Lymphatic Vessel Endothelial Hyaluronate Receptor 1 (LYVE-1)–Positive Macrophages. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5222–5229. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, M.; Reid, D.M.; Forrester, J.V. LYVE-1-positive macrophages are present in normal murine eyes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2162–2171. [Google Scholar] [CrossRef]
- Chen, L.; Cursiefen, C.; Barabino, S.; Zhang, Q.; Dana, M.R. Novel Expression and Characterization of Lymphatic Vessel Endothelial Hyaluronate Receptor 1 (LYVE-1) by Conjunctival Cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4536–4540. [Google Scholar] [CrossRef][Green Version]
- Schlereth, S.L.; Neuser, B.; Herwig, M.C.; Müller, A.M.; Koch, K.R.; Reitsamer, H.A.; Schrödl, F.; Cursiefen, C.; Heindl, L.M. Absence of lymphatic vessels in the developing human sclera. Exp. Eye Res. 2014, 125, 203–209. [Google Scholar] [CrossRef]
- Killer, H.E.; Jaggi, G.P.; Miller, N.R.; Flammer, J.; Meyer, P. Does immunohistochemistry allow easy detection of lymphatics in the optic nerve sheath? J. Histochem. Cytochem. 2008, 56, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Killer, H.E.; Laeng, H.R.; Groscurth, P. Lymphatic capillaries in the meninges of the human optic nerve. J. Neuroophthalmol. 1999, 19, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Gausas, R.E.; Daly, T.; Fogt, F. D2-40 expression demonstrates lymphatic vessel characteristics in the dural portion of the optic nerve sheath. Ophthalmic Plast. Reconstr. Surg. 2007, 23, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lou, N.; Eberhardt, A.; Yang, Y.; Kusk, P.; Xu, Q.; Förstera, B.; Peng, S.; Shi, M.; Ladrón-de-Guevara, A.; et al. An ocular glymphatic clearance system removes β-amyloid from the rodent eye. Sci. Transl. Med. 2020, 12, eaaw3210. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The glymphatic system: A beginner’s guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef]
- Taoka, T.; Naganawa, S. Gadolinium-based Contrast Media, Cerebrospinal Fluid and the Glymphatic System: Possible Mechanisms for the Deposition of Gadolinium in the Brain. Magn. Reson. Med. Sci. 2018, 17, 111–119. [Google Scholar] [CrossRef]
- Mestre, H.; Tithof, J.; Du, T.; Song, W.; Peng, W.; Sweeney, A.M.; Olveda, G.; Thomas, J.H.; Nedergaard, M.; Kelley, D.H. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Nedergaard, M. Garbage truck of the brain. Science 2013, 28, 1529–1530. [Google Scholar] [CrossRef]
- Rennels, M.L.; Gregory, T.F.; Blaumanis, O.R.; Fujimoto, K.; Grady, P.A. Evidence for a ‘Paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985, 326, 47–63. [Google Scholar] [CrossRef]
- Rennels, M.L.; Blaumanis, O.R.; Grady, P.A. Rapid solute transport throughout the brain via paravascular fluid pathways. Adv. Neurol. 1990, 52, 431–439. [Google Scholar] [PubMed]
- Cserr, H.F. Relationship between cerebrospinal fluid and interstitial fluid of brain. Fed. Proc. 1974, 33, 2075–2078. [Google Scholar]
- Szentistvanyi, I.; Patlak, C.S.; Ellis, R.A.; Cserr, H.F. Drainage of interstitial fluid from different regions of rat brain. Am. J. Physiol. Ren. Physiol. 1984, 246, F835–F844. [Google Scholar] [CrossRef] [PubMed]
- Cserr, H.F.; Cooper, D.N.; Suri, P.K.; Patlak, C.S. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am. J. Physiol. Ren. Physiol. 1981, 240, F319–F328. [Google Scholar] [CrossRef] [PubMed]
- Groothuis, D.R.; Vavra, M.W.; Schlageter, K.E.; Kang, E.W.; Itskovich, A.C.; Hertzler, S.; Allen, C.V.; Lipton, H.L. Efflux of drugs and solutes from brain: The interactive roles of diffusional transcapillary transport, bulk flow and capillary transporters. J. Cereb. Blood Flow Metab. 2007, 27, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.J.; Iliff, J.J. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim. Biophys. Acta 2016, 1862, 442–451. [Google Scholar] [CrossRef]
