miRNA-10a-5p Targeting the BCL6 Gene Regulates Proliferation, Differentiation and Apoptosis of Chicken Myoblasts
Abstracts
1. Introduction
2. Results
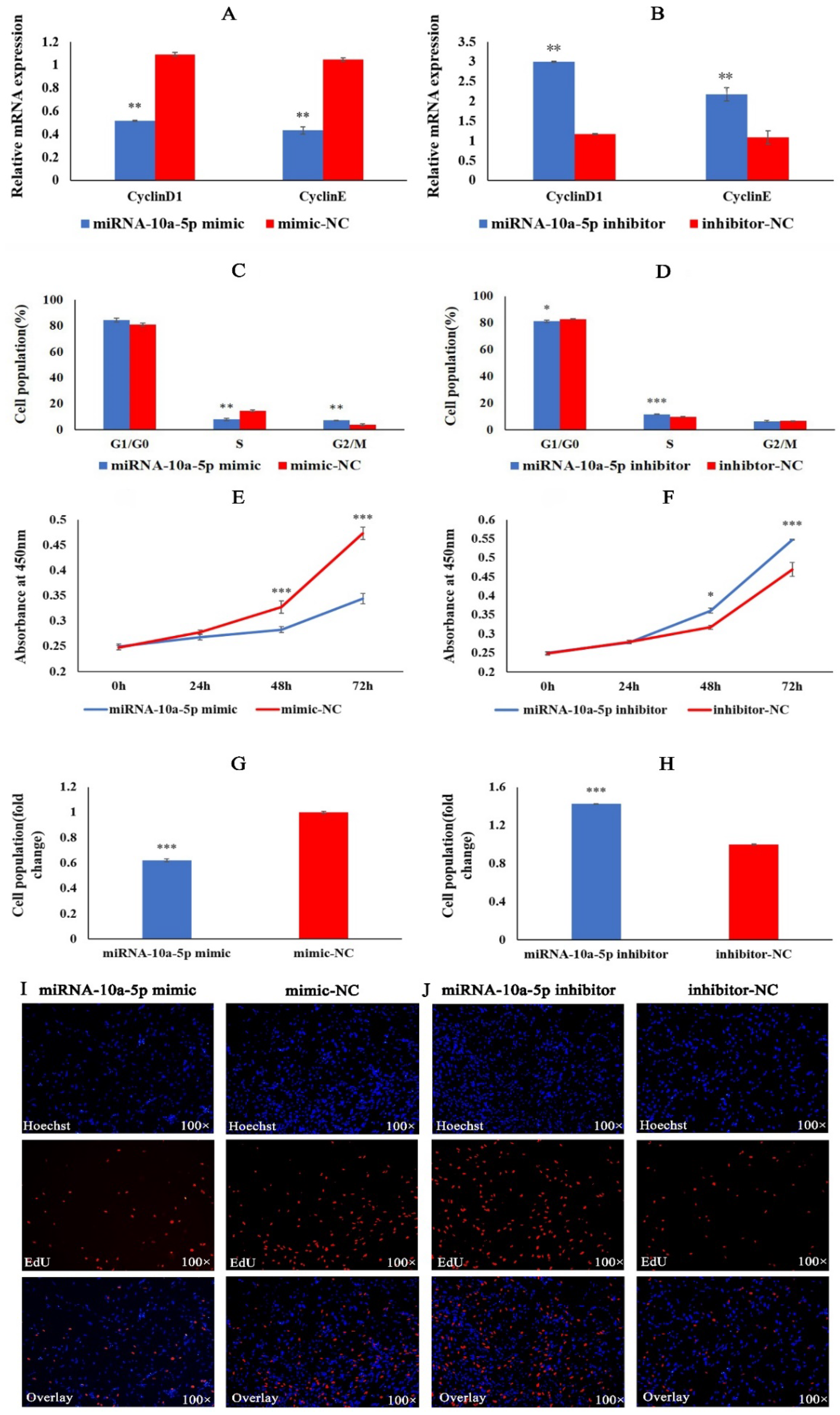
2.1. miRNA-10a-5p Inhibits the Proliferation of Chicken Myoblasts
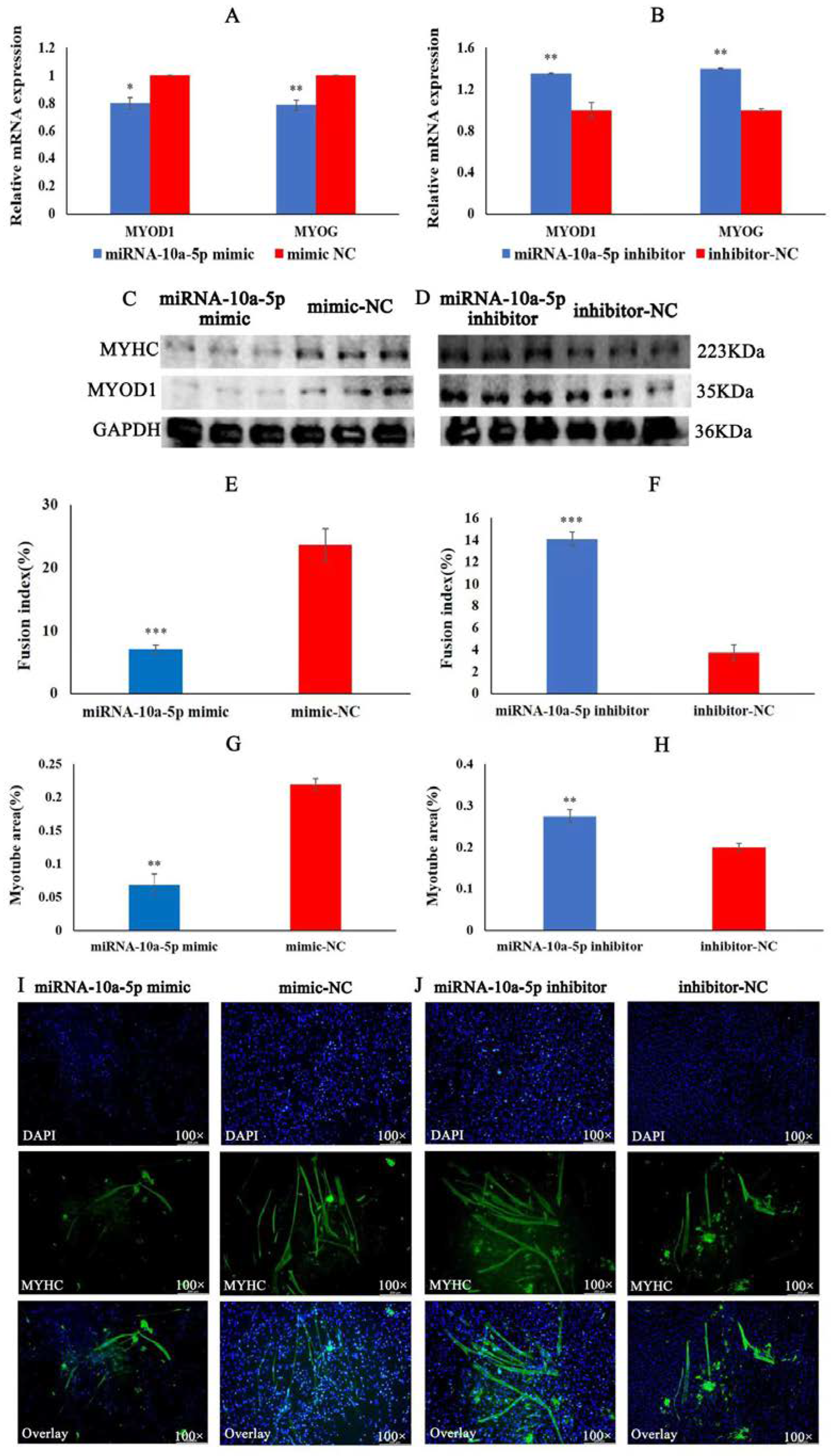
2.2. miRNA-10a-5p Inhibits the Differentiation of Chicken Myoblasts
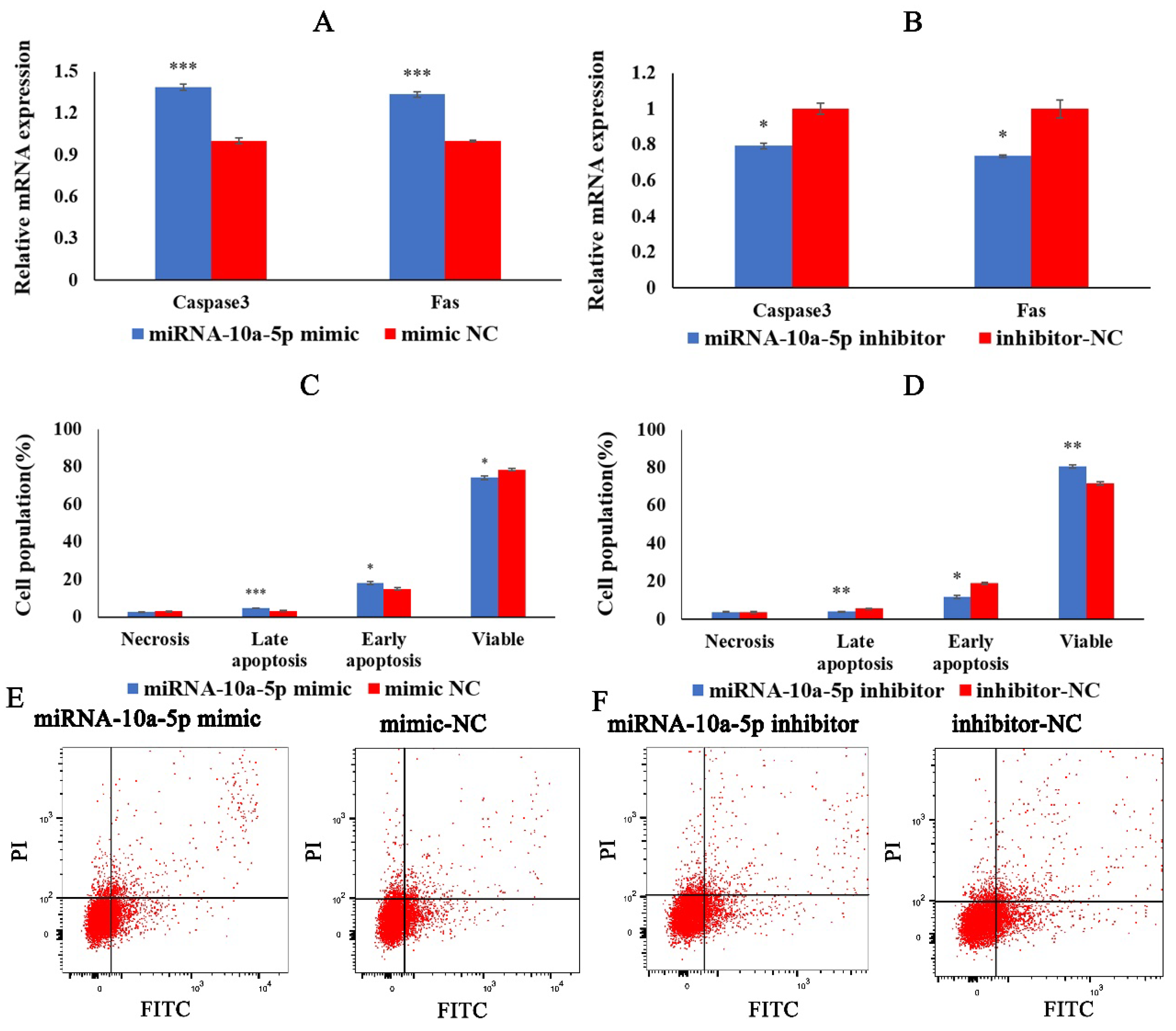
2.3. miRNA-10a-5p Promotes the Apoptosis of Chicken Myoblasts
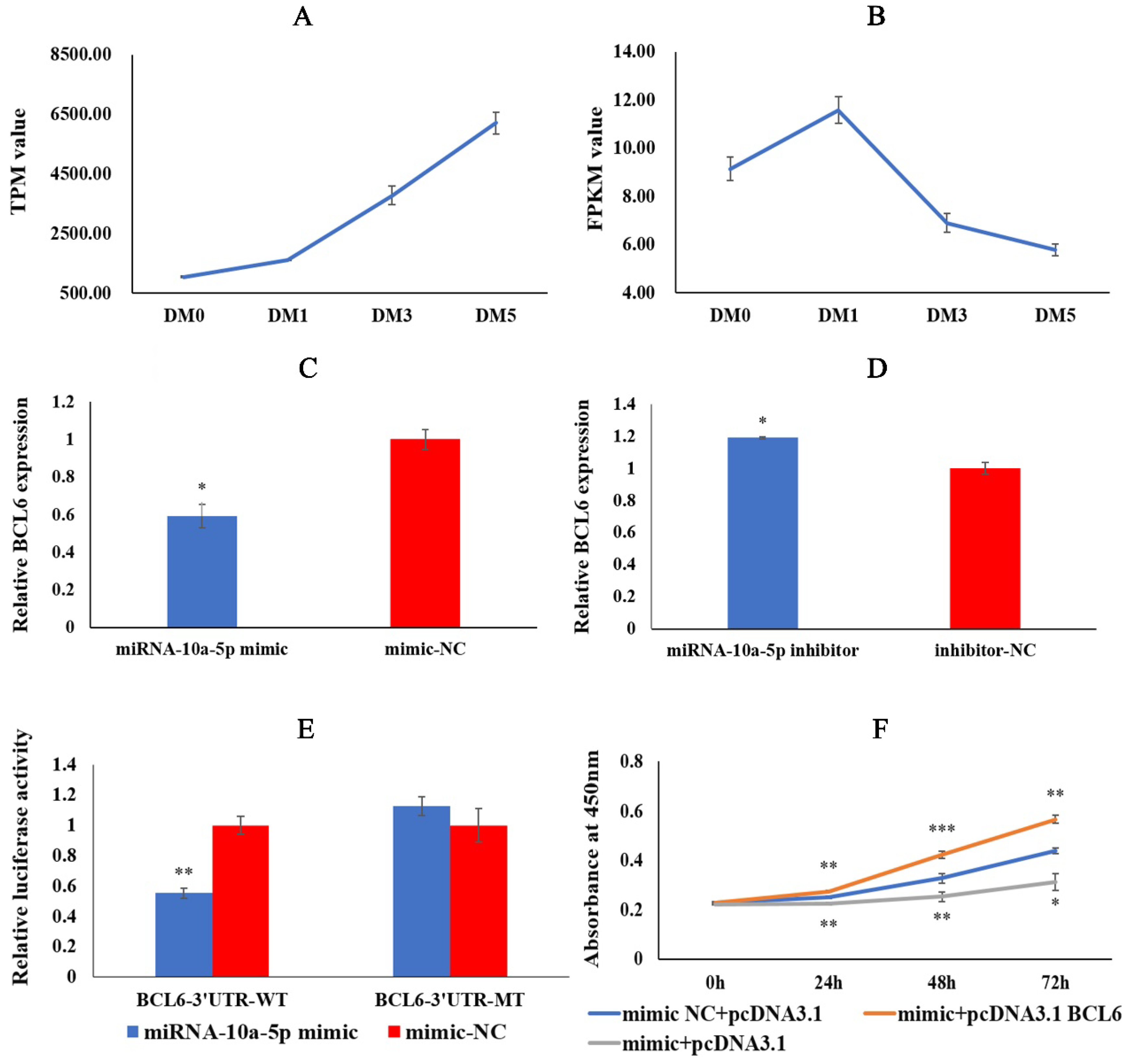
2.4. miRNA-10a-5p Has a Targeting Relationship with BCL6
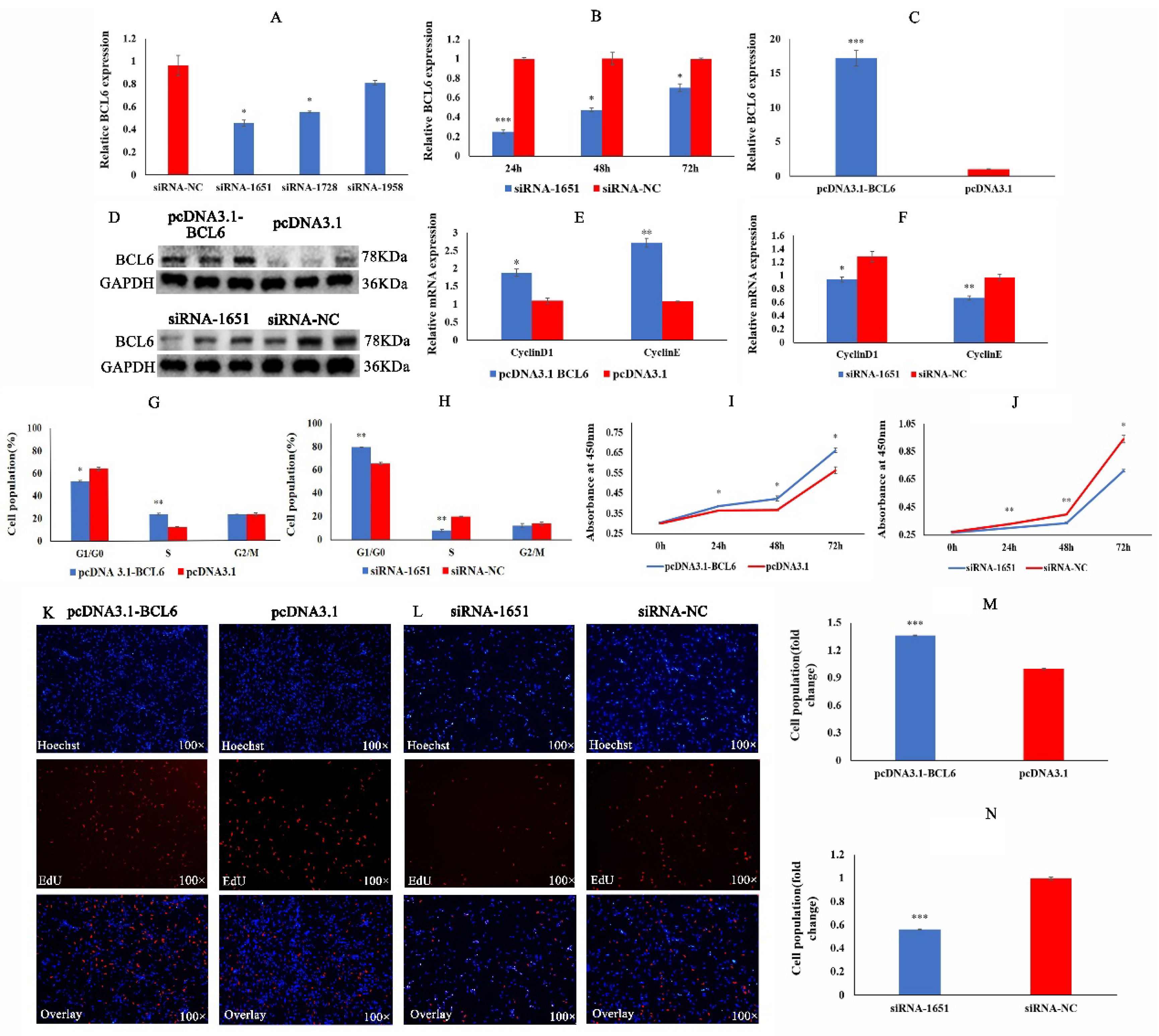
2.5. BCL6 Gene Promotes the Proliferation of Chicken Myoblasts
2.6. BCL6 Gene Promotes the Differentiation of Chicken Myoblasts
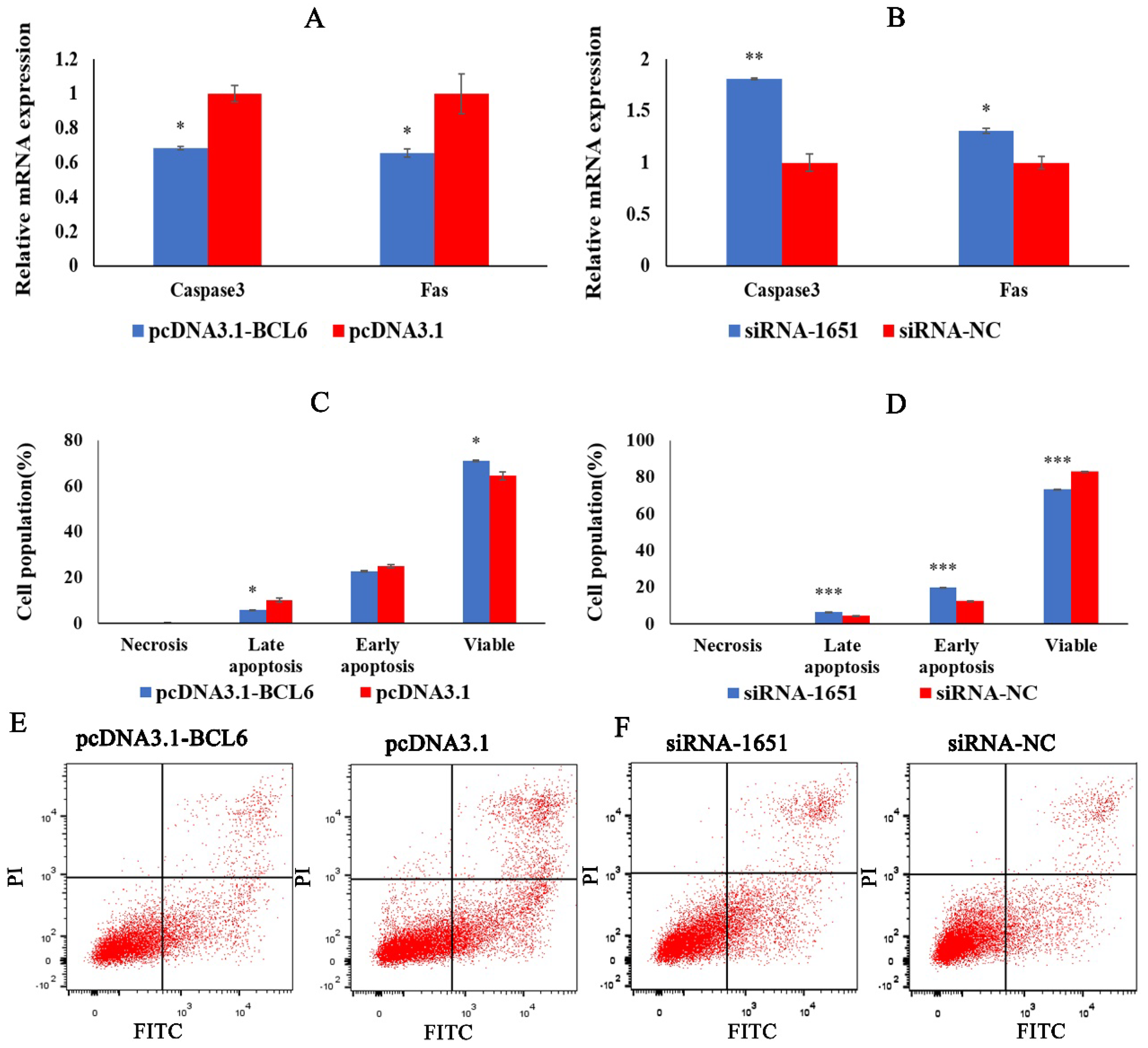
2.7. BCL6 Gene Inhibits the Apoptosis of Chicken Myoblasts
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Cell Isolation, Culture, and Total RNA Extraction
4.3. Primer Design for Quantitative Real-Time PCR (qRT-PCR)
4.4. cDNA Synthesis and Quantitative Real-Time PCR (qRT-PCR)
4.5. Sequence Synthesis and Vector Construction
4.6. Cell Transfection and Dual-Luciferase Reporter Assay
4.7. CCK-8 Assay and Edu Assay
4.8. Cell Cycle Assay
4.9. Apoptosis Assay
4.10. Indirect Immunofluorescence Assay (IFA)
4.11. Western Blot Assay
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mohr, A.M.; Mott, J.L. Overview of microRNA biology. Semin. Liver Dis. 2015, 35, 3–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bushati, N.; Cohen, S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007, 23, 175–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stähler, C.; Meese, E.; et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016, 44, 3865–3877. