Abstract
Plants frequently experience hypoxia due to flooding caused by intensive rainfall or irrigation, when they are partially or completely submerged under a layer of water. In the latter case, some resistant plants implement a hypoxia avoidance strategy by accelerating shoot elongation, which allows lifting their leaves above the water surface. This strategy is achieved due to increased water uptake by shoot cells through water channels (aquaporins, AQPs). It remains a puzzle how an increased flow of water through aquaporins into the cells of submerged shoots can be achieved, while it is well known that hypoxia inhibits the activity of aquaporins. In this review, we summarize the literature data on the mechanisms that are likely to compensate for the decline in aquaporin activity under hypoxic conditions, providing increased water entry into cells and accelerated shoot elongation. These mechanisms include changes in the expression of genes encoding aquaporins, as well as processes that occur at the post-transcriptional level. We also discuss the involvement of hormones, whose concentration changes in submerged plants, in the control of aquaporin activity.
1. Introduction
Oxygen is necessary for the normal metabolism of plants, and its deficiency negatively affects almost all biochemical and physiological processes, ultimately leading to inhibition of plants growth and a decrease in agricultural crop yield. There are several native scenarios that are triggered by oxygen deficiency of different intensity (hypoxia and anoxia). Among main reasons are the formation of an ice crust, asphalt pavement, or, more often, flooding of plants as a result of heavy rain or excessive irrigation. Due to the poor solubility of gases in water (including oxygen), waterlogging of the rhizosphere leads to local root hypoxia, while complete submergence of a plant more often causes a much more severe effect such as total anoxia [1]. Under normal conditions, the availability of oxygen in the soil is ensured by the diffusion through well-developed air-filled pores and channels. Compaction and water saturation of soils are suggested to be the main barriers to soil oxygen transport [2]. In the case of local soil flooding when only the roots are under direct oxygen deprivation, O2 can come from the shoot through aerenchyma, whose enhanced development is one of the characteristic adaptive reactions of plants to flooding [3,4,5]. It is more difficult to survive submergence when the entire plant (root and shoot) is under water. In rare cases (transparent H2O), light can pass through a water layer, enabling photosynthesis and, thus, oxygen production by the leaves of fully submerged plants. This rarely occurs in natural conditions, since the water is most often opaque due to the presence of a large amount of suspended solids and dissolved colored material reducing water clarity [6]. The lack of oxygen can be also aggravated by the pathogenic action of soil biota [1].
As a result, plants experience disturbances in respiration and photosynthesis, and intoxication develops due to the accumulation of toxic metabolic products, particularly acetaldehyde and ethanol [7,8]. A lack of oxygen stimulates glycolysis, as well as lactic and alcoholic fermentation [8,9,10]. At the same time, the rapid activation of lactate dehydrogenase, observed in many plants, may be the cause of the initial acidification of the cytosol, and then, with time, alcoholic fermentation begins to predominate [10,11].
Under such conditions, plants adapt to hypoxia through two alternative strategies. The first relies on the decrease in the growth and metabolic rate, with a switch to anaerobic metabolism (low-oxygen quiescence syndrome, LOQS) [10,12]. Energy generated due to glycolysis and fermentation is mostly used for the synthesis of stress proteins involved in chaperone activity, membrane transport, and antioxidant defense [9]. The second alternative pathway for plants to tolerate their shoot submergence, on the contrary, depends on intensification of the shoot growth that serves to elevate the photosynthetic tissues above the water surface [6,12,13]. This strategy (low-oxygen escape syndrome, LOES) is implemented by many varieties of rice (deep-water), as well as the majority of wild hydrophytes adapted to grow in wetlands (e.g., Callitriche platycarpa, Hydrocharis morsus-ranae, Nymphoides peltata, Ranunculus repens, R. sceleratus, Rumex crispus, and R. palustris), as reviewed in [14,15]. The study of the mechanisms involved in plant growth activation under hypoxic conditions is important not only to find ways to improve plant resistance to oxygen deficiency, but also for a better understanding plant growth regulation in general. In this review, emphasis is placed on an aspect of this problem, which, in our opinion, has received insufficient attention. It consists in elucidating the role of water channels, aquaporins (AQPs), in the activation of plant shoot growth under conditions of oxygen deficiency.
2. Aquaporin Structure and Plant Growth
Plants, unlike animals, have the ability to rapidly and significantly increase their cell volume due to considerable water uptake. The length of the cells in this case can increase manifold. The dependence of the rate of cell elongation on the uptake of water is described by the equation R = L(ΔΨ), where R is the cell growth rate, L is the hydraulic conductance, and ΔΨ is the difference in water potential between the cell and external medium, which is in turn broken up into its two components, osmotic pressure and hydrostatic pressure [16]. Numerous investigations of cell elongation growth regulation focused on the mechanisms maintaining the level of osmotically active substances against the background of their dilution resulting from water uptake [17,18], and (perhaps even to a greater extent) attention was paid to the state of the cell wall, which provides its extension under the influence of the hydrostatic component of the water potential, i.e., turgor [19,20,21]. It is well proven that growth is a complex process in which all cell compartments are involved. Elongation is always accompanied by vacuolization. This highlights the importance of water flux not only through plasma membrane but also through the tonoplast. Since vacuoles occupy up to 90% of the plant cell volume, water is transported constantly through the tonoplast, and the intensity of the osmotic flow and the volume of vacuoles depend on the permeability of the vacuolar membrane [22,23].
Water can penetrate cell membranes both directly through the phospholipid bilayer [24] and indirectly through specialized water channels, aquaporins [25,26]. The concept of the ability of small molecules, such as water, to freely diffuse through the membrane is based on the ability of liposomes (artificial vesicles containing only lipids) to absorb water [27]. However, experiments with the expression of Arabidopsis aquaporin genes in a heterologous system (e.g., in Xenopus oocytes) showed an increase in permeability of cell membrane for water due to aquaporins [25]. It was revealed that, with an increase in the expression of genes encoding them, aquaporins are able to accelerate cell lysis in a hypotonic medium detected by changes in the optical density of the suspension [28,29]. This approach is widely used to demonstrate the ability of certain aquaporins to conduct water [30,31]. Modulation of AQP abundance through transcriptional approaches affects the hydraulic conductivity of plant biological membranes [32,33]. In comparison with mammals having 15 types of AQPs described so far (displaying 18 paralogs), a single plant species can represent more than 120 isoforms, providing the transport of different types of solutes [34]. In plants, water transporters have been found in all tested organs including roots, leaves, stems, flowers, fruits, and seeds [35]. AQPs in higher plants are classified into five subfamilies: plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), nodulin-26 like intrinsic proteins (NIPs), small basic intrinsic proteins (SIPs), and X intrinsic proteins/uncharacterized-intrinsic proteins (XIPs). AQPs are known as low-molecular-weight proteins of about 23–31 kDa [36] containing six transmembrane helices. Four AQP monomers assemble to form homo- or heterotetrameric complexes [31,34], with each monomer acting as an independent water channel. Features of the aquaporin tertiary structure enable the incorporation of these proteins into the hydrophobic layer of membranes due to the exposure of hydrophobic domains on the surface of the protein molecule of AQPs. At the same time, the presence in the molecule of a channel with a small radius lined with hydrophilic domains ensures the movement of small hydrophilic molecules along it (not only water, but also glycerol, ammonia, urea, lactic acid, hydrogen peroxide, different ions such as boron, silicon, and arsenate, and even some gases such as CO2 and O2 [35]). Intensive investigation of the structures of AQPs proteins and the genes encoding them revealed that AQPs are conserved transport proteins identified in each kingdom [37].
There is extensive evidence proving the importance of AQPs in growth and development in plants and even determining health and disease in the case of animals. A study of the localization of aquaporins in animals and plants revealed a high level of their presence in sites characterized by a high level of water flow (e.g., in the kidneys of animals [38] or in the cells around plant vascular bundles [39]. It has been noted that AQPs are not only important at a cellular level but also involved in the whole-plant functional water transport between shoot and root. Tobacco plants with antisense transformation with NtAQP1 expression provided evidence for NtAQP1 function in cellular and whole-plant water relations. A decrease in cellular water permeability due to antisense construct positively correlated with transpiration rate, stem and leaf water potential changes, and decrement in growth intensity under extreme soil water depletion [40]. On the contrary, the increased levels of AQPs and the expression of aquaporin-encoding genes in roots correlated with higher water flow and elevated hydraulic conductivity of roots, e.g., during the photoperiod [41] or when air heating increases transpiration rate [42]. Changes in PIP transcript amounts in the morning were synchronous with the changes in plant hydraulic conductance [43].
3. The Growth under Normoxic Conditions and the Role of Water Transporting Aquaporins
A constant water supply is important for growing tissues required for maintenance of turgor pressure upon cell enlargement [44]. Water entering the cells during growth passes through the plasma membrane and tonoplast (vacuolar membrane). Plasma membrane (PIP) and tonoplast (TIP) aquaporins are involved in this process [45]. PIP aquaporins are divided into two groups: PIP2 and PIP1. Expression of class II aquaporin genes in a heterologous system increased membrane permeability for water, while PIP1 aquaporins showed weak ability to conduct water. Nevertheless, it was demonstrated that PIP1;1 has high water channel activity when co-expressed with PIP2, and that PIP1–PIP2 random heterotetramerization not only allows PIP1;1 to arrive at the plasma membrane, but also results in an enhanced activity of PIP2;1 [31,46]. These results suggested the importance of both PIP1 and PIP2 aquaporins for the control of water movement across the plasma membrane.