- Lundgaard, I.; Li, B.; Xie, L.; Kang, H.; Sanggaard, S.; Haswell, J.D.; Sun, W.; Goldman, S.; Blekot, S.; Nielsen, M.; et al. Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat. Commun. 2015, 6, 6807. [Google Scholar] [CrossRef]
- Rangroo Thrane, V.; Thrane, A.S.; Plog, B.A.; Thiyagarajan, M.; Iliff, J.J.; Deane, R.; Nagelhus, E.A.; Nedergaard, M. Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci. Rep. 2013, 3, 2582. [Google Scholar] [CrossRef]
- Plog, B.A.; Nedergaard, M. The glymphatic system in central nervous system health and disease: Past, present, and future. Annu. Rev. Pathol. 2018, 13, 379–394. [Google Scholar] [CrossRef]
- Reeves, B.C.; Karimy, J.K.; Kundishora, A.J.; Mestre, H.; Cerci, H.M.; Matouk, C.; Alper, S.L.; Lundgaard, I.; Nedergaard, M.; Kahle, K.T. Glymphatic System Impairment in Alzheimer’s Disease and Idiopathic Normal Pressure Hydrocephalus. Trends Mol. Med. 2020, 26, 285–295. [Google Scholar] [CrossRef]
- Iliff, J.J.; Nedergaard, M. Is there a cerebral lymphatic system? Stroke 2013, 44, S93–S95. [Google Scholar] [CrossRef] [PubMed]
- Gaberel, T.; Gakuba, C.; Goulay, R.; Martinez De Lizarrondo, S.; Hanouz, J.L.; Emery, E.; Touze, E.; Vivien, D.; Gauberti, M. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: A new target for fibrinolysis? Stroke 2014, 45, 3092–3096. [Google Scholar] [CrossRef]
- Pu, T.; Zou, W.; Feng, W.; Zhang, Y.; Wang, L.; Wang, H.; Xiao, M. Persistent Malfunction of Glymphatic and Meningeal Lymphatic Drainage in a Mouse Model of Subarachnoid Hemorrhage. Exp. Neurobiol. 2019, 28, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Sakka, L.; Coll, G.; Chazal, J. Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 309–316. [Google Scholar] [CrossRef]
- Benveniste, H.; Lee, H.; Volkow, N.D. The Glymphatic Pathway: Waste Removal from the CNS via Cerebrospinal Fluid Transport. Neuroscientist 2017, 23, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; Barrand, M.A. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids Barriers CNS 2014, 11, 26. [Google Scholar] [CrossRef]
- Kao, Y.H.; Guo, W.Y.; Liou, A.J.; Hsiao, Y.H.; Chou, C.C. The respiratory modulation of intracranial cerebrospinal fluid pulsation observed on dynamic echo planar images. Magn. Reson. Imaging 2008, 26, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, K.G.; Katz, S.E.; Grzybowski, D.M.; Lubow, M. Cerebrospinal fluid outflow: An evolving perspective. Brain Res. Bull. 2008, 77, 327–334. [Google Scholar] [CrossRef]
- Iliff, J.J.; Lee, H.; Yu, M.; Feng, T.; Logan, J.; Nedergaard, M.; Benveniste, H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Investig. 2013, 123, 1299–1309. [Google Scholar] [CrossRef]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef]
- Mastorakos, P.; McGavern, D. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 2019, 4, eaav0492. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Benveniste, H.; Nedergaard, M.; Zlokovic, B.V.; Mestre, H.; Lee, H.; Doubal, F.N.; Brown, R.; Ramirez, J.; MacIntosh, B.J.; et al. Colleagues from the Fondation Leducq Transatlantic Network of Excellence on the Role of the Perivascular Space in Cerebral Small Vessel, D. Perivascular spaces in the brain: Anatomy, physiology and pathology. Nat. Rev. Neurol. 2020, 16, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.; Benveniste, H.; Black, S.E.; Charpak, S.; Dichgans, M.; Joutel, A.; Nedergaard, M.; Smith, K.J.; Zlokovic, B.V.; Wardlaw, J.M. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc. Res. 2018, 114, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Wuerfel, J.; Haertle, M.; Waiczies, H.; Tysiak, E.; Bechmann, I.; Wernecke, K.D.; Zipp, F.; Paul, F. Perivascular spaces—MRI marker of inflammatory activity in the brain? Brain 2008, 131, 2332–2340. [Google Scholar] [CrossRef] [PubMed]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef]