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; He, M.; Wu, P.; Zhang, X.; Zhou, K.; Li, T.; Zhang, T.; Xie, K.; Dai, G.; Wang, J. MicroRNA-27b-3p Targets the Myostatin Gene to Regulate Myoblast Proliferation and Is Involved in Myoblast Differentiation. Cells 2021, 10, 423. [Google Scholar] [CrossRef]
- Giusti, J.; Pinhal, D.; Moxon, S.; Campos, C.L.; Münsterberg, A.; Martins, C. MicroRNA-10 modulates Hox genes expression during Nile tilapia embryonic development. Mech. Dev. 2016, 140, 12–18. [Google Scholar] [CrossRef]
- Ma, L.; Teruya-Feldstein, J.; Weinberg, R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 2007, 449, 682–688. [Google Scholar] [CrossRef]
- Liu, L.J.; Sun, X.Y.; Yang, C.X.; Zou, X.Y. MiR-10a-5p restrains the aggressive phenotypes of ovarian cancer cells by inhibiting HOXA1. Kaohsiung J. Med. Sci. 2021, 37, 276–285. [Google Scholar] [CrossRef]
- Worst, T.S.; Previti, C.; Nitschke, K.; Diessl, N.; Gross, J.C.; Hoffmann, L.; Frey, L.; Thomas, V.; Kahlert, C.; Bieback, K.; et al. miR-10a-5p and miR-29b-3p as Extracellular Vesicle-Associated Prostate Cancer Detection Markers. Cancers 2019, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.F. The Role and Mechanism of miR-10a-5p in the Proliferation and Differentiation of Preadipocytes. Master’s Thesis, Northwest A&F University, Xianyang, China, 2019. [Google Scholar]
- Ye, B.H.; Lista, F.; Lo Coco, F.; Knowles, D.M.; Offit, K.; Chaganti, R.S.; Dalla-Favera, R. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science 1993, 262, 747–750. [Google Scholar] [CrossRef]
