Topographical Distribution and Phenotype of Resident Meibomian Gland Orifice Immune Cells (MOICs) in Mice and the Effects of Topical Benzalkonium Chloride (BAK)
Abstract
1. Introduction
2. Results
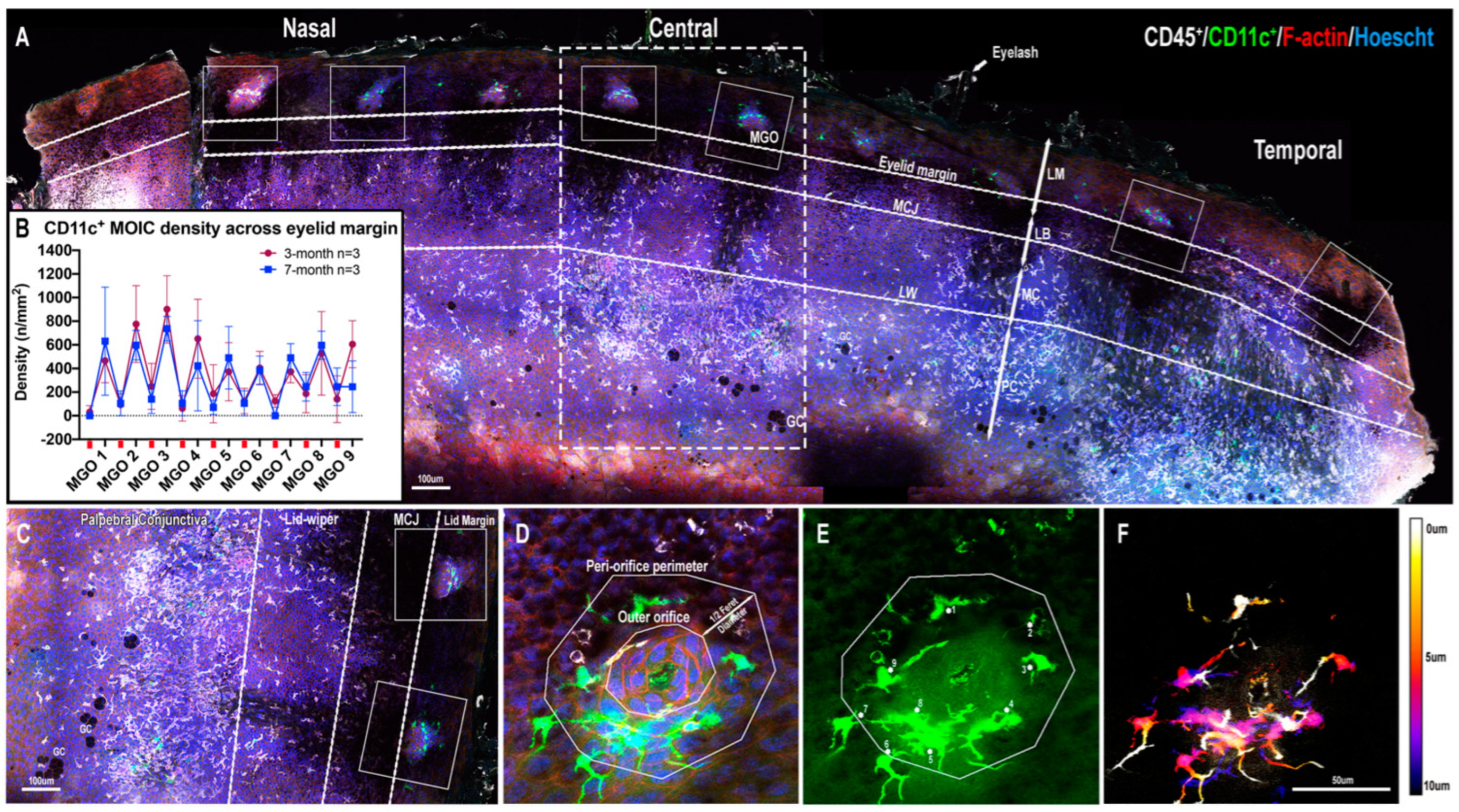
2.1. CD11c+ MOICs
2.1.1. CD11c+ MOICs Surround MGOs along the Eyelid Margin
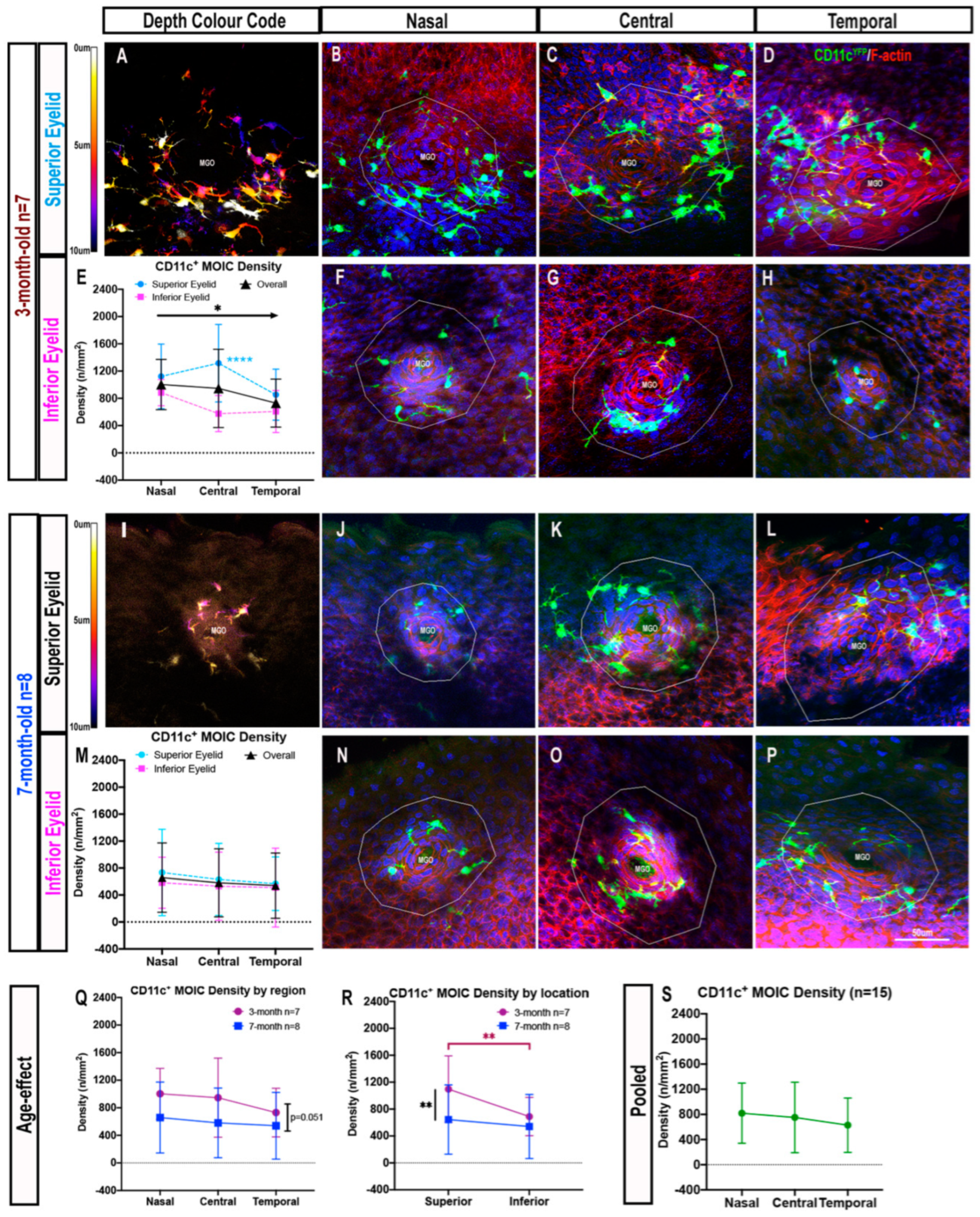
2.1.2. Decreasing Distribution Gradient of CD11c+ MOIC Density across the Eyelid Margin in Three-Month-Old Mice
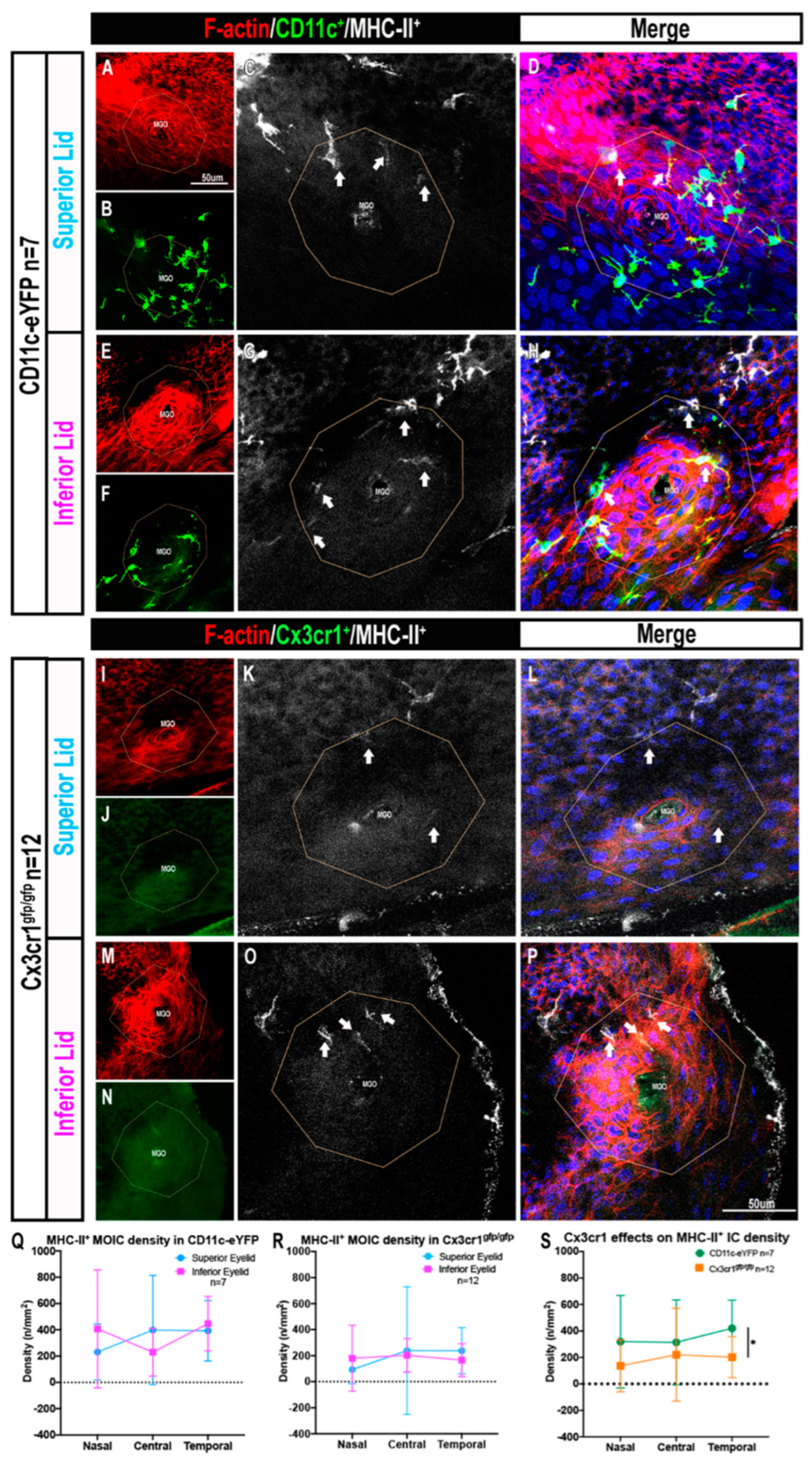
2.2. Cx3cr1+ MOIC Density Is Lower Than CD11c+ MOIC Density
2.3. The Presence of MHC-II+ MOICs Is Partially Dependent on the Cx3cr1 Receptor
2.4. MOICs Express CD11c and Cx3cr1 in CD11c-eYFP Mice
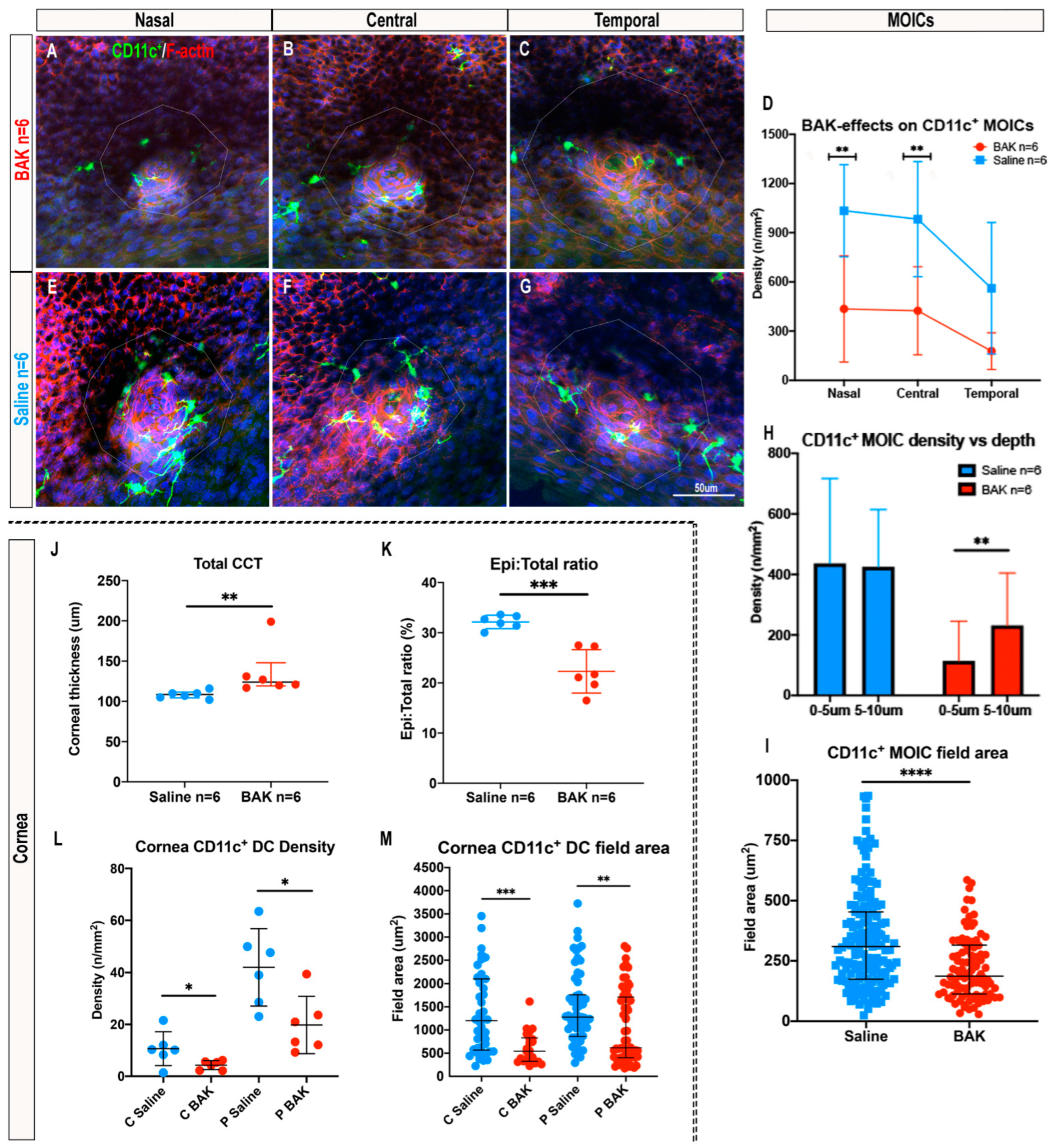
2.5. Topical Benzalkonium Chloride (BAK) Reduces CD11c+ MOIC Density
3. Discussion
4. Materials and Methods
4.1. Ethics and Animals