- Zong, X.; Lian, C.; Jimenez, J.; Yamashita, K.; Shen, D.; Lin, W. Morphology of perivascular spaces and enclosed blood vessels in young to middle-aged healthy adults at 7T: Dependences on age, brain region, and breathing gas. Neuroimage 2020, 218, 116978. [Google Scholar] [CrossRef]
- Gleiser, C.; Wagner, A.; Fallier-Becker, P.; Wolburg, H.; Hirt, B.; Mack, A.F. Aquaporin-4 in Astroglial Cells in the CNS and Supporting Cells of Sensory Organs-A Comparative Perspective. Int. J. Mol. Sci. 2016, 17, 1411. [Google Scholar] [CrossRef]
- Mestre, H.; Hablitz, L.M.; Xavier, A.L.; Feng, W.; Zou, W.; Pu, T.; Monai, H.; Murlidharan, G.; Rivera, R.M.C.; Simon, M.J. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 2018, 7, e40070. [Google Scholar] [CrossRef]
- Haj-Yasein, N.N.; Vindedal, G.F.; Eilert-Olsen, M.; Gundersen, G.A.; Skare, Ø.; Laake, P.; Klungland, A.; Thorén, A.E.; Burkhardt, J.M.; Ottersen, O.P.; et al. Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc. Natl. Acad. Sci. USA 2011, 108, 17815–17820. [Google Scholar] [CrossRef]
- Harrison, I.F.; Ismail, O.; Machhada, A.; Colgan, N.; Ohene, Y.; Nahavandi, P.; Ahmed, Z.; Fisher, A.; Meftah, S.; Murray, T.K.; et al. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain 2020, 143, 2576–2593. [Google Scholar] [CrossRef]
- Iliff, J.J.; Chen, M.J.; Plog, B.A.; Zeppenfeld, D.M.; Soltero, M.; Yang, L.; Singh, I.; Deane, R.; Nedergaard, M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014, 34, 16180–16193. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Iliff, J.J.; Yang, L.; Yang, J.; Chen, X.; Chen, M.J.; Giese, R.N.; Wang, B.; Shi, X.; Nedergaard, M. ‘Hit & Run’ model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J. Cereb. Blood Flow Metab. 2013, 33, 834–845. [Google Scholar]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain—from eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Ichhpujani, P.; Kumar, S. What’s New in Pathogenesis of Glaucoma. In Glaucoma; Ichhpujani, P., Ed.; Springer: Singapore, 2019; pp. 1–6. [Google Scholar]
- Nagelhus, E.; Mathiisen, T.; Ottersen, O. Aquaporin-4 in the central nervous system: Cellular and subcellular distribution and coexpression with KIR4. 1. Neuroscience 2004, 129, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin water channels in the nervous system. Nat. Rev. Neurosci. 2013, 14, 265–277. [Google Scholar] [CrossRef]
- Denniston, A.K.; Keane, P.A. Paravascular pathways in the eye: Is there an ‘ocular glymphatic system’? Investig. Ophthalmol. Vis. Sci. 2015, 56, 3955–3956. [Google Scholar] [CrossRef]
- Petzold, A. Retinal glymphatic system: An explanation for transient retinal layer volume changes? Brain 2016, 139, 2816–2819. [Google Scholar] [CrossRef]
- Wostyn, P.; De Groot, V.; Van Dam, D.; Audenaert, K.; Killer, H.E.; De Deyn, P.P. The glymphatic hypothesis of glaucoma: A unifying concept incorporating vascular, biomechanical, and biochemical aspects of the disease. Biomed. Res. Int. 2017, 2017, 5123148. [Google Scholar] [CrossRef]
- Wostyn, P. Retinal nerve fiber layer thinning in chronic fatigue syndrome as a possible ocular biomarker of underlying glymphatic system dysfunction. Med. Hypotheses 2019, 134, 109416. [Google Scholar] [CrossRef]
- Wostyn, P.; De Groot, V.; Van Dam, D.; Audenaert, K.; Killer, H.E.; De Deyn, P.P. Age-related macular degeneration, glaucoma and Alzheimer’s disease: Amyloidogenic diseases with the same glymphatic background? Cell. Mol. Life Sci. 2016, 73, 4299–4301. [Google Scholar] [CrossRef]