TIP aquaporins have a special function in the process of cell elongation growth, since they ensure the absorption of water by vacuoles, providing an increase in cell volume. TIPs basically act as regulators of the intracellular water flow, thereby defining cell turgor [47]. Therefore, they are given special attention when debating the role of aquaporins in the process of cell growth [44,48]. TIP aquaporins play an important role in seed germination [49]. Their abundance is positively correlated with the formation of leaf vacuoles [50] and is typical for rapidly growing cells, especially in roots [51]. Multiple TIP isoforms with water channel activity have been shown to co-express abundantly in root cells, but it remains to be shown which (if any) of these TIP isoforms carries a growth-specific function [52].
The admitted discrepancy of results focusing on plant growth ability (see below) can be explained by the presence of a large number of representatives of the family of both PIP and TIP aquaporins in different plant species, which differ in their localization in tissues of shoots and roots. For example, the Arabidopsis genome contains 38 sequences with homology to aquaporin [53], while 33 aquaporin genes were identified in rice genome [54], and a comprehensive search revealed that the barley MIP family comprises at least 40 aquaporins [55]. The diversity of aquaporins may be the reason for the difference in the results of their detection in growing cells when only some of the genes and proteins of this family were analyzed. Elucidation of the role of aquaporins in the regulation of cell growth is important in connection with the debate about the involvement of membrane water permeability in the regulation of cell elongation [43]. Since ΔΨ (the difference between the water potentials of the cell and its environment) depends on the hydraulic conductivity of cell membranes, it is considered as an indicator of membrane permeability to water. High ΔΨ values indicate that hydraulic conductivity is low and water uptake limits cell growth, while low ΔΨ values indicate high hydraulic conductivity and that growth is primarily limited by cell-wall parameters [56]. Measurements carried out on individual cells showed low ΔΨ values, indicating that their membranes are highly permeable for water (cell Lp), and that hydraulic conductivity does not limit cell elongation [16]. However, a study on elongating barley leaf cells revealed the existence of a significant (>0.1 MPa) ΔΨ, being indicative of some hydraulic (co-)limitation of cell expansion, in growing cells [57]. The model proposed by Calderia and co-authors suggests that a hydraulic process may account for the rapid changes in LER upon changes in evaporative demand or soil water content [43]. The hydraulic conductivity of cortical cells decreased in the elongating tissue and increased slightly during growth recovery in response to changes in availability of water and elongation rate [58]. The role of aquaporins increases during the transport of water from its source (xylem and phloem vessels) to individual growing cells, since water overcomes a large number of membranes along this path, and the hydraulic resistance of all crossed membranes is summed up [52]. The authors suggested that cell Lp and growth-dependent expression of AQPs in leaves match more the need for water transport through tissue than for expansion of an individual cell.
The original investigation, which employed growing maize suspension cultured cells, showed that the Pf (membrane osmotic water permeability coefficient) was significantly increased at the end of the logarithmic growth phase and during the steady-state phase compared to the lag phase. A positive correlation between AQP abundance in the plasma membrane and the cell Pf was elucidated at cellular level [59].
Manifold elevation of ZmTIP expression during the post-germination period was much more intensive in comparison with the increase in ZmPIP abundance [60]. The analysis of early maize seedling growth revealed that overdevelopment TIPs might be detected in the plasma membrane. Moreover, this effect corresponded to a higher intensity of water flow through cell membranes. Such a complicated profile of PIP and TIP expression, as well as its different redistribution between plasma membrane and tonoplast over development, demands additional investigation.
Roots are the most convenient and widely used objects for studying growth, since cell division and elongation occur in different zones of the root tip. Roots are dominant organs in AQPs expression [61]. PIPs and TIPs are enriched in the growing regions of the root tip, where more growing cells and tissues exist [50]. It was demonstrated with in situ hybridization and immunolocalization that VvPIP1 and VvPIP2 proteins and expression of their genes were localized evenly in the cortex and vascular tissues of the root tip, but showed lower signals in the cortex of mature root regions [62]. However, the relationship between aquaporin gene expression and growth cannot always be traced. It was shown that overexpression of PIP1 aquaporin of Vicia faba in Arabidopsis promoted the growth of primary and lateral roots (LRs) [63]. Overexpression of poplar PtoPIP1;1 in Arabidopsis accelerated cell growth in both the leaf and root [64]. On the contrary, Arabidopsis antisense plants with decreased expression of AtPIP1a and AtPIP1b had more abundant roots compared to the control plants under normal condition [65].
Stem growth, including juvenile hypocotyls, is significantly based on a cell’s ability to elongate. This process was shown to be regulated by AQPs. The importance of PIPs and TIPs was shown for different plant species such as Arabidopsis, pea, maize, tulip, castor bean, and rice [50]. Moreover, ectopic expression of APQs genes from one plant species in another was followed by intensification of shoot growth. As an example, transgenic tobacco (Nicotiana benthamiana), expressing rice OsPIP1;3, was characterized not only by intensive shoot growth but higher photosynthesis rates, Lpr, and water-use efficiency [66]. A similar positive role was determined for vacuolar AQPs. Accordingly, the overexpression of AtTIP5;1 in Arabidopsis resulted in hypocotyl cell elongation [67]. In adult plants, the ratio between root and shoot growth is an important parameter which was found to be under control of AQPs. This phenomenon was proven by 1.5-fold elevation of the shoot/root ratio in rice plants with overexpression of barley HvPIP2;1 [68]. This coincides with the suggested aquaporin involvement in long-distance water transport (xylem function), as well as in short-distance transcellular water flow, and in intracellular osmotic adjustment.
The importance of leaf growth is based on a variety of biochemical and physiological processes such as photosynthesis, respiration, and transpiration. A number of investigations have demonstrated that genes encoding PIPs and TIPs are natively expressed more intensively in the most elongated zones of Arabidopsis and maize leaves [51,69]. Ectopic overexpression of Vicia faba VfPIP1 and Panax ginseng PgTIP1 in Arabidopsis, as well as citrus CsTIP2;1 in tobacco, significantly intensified leaf growth and increased the size of leaf mesophyll cells in all cases [50]. Such an increase is known to be dependent on the AQP-induced elevation of the hydraulic conductivity of leaves (Lpl) characterizing leaf elongation zones [70].
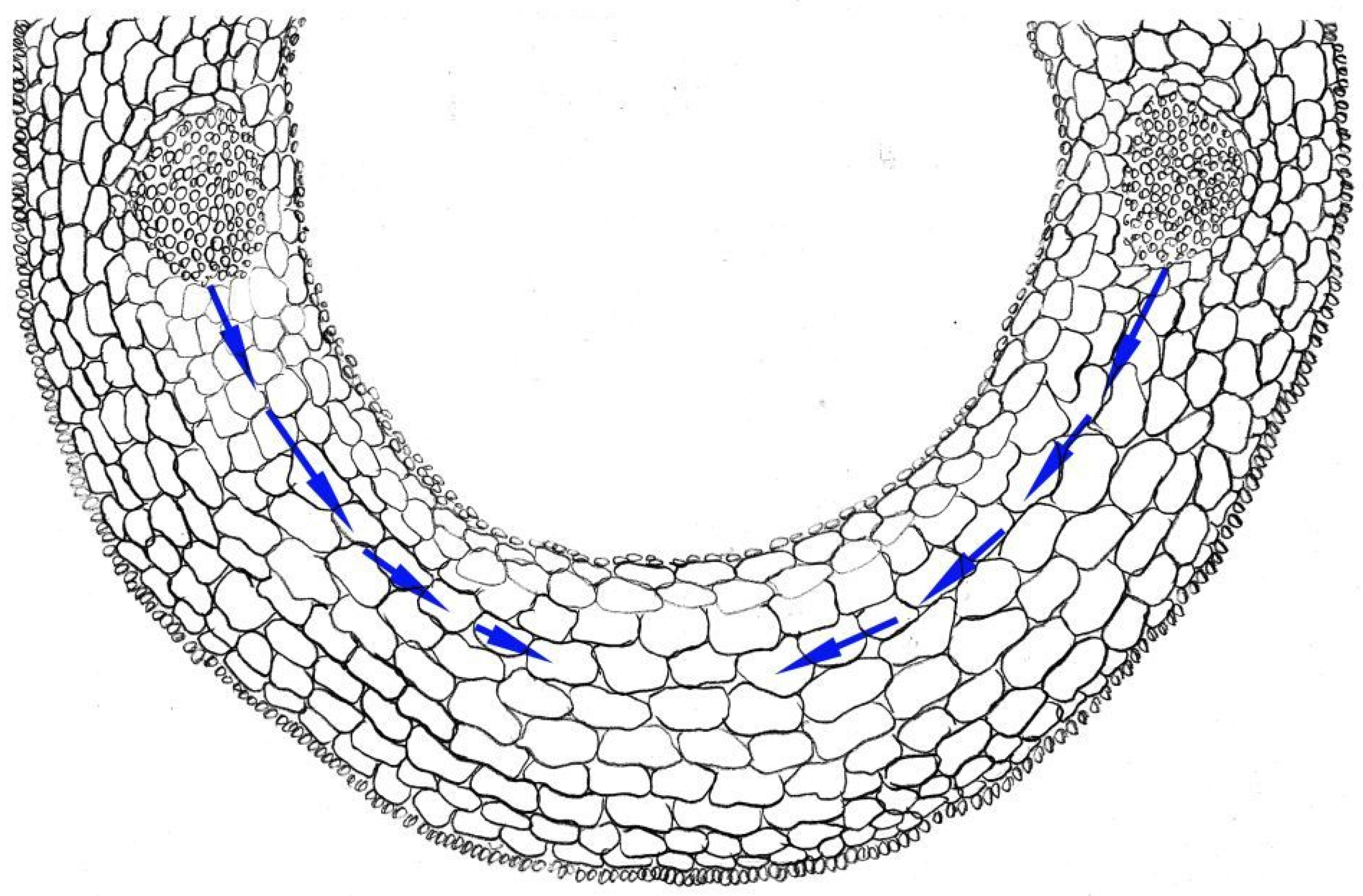
Another classic model for studying elongation growth is cereal coleoptiles (Figure 1). These are juvenile organs that protect the leaf during germination. Inherently longer coleoptiles are supposed to be an advantage in many cases, such as the protection from high temperature and dense environment [71,72]. Currently, a large number of factors have been identified that regulate coleoptile elongation at the transcriptional and post-translational levels [73,74].
