In Vitro and In Vivo Activities of Tilmicosin and Acetylisovaleryltylosin Tartrate against Toxoplasma gondii
Abstract
1. Introduction
2. Results
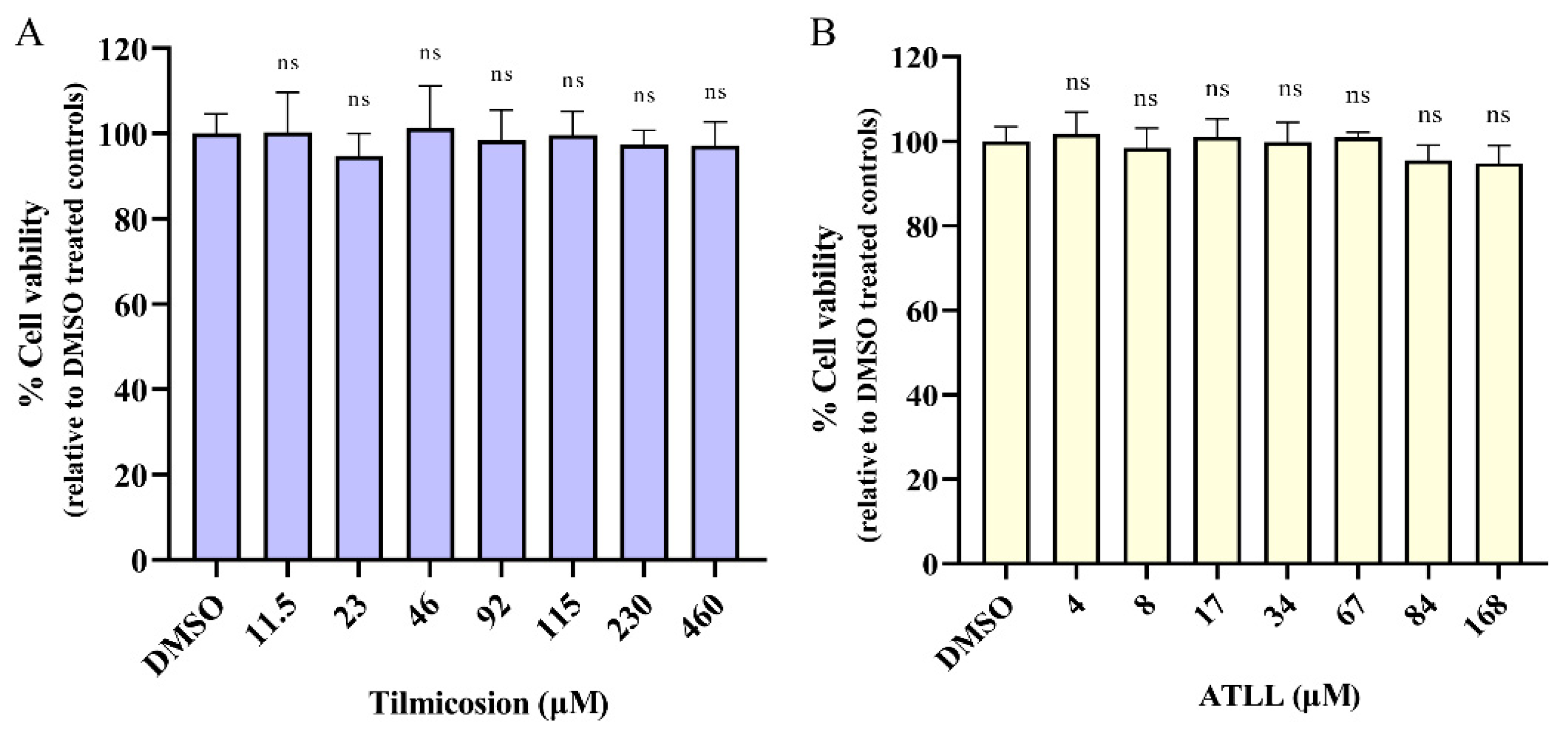
2.1. Cytotoxicity Activity of Tilmicosin and ATLL
2.2. Tilmicosin and ATLL Have Good Anti-T. gondii Effect In Vitro
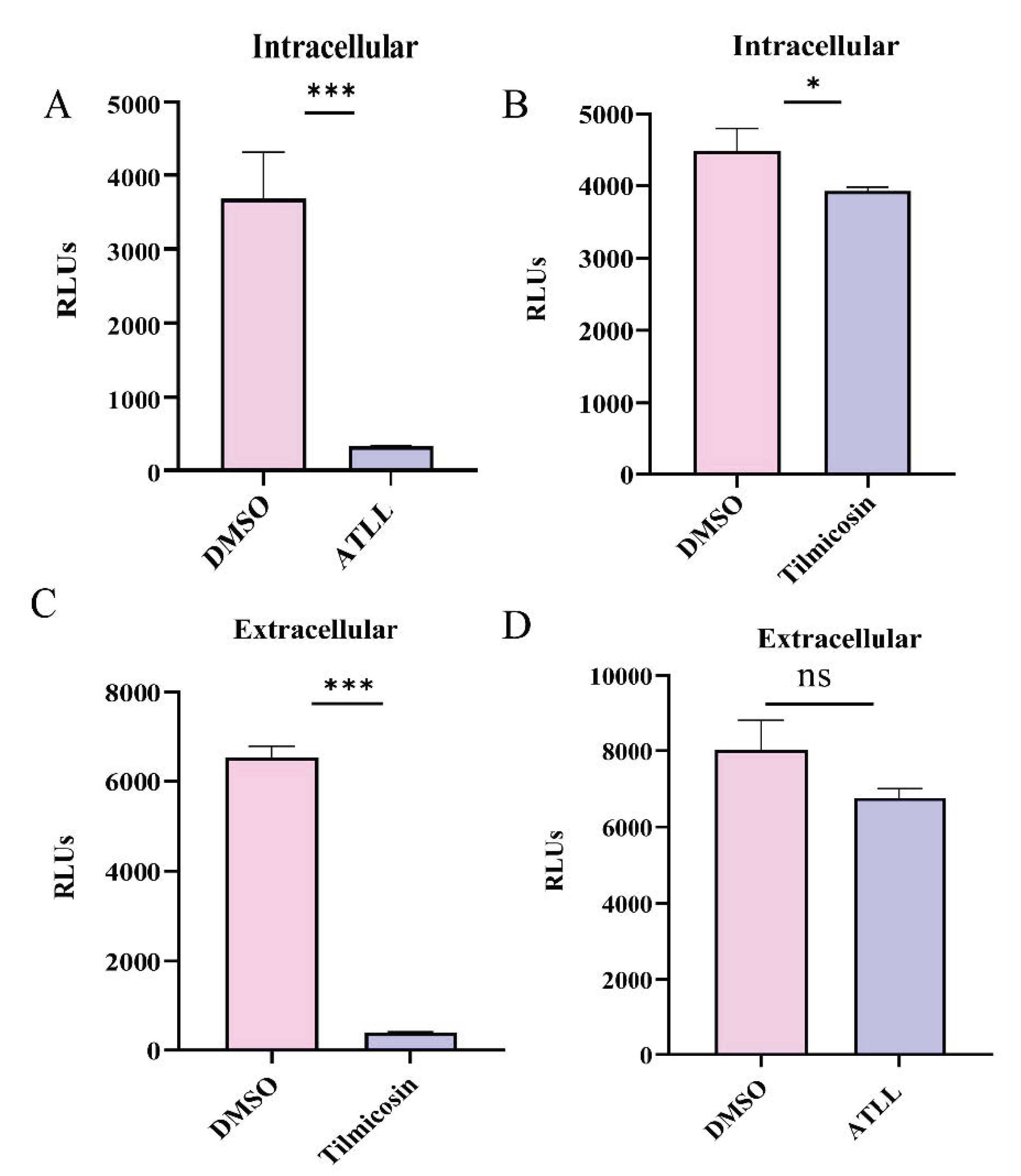
2.3. Differential Effects of Tilmicosin and ATLL on Tachyzoite Invasion and Proliferation
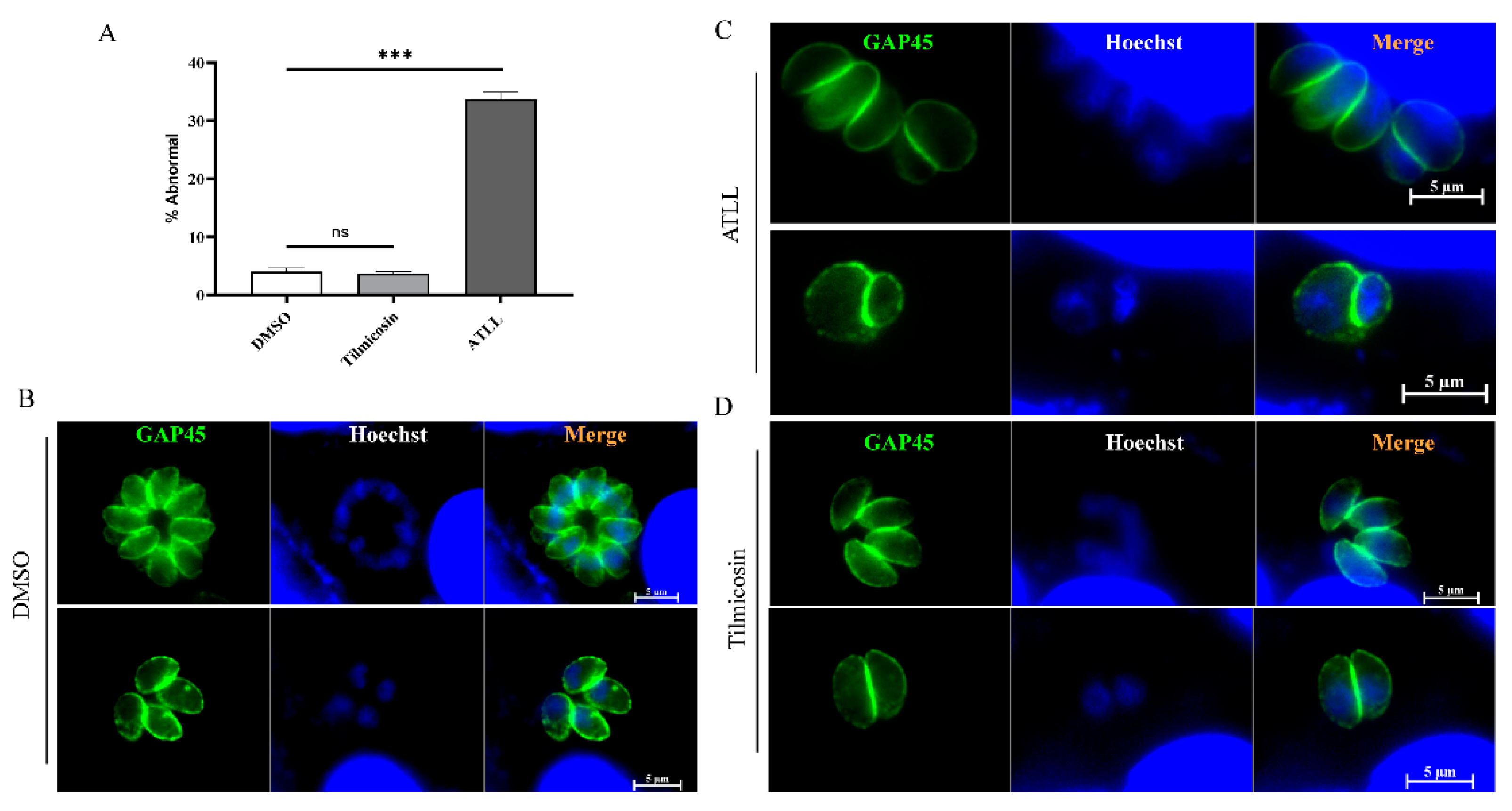
2.4. Effect of ATLL on the Morphology and Division of Tachyzoites
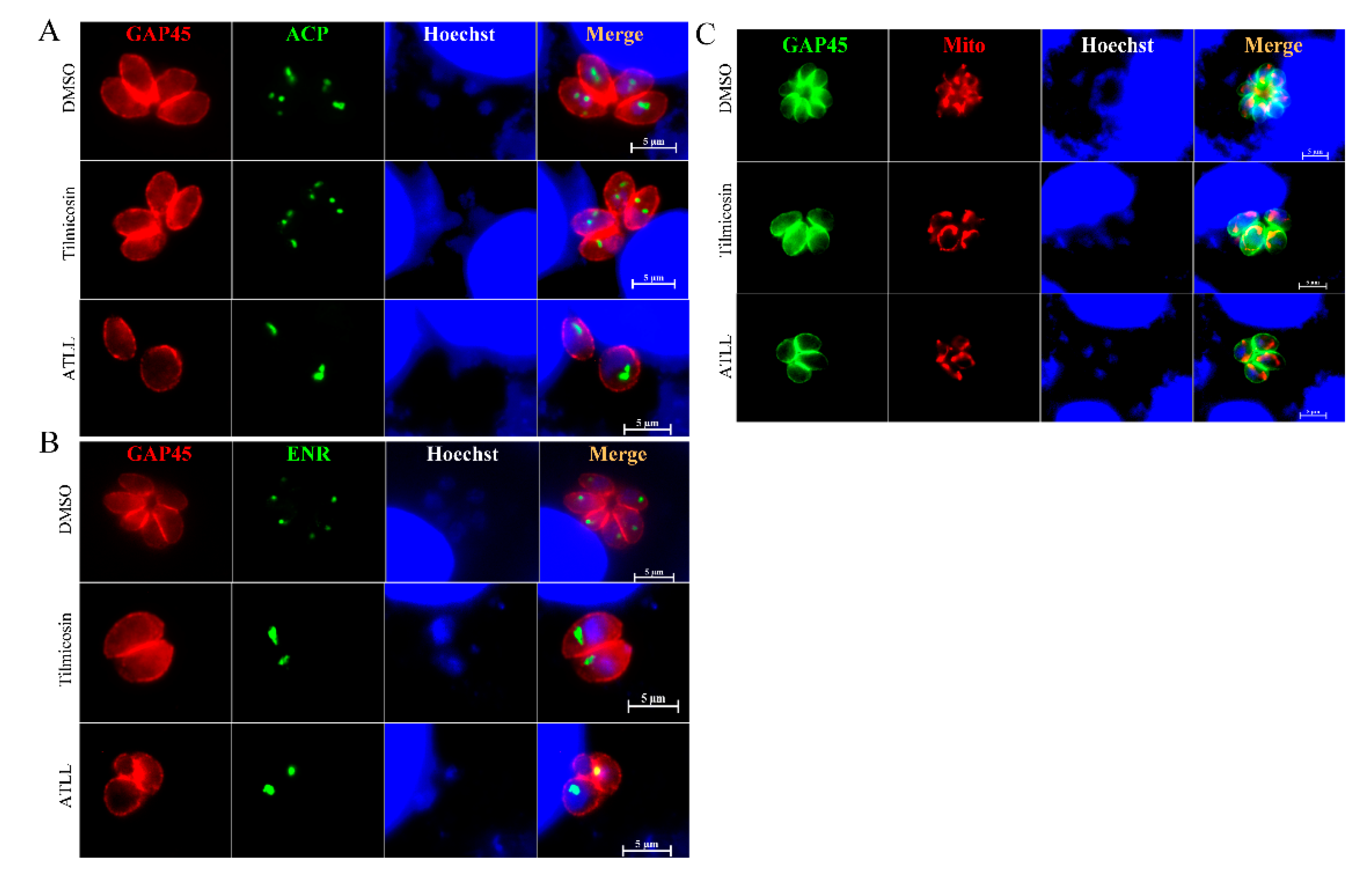
2.5. Tilmicosin and ATLL Do Not Affect the Mitochondria and Apicoplast of Tachyzoites
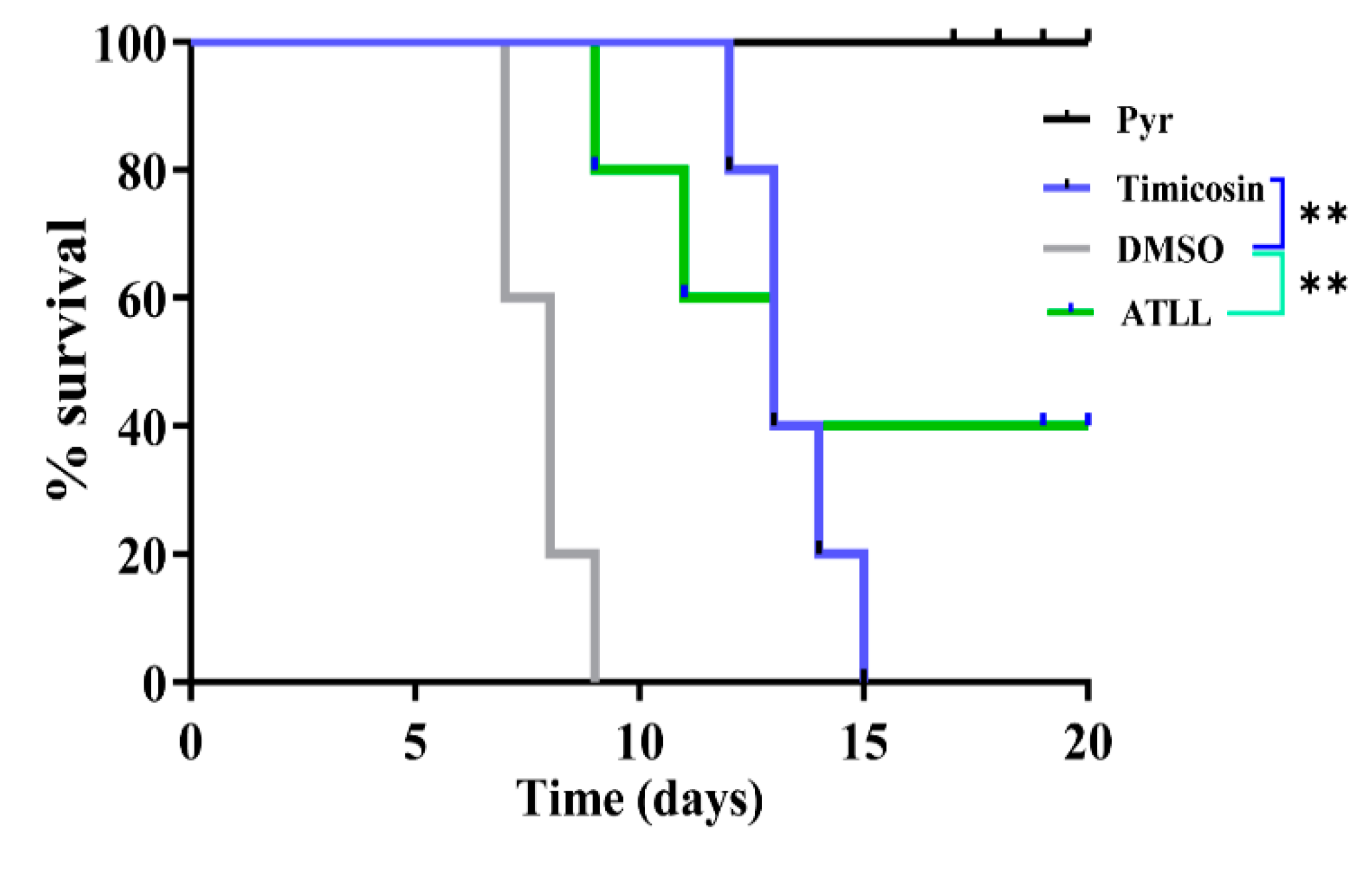
2.6. Tilmicosin and ATLL Delay Mortality in Mice Caused by Acute Toxoplasmosis
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Parasites and Cell Culture
4.3. Compounds
4.4. Cytotoxicity Assay
4.5. Plaque Assay
4.6. Immunofluorescence Assays
4.7. Invasion Assay and Intracellular Replication Assay
4.8. In Vitro Drug Inhibition Assay for T. gondii
4.9. T. gondii Intracellular and Extracellular Inhibition Assay
