Quantitative Chemical Exchange Saturation Transfer Imaging of Amide Proton Transfer Differentiates between Cerebellopontine Angle Schwannoma and Meningioma: Preliminary Results
Abstract
:1. Introduction
2. Results
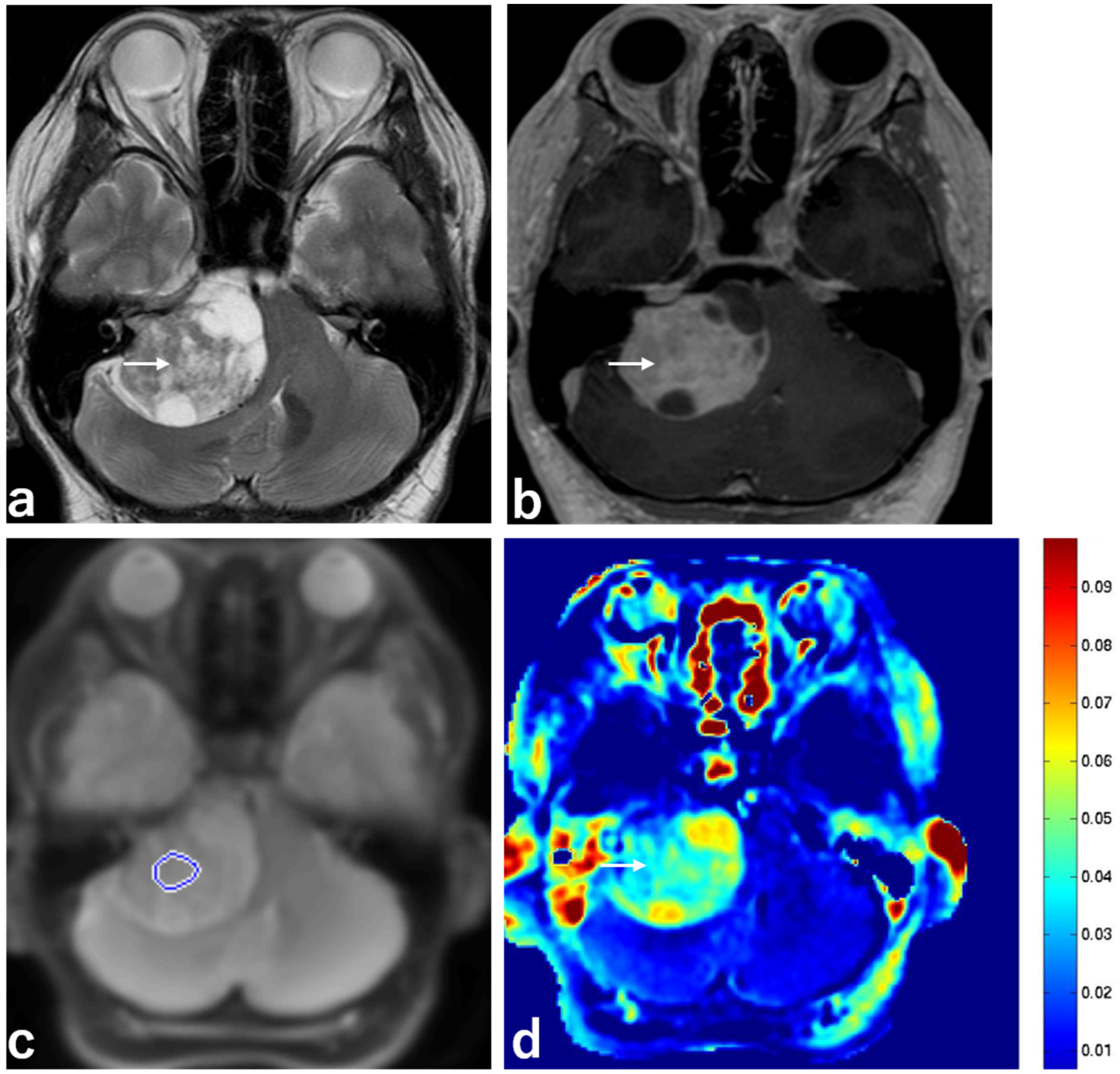
2.1. Clinical Characteristics and Imaging Findings of Schwannomas and Meningiomas
2.2. Schwannomas with T2 Hyperintensity and T2 Low Intensity
2.3. Pathological Findings
2.4. ROC Analysis in Patients in the Schwannoma and Meningioma Groups
2.5. Interobserver Agreement
3. Discussion
Limitations
4. Materials and Methods
4.1. Patients
4.2. MRI Protocol
4.3. APT Image Processing
4.4. Image Analysis
4.5. Pathological Diagnosis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | two-dimensional |
| 3D | three-dimensional |
| APT | amide proton transfer |
| CEST | chemical exchange saturation transfer |
| CI | confidence interval |
| CPA | cerebellopontine angle |
| EPI | echo-planar imaging |
| FOV | field of view |
| ICC | intraclass coefficient |
| MRI | magnetic resonance imaging |
| MTD | maximum tumor diameter |
| MTR | magnetization transfer ratio |
| NEX | number of excitations |
| ROC | receiver operating characteristic |
| ROI | region of interest |
| SD | standard deviation |
| SI | signal intensity |
| Ssat | signal intensity with selective imaging |
| T1WI | T1-weighted imaging |
| T2WI | T2-weighted imaging |
| TE | echo time |
| TR | repetition time |
| VS | vestibular schwannoma |
| WASSR | water saturation reference map |
| WHO | World Health Organization |
References
- Van Zijl, P.C.M.; Yadav, N.N. Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn. Reson. Med. 2011, 65, 927–948. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.M.; Aletras, A.H.; Balaban, R.S. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J. Magn. Reson. 2000, 143, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lal, B.; Wilson, D.A.; Laterra, J.; van Zijl, P.C. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn. Reson. Med. 2003, 50, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Payen, J.-F.; Wilson, D.A.; Traystman, R.J.; Van Zijl, P.C.M. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat. Med. 2003, 9, 1085–1090. [Google Scholar] [CrossRef]
- Zhou, J.; Tryggestad, E.; Wen, Z.-B.; Lal, B.; Zhou, T.; Grossman, R.; Wang, S.; Yan, K.; Fu, D.-X.; Ford, E.; et al. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat. Med. 2011, 17, 130–134. [Google Scholar] [CrossRef]