- Duan, S.; Cermak, L.; Pagan, J.K.; Rossi, M.; Martinengo, C.; di Celle, P.F.; Chapuy, B.; Shipp, M.; Chiarle, R.; Pagano, M. FBXO11 targets BCL6 for degradation and is inactivated in diffuse large B-cell lymphomas. Nature 2012, 481, 90–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Liu, Y.; Lv, J.; Zou, B.; Chen, Z.; Li, K.; Feng, J.; Cai, Z.; Wei, L.; Liu, M.; et al. BCL6 confers KRAS-mutant non-small-cell lung cancer resistance to BET inhibitors. J. Clin. Investig. 2021, 131, e133090. [Google Scholar] [CrossRef] [PubMed]
- Albagli-Curiel, O.; Dhordain, P.; Lantoine, D.; Auradé, F.; Quief, S.; Kerckaert, J.P.; Montarras, D.; Pinset, C. Increased expression of the LAZ3 (BCL6) proto-oncogene accompanies murine skeletal myogenesis. Differentiation 1998, 64, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Liou, L.B.; Wu, M.W.; Chao, W.J. Macrophages and abnormal BCL-6 or c-MYC gene expression affect the resistance of peritoneal B cells to induction of hyporesponsiveness. Scand. J. Immunol. 2009, 69, 447–456. [Google Scholar] [CrossRef]
- Guermonprez, P.; Gerber-Ferder, Y.; Vaivode, K.; Bourdely, P.; Helft, J. Origin and development of classical dendritic cells. Int. Rev. Cell Mol. Biol. 2019, 349, 1–54. [Google Scholar] [CrossRef]
- Mitra, A.; Luo, J.; Zhang, H.; Cui, K.; Zhao, K.; Song, J. Marek’s disease virus infection induces widespread differential chromatin marks in inbred chicken lines. BMC Genom. 2012, 13, 557. [Google Scholar] [CrossRef] [Green Version]
- Han, W. Global Changes of Hypothalamic Gene Transcription and Microrna Profile before and after Puberty Onset in Wenchang Chicken. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2015. [Google Scholar]