4.10. Parasite Division and Morphological Observation by Immunofluorescence Assays
4.11. Therapeutic Effect of Tilmicosin and ATLL on Acute Toxoplasmosis in Mice
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Nayeri, T.; Sarvi, S.; Moosazadeh, M.; Amouei, A.; Hosseininejad, Z.; Daryani, A. The global seroprevalence of anti-Toxoplasma gondii antibodies in women who had spontaneous abortion: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2020, 14, e0008103. [Google Scholar] [CrossRef]
- Wang, Z.D.; Wang, S.C.; Liu, H.H.; Ma, H.Y.; Li, Z.Y.; Wei, F.; Zhu, X.Q.; Liu, Q. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: A systematic review and meta-analysis. Lancet HIV 2017, 4, e177–e188. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis—A waterborne zoonosis. Vet. Parasitol. 2004, 126, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Halonen, S.K.; Weiss, L.M. Toxoplasmosis. Handb. Clin. Neurol. 2013, 114, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Cerqueira-Cézar, C.K.; Murata, F.H.A.; Kwok, O.C.H.; Hill, D.; Yang, Y.; Su, C. All about Toxoplasma gondii infections in pigs: 2009–2020. Vet. Parasitol. 2020, 288, 109185. [Google Scholar] [CrossRef] [PubMed]