- Togao, O.; Yoshiura, T.; Keupp, J.; Hiwatashi, A.; Yamashita, K.; Kikuchi, K.; Suzuki, Y.; Suzuki, S.O.; Iwaki, T.; Hata, N.; et al. Amide proton transfer imaging of adult diffuse gliomas: Correlation with histopathological grades. Neuro. Oncol. 2014, 16, 441–448. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, H.; Lim, M.; Blair, L.; Quinones-Hinojosa, A.; Messina, S.A.; Eberhart, C.G.; Pomper, M.G.; Laterra, J.; Barker, P.B.; et al. Three-dimensional amide proton transfer MR imaging of gliomas: Initial experience and comparison with gadolinium enhancement. J. Magn. Reson. Imaging 2013, 38, 1119–1128. [Google Scholar] [CrossRef]
- Wen, Z.; Hu, S.; Huang, F.; Wang, X.; Guo, L.; Quan, X.; Wang, S.; Zhou, J. MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuroimage 2010, 51, 616–622. [Google Scholar] [CrossRef]
- Jones, C.K.; Schlosser, M.J.; van Zijl, P.C.; Pomper, M.G.; Golay, X.; Zhou, J. Amide proton transfer imaging of human brain tumors at 3T. Magn. Reson. Med. 2006, 56, 585–592. [Google Scholar] [CrossRef]
- Sagiyama, K.; Mashimo, T.; Togao, O.; Vemireddy, V.; Hatanpaa, K.J.; Maher, E.A.; Mickey, B.E.; Pan, E.; Sherry, A.D.; Bachoo, R.M.; et al. In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proc. Natl. Acad. Sci. USA 2014, 111, 4542–4547. [Google Scholar] [CrossRef] [Green Version]
- Scheidegger, R.; Wong, E.T.; Alsop, D.C. Contributors to contrast between glioma and brain tissue in chemical exchange saturation transfer sensitive imaging at 3Tesla. Neuroimage 2014, 99, 256–268. [Google Scholar] [CrossRef]
- Laird, F.J.; Harner, S.G.; Laws, E.R.; Reese, D.F. Meningioma of the cerebellopontine angle. Otolaryngol. Head Neck Surg. 1985, 93, 163–167. [Google Scholar] [CrossRef]
- Samii, M.; Matthies, C. Management of 1000 vestibular schwannomas (acoustic neuromas): Hearing function in 1000 tumor resection. Neurosurgery 1997, 40, 248–260. [Google Scholar] [CrossRef]
- Roser, F.; Nakamura, M.; Dormiani, M.; Matthies, C.; Vorkapic, P.; Samii, M. Meningiomas of the cerebellopontine angle with extension into the internal auditory canal. J. Neurosurg. 2005, 102, 17–23. [Google Scholar] [CrossRef]
- McLendon, R.E.; Rosenblum, M.K.; Bigner, D.D. (Eds.) Russell and Rubinstein’s Pathology of Tumors of the Nervous System, 7th ed.; Hodder Arnold: London, UK, 2006. [Google Scholar]
- Gao, K.; Ma, H.; Cui, Y.; Chen, X.; Ma, J.; Dai, J. Meningiomas of the cerebellopontine angle: Radiological differences in tumors with internal auditory canal involvement and their influence on surgical outcome. PLoS ONE 2015, 10, e0122949. [Google Scholar] [CrossRef]