- Kumagai, T.; Miki, T.; Kikuchi, M.; Fukuda, T.; Miyasaka, N.; Kamiyama, R.; Hirosawa, S. The proto-oncogene Bc16 inhibits apoptotic cell death in differentiation-induced mouse myogenic cells. Oncogene 1999, 18, 467–475. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Ding, Y.; Zhao, Y.; So, K.K.; Peng, X.L.; Li, Y.; Yuan, J.; He, Z.; Chen, X.; Sun, H.; et al. CRISPR/Cas9/AAV9-mediated in vivo editing identifies MYC regulation of 3D genome in skeletal muscle stem cell. Stem Cell Rep. 2021, 16, 2442–2458. [Google Scholar] [CrossRef]
- Chal, J.; Pourquié, O. Making muscle: Skeletal myogenesis in vivo and In Vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef] [Green Version]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Jia, B.; Liu, Y.; Li, Q.; Zhang, J.; Ge, C.; Wang, G.; Chen, G.; Liu, D.; Yang, F. Altered miRNA and mRNA Expression in Sika Deer Skeletal Muscle with Age. Genes 2020, 11, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T. Screening of Key mRNAs, LncRNAs, and miRNAs Related to Myogenic Differentiation of Jinghai Yellow Chicken. Ph.D. Thesis, Yangzhou University, Yangzhou, China, 2018. [Google Scholar]
- Xiao, B.; Spencer, J.; Clements, A.; Ali-Khan, N.; Mittnacht, S.; Broceño, C.; Burghammer, M.; Perrakis, A.; Marmorstein, R.; Gamblin, S.J. Crystal structure of the retinoblastoma tumor suppressor protein bound to E2F and the molecular basis of its regulation. Proc. Natl. Acad. Sci. USA 2003, 100, 2363–2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhang, H.; Xu, M.; Shi, X.; Yang, G.; Sun, S.; Li, X. Elevated miR-10a-5p facilitates cell cycle and restrains adipogenic differentiation via targeting Map2k6 and Fasn, respectively. Acta. Biochim. Biophys. Sin. (Shanghai) 2020, 52, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Li, Y.; Lan, X.; Ai, L. MicroRNA-10a-5p suppresses cancer proliferation and division in human cervical cancer by targeting BDNF. Exp Ther. Med. 2017, 14, 6147–6151. [Google Scholar] [CrossRef] [Green Version]