- García-Bocanegra, I.; Simon-Grifé, M.; Dubey, J.P.; Casal, J.; Martín, G.E.; Cabezón, O.; Perea, A.; Almería, S. Seroprevalence and risk factors associated with Toxoplasma gondii in domestic pigs from Spain. Parasitol. Int. 2010, 59, 421–426. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Marra, C.M.; Zhu, X.Q. Epidemiology, Pathophysiology, Diagnosis, and Management of Cerebral Toxoplasmosis. Clin. Microbiol. Rev. 2021, 34, e00115-19. [Google Scholar] [CrossRef]
- Ben-Harari, R.R.; Goodwin, E.; Casoy, J. Adverse Event Profile of Pyrimethamine-Based Therapy in Toxoplasmosis: A Systematic Review. Drugs R&D 2017, 17, 523–544. [Google Scholar] [CrossRef]
- Shammaa, A.M.; Powell, T.G.; Benmerzouga, I. Adverse outcomes associated with the treatment of Toxoplasma infections. Sci. Rep. 2021, 11, 1035. [Google Scholar] [CrossRef]
- Alday, P.H.; Doggett, J.S. Drugs in development for toxoplasmosis: Advances, challenges, and current status. Drug Des. Dev. Ther. 2017, 11, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.D.; Teixeira, C.; Gomes, P.; Borges, M. Promising Drug Targets and Compounds with Anti-Toxoplasma gondii Activity. Microorganisms 2021, 9, 1960. [Google Scholar] [CrossRef]