- Nakamura, M.; Roser, F.; Dormiani, M.; Matthies, C.; Vorkapic, P.; Samii, M. Facial and cochlear nerve function after surgery of cerebellopontine angle meningiomas. Neurosurgery 2005, 57, 77–90. [Google Scholar] [CrossRef]
- Mallucci, C.L.; Ward, V.; Carney, A.S.; O’Donoghue, G.M.; Robertson, I. Clinical features and outcomes in patients with non-acoustic cerebellopontine angle tumors. J. Neurol. Neurosurg. Psychiatry 1999, 66, 768–771. [Google Scholar] [CrossRef]
- Joo, B.; Han, K.; Choi, Y.S.; Lee, S.-K.; Ahn, S.S.; Chang, J.H.; Kang, S.-G.; Kim, S.H.; Zhou, J. Amide proton transfer imaging for differentiation of benign and atypical meningiomas. Eur. Radiol. 2018, 28, 331–339. [Google Scholar] [CrossRef]
- Koike, H.; Morikawa, M.; Ideguchi, R.; Uetani, M.; Hiu, T.; Matsuo, T. Amide proton transfer and chemical exchange saturation transfer MRI differentiates between growing and non-growing intracranial meningiomas: A pilot study. Clin. Radiol. 2022, 77, e295–e301. [Google Scholar] [CrossRef]
- Moffat, D.A.; Ballagh, R.H. Rare tumours of the cerebellopontine angle. Clin. Oncol. 1995, 7, 28–41. [Google Scholar] [CrossRef]
- Charabi, S.; Tos, M.; Thomsen, J.; Rygaard, J.; Fundova, P.; Charabi, B. Cystic vestibular schwannomaclinical and experimental studies. Acta Otolaryngol. Suppl. 2000, 543, 11–13. [Google Scholar] [CrossRef]
- Gomez-Brouchet, A.; Delisle, M.B.; Cognard, C.; Bonafe, A.; Charlet, J.P.; Deguine, O.; Fraysse, B. Vestibular schwannomas: Correlations between magnetic resonance imaging and histopathologic appearance. Otol. Neurotol. 2001, 22, 79–86. [Google Scholar] [CrossRef]
- Burger, P.C.; Scheithauer, B.W. Tumors of the central nervous system. In Atlas of Tumor Pathology, Third Series, Fasc 10; Armed Forces Institute of Pathology: Washington, DC, USA, 1994; pp. 333–343. [Google Scholar]
- Duvoisin, B.; Fernandes, J.; Doyon, D.; Denys, A.; Sterkers, J.M.; Bobin, S. Magnetic resonance findings in 92 acoustic neuromas. Eur J. Radiol 1991, 13, 96–102. [Google Scholar] [CrossRef]
- Sarrazin, J.L. Infra tentorial tumors. J. Radiol. 2006, 87, 748–763. [Google Scholar] [CrossRef]
- Guermazi, A.; Lafitte, F.; Miaux, Y.; Adem, C.; Bonneville, J.F.; Chiras, J. The dural tail sign-beyond meningioma. Clin. Radiol. 2005, 60, 171–188. [Google Scholar] [CrossRef]
- Cho, Y.D.; Choi, G.H.; Lee, S.P.; Kim, J.K. (1)H-MRS metabolic patterns for distinguishing between meningiomas and other brain tumors. Magn. Reson. Imaging 2003, 21, 663–672. [Google Scholar] [CrossRef]