- Wostyn, P.; Van Dam, D.; Audenaert, K.; Killer, H.E.; De Deyn, P.P.; De Groot, V. A new glaucoma hypothesis: A role of glymphatic system dysfunction. Fluids Barriers CNS 2015, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Gupta, N.; Ahari, A.; Zhou, X.; Hanna, J.; Yücel, Y.H. Evidence for cerebrospinal fluid entry into the optic nerve via a glymphatic pathway. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4784–4791. [Google Scholar] [CrossRef]
- Wostyn, P.; Killer, H.E.; De Deyn, P.P. Glymphatic stasis at the site of the lamina cribrosa as a potential mechanism underlying open-angle glaucoma. Clin. Exp. Ophthalmol. 2017, 45, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Thomson, B.R.; Heinen, S.; Jeansson, M.; Ghosh, A.K.; Fatima, A.; Sung, H.K.; Onay, T.; Chen, H.; Yamaguchi, S.; Economides, A.N.; et al. A lymphatic defect causes ocular hypertension and glaucoma in mice. J. Clin. Investig. 2014, 124, 4320–4324. [Google Scholar] [CrossRef] [PubMed]
- Yucel, Y.; Gupta, N. Lymphatic drainage from the eye: A new target for therapy. Prog. Brain Res. 2015, 220, 185–198. [Google Scholar] [PubMed]
- Patel, T.K.; Habimana-Griffin, L.; Gao, X.; Xu, B.; Achilefu, S.; Alitalo, K.; McKee, C.A.; Sheehan, P.W.; Musiek, E.S.; Xiong, C.; et al. Dural lymphatics regulate clearance of extracellular tau from the CNS. Mol. Neurodegener. 2019, 14, 11. [Google Scholar] [CrossRef]
- Mathieu, E.; Gupta, N.; Paczka-Giorgi, L.A.; Zhou, X.; Ahari, A.; Lani, R.; Hanna, J.; Yücel, Y.H. Reduced Cerebrospinal Fluid Inflow to the Optic Nerve in Glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5876–5884. [Google Scholar] [CrossRef]
- Natoli, R.; Fernando, N.; Jiao, H.; Racic, T.; Madigan, M.; Barnett, N.L.; Chu-Tan, J.A.; Valter, K.; Provis, J.; Rutar, M. Retinal Macrophages Synthesize C3 and Activate Complement in AMD and in Models of Focal Retinal Degeneration. Investig. Ophthalmol Vis. Sci 2017, 58, 2977–2990. [Google Scholar] [CrossRef]
- Bowes Rickman, C.; Farsiu, S.; Toth, C.A.; Klingeborn, M. Dry age-related macular degeneration: Mechanisms, therapeutic targets, and imaging. Investig. Ophthalmol. Vis. Sci. 2013, 54, Orsf68-80. [Google Scholar] [CrossRef]
- Penfold, P.L.; Madigan, M.C.; Gillies, M.C.; Provis, J.M. Immunological and aetiological aspects of macular degeneration. Prog Retin Eye Res. 2001, 20, 385–414. [Google Scholar] [CrossRef]
- Anderson, D.H.; Radeke, M.J.; Gallo, N.B.; Chapin, E.A.; Johnson, P.T.; Curletti, C.R.; Hancox, L.S.; Hu, J.; Ebright, J.N.; Malek, G.; et al. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Prog Retin Eye Res. 2010, 29, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Salminen, A.; Haapasalo, A.; Soininen, H.; Hiltunen, M. Age-related macular degeneration (AMD): Alzheimer’s disease in the eye? J. Alzheimers Dis 2011, 24, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Akinsoji, E.; Goldhardt, R.; Galor, A. A Glimpse into Uveitis in the Aging Eye: Pathophysiology, Clinical Presentation and Treatment Considerations. Drugs Aging 2018, 35, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Yücel, Y.H.; Cheng, F.; Cardinell, K.; Zhou, X.; Irving, H.; Gupta, N. Age-related decline of lymphatic drainage from the eye: A noninvasive in vivo photoacoustic tomography study. Exp. Eye Res. 2020, 194, 108029. [Google Scholar] [CrossRef]
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Rothschild, P.-R.; Omri, S.; Gélizé, E.; Jonet, L.; et al. Mechanisms of macular edema: Beyond the surface. Prog. Retin. Eye Res. 2018, 63, 20–68. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Nedergaard, M.; Goldman, S.A. Glymphatic failure as a final common pathway to dementia. Science 2020, 370, 50–56. [Google Scholar] [CrossRef]