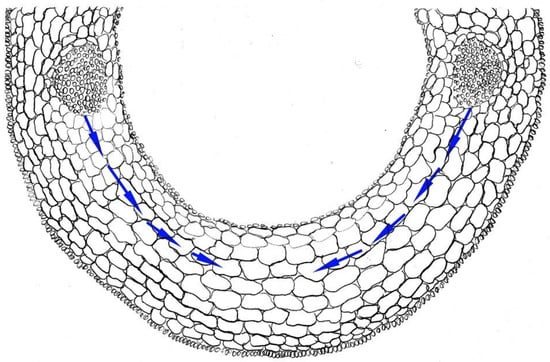
Figure 1.
Scheme of cross-section of rice coleoptile. Arrows indicate the water pathway from vascular bundles to elongating cells. This figure was drawn by Dr. G. Sharipova on the basis of microscope observation.
To clarify the molecular mechanisms of elongation growth in these cells, the theory of acidic growth was proposed back in the 1970s [75,76,77]. It implies the activation of the proton ATPase of the plasma membrane and subsequent acidification of the cell wall, leading to an increase in its plasticity. It coincides with an alteration in the membrane potential value, as well as the direction of transport of different ions through plasma membrane. Subsequently, this primary stage is accompanied and followed by the activation of a number of cell systems, which leads to intensive elongation growth [78,79]. Unfortunately, we failed to find clear evidence revealing the importance of AQP activity, especially in the determination of coleoptile growth.
Given examples strictly define the role of water-transporting AQPs (PIPs and TIPs), located in the plasma membrane and tonoplast, in the determination of water flow over elongation growth.
4. Shoot Growth under Hypoxia and the Role of Aquaporins
Aquaporins may be considered as factors limiting growth during hypoxia. The decrease in hydraulic conductivity is one of the first effects recorded during flooding [80]. This reaction leads to a decrease in stomatal conductance and transpiration. A decline in hydraulic conductivity allows explaining the paradoxical nature of stomatal closure in plants experiencing water excess during flooding, which is normally a characteristic response of plants to water deficiency. The decline in hydraulic conductivity is believed to be due to acidification of tissues brought about by the changes in metabolism resulting from hypoxia. Cytosolic acidification closes the AQP pore and reduces the membrane permeability to water [81] due to the protonation of a conserved histidine residue in aquaporin molecules following a drop in cytoplasmic pH [82]. Inhibitors of cytochrome pathway respiration can be used to mimic oxygen deprivation [83]. Although this effect has been registered in differentiated cells, it is obvious that hypoxia should reduce the hydraulic conductivity of growing cells in the same way. Consequently, the opinion that the membrane permeability of individual cells is too high and, thus, cannot limit the growth of cells by elongation [56] is obviously incorrect under hypoxic conditions. Hypoxia-induced decline in hydraulic conductance makes it a real limiting factor for elongation growth.
Meanwhile, shoot elongation is an important stage of plant survival known as the LOES strategy under submergence. Limited data have been obtained with a focus on coleoptile elongation growth under conditions of oxygen deficiency. In the case of flooding, the initial step of rice seedling development is intensification of coleoptile elongation [84]. Once coleoptiles reach the water surface, the formation of the aerenchyma occurs, which provides the developed seedling (root and primary leaf) with oxygen [85]. Some data indicated a typical quantitative characteristic of coleoptile elongation intensity [84,86]. Further genetic mapping analyses identified several quantitative trait loci (QTL) associated with anaerobic germination and early seedling growth under submergence [87,88]. Several QTLs (4–13 at different chromosomes) responsible for germination and further coleoptile growth under anoxia were found in a number of investigations [88]. Among candidate genes involved in anaerobic germination of tolerant rice were genes encoding anaerobic metabolism, cell-wall plasticity, hormone signaling, etc. [87,88,89]. Nevertheless, AQPs were not listed among them.
Furthermore, it is necessary to take into account that, during elongation of submerged shoot organs (e.g., coleoptiles), absorption of water by the cells directly from the solution is complicated due to the low water permeability of the layer of the cuticle covering coleoptiles or stem [90]. Therefore, water is flows to growing cells from the vascular bundles (there are only two of them in a coleoptile of rice), while crossing the membranes of a large number of cells, which leads to an increase in the contribution of aquaporins to the control of cell extension growth.
The hypoxia-induced decline in the activity of aquaporins raises the question of how acceleration of elongation growth, enabling the lifting of photosynthetic tissues above the water surface, can occur during whole-plant submergence. It can be assumed that the flow of water into growing cells during hypoxia may be facilitated by compensatory mechanisms that can maintain activity of aquaporins. Although an answer to this question requires additional experiments, in this review, we discuss the mechanisms that affect the ability of aquaporins to conduct water. To achieve this, we consider in more detail the factors influencing the activity of aquaporins.
5. Factors Affecting the Activity of Aquaporins
According to conventional wisdom, the activity of aquaporins as representatives of membrane transporters is generally controlled at the transcriptional and post-translational level. It is necessary to admit that quite a number of investigations have reported that the ability to modulate the expression of genes encoding aquaporins is the most powerful mechanism to control their activity. Transcription upregulation resulted in increased abundance of AQP proteins [33,50,91]. However, a direct correlation between the expression of aquaporin genes and abundance of proteins encoded by them could not always be traced [92]. Thus, under osmotic stress, the expression of a number of PIP aquaporins in the roots of maize plants increased tenfold, while the level of proteins encoded by them increased no more than 1.5 times [93]. It can be assumed that a high expression level of aquaporins compensates for the possible destruction of proteins induced by stress.
There are few and contradictory data on the effect of hypoxia on the expression of aquaporin genes, which can be explained by the fact that the differences in the strategies of plant adaptation to hypoxia were not always taken into account. A stable expression of aquaporins and hypoxia responsive genes in adventitious roots was linked to maintaining hydraulic conductance in tobacco (Nicotiana tabacum) exposed to root hypoxia [94]. In Sorghum, the transcript abundance of genes encoding several TIP and PIP aquaporins was differentially altered in response to waterlogging stress due to their tissue-specific roles [95]. AQPs were found to be downregulated in some studies [96]. However, higher transcript abundance of one PIP and two TIP aquaporins coupled with higher Lpr was detected in root tips of Agave deserti, in wet soil [97].
Even fewer studies have investigated the expression of aquaporin genes in elongating shoot cells during hypoxia. Aquaporins have been shown to be involved in rapid internode elongation of deep-water rice. Expression of two OsTIP and three OsPIP aquaporins was significantly enhanced by submergence, supporting the quick elongation of internodes [98]. However, it is still questionable which factors are involved in this up- or downregulation.
Coming to the post-translational level of regulation, one can notice that the employment of a number of model objects convincingly demonstrated that the permeability of water channels may be regulated primarily as a result of phosphorylation and dephosphorylation of serine and threonine residues of aquaporins [33,99,100]. These processes are controlled by certain protein kinases [101,102] and affect the tertiary structure of proteins and the size of the water channel (pore). Several sites for phosphorylation were determined in aquaporin protein. However, it was demonstrated that Ser256 phosphorylation was necessary for normal water transport across the plasma membrane, while phosphorylation of Ser264 and Ser269 did not show the same requirement [103]. Phosphorylation and dephosphorylation of plasma membrane aquaporins (and, thus, AQP activity) have been shown to be Ca2+- and pH-dependent [35,104]. In some investigations, AQPs were even suggested to have pH-sensing qualities [105]. Changes in cytosolic pH and Ca2+ concentration during oxygen shortage are well documented [106,107,108,109].
The presence of disulfide bridges between cysteine residues also plays an important role in maintaining the activity of aquaporins. One way to experimentally reduce the activity of aquaporins is to destroy disulfide bridges with heavy metals (mercury, silver) [110,111] or reactive oxygen species (ROS) [112]. The latter mechanism is considered to be more specific. This was demonstrated in experiments where an increase in the ROS level was modeled using Fenton’s reagent (a mixture of peroxide and divalent iron ions), which provides the formation of a hydroxyl radical as a result of the reaction of peroxide with iron ions [112]. A decrease in the activity of aquaporins induced by Fenton’s reagent led to reduction of hydraulic conductivity and a drop in water potential under air heating [42]. Oxygen deprivation, as well as reoxygenation, is known to stimulate ROS production [113].
Nonetheless, the effect of peroxide and other reactive oxygen species depends on their concentration: high concentrations of ROS inhibited aquaporins [114,115], while low concentrations of peroxides increased their activity [116]. Inactivation of ROS prevented increases in hydraulic conductivity and the level of aquaporins under the influence of abscisic acid (ABA) [117].