- Zhao, X.; Wen, Z.; Huang, F.; Lu, S.; Wang, X.; Hu, S.; Zu, D.; Zhou, J. Saturation power dependence of amide proton transfer image contrasts in human brain tumors and strokes at 3 T. Magn. Reson. Med. 2011, 66, 1033–1041. [Google Scholar] [CrossRef]
- Lingl, J.P.; Wunderlich, A.; Goerke, S. The Value of APTw CEST MRI in Routine Clinical Assessment of Human Brain Tumor Patients at 3T. Diagnostics 2022, 12, 490. [Google Scholar] [CrossRef]
- Goldenberg, J.M.; Pagel, M.D. Assessments of tumor metabolism with CEST MRI. NMR Biomed. 2019, 32, e3943. [Google Scholar] [CrossRef]
- Cho, N.S.; Hagiwara, A.; Yao, J.; Nathanson, D.A.; Prins, R.M.; Wang, C.; Raymond, C.; Desousa, B.R.; Divakaruni, A.; Morrow, D.H.; et al. Amine-weighted chemical exchange saturation transfer magnetic resonance imaging in brain tumors. NMR Biomed. 2022, 15, e4785. [Google Scholar] [CrossRef]
- Zhou, J.; Heo, H.-Y.; Knutsson, L.; Van Zijl, P.C.; Jiang, S. APT-weighted MRI: Techniques, current neuro applications, and challenging issues. J. Magn. Reson. Imaging 2019, 50, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hong, X.; Zhao, X.; Gao, J.-H.; Yuan, J. APT-weighted and NOE-weighted image contrasts in glioma with different RF saturation powers based on magnetization transfer ratio asymmetry analyses. Magn. Reson. Med. 2013, 70, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Gillen, J.; Landman, B.A.; Zhou, J.; van Zijl, P.C. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn. Reson. Med. 2009, 61, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Goldsher, D.; Litt, A.W.; Pinto, R.S.; Bannon, K.R.; Kricheff, I.I. Dural “tail” associated with meningiomas on Gd-DTPA-enhanced MR images: Characteristics, differential diagnostic value, and possible implications for treatment. Radiology 1990, 176, 447–450. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K. (Eds.) World Health Organization Histological Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Fleiss, J.L.; Levin, B.; Paik, M.C. Statistical Methods for Rates and Proportions, 2nd ed.; Wiley: New York, NY, USA, 1981. [Google Scholar]



| Variable | Schwannoma (n = 9) | Meningioma (n = 9) | p |
|---|---|---|---|
| Age (years) | 57.1 ± 12.1 | 61.7 ± 14.5 | 0.506 |
| Male–female | 2:7 | 2:7 | 1.000 |
| Mean MTRasym | 0.033 ± 0.012 | 0.021 ± 0.004 | 0.007 |
| Mean MTD values (mm) | 30.0 ± 7.2 | 36.6 ± 10.8 | 0.173 |
| T2 hyperintensity | 6 (66.7%) | 2 (22.2%) | 0.058 |
| Peritumoral brain edema | 2 (22.2%) | 3 (33.3%) | 0.599 |
| Irregular tumor margin | 2 (22.2%) | 4 (44.4%) | 0.137 |
| Heterogeneous enhancement | 6 (66.7%) | 2 (22.2%) | 0.058 |