- Montazeri, M.; Sharif, M.; Sarvi, S.; Mehrzadi, S.; Ahmadpour, E.; Daryani, A. A Systematic Review of In Vitro and In Vivo Activities of Anti-Toxoplasma Drugs and Compounds (2006–2016). Front. Microbiol. 2017, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, S.; Chrishan Shivanthan, M.; Samaranayake, N.; Rodrigo, C.; Deepika Fernando, S. Antibiotics for human toxoplasmosis: A systematic review of randomized trials. Pathog. Glob. Health 2013, 107, 162–169. [Google Scholar] [CrossRef]
- Wei, H.X.; Wei, S.S.; Lindsay, D.S.; Peng, H.J. A Systematic Review and Meta-Analysis of the Efficacy of Anti-Toxoplasma gondii Medicines in Humans. PLoS ONE 2015, 10, e0138204. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Ma, S. Recent advances in the field of 16-membered macrolide antibiotics. Mini Rev. Med. Chem. 2011, 11, 1009–1018. [Google Scholar] [CrossRef]
- Arsic, B.; Barber, J.; Čikoš, A.; Mladenovic, M.; Stankovic, N.; Novak, P. 16-membered macrolide antibiotics: A review. Int. J. Antimicrob. Agents 2018, 51, 283–298. [Google Scholar] [CrossRef]