Another way to modulate the activity of aquaporins is their rapid traffic to the proper membranes [118]. This mechanism procures a rapid increase in the permeability of membranes for water without contribution to its synthesis or modification. The general mechanisms controlling AQPs traffic to the target membrane and their export out of the ER have not truly been discovered. A recent discovery elucidated that PIP2 primary protein sequences were found to carry the so-called diacidic motifs involved in the mechanisms of exit from the ER [119]. Another possible method of regulation is that the path of PIPs to the PM is under the control of SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptor, SYP121 isoform) of the syntaxin family [120,121]. This type of AQPs traffic was found to be very sensitive to stress factors, such as salinity, for both PIP and TIP representatives [118]. Moreover, intercellular traffic of PIP showed the ability to undergo lateral diffusion in the plasma membrane [122]. Such diffusion might correspond to the plasma membrane interaction with the cell wall and actin filaments [123,124]. Here, a complicated mechanism of endocytosis followed by AQPs degradation also has to be mentioned. The functioning of these complex multicomponent systems will inevitably be different according to tissue type, developmental stage, stress, etc.
Thus, a significant number of mechanisms might be involved in AQP regulation, and almost all of them are likely to occur under oxygen deficiency. Hypoxia and stronger anoxia induce cytosolic acidification, elevation of Ca2+ cytoplasmic concentration, alteration in ROS level, etc. [106,107,108,109,113]. However, available data are insufficient to conclude which of them is the most important.
6. Hypoxia, Hormones, Growth, and the Role of Aquaporins
Taking into account that growth is a process intensively regulated by plant hormones, the final part of our review is focused on the possible involvement of plant hormones in the regulation of AQP activity under oxygen deficiency. The role of hormones in the regulation of cell elongation during hypoxia has been discussed [10,15,125]. However, the involvement of hormones in the regulation of aquaporin activity in connection with the activation of shoot elongation during hypoxia has received little attention. Therefore, in discussing this problem, we involve data on the effects of hormones on the level of aquaporins in a broader aspect.
6.1. Effects of Ethylene under Hypoxia
The greatest amount of information about the role of hormones in the implementation of low-oxygen escape syndrome during hypoxia concerns ethylene [13]. Promotion of shoot extension by ethylene is mostly linked with cell-wall loosening provided by ethylene-dependent stimulation of enzymes involved in the process [15]. In the context of the problem we are discussing, it is important that, according to some data, ethylene is able to increase the expression of aquaporin genes [92,126], which can contribute to the activation of shoot growth. Ethylene significantly enhances root hydraulic conductivity by increasing plasma membrane permeability, permitting more water to cross the cells [127]. Increased water transport in hypoxic seedlings exposed to ethylene was explained in terms of enhanced aquaporin activity, probably due to a direct effect of ethylene on the phosphorylation of aquaporins [128]. The problem is that during hypoxia, ethylene accumulates not only in plants through implementing the strategy of avoiding oxygen deficiency by activating shoot elongation (LOES plants), but also in those using the opposite strategy of growth inhibition [129]. In addition, ethylene-sensitive factors (ERF) are involved in a recently discovered mechanism of oxygen sensing and response to its deficiency [130]. This mechanism is implemented via the Cys–Arg/N-end rule pathway through the oxygen-dependent degradation of a number of transcription factors, primarily group VII ethylene response factors (ERFVII). These transcription factors are recognized by the E3 ligase, which leads to their degradation with the participation of the 26S proteasome complex. A low oxygen concentration increases the stability of ERFVII, which leads to the launch of reactions that provide plant adaptation to flooding [131]. The problem is that this mechanism can upregulate the HCR1 gene (hydraulic conductivity of root 1-Raf-like MAPKKK) that negatively controls hydraulic conductance, which is likely to be due to changes in AQPs activity. HCR1 accumulates and is functional under combined O2 limitation and potassium (K+) sufficiency [132]. The ambiguity of the effect of ethylene on hydraulic conductivity during flooding is explained by the fact that the response to oxygen deficiency depends on many concomitant factors, e.g., on the concentration of potassium or ethylene, as well as on the ability of ethylene to inactivate nitric oxide, which is also involved in the response of plants to hypoxia [133]. One way or another, the available information indicates the prospects for further study of the role of ethylene in the regulation of aquaporin activity under oxygen deficiency. The mechanism of action of ethylene during hypoxia may also be due to the interaction of ethylene with other hormones, whose concentration can be influenced by ethylene [134]. Thus, ethylene increases the concentration of gibberellins (GAs) [135], reduces ABA [13], and affects the distribution of auxins [136].
6.2. Gibberellins under Hypoxia
The role of gibberellins in the activation of stem elongation during hypoxia has been discussed in connection with the data on the accumulation of these hormones under flooding conditions, as well as their ability to accelerate shoot elongation due to the inactivation of DELLA (a known inhibitor of cell elongation growth [60]). Thus, GAs are credited with an important role in stimulating shoot elongation under hypoxia, but not at the expense of their possible effect on hydraulic conductivity or AQP activity. Although a review addressing functional aquaporin diversity in plants [137] gave many examples of the effects of GAs on aquaporins, there is little information about their role in the regulation of AQPs activity during flooding. Recently published work also showed that aquaporin AtTIP5;1 is an essential target of GAs promoting hypocotyl cell elongation in Arabidopsis thaliana under excess boron stress [138]. However, we failed to find information on the effect of GAs on aquaporins during hypoxia. At the same time, this does not mean that the role of GAs in the regulation of aquaporin activity during hypoxia is insignificant. Rather, this is evidence of insufficient knowledge of this issue, highlighting prospects for further research.
6.3. Abscisic Acid (ABA) and Hypoxia
By analogy with the role of ABA in the regulation of stomatal closure during drought [139], abscisic acid is supposed to be involved in the inhibition of transpiration during flooding. However, it was not possible to register an increase in the concentration of this hormone in the xylem during flooding, and its role in the regulation of stomatal behavior during flooding was not proven [140]. However, the involvement of ABA in the response of plants to flooding has been actively discussed [15,141]. In flooded LOES plants, a decrease in the concentration of ABA was detected [13], and the known ability of this hormone to inhibit growth, acting as an antagonist of GAs and maintaining the stability of DELLA, made it possible to associate a reduced concentration of ABA with growth activation [142]. The decrease in ABA concentration during hypoxia may be due to the action of ethylene, which can inactivate ABA via its conjugation [143] and downregulate its biosynthesis [15,125]. Nevertheless, in plants that are not characterized by stimulation of shoot elongation during hypoxia, a decrease in ABA concentration was not recorded [6], although (see above) ethylene accumulation occurred. Plants sensitive to oxygen deprivation accumulate a higher amount of more ABA during submergence or total anoxia [125,144].
In the context of the discussed problem of the regulation of aquaporin activity, it is of interest that there is more information about the effect of ABA on the activity of aquaporins than about any other hormone. An ABA-induced increase in both the level of aquaporins [145] and the expression of the genes encoding them, as well as their activation as a result of phosphorylation and traffic from the cytoplasm to the membranes, has been repeatedly shown [26]. However, during flooding, ABA is not likely to be involved in the activation of aquaporins during the acceleration of shoot elongation, since its concentration decreases in this case (see above). Nevertheless, uncertainties regarding this issue remain. Despite the decrease in the level of ABA, the level of its receptor increases during flooding [146].
6.4. Auxins and Flooding
Data on the effect of submergence on the content of auxins are contradictory. There is information on the submergence-induced decline in the level of indoleacetic acid (IAA) in petioles of R. palustris [147]. At the same time, anoxia-induced accumulation of IAA in wheat and rice seedlings has been shown, as well as the relationship of plant tolerance with oxygen deprivation [144,148,149]. The role of auxins in the activation of shoot elongation during flooding has been discussed mainly in connection with the ability of this hormone to influence the accumulation of osmotically active substances and the extensibility of the cell wall [150,151]. The possible effect of auxins on the activity of aquaporins is difficult to discuss, since there is very limited information on this subject. Most often, the same study is cited, where a decrease in the expression of aquaporins under the influence of auxins was demonstrated [152]. However, it was shown that treatment of plants with an auxin transport inhibitor prevented the increase in aquaporin expression registered during hypoxia in the absence of this inhibitor [153]. These results indicate that the normal distribution of auxins during hypoxia plays an important role in the induction of aquaporin expression.
7. Conclusions
A decrease in hydraulic conductivity, which is one of the characteristic consequences of hypoxia, indicates that this factor is limiting for cell growth by elongation during flooding. Since low-oxygen escape syndrome is based on the stimulation of shoot elongation during flooding, it is obvious that there must be a mechanism to compensate for the decline in hydraulic conductivity during hypoxia. The role of such a compensatory mechanism may consist of an increase of the ability of aquaporins to conduct water, which is indirectly evidenced by the little data on an increase in the expression of some aquaporin-coding genes during hypoxia and a change in the concentration of phytohormones under hypoxia, potentially capable of influencing the activity of aquaporins. At the same time, the amount of experimental data in this regard is extremely limited, which dictates the need for further research in this direction. Expanding our understanding of the regulation of aquaporin activity during hypoxia is a promising approach, not only for deepening knowledge about the ways to increase plant resistance to flooding, but also for a general understanding of the regulation of cell extension growth.