- Vaher, H.; Runnel, T.; Urgard, E.; Aab, A.; Carreras Badosa, G.; Maslovskaja, J.; Abram, K.; Raam, L.; Kaldvee, B.; Annilo, T.; et al. miR-10a-5p is increased in atopic dermatitis and has capacity to inhibit keratinocyte proliferation. Allergy 2019, 74, 2146–2156. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Zhang, Z.; Ren, F.; Chen, L.; Lan, Z. miR-10a-5p inhibits osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Mol. Med. Rep. 2020, 22, 135–144. [Google Scholar] [CrossRef]
- Zhang, J. MiRNA-10a-5p Targets CHL1 to Regulate the Biological Behavior of Neural Stem Cells and Its Mechanism Study in the Occurrence of Neural Tube Defect; Shanxi Medical University: Shanxi, China, 2020. [Google Scholar]
- Li, C.; Huang, H.; Zhao, Z.; Li, S.; Wang, Q.; Huang, Z.; Xue, L. Regulation of Proliferation or Apoptosis of Granulosa Cells in Follicular Development in Poultry. China Poult. 2020, 42, 88–96. [Google Scholar] [CrossRef]
- Li, H.; Hu, B.; Hu, S.; Luo, W.; Sun, D.; Yang, M.; Liao, Z.; Wei, H.; Zhao, C.; Li, D.; et al. High expression of BCL6 inhibits the differentiation and development of hematopoietic stem cells and affects the growth and development of chickens. J. Anim Sci Biotechnol. 2021, 12, 18. [Google Scholar] [CrossRef]
- Chen, J. BCL6 Regulation of Skeletal Muscle Glucose Metabolism and its Mechanism. Ph.D. Thesis, Chongqing Medical University, Chongqing, China, 2020. [Google Scholar]
- Chen, D.; Zang, Y.H.; Qiu, Y.; Zhang, F.; Chen, A.D.; Wang, J.J.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Zhu, G.Q. BCL6 Attenuates Proliferation and Oxidative Stress of Vascular Smooth Muscle Cells in Hypertension. Oxid. Med. Cell Longev. 2019, 2019, 5018410. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Song, Q.L.; Ji, R.; Wang, J.Y.; Li, Z.H.; Guo, D.; Yin, T.L.; Wang, S.J.; Yang, J. MiR-187 regulates the proliferation, migration and invasion of human trophoblast cells by repressing BCL6-mediated activation of PI3K/AKT signaling. Placenta 2022, 118, 20–31. [Google Scholar] [CrossRef]
- Cardenas, M.G.; Oswald, E.; Yu, W.; Xue, F.; MacKerell, A.D., Jr.; Melnick, A.M. The Expanding Role of the BCL6 Oncoprotein as a Cancer Therapeutic Target. Clin. Cancer Res. 2017, 23, 885–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, R.J.; Poholek, A.C.; DiToro, D.; Yusuf, I.; Eto, D.; Barnett, B.; Dent, A.L.; Craft, J.; Crotty, S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009, 325, 1006–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Crotty, S. Bcl6-Mediated Transcriptional Regulation of Follicular Helper T cells (TFH). Trends Immunol. 2021, 42, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kumar Mishra, S.; Wang, T.; Xu, Z.; Zhao, X.; Wang, Y.; Yin, H.; Fan, X.; Zeng, B.; Yang, M.; et al. AFB1 Induced Transcriptional Regulation Related to Apoptosis and Lipid Metabolism in Liver of Chicken. Toxins 2020, 12, 290. [Google Scholar] [CrossRef]
- Rao, P.K.; Missiaglia, E.; Shields, L.; Hyde, G.; Yuan, B.; Shepherd, C.J.; Shipley, J.; Lodish, H.F. Distinct roles for miR-1 and miR-133a in the proliferation and differentiation of rhabdomyosarcoma cells. FASEB J. 2010, 24, 3427–3437. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Dong, X.D.E.; Chen, X.; Wang, L.; Lu, C.; Wang, J.; Qu, J.; Tu, L. MicroRNA-1/206 targets c-Met and inhibits rhabdomyosarcoma development. J. Biol. Chem. 2009, 284, 29596–29604. [Google Scholar] [CrossRef] [Green Version]