4.2. Model of Benzalkonium Chloride (BAK)-Induced Inflammation
4.3. Sample Size
4.4. Wholemount Immunofluorescence
4.5. Spectral Domain Optical Coherence Tomography (SD-OCT)
4.6. Image Collection and Analysis
4.7. Corneal Imaging
4.8. Image Analysis
4.9. Flow Cytometry
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Downie, L.E.; Bandlitz, S.; Bergmanson, J.P.G.; Craig, J.P.; Dutta, D.; Maldonado-Codina, C.; Ngo, W.; Siddireddy, J.S.; Wolffsohn, J.S. BCLA CLEAR—Anatomy and physiology of the anterior eye. Contact Lens Anterior Eye 2021, 44, 132–156. [Google Scholar] [CrossRef]
- Jester, J.V.; Nicolaides, N.; Smith, R.E. Meibomian gland studies: Histologic and ultrastructural investigations. Investig. Ophthalmol. Vis. Sci. 1981, 20, 537–547. [Google Scholar]
- Green-Church, K.B.; Butovich, I.; Willcox, M.; Borchman, D.; Paulsen, F.; Barabino, S.; Glasgow, B.J. The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Tear Film Lipids and Lipid–Protein Interactions in Health and Disease. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1979–1993. [Google Scholar] [CrossRef]
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef]
- Dietrich, J.; Garreis, F.; Paulsen, F. Pathophysiology of Meibomian Glands—An Overview. Ocul. Immunol. Inflamm. 2021, 29, 803–810. [Google Scholar] [CrossRef]
- Suzuki, T.; Teramukai, S.; Kinoshita, S. Meibomian glands and ocular surface inflammation. Ocul. Surf. 2015, 13, 133–149. [Google Scholar] [CrossRef]
- Blackie, C.A.; Korb, D.R. The diurnal secretory characteristics of individual meibomian glands. Cornea 2010, 29, 34–38. [Google Scholar] [CrossRef]
- Korb, D.R.; Blackie, C.A. Meibomian gland diagnostic expressibility: Correlation with dry eye symptoms and gland location. Cornea 2008, 27, 1142–1147. [Google Scholar] [CrossRef]