Author Contributions
Conceptualization, G.K. and M.S.; writing—original draft preparation, G.K., D.V., V.Y. and M.S.; writing—review and editing, G.K., V.Y. and M.S.; supervision, M.S.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 22-14-00096.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank G. Sharipova for help with the preparation of the figure.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gupta, K.J.; Zabalza, A.; Van Dongen, J.T. Regulation of respiration when the oxygen availability changes. Physiol. Plant. 2009, 137, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Neira, J.; Ortiz, M.; Morales, L.; Acevedo, E. Oxygen diffusion in soils: Understanding the factors and processes needed for modeling. Chil. J. Agric. Res. 2015, 75, 35–44. [Google Scholar] [CrossRef]
- Colmer, T.D. Long-distance transport of gases in plants: A perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ. 2003, 26, 17–36. [Google Scholar] [CrossRef]
- Evans, D.E. Aerenchyma formation. New Phytol. 2004, 161, 35–49. [Google Scholar] [CrossRef]
- Veldhuis, E.R.; Schrama, M.; Staal, M.; Elzenga, J.T.M. Plant stress-tolerance traits predict salt marsh vegetation patterning. Front. Mar. Sci. 2019, 5, 501. [Google Scholar] [CrossRef]
- van Veen, H.; Mustroph, A.; Barding, G.A.; Vergeer-van Eijk, M.; Welschen-Evertman, R.A.M.; Pedersen, O.; Visser, E.J.W.; Larive, C.K.; Pierik, R.; Bailey-Serres, J.; et al. Two Rumex species from contrasting hydrological niches regulate flooding tolerance through distinct mechanisms. Plant Cell 2013, 25, 4691–4707. [Google Scholar] [CrossRef]
- Chirkova, T.V. Rastenie i anaerobioz [Plant and anaerobiosis]. Biol. Commun. 1998, 43, 41–52. (In Russian) [Google Scholar]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Chirkova, T.; Yemelyanov, V. The study of plant adaptation to oxygen deficiency in Saint Petersburg University. Biol. Commun. 2018, 63, 17–31. [Google Scholar] [CrossRef]
- Nakamura, M.; Noguchi, K. Tolerant mechanisms to O2 deficiency under submergence conditions in plants. J. Plant Res. 2020, 133, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flooding tolerance: O2 sensing and survival strategies. Curr. Opin. Plant Biol. 2013, 16, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M. Ethylene-promoted elongation: An adaptation to submergence stress. Ann. Bot. 2008, 101, 229–248. [Google Scholar] [CrossRef]
- Peeters, A.J.M.; Cox, M.C.H.; Benschop, J.J.; Vreeburg, R.A.M.; Bou, J.; Voesenek, L.A.C.J. Submergence research using Rumex palustris as a model; looking back and going forward. J. Exp. Bot. 2002, 53, 391–398. [Google Scholar] [CrossRef]
- Yemelyanov, V.V.; Shishova, M.F. The role of phytohormones in the control of plant adaptation to oxygen depletion. In Phytohormones and Abiotic Stress Tolerance in Plants; Khan, N.A., Nazar, R., Iqbal, A., Anjum, N.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 229–248. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Water uptake by growing cells: An assessment of the controlling roles of wall relaxation, solute uptake and hydraulic conductance. Int. J. Plant Sci. 1993, 154, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Fricke, W. Biophysical limitation of leaf cell elongation in source-reduced barley. Planta 2002, 215, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C. Turgor maintenance by osmotic adjustment: 40 years of progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef]
- Sabirzhanova, I.B.; Sabirzhanov, B.E.; Chemeris, A.V.; Veselov, D.S.; Kudoyarova, G.R. Fast changes in expression of expansin gene and leaf extensibility in osmotically stressed maize plants. Plant Physiol. Biochem. 2005, 43, 419–422. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Plant cell wall extensibility: Connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 2016, 67, 463–476. [Google Scholar] [CrossRef]
- Liu, J.; Shao, Y.; Feng, X.; Otie, V.; Matsuura, A.; Irshad, M.; Zheng, Y.; An, P. Cell wall components and extensibility regulate root growth in Suaeda salsa and Spinacia oleracea under salinity. Plants 2022, 11, 900. [Google Scholar] [CrossRef]
- Kuwagata, T.; Murai-Hatano, M. Osmotic water permeability of plasma and vacuolar membranes in protoplasts II: Theoretical basis. J. Plant Res. 2007, 120, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Vitali, V.; Sutka, M.; Amodeo, G.; Chara, O.; Ozu, M. The water to solute permeability ratio governs the osmotic volume dynamics in beet root vacuoles. Front. Plant Sci. 2016, 7, 1388. [Google Scholar] [CrossRef]
- Deamer, D.W.; Bramhall, J. Permeability of lipid bilayers to water and ionic solutes. Chem. Phys. Lipids. 1986, 40, 167–188. [Google Scholar] [CrossRef]
- Maurel, C.; Reizer, J.; Schroeder, J.I.; Chrispeels, M.J. The vacuolar membrane protein γ-TIP creates water specific channels in Xenopus oocytes. EMBO J. 1993, 12, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Boursiac, Y.; Luu, D.-T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef] [PubMed]
- Huster, D.; Jin, A.J.; Arnold, K.; Gawrisch, K. Water permeability of polyunsaturated lipid membranes measured by 17O NMR. Biophys. J. 1997, 73, 855–864. [Google Scholar] [CrossRef]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Kabutomori, J.; Beloto-Silva, O.; Geyer, R.; Musa-Aziz, R. Lithobates catesbeianus (American Bullfrog) oocytes: A novel heterologous expression system for aquaporins. Biol. Open. 2018, 7, bio031880. [Google Scholar] [CrossRef]
- Lu, M.-X.; Pan, D.-D.; Xu, J.; Liu, Y.; Wang, G.-R.; Du, Y.-Z. Identification and functional analysis of the first aquaporin from striped stem borer, Chilo suppressalis. Front. Physiol. 2018, 9, 57. [Google Scholar] [CrossRef]
- Bienert, M.D.; Diehn, T.A.; Richet, N.; Chaumont, F.; Bienert, G.P. Heterotetramerization of Plant PIP1 and PIP2 aquaporins is an evolutionary ancient feature to guide PIP1 plasma membrane localization and function. Front. Plant Sci. 2018, 9, 382. [Google Scholar] [CrossRef]
- Martre, P.; Morillon, R.; Barrieu, F.; North, G.; Nobel, P.; Chrispeels, M. Plasma membrane aquaporin play a significant role during recovery from water deficit. Plant Physiol. 2002, 130, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Hachez, C.; Zelazny, E.; Chaumont, F. Modulating the expression of aquaporin genes in planta: A key to understand their physiological functions? Biochim. Biophys. Acta 2006, 1758, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Bezerra-Neto, J.P.; Czekalski de Araújo, F.; Ferreira-Neto, J.R.C.; da Silva, M.D.; Pandolfi, V.; Aburjaile, F.F.; Sakamoto, T.; de Oliveira Silva, R.L.; Kido, E.A.; Barbosa Amorim, L.L.; et al. Plant aquaporins: Diversity, evolution and biotechnological applications. Curr. Protein Pept. Sci. 2019, 20, 368–395. [Google Scholar] [CrossRef] [PubMed]
- Kapilan, R.; Vaziri, M.; Zwiazek, J.J. Regulation of aquaporins in plants under stress. Biol. Res. 2018, 51, 4. [Google Scholar] [CrossRef]
- Gomes, D.; Agasse, A.; Thiebaud, P.; Delrot, S.; Geros, H.; Chaumont, F. Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim. Biophys. Acta 2009, 1788, 1213–1228. [Google Scholar] [CrossRef]
- Sutka, M.; Amodeo, G.; Ozu, M. Plant and animal aquaporins crosstalk: What can be revealed from distinct perspectives. Biophys. Rev. 2017, 9, 545–562. [Google Scholar] [CrossRef]
- Su, W.; Cao, R.; Zhang, X.-Y.; Guan, Y. Aquaporins in the kidney: Physiology and pathophysiology. Am. J. Physiol. Renal. Physiol. 2020, 318, F193–F203. [Google Scholar] [CrossRef]
- Yaaran, A.; Moshelion, M. Role of aquaporins in a composite model of water transport in the leaf. Int. J. Mol. Sci. 2016, 17, 1045. [Google Scholar] [CrossRef]
- Siefritz, F.; Tyree, M.T.; Lovisolo, C.; Schubert, A.; Kaldenhoff, R. PIP1 plasma membrane aquaporins in tobacco: From cellular effects to function in plants. Plant Cell 2002, 14, 869–876. [Google Scholar] [CrossRef]
- Cochard, H.; Venisse, J.S.; Barigah, T.S.; Brunel, N.; Herbette, S.; Guilliot, A.; Sakr, S. Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiol. 2007, 143, 122–133. [Google Scholar] [CrossRef]
- Veselov, D.S.; Sharipova, G.V.; Veselov, S.Y.; Dodd, I.C.; Ivanov, I.; Kudoyarova, G.R. Rapid changes in root HvPIP2;2 aquaporins abundance and ABA concentration are required to enhance root hydraulic conductivity and maintain leaf water potential in response to increased evaporative demand. Funct. Plant Biol. 2018, 45, 143–149. [Google Scholar] [CrossRef]