- Cai, B.; Ma, M.; Chen, B.; Li, Z.; Abdalla, B.A.; Nie, Q.; Zhang, X. MiR-16-5p targets SESN1 to regulate the p53 signaling pathway, affecting myoblast proliferation and apoptosis, and is involved in myoblast differentiation. Cell Death Dis. 2018, 9, 367. [Google Scholar] [CrossRef] [Green Version]

| Name | Primer Sequence (5′-3′) | Annealing Temperature (°C) |
|---|---|---|
| Gga-miRNA-10a-5p | F: CGCGTACCCTGTAGATCCGA R: AGTGCAGGGTCCGAGGTATT | 60 |
| U6 | F: GTCACTTCTGGTGGCGGTAA R: GTTCAGTAGAGGGTCAAA | 60 |
| Stem-loop primer | GTCGTATCCAGTGCAGGGTCC GAGGTATTCGCACTGGATACGAC |
| Gene | Primer Sequence (5′-3′) | Product Size(bp) | Annealing Temperature (°C) | Accession Number |
|---|---|---|---|---|
| BCL6 | F: CTCTGGCTCAGCCTTTGGA R: GCACTTGTAGGGTTTGTCGC | 169 | 60 | NM_00102930.1 |
| β-actin | F: CAGCCATCTTTCTTGGGTAT R: CTGTGATCTCCTTCTGCA TCC | 169 | 60 | NM_205518.1 |
| MYOD 1 | F: GCTACTACACGGAATCACCAAAT R: CTGGGCTCCACTGTCACTCA | 200 | 60 | NM_204214.2 |
| MYOG | F: CGGAGGCTGAAGAAGGTGAA R: CGGTCCTCTGCCTGGTCAT | 320 | 60 | NM_204184.1 |
| Caspase3 | F: TGGCCCTCTTGAACTGAAAG R: TCCACTGTCTGCTTCAATACC | 139 | 60 | NM_204725.1 |
| Fas | F: TCCACCTGCTCCTCGTCATT R: GTGCAGTGTGTGTGGGAACT | 78 | 60 | NM_001199487.1 |
| Cyclin D1 | F: CTGCTCAATGACAGGGTGC R: TCGGGTCTGATGGAGTTG | 341 | 60 | NM_205381 |
| CyclinE | F: ACCTAAAATGAGAACAATCC R: GGCAACAATACCTCCTAAA | 381 | 60 | NM_001031358.2 |
| Fragment Name | Sequence(5′-3′) |
|---|---|
| miRNA-10a-5p mimic | UACCCUGUAGAUCCGAAUUUGU AAAUUCGGAUCUACAGGGUAUU |
| mimic-NC | UUCUCCGAACGUGUCACGUTT ACGUGACACGUUCGGAGAATT |
| miRNA-10a-5p inhibitor | ACAAAUUCGGAUCUACAGGGUA |
| inhibitor-NC | CAGUACUUUUGUGUAGUACAA |
| siRNA-1651 | GCGAGAACGGAGCCUUCUUTT AAGAAGGCUCCGUUCUCGCTT |
| siRNA-1727 | GCAAGUCCACAGCGACAAATT UUUGUCGCUGUGGACUUGCTT |