- Elbadawy, M.; Aboubakr, M.; Abugomaa, A. Pharmacokinetics of Tylvalosin in Broiler Turkeys (Meleagris gallopavo) After Single Intravenous and Oral Administration. Front. Vet. Sci. 2019, 6, 355. [Google Scholar] [CrossRef]
- Lemli, B.; Derdák, D.; Laczay, P.; Kovács, D.; Kunsági-Máté, S. Noncovalent Interaction of Tilmicosin with Bovine Serum Albumin. Molecules 2018, 23, 1915. [Google Scholar] [CrossRef]
- Tsutsui, A.; Hirose, T.; Ishiyama, A.; Iwatsuki, M.; Yokota, A.; Maruyama, H.; Matsui, H.; Otoguro, K.; Hanaki, H.; Omura, S.; et al. Antimalarial C-9 oxime derivatives from desmycosin, produced by click chemistry. J. Antibiot. 2013, 66, 191–194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blader, I.J.; Coleman, B.I.; Chen, C.T.; Gubbels, M.J. Lytic Cycle of Toxoplasma gondii: 15 Years Later. Annu. Rev. Microbiol. 2015, 69, 463–485. [Google Scholar] [CrossRef]
- Neville, A.J.; Zach, S.J.; Wang, X.; Larson, J.J.; Judge, A.K.; Davis, L.A.; Vennerstrom, J.L.; Davis, P.H. Clinically Available Medicines Demonstrating Anti-Toxoplasma Activity. Antimicrob. Agents Chemother. 2015, 59, 7161–7169. [Google Scholar] [CrossRef] [PubMed]