- Caldeira, C.F.; Bosio, M.; Parent, B.; Jeanguenin, L.; Chaumont, F.; Tardieu, F. A hydraulic model is compatible with rapid changes in leaf elongation under fluctuating evaporative demand and soil water status. Plant Physiol. 2014, 164, 1718–1730. [Google Scholar] [CrossRef] [PubMed]
- Obroucheva, N.V.; Sin’kevich, I.A. Aquaporins and cell growth. Russ. J. Plant Physiol. 2010, 57, 153–165. [Google Scholar] [CrossRef]
- Li, Q.; Tong, T.; Jiang, W.; Cheng, J.; Deng, F.; Wu, X.; Chen, Z.-H.; Ouyang, Y.; Zeng, F. Highly conserved evolution of aquaporin PIPs and TIPs confers their crucial contribution to flowering process in plants. Front. Plant Sci. 2022, 12, 761713. [Google Scholar] [CrossRef]
- Yaneff, A.; Vitali, V.; Amodeo, G. PIP1 aquaporins: Intrinsic water channels or PIP2 aquaporin modulators? FEBS Lett. 2015, 589, 3508–3515. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, M.M. TIP aquaporins in plants: Role in abiotic stress tolerance. In Abiotic Stress in Plants; Fahad, S., Saud, S., Chen, Y., Wu, C., Wang, D., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Kurowska, M.M.; Wiecha, K.; Gajek, K.; Szarejko, I. Drought stress and re-watering affect the abundance of TIP aquaporin transcripts in barley. PLoS ONE 2019, 14, e0226423. [Google Scholar] [CrossRef] [PubMed]
- Béré, E.; Lahbib, K.; Merceron, B.; Fleurat-Lessard, P.; Boughanmi, N.G. α-TIP aquaporin distribution and size tonoplast variation in storage cells of Vicia faba cotyledons at seed maturation and germination stages. J. Plant Physiol. 2017, 216, 145–151. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Liu, F.; Sun, L.; Hao, F. Versatile roles of aquaporins in plant growth and development. Int. J. Mol. Sci. 2020, 21, 9485. [Google Scholar] [CrossRef]
- Ludevid, D.; Höfte, H.; Himelblau, E.; Chrispeels, M.J. The expression pattern of the tonoplast intrinsic protein γ-TIP in Arabidopsis thaliana is correlated with cell enlargement. Plant Physiol. 1992, 100, 1633–1639. [Google Scholar] [CrossRef]
- Fricke, W.; Knipfer, T. Plant aquaporins and cell elongation. In Plant Aquaporins, Signaling and Communication in Plants; Chaumont, F., Tyerman, S.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 107–131. [Google Scholar] [CrossRef]
- Quigley, F.; Rosenberg, J.M.; Shachar-Hill, Y.; Bohnert, H.J. From genome to function: The Arabidopsis aquaporins. Genome Biol. 2001, 3, research0001.1. [Google Scholar] [CrossRef]
- Sakurai, J.; Ishikawa, F.; Yamaguchi, T.; Uemura, M.; Maeshima, M. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol. 2005, 46, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Hove, R.M.; Ziemann, M.; Bhave, M. Identification and expression analysis of the barley (Hordeum vulgare L.) aquaporin gene family. PLoS ONE 2015, 10, e0128025. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Plant cell growth and elongation. In eLS.; John Wiley & Sons, Ltd.: Chichester, UK, 2014. [Google Scholar] [CrossRef]
- Touati, M.; Knipfer, T.; Visnovitz, T.; Kameli, A.; Fricke, W. Limitation of cell elongation in barley (Hordeum vulgare L.) leaves through mechanical and tissue-hydraulic properties. Plant Cell Physiol. 2015, 56, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Nonami, H.; Boyer, J.S. Wall extensibility and cell hydraulic conductivity decrease in enlarging stem tissues at low water potentials. Plant Physiol. 1990, 93, 1610–1619. [Google Scholar] [CrossRef]
- Moshelion, M.; Hachez, C.; Ye, Q.; Cavez, D.; Bajji, M.; Jung, R.; Chaumont, F. Membrane water permeability and aquaporin expression increase during growth of maize suspension cultured cells. Plant Cell Environ. 2009, 32, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Zhu, T.; Su, J.; Li, J.; Zhao, Q.; Kang, X.; Zheng, R. Transcriptome analysis of gibberellins and abscisic acid during the flooding response in Fokienia hodginsii. PLoS ONE 2022, 17, e0263530. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar] [CrossRef] [PubMed]
- Vandeleur, R.K.; Mayo, G.; Shelden, M.C.; Gilliham, M.; Kaiser, B.N.; Tyerman, S.D. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: Diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol. 2009, 149, 445–460. [Google Scholar] [CrossRef]
- Cui, X.H.; Hao, F.S.; Chen, H.; Chen, J.; Wang, X.C. Expression of the Vicia faba VfPIP1 gene in Arabidopsis thaliana plants improves their drought resistance. J. Plant Res. 2008, 121, 207–214. [Google Scholar] [CrossRef]
- Leng, H.; Jiang, C.; Song, X.; Lu, M.; Wan, X. Poplar aquaporin PIP1;1 promotes Arabidopsis growth and development. BMC Plant Biol. 2021, 21, 253. [Google Scholar] [CrossRef]
- Kaldenhoff, R.; Grote, K.; Zhu, J.J.; Zimmermann, U. Significance of plasmalemma aquaporins for water-transport in Arabidopsis thaliana. Plant J. 1998, 14, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fukumoto, T.; Gena, P.; Feng, P.; Sun, Q.; Li, Q.; Matsumoto, T.; Kaneko, T.; Zhang, H.; Zhang, Y.; et al. Ectopic expression of a rice plasma membrane intrinsic protein (OsPIP1;3) promotes plant growth and water uptake. Plant J. 2020, 102, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.Q.; Li, L.J.; Ren, F.; Lu, P.L.; Wei, P.C.; Cai, J.H.; Xin, L.G.; Zhang, J.; Chen, J.; Wang, X.C. Overexpression of the tonoplast aquaporin AtTIP5;1 conferred tolerance to boron toxicity in Arabidopsis. J. Genet. Genom. 2010, 37, 389–397. [Google Scholar] [CrossRef]
- Katsuhara, M.; Koshio, K.; Shibasaka, M.; Hayashi, Y.; Hayakawa, T.; Kasamo, K. Over-expression of a barley aquaporin increased the shoot/root ratio and raised salt sensitivity in transgenic rice plants. Plant Cell Physiol. 2003, 44, 1378–1383. [Google Scholar] [CrossRef]
- Hachez, C.; Heinen, R.B.; Draye, X.; Chaumont, F. The expression pattern of plasma membrane aquaporins in maize leaf highlights their role in hydraulic regulation. Plant Mol. Biol. 2008, 68, 337–353. [Google Scholar] [CrossRef]
- Heinen, R.B.; Ye, Q.; Chaumont, F. Role of aquaporins in leaf physiology. J. Exp. Bot. 2009, 60, 2971–2985. [Google Scholar] [CrossRef]
- Takahashi, H.; Sato, K.; Takeda, K. Mapping genes for deep-seeding tolerance in barley. Euphytica 2001, 122, 37–43. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Zheng, B.; Chapman, S.C. Do wheat breeders have suitable genetic variation to overcome short coleoptiles and poor establishment in the warmer soils of future climates? Funct. Plant Biol. 2016, 43, 961–972. [Google Scholar] [CrossRef]
- Bovill, W.D.; Hyles, J.; Zwart, A.B.; Ford, B.A.; Perera, G.; Phongkham, T.; Brooks, B.J.; Rebetzke, G.J.; Hayden, M.J.; Hunt, J.R.; et al. Increase in coleoptile length and establishment by Lcol-A1, a genetic locus with major effect in wheat. BMC Plant Biol. 2019, 19, 332. [Google Scholar] [CrossRef]
- Luo, H.; Hill, C.B.; Zhou, G.; Zhang, X.-Q.; Li, C. Genome-wide association mapping reveals novel genes associated with coleoptile length in a worldwide collection of barley. BMC Plant Biol. 2020, 20, 346. [Google Scholar] [CrossRef]
- Cleland, R.E. Cell wall extension. Annu. Rev. Plant Physiol. 1971, 22, 197–222. [Google Scholar] [CrossRef]
- Polevoi, V.V.; Salamatova, T.A. Auxin, proton pump and cell trophics. In Regulation of Cell Membrane Activities in Plants; Marre, E., Ciferri, O., Eds.; Elsevier: Amsterdam, The Netherlands, 1977; pp. 209–216. [Google Scholar]
- Hager, A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J. Plant Res. 2003, 116, 483–505. [Google Scholar] [CrossRef] [PubMed]
- Kirpichnikova, A.; Chen, T.; Teplyakova, S.; Shishova, M. Proton pump and plant cell elongation. Biol. Commum. 2018, 63, 32–42. [Google Scholar] [CrossRef]
- Polak, M.; Karcz, W. Some new methodological and conceptual aspects of the “Acid growth theory” for the auxin action in maize (Zea mays L.) coleoptile segments: Do acid- and auxin-induced rapid growth differ in their mechanisms? Int. J. Mol. Sci. 2021, 22, 2317. [Google Scholar] [CrossRef]
- Jackson, M.B.; Davies, W.J.; Else, M. Pressure –flow relationships, xylem solutes and root hydraulic conductance in flooded tomato plants. Ann. Bot. 1996, 77, 17–24. [Google Scholar] [CrossRef]
- Bramley, H.; Turner, D.W.; Tyerman, S.D.; Turner, N.C. Water flow in the roots of crop species: The influence of root structure, aquaporin activity, and waterlogging. Adv. Agron. 2007, 96, 134–193. [Google Scholar] [CrossRef]
- Törnroth-Horsefield, S.; Wang, Y.; Kjellbom, K.P. Structural mechanism of plant aquaporin gating. Nature 2006, 439, 688–694. [Google Scholar] [CrossRef]