| siRNA-1968 | GCUCAUGUGCUCAUUCAUATT UAUGAAUGAGCACAUGAGCTT |
| siRNA-NC | UUCUCCGAACGUGUCACGUTT ACGUGACACGUUCGGAGAATT |
| Primer Name | Primer Sequence | Product Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| BCL6-3′UTR-WT | F:AAAGATCCTTTATTAAGCTTTTTTCCCCAGTCTTATTT R:ATAGGCCGGCATAGACGCGTTTGGCGCTTCACTTTTCT | 143 | 62 |
| BCL6-3′UTR-MT | F:CGGGAGACGTAGTTTGGGCTGTGTCTAAACTGCAT R:CAAACTACGTCTCCCGAGATTGTTTATAACACAAGC | 6581 | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Zhang, X.; Zhou, K.; Ling, X.; Zhang, J.; Wu, P.; Zhang, T.; Xie, K.; Dai, G. miRNA-10a-5p Targeting the BCL6 Gene Regulates Proliferation, Differentiation and Apoptosis of Chicken Myoblasts. Int. J. Mol. Sci. 2022, 23, 9545. https://doi.org/10.3390/ijms23179545
Zhang G, Zhang X, Zhou K, Ling X, Zhang J, Wu P, Zhang T, Xie K, Dai G. miRNA-10a-5p Targeting the BCL6 Gene Regulates Proliferation, Differentiation and Apoptosis of Chicken Myoblasts. International Journal of Molecular Sciences. 2022; 23(17):9545. https://doi.org/10.3390/ijms23179545
Chicago/Turabian StyleZhang, Genxi, Xinchao Zhang, Kaizhi Zhou, Xuanze Ling, Jin Zhang, Pengfei Wu, Tao Zhang, Kaizhou Xie, and Guojun Dai. 2022. "miRNA-10a-5p Targeting the BCL6 Gene Regulates Proliferation, Differentiation and Apoptosis of Chicken Myoblasts" International Journal of Molecular Sciences 23, no. 17: 9545. https://doi.org/10.3390/ijms23179545
APA StyleZhang, G., Zhang, X., Zhou, K., Ling, X., Zhang, J., Wu, P., Zhang, T., Xie, K., & Dai, G. (2022). miRNA-10a-5p Targeting the BCL6 Gene Regulates Proliferation, Differentiation and Apoptosis of Chicken Myoblasts. International Journal of Molecular Sciences, 23(17), 9545. https://doi.org/10.3390/ijms23179545