| Capsular enhancement | 2 (22.2%) | 3 (33.3%) | 0.599 |
| Dural tail sign | 0 (0%) | 6 (66.7%) | 0.003 |
| Progress to the internal auditory canal | 6 (66.7%) | 5 (55.6%) | 0.629 |
| Eccentricity of tumor compared to nerve | 6 (66.7%) | 2(22.2%) | 0.058 |
| Variable | Schwannoma with Hyperintensity (n = 6) | Schwannoma with Low Intensity (n = 3) | p |
|---|---|---|---|
| Age (years) | 60.7 ± 10.8 | 50.0 ± 11.6 | 0.267 |
| Male–female | 1:5 | 1:2 | 0.571 |
| Mean MTRasym | 0.039 ± 0.006 | 0.021 ± 0.008 | 0.014 |
| Mean MTD values (mm) | 32.8 ± 6.9 | 24.4 ± 3.2 | 0.121 |
| Peritumoral brain edema | 1 (16.7%) | 1 (33.3%) | 0.571 |
| Irregular tumor margin | 1 (16.7%) | 1 (33.3%) | 0.571 |
| Heterogeneous enhancement | 6 (100%) | 0 (0%) | 0.003 |
| Capsular enhancement | 4 (66.7%) | 1 (33.3%) | 0.343 |
| Dural tail sign | 0 (0%) | 0 (0%) | N.S. |
| Progress to the internal auditory canal | 4 (66.7%) | 2 (66.7%) | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koike, H.; Morikawa, M.; Ishimaru, H.; Ideguchi, R.; Uetani, M.; Hiu, T.; Matsuo, T.; Miyoshi, M. Quantitative Chemical Exchange Saturation Transfer Imaging of Amide Proton Transfer Differentiates between Cerebellopontine Angle Schwannoma and Meningioma: Preliminary Results. Int. J. Mol. Sci. 2022, 23, 10187. https://doi.org/10.3390/ijms231710187
Koike H, Morikawa M, Ishimaru H, Ideguchi R, Uetani M, Hiu T, Matsuo T, Miyoshi M. Quantitative Chemical Exchange Saturation Transfer Imaging of Amide Proton Transfer Differentiates between Cerebellopontine Angle Schwannoma and Meningioma: Preliminary Results. International Journal of Molecular Sciences. 2022; 23(17):10187. https://doi.org/10.3390/ijms231710187
Chicago/Turabian StyleKoike, Hirofumi, Minoru Morikawa, Hideki Ishimaru, Reiko Ideguchi, Masataka Uetani, Takeshi Hiu, Takayuki Matsuo, and Mitsuharu Miyoshi. 2022. "Quantitative Chemical Exchange Saturation Transfer Imaging of Amide Proton Transfer Differentiates between Cerebellopontine Angle Schwannoma and Meningioma: Preliminary Results" International Journal of Molecular Sciences 23, no. 17: 10187. https://doi.org/10.3390/ijms231710187
APA StyleKoike, H., Morikawa, M., Ishimaru, H., Ideguchi, R., Uetani, M., Hiu, T., Matsuo, T., & Miyoshi, M. (2022). Quantitative Chemical Exchange Saturation Transfer Imaging of Amide Proton Transfer Differentiates between Cerebellopontine Angle Schwannoma and Meningioma: Preliminary Results. International Journal of Molecular Sciences, 23(17), 10187. https://doi.org/10.3390/ijms231710187