- Ji, C.; Yu, X.; Xu, W.; Lenahan, C.; Tu, S.; Shao, A. The role of glymphatic system in the cerebral edema formation after ischemic stroke. Exp. Neurol. 2021, 340, 113685. [Google Scholar] [CrossRef]
- Ayasoufi, K.; Kohei, N.; Nicosia, M.; Fan, R.; Farr, G.W.; McGuirk, P.R.; Pelletier, M.F.; Fairchild, R.L.; Valujskikh, A. Aquaporin 4 blockade improves survival of murine heart allografts subjected to prolonged cold ischemia. Am. J. Transplant. 2018, 18, 1238–1246. [Google Scholar] [CrossRef]
- Kasi, A.; Liu, C.; Faiq, M.A.; Chan, K.C. Glymphatic imaging and modulation of the optic nerve. Neural. Regen Res. 2022, 17, 937–947. [Google Scholar]
- Huber, V.J.; Igarashi, H.; Ueki, S.; Kwee, I.L.; Nakada, T. Aquaporin-4 facilitator TGN-073 promotes interstitial fluid circulation within the blood-brain barrier: [17O]H2O JJVCPE MRI study. Neuroreport 2018, 29, 697–703. [Google Scholar] [CrossRef] [PubMed]
- von Holstein-Rathlou, S.; Petersen, N.C.; Nedergaard, M. Voluntary running enhances glymphatic influx in awake behaving, young mice. Neurosci Lett 2018, 662, 253–258. [Google Scholar] [CrossRef]
- He, X.F.; Liu, D.X.; Zhang, Q.; Liang, F.Y.; Dai, G.Y.; Zeng, J.S.; Pei, Z.; Xu, G.Q.; Lan, Y. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front. Mol. Neurosci 2017, 10, 144. [Google Scholar] [CrossRef]
- Hou, Y.; Bock, F.; Hos, D.; Cursiefen, C. Lymphatic Trafficking in the Eye: Modulation of Lymphatic Trafficking to Promote Corneal Transplant Survival. Cells 2021, 10, 1661. [Google Scholar] [CrossRef]
- Cursiefen, C.; Maruyama, K.; Bock, F.; Saban, D.; Sadrai, Z.; Lawler, J.; Dana, R.; Masli, S. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J. Exp. Med. 2011, 208, 1083–1092. [Google Scholar] [CrossRef]
- Detry, B.; Blacher, S.; Erpicum, C.; Paupert, J.; Maertens, L.; Maillard, C.; Munaut, C.; Sounni, N.E.; Lambert, V.; Foidart, J.-M.; et al. Sunitinib Inhibits Inflammatory Corneal Lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3082–3093. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Tiem, M.; Watkins, R.; Cho, Y.K.; Wang, Y.; Olsen, T.; Uehara, H.; Mamalis, C.; Luo, L.; Oakey, Z.; et al. Soluble vascular endothelial growth factor receptor 3 is essential for corneal alymphaticity. Blood 2013, 121, 4242–4249. [Google Scholar] [CrossRef]
- Hanna, J.; Yücel, Y.H.; Zhou, X.; Kim, N.; Irving, H.; Gupta, N. Beta-adrenergic glaucoma drugs reduce lymphatic clearance from the eye: A sequential photoacoustic imaging study. Exp. Eye Res. 2021, 212, 108775. [Google Scholar] [CrossRef]
- Shah, B.; Arora, V.; Wadhwani, M.; Mishra, S.K. Prostaglandin analogs. J. Curr. Glaucoma Pract. 2011, 5, 15–20. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, N.; Rutar, M. Ocular Lymphatic and Glymphatic Systems: Implications for Retinal Health and Disease. Int. J. Mol. Sci. 2022, 23, 10139. https://doi.org/10.3390/ijms231710139
Uddin N, Rutar M. Ocular Lymphatic and Glymphatic Systems: Implications for Retinal Health and Disease. International Journal of Molecular Sciences. 2022; 23(17):10139. https://doi.org/10.3390/ijms231710139
Chicago/Turabian StyleUddin, Nasir, and Matt Rutar. 2022. "Ocular Lymphatic and Glymphatic Systems: Implications for Retinal Health and Disease" International Journal of Molecular Sciences 23, no. 17: 10139. https://doi.org/10.3390/ijms231710139
APA StyleUddin, N., & Rutar, M. (2022). Ocular Lymphatic and Glymphatic Systems: Implications for Retinal Health and Disease. International Journal of Molecular Sciences, 23(17), 10139. https://doi.org/10.3390/ijms231710139