- Tournaire-Roux, C.; Sutka, M.; Javot, H.; Gout, E.; Gerbeau, P.; Luu, D.-T.; Bligny, R.; Maurel, C. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 2003, 425, 393–397. [Google Scholar] [CrossRef]
- Magneschi, L.; Perata, P. Rice germination and seedling growth in the absence of oxygen. Ann. Bot. 2009, 103, 181–196. [Google Scholar] [CrossRef]
- Alpi, A.; Beevers, H. Effects of O2 concentration on rice seedlings. Plant Physiol. 1983, 71, 30–34. [Google Scholar] [CrossRef]
- Magneschi, L.; Kudahettige, R.L.; Alpi, A.; Perata, P. Comparative analysis of anoxic coleoptile elongation in rice varieties: Relationship between coleoptile length and carbohydrate levels, fermentative metabolism and anaerobic gene expression. Plant Biol. 2009, 11, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-K.; Tung, C.-W. Genetic mapping of anaerobic germination-associated QTLs controlling coleoptile elongation in rice. Rice 2015, 8, 38. [Google Scholar] [CrossRef]
- Su, L.; Yang, J.; Li, D.; Peng, Z.; Xia, A.; Yang, M.; Luo, L.; Huang, C.; Wang, J.; Wang, H.; et al. Dynamic genome-wide association analysis and identification of candidate genes involved in anaerobic germination tolerance in rice. Rice 2021, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-K.; Tung, C.-W. RNA-Seq analysis of diverse rice genotypes to identify the genes controlling coleoptile growth during submerged germination. Front. Plant Sci. 2017, 8, 762. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, K.Y.; Zhang, J.; Yamaoka, Y.; Gao, P.; Kim, H.; Hwang, J.U.; Suh, M.C.; Kang, B.; Lee, Y. Arabidopsis seedling establishment under waterlogging requires ABCG5-mediated formation of a dense cuticle layer. New Phytol. 2021, 229, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Aroca, R.; Ferrante, A.; Vernieri, P.; Chrispeels, M.J. Drought, abscisic acid and transpiration rate effects on the regulation of PIP aquaporin gene expression and abundance in Phaseolus vulgaris plants. Ann. Bot. 2006, 98, 1301–1310. [Google Scholar] [CrossRef]
- Tan, X.; Xu, H.; Khan, S.; Equiza, M.A.; Lee, S.H.; Vaziriyeganeh, M.; Zwiazek, J.J. Plant water transport and aquaporins in oxygen-deprived environments. J. Plant Physiol. 2018, 227, 20–30. [Google Scholar] [CrossRef]
- Hachez, C.; Veselov, D.; Ye, Q.; Reinhardt, H.; Knipfer, T.; Fricke, W.; Chamout, F. Short-term control of maize cell and root water permeability through plasma membrane aquaporin isoforms. Plant Cell Environ. 2012, 35, 185–198. [Google Scholar] [CrossRef]
- Tan, X.; Zwiazek, J.J. Stable expression of aquaporins and hypoxia-responsive genes in adventitious roots are linked to maintaining hydraulic conductance in tobacco (Nicotiana tabacum) exposed to root hypoxia. PLoS ONE 2019, 14, e0212059. [Google Scholar] [CrossRef]
- Kadam, S.; Abril, A.; Dhanapal, A.P.; Koester, R.P.; Vermerris, W.; Jose, S.; Fritschi, F.B. Characterization and regulation of aquaporin genes of sorghum [Sorghum bicolor (L.) Moench] in response to waterlogging stress. Front. Plant Sci. 2017, 8, 862. [Google Scholar] [CrossRef]
- Reeksting, B.J.; Olivier, N.A.; van den Berg, N. Transcriptome responses of an ungrafted Phytophthora root rot tolerant avocado (Persea americana) rootstock to flooding and Phytophthora cinnamomi. BMC Plant Biol. 2016, 16, 205. [Google Scholar] [CrossRef] [PubMed]
- North, G.B.; Martre, P.; Nobel, P.S. Aquaporins account for variations in hydraulic conductance for metabolically active root regions of Agave deserti in wet, dry, and rewetted soil. Plant Cell Environ. 2004, 27, 219–228. [Google Scholar] [CrossRef]
- Muto, Y.; Segami, S.; Hayashi, H.; Sakurai, J.; Hatan, M.; Hattori, Y.; Ashikari, M.; Maeshima, M. Vacuolar proton pumps and aquaporins involved in rapid internode elongation of deepwater rice. Biosci. Biotechnol. Biochem. 2011, 75, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Verdoucq, L.; Rodrigues, O.; Martinière, A.; Luu, D.T.; Maurel, C. Plant aquaporins on the move: Reversible phosphorylation, lateral motion and cycling. Curr. Opin. Plant Biol. 2014, 22, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, W. Molecular biology of aquaporins. Adv. Exp. Med. Biol. 2017, 969, 1–34. [Google Scholar] [CrossRef]
- Wu, X.N.; Rodriguez, C.S.; Pertl-Obermeyer, H.; Obermeyer, G.; Schulze, W.X. Sucrose-induced receptor kinase SIRK1 regulates a plasma membrane aquaporin in Arabidopsis. Mol. Cell. Proteom. 2013, 12, 2856–2873. [Google Scholar] [CrossRef]
- Bellati, J.; Champeyroux, C.; Hem, S.; Rofidal, V.; Krouk, G.; Maurel, C.; Santoni, V. Novel aquaporin regulatory mechanisms revealed by interactomics. Mol. Cell. Proteom. 2016, 15, 3473–3487. [Google Scholar] [CrossRef]
- Fushimi, K.; Sasaki, S.; Marumo, F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J. Biol. Chem. 1997, 272, 14800–14804. [Google Scholar] [CrossRef]
- Gerbeau, P.; Amodeo, G.; Henzler, T.; Santoni, V.; Ripoche, P.; Maurel, C. The water permeability of Arabidopsis plasma membrane is regulated by divalent cations and pH. Plant J. 2002, 30, 71–81. [Google Scholar] [CrossRef]
- Yaneff, A.; Sigaut, L.; Gómez, N.; Fandiño, C.A.; Alleva, K.; Pietrasanta, L.I.; Amodeo, G. Loop B serine of a plasma membrane aquaporin type PIP2 but not PIP1 plays a key role in pH sensing. Biochim. Biophys. Acta 2016, 1858, 2778–2787. [Google Scholar] [CrossRef]
- Roberts, J.K.M.; Callis, J.; Jardetzky, O.; Walbot, V.; Freeling, M. Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proc. Natl. Acad. Sci. USA 1984, 81, 6029–6033. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, C.; Bush, D.S.; Sachs, M. Elevation of cytosolic calcium precedes anoxic gene expression in maize suspension cultured cells. Plant Cell. 1994, 6, 1747–1762. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yemelyanov, V.V.; Shishova, M.F.; Chirkova, T.V.; Lindberg, S.M. Anoxia-induced elevation of cytosolic Ca2+ concentration depends on different Ca2+ sources in rice and wheat protoplasts. Planta 2011, 234, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Yemelyanov, V.V.; Chirkova, T.V.; Lindberg, S.M.; Shishova, M.F. Potassium efflux and cytosol acidification as primary anoxia-induced events in wheat and rice seedlings. Plants 2020, 9, 1216. [Google Scholar] [CrossRef] [PubMed]
- Javot, H.; Maurel, C. The role of aquaporins in water uptake. Ann. Bot. 2002, 90, 301–313. [Google Scholar] [CrossRef]
- Przedpelska-Wasowicz, E.M.; Wierzbicka, M. Gating of aquaporins by heavy metals in Allium cepa L. epidermal cells. Protoplasma 2011, 248, 663–671. [Google Scholar] [CrossRef]
- Henzler, T.; Ye, Q.; Steudle, E. Oxidative of water channels (aquaporins) in Chara by hydroxyl radicals. Plant Cell Environ. 2004, 27, 1184–1195. [Google Scholar] [CrossRef]
- Blokhina, O.B.; Chirkova, T.V.; Fagerstedt, K.V. Anoxic stress leads to hydrogen peroxide formation in plant cells. J. Exp. Bot. 2001, 52, 1179–1190. [Google Scholar] [CrossRef]
- Kim, Y.X.; Steudle, E. Gating of aqùaporins by light and reactive oxygen species in leaf parenchyma cells of the midrib of Zea mays. J. Exp. Bot. 2009, 60, 547–556. [Google Scholar] [CrossRef]
- Cordeiro, R.M. Molecular dynamics simulations of the transport of reactive oxygen species by mammalian and plant aquaporins. Biochim. Biophys. Acta 2015, 1850, 1786–1794. [Google Scholar] [CrossRef]
- Aroca, R. Exogenous catalase and ascorbate modify the effects of abscisic acid (ABA) on root hydraulic properties in Phaseolus vulgaris L. plants. J. Plant Growth Regul. 2006, 25, 10–17. [Google Scholar] [CrossRef]
- Sharipova, G.; Ivanov, R.; Veselov, D.; Akhiyarova, G.; Shishova, M.; Nuzhnaya, T.; Kudoyarova, G. Involvement of reactive oxygen species in ABA-induced increase in hydraulic conductivity and aquaporin abundance. Int. J. Mol. Sci. 2021, 22, 9144. [Google Scholar] [CrossRef] [PubMed]
- Luu, D.-T.; Maurel, C. Aquaporin trafficking in plant cells: An emerging membrane-protein model. Traffic 2013, 14, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Zelazny, E.; Miecielica, U.; Borst, J.W.; Hemminga, M.A.; Chaumont, F. An N-terminal diacidic motif is required for the trafficking of maize aquaporins ZmPIP2;4 and ZmPIP2;5 to the plasma membrane. Plant J. 2009, 57, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Besserer, A.; Burnotte, E.; Bienert, G.P.; Chevalier, A.S.; Errachid, A.; Grefen, C.; Blatt, M.R.; Chaumont, F. Selective regulation of maize plasma membrane aquaporin trafficking and activity by the SNARE SYP121. Plant Cell 2012, 24, 3463–3481. [Google Scholar] [CrossRef]