- Julliac, B.; Théophile, H.; Begorre, M.; Richez, B.; Haramburu, F. Side effects of spiramycin masquerading as local anesthetic toxicity during labor epidural analgesia. Int. J. Obstet. Anesth. 2010, 19, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Thiébaut, R.; Leproust, S.; Chêne, G.; Gilbert, R. Effectiveness of prenatal treatment for congenital toxoplasmosis: A meta-analysis of individual patients’ data. Lancet 2007, 369, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Alder, J.; Hutch, T.; Meulbroek, J.A.; Clement, J.C. Treatment of experimental Toxoplasma gondii infection by clarithromycin-based combination therapy with minocycline or pyrimethamine. J. Acquir. Immune Defic. Syndr. 1994, 7, 1141–1148. [Google Scholar]
- Araujo, F.G.; Shepard, R.M.; Remington, J.S. In vivo activity of the macrolide antibiotics azithromycin, roxithromycin and spiramycin against Toxoplasma gondii. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 519–524. [Google Scholar] [CrossRef]
- Fichera, M.E.; Roos, D.S. A plastid organelle as a drug target in apicomplexan parasites. Nature 1997, 390, 407–409. [Google Scholar] [CrossRef]
- Li, M.; Hui, W.; Jing, L.; Pan, H.; Lei, M.; Qun, L. The Apoptotic Role of Metacaspase in Toxoplasma gondii. Front. Microbiol. 2016, 6, 1560. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Cao, X.; Wang, H.; Song, X.; Hu, D. In Vitro and In Vivo Activities of Tilmicosin and Acetylisovaleryltylosin Tartrate against Toxoplasma gondii. Int. J. Mol. Sci. 2022, 23, 9586. https://doi.org/10.3390/ijms23179586
Ma Y, Cao X, Wang H, Song X, Hu D. In Vitro and In Vivo Activities of Tilmicosin and Acetylisovaleryltylosin Tartrate against Toxoplasma gondii. International Journal of Molecular Sciences. 2022; 23(17):9586. https://doi.org/10.3390/ijms23179586
Chicago/Turabian StyleMa, Yazhen, Xinru Cao, Hui Wang, Xingju Song, and Dandan Hu. 2022. "In Vitro and In Vivo Activities of Tilmicosin and Acetylisovaleryltylosin Tartrate against Toxoplasma gondii" International Journal of Molecular Sciences 23, no. 17: 9586. https://doi.org/10.3390/ijms23179586
APA StyleMa, Y., Cao, X., Wang, H., Song, X., & Hu, D. (2022). In Vitro and In Vivo Activities of Tilmicosin and Acetylisovaleryltylosin Tartrate against Toxoplasma gondii. International Journal of Molecular Sciences, 23(17), 9586. https://doi.org/10.3390/ijms23179586