- Hachez, C.; Laloux, T.; Reinhardt, H.; Cavez, D.; Degand, H.; Grefen, C.; De Rycke, R.; Inzé, D.; Blatt, M.R.; Russinova, E.; et al. Arabidopsis SNAREs SYP61 and SYP121 coordinate the trafficking of plasma membrane aquaporin PIP2;7 to modulate the cell membrane water permeability. Plant Cell 2014, 26, 3132–3147. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Yang, Y.; Li, R.; He, Q.; Fang, X.; Luu, D.T.; Maurel, C.; Lin, J. Single-molecule analysis of PIP2;1 dynamics and partitioning reveals multiple modes of Arabidopsis plasma membrane aquaporin regulation. Plant Cell 2011, 23, 3780–3797. [Google Scholar] [CrossRef]
- Martiniere, A.; Lavagi, I.; Nageswaran, G.; Rolfe, D.J.; Maneta-Peyret, L.; Luu, D.-T.; Botchway, S.W.; Webb, S.E.D.; Mongrand, S.; Maurel, C.; et al. Cell wall constrains lateral diffusion of plant plasma-membrane proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 12805–12810. [Google Scholar] [CrossRef]
- Hosy, E.; Martiniere, A.; Choquet, D.; Maurel, C.; Luu, D.-T. Super-resolved and dynamic imaging of membrane proteins in plant cells reveal contrasting kinetic profiles and multiple confinement mechanisms. Mol. Plant 2015, 8, 339–342. [Google Scholar] [CrossRef]
- Hu, Z.; Qi, X.; Zhang, M.; Chen, X.; Nakazono, M. Roles of phytohormones in morphological and anatomical responses of plants to flooding stress. In Plant Hormones under Challenging Environmental Factors; Ahammed, G.J., Yu, J.-Q., Eds.; Springer Science+Business Media: Dordrecht, Germany, 2016; pp. 117–132. [Google Scholar] [CrossRef]
- Tan, X.; Liu, M.; Du, N.; Zwiazek, J.J. Ethylene enhances root water transport and aquaporin expression in trembling aspen (Populus tremuloides) exposed to root hypoxia. BMC Plant Biol. 2021, 21, 227. [Google Scholar] [CrossRef]
- Shao, H.-B.; Chu, L.-Y.; Shao, M.-A.; Zhao, C.-X. Advances in functional regulation mechanisms of plant aquaporins: Their diversity, gene expression, localization, structure and roles in plant soil-water relations (Review). Mol. Membr. Biol. 2008, 25, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Qing, D.; Yang, Z.; Li, M.; Wong, W.S.; Guo, G.; Liu, S.; Guo, H.; Li, N. Quantitative and functional phosphoproteomic analysis reveals that ethylene regulates water transport via the C-terminal phosphorylation of aquaporin PIP2;1 in Arabidopsis. Mol. Plant. 2016, 9, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.B. Ethylene and responses of plants to soil waterlogging and submergence. Annu. Rev. Plant Physiol. 1985, 36, 145–174. [Google Scholar] [CrossRef]
- van Dongen, J.T.; Licausi, F. Oxygen sensing and signaling. Annu. Rev. Plant Biol. 2015, 66, 345–367. [Google Scholar] [CrossRef]
- Weits, D.A.; van Dongen, J.T.; Licausi, F. Molecular oxygen as a signaling component in plant development. New Phytol. 2021, 229, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, Z.; Canut, M.; Tournaire-Roux, C.; Martinière, A.; Boursiac, Y.; Loudet, O.; Maurel, C. A potassium-dependent oxygen sensing pathway regulates plant root hydraulics. Cell 2016, 167, 87–98. [Google Scholar] [CrossRef]
- Hartman, S.; Liu, Z.; van Veen, H.; Vicente, J.; Reinen, E.; Martopawiro, S.; Zhang, H.; van Dongen, N.; Bosman, F.; Bassel, G.W.; et al. Ethylene-mediated nitric oxide depletion pre-adapts plants to hypoxia stress. Nat. Commun. 2019, 10, 4020. [Google Scholar] [CrossRef]
- Wang, X.; Komatsu, S. The role of phytohormones in plant response to flooding. Int. J. Mol. Sci. 2022, 23, 6383. [Google Scholar] [CrossRef]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of waterlogging tolerance in plants: Research progress and prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef]
- Sasidharan, R.; Voesenek, L.A.C.J. Ethylene-mediated acclimations to flooding stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef]
- Kaldenhoff, R.; Fischer, M. Functional aquaporin diversity in plants. Biochim. Biophys. Acta 2006, 1758, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Li, J.; Qi, B.; Tian, M.; Sun, L.; Wang, X.; Hao, F. Aquaporin AtTIP5;1 as an essential target of gibberellins promotes hypocotyl cell elongation in Arabidopsis thaliana under excess boron stress. Funct. Plant Biol. 2018, 45, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Davies, W.J.; Kudoyarova, G.; Hartung, W. Long-distance ABA signaling and its relation to other signaling pathways in the detection of soil drying and the mediation of the plant’s response to drought. J. Plant Growth Regul. 2005, 24, 285–295. [Google Scholar] [CrossRef]
- Else, M.A.; Tiekstra, A.E.; Croker, S.J.; Davies, W.J.; Jackson, M.B. Stomatal closure in flooded tomato plants involves abscisic acid and a chemically unidentified anti-transpirant in xyle sap. Plant Physiol. 1996, 11, 239–247. [Google Scholar] [CrossRef]
- González-Guzmán, M.; Gómez-Cadenas, A.; Arbona, V. Abscisic acid as an emerging modulator of the responses of plants to low oxygen conditions. Front. Plant Sci. 2021, 12, 661789. [Google Scholar] [CrossRef]
- Chen, X.; Pierik, R.; Peeters, A.J.M.; Poorter, H.; Visser, E.J.W.; Huber, H.; de Kroon, H.; Voesenek, L.A.C.J. Endogenous abscisic acid as a key switch for natural variation in flooding-induced shoot elongation. Plant Physiol. 2010, 154, 969–977. [Google Scholar] [CrossRef]
- Arbona, V.; Zandalinas, S.I.; Manzi, M.; González-Guzmán, M.; Rodriguez, P.L.; Gómez-Cadenas, A. Depletion of abscisic acid levels in roots of flooded Carrizo citrange (Poncirus trifoliata L. Raf. × Citrus sinensis L. Osb.) plants is a stress-specific response associated to the differential expression of PYR/PYL/RCAR receptors. Plant Mol. Biol. 2017, 93, 623–640. [Google Scholar] [CrossRef]
- Emel’yanov, V.V.; Kirchikhina, N.A.; Lastochkin, V.V.; Chirkova, T.V. Hormonal status in wheat and rice seedlings under anoxia. Russ. J. Plant Physiol. 2003, 50, 827–834. [Google Scholar] [CrossRef]
- Sharipova, G.; Veselov, D.; Kudoyarova, G.; Fricke, W.; Dodd, I.; Katsuhara, M.; Furuichi, T.; Ivanov, I.; Veselov, S. Exogenous application of abscisic acid (ABA) increases root and cell hydraulic conductivity and abundance of some aquaporin isoforms in the ABA deficient barley mutant Az34. Ann. Bot. 2016, 118, 777–785. [Google Scholar] [CrossRef]
- De Ollas, C.; González-Guzmán, M.; Pitarch, Z.; Matus, J.T.; Candela, H.; Rambla, J.L.; Granell, A.; Gómez-Cadenas, A.; Arbona, V. Identification of ABA-mediated genetic and metabolic responses to soil flooding in tomato (Solanum lycopersicum L. Mill). Front. Plant Sci. 2021, 12, 613059. [Google Scholar] [CrossRef]
- Cox, M.C.H.; Benschop, J.J.; Vreeburg, R.A.M.; Wagemaker, C.A.M.; Moritz, T.; Peeters, A.J.M.; Voesenek, L.A.C.J. The roles of ethylene, auxin, abscisic acid, and gibberellin in the hyponastic growth of submerged Rumex palustris petioles. Plant Physiol. 2004, 136, 2948–2960. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, S.; Rocchi, P.; Bertany, A. ABA and IAA in rice seedlings under anaerobic conditions. Biol. Plant. 1986, 28, 57–61. [Google Scholar] [CrossRef]
- Yemelyanov, V.V.; Lastochkin, V.V.; Chirkova, T.V.; Lindberg, S.M.; Shishova, M.F. Indoleacetic acid levels in wheat and rice seedlings under oxygen deficiency and subsequent reoxygenation. Biomolecules 2020, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, I.; Bertani, A.; Mapelli, S.; Reggiani, R. Effects of anoxia and indoleacetic acid on gravitropic responsiveness of rice seedlings. Russ. J. Plant Physiol. 1996, 43, 26–30. [Google Scholar]
- Vreeburg, R.A.; Benschop, J.J.; Peeters, A.J.M.; Colmer, T.D.; Ammerlaan, A.H.; Staal, M.; Elzenga, T.M.; Staals, R.H.; Darley, C.P.; McQueen-Mason, S.J.; et al. Ethylene regulates fast apoplastic acidification and expansin A transcription during submergence-induced petiole elongation in Rumex palustris. Plant J. 2005, 43, 597–610. [Google Scholar] [CrossRef]
- Péret, B.; Li, G.; Zhao, J.; Band, L.R.; Voß, U.; Postaire, O.; Luu, D.T.; Da Ines, O.; Casimiro, I.; Lucas, M.; et al. Auxin regulates aquaporin function to facilitate lateral root emergence. Nat. Cell. Biol. 2012, 14, 991–998. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Yang, C.-Y. Comprehensive transcriptomic analysis of auxin responses in submerged rice coleoptile growth. Int. J. Mol. Sci. 2020, 21, 1292. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).