- Nien, C.J.; Massei, S.; Lin, G.; Nabavi, C.; Tao, J.; Brown, D.J.; Paugh, J.R.; Jester, J.V. Effects of age and dysfunction on human meibomian glands. Arch. Ophthalmol. 2011, 129, 462–469. [Google Scholar] [CrossRef]
- Qazi, Y.; Kheirkhah, A.; Blackie, C.; Trinidad, M.; Williams, C.; Cruzat, A.; Korb, D.R.; Hamrah, P. Clinically Relevant Immune-Cellular Metrics of Inflammation in Meibomian Gland Dysfunction. Investig. Ophthalmol. Vis. Sci. 2018, 59, 6111–6123. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.D.; Shimazaki, J.; Benitez-del-Castillo, J.M.; Craig, J.P.; McCulley, J.P.; Den, S.; Foulks, G.N. The international workshop on meibomian gland dysfunction: Report of the definition and classification subcommittee. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Knop, E.; Knop, N. The role of eye-associated lymphoid tissue in corneal immune protection. J. Anat. 2005, 206, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Labrecque, N.; Cermakian, N. Circadian Clocks in the Immune System. J. Biol. Rhythm. 2015, 30, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, A.; Godefroy, D.; Célérier, I.; Frugier, J.; Riancho, L.; Baudouin, F.; Rostène, W.; Baudouin, C. CX3CL1 expression in the conjunctiva is involved in immune cell trafficking during toxic ocular surface inflammation. Mucosal Immunol. 2012, 5, 702–711. [Google Scholar] [CrossRef]
- Goldstein, M.H.; Silva, F.Q.; Blender, N.; Tran, T.; Vantipalli, S. Ocular benzalkonium chloride exposure: Problems and solutions. Eye 2022, 36, 361–368. [Google Scholar] [CrossRef]
- Chinnery, H.R.; Ruitenberg, M.J.; Plant, G.W.; Pearlman, E.; Jung, S.; McMenamin, P.G. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1568–1574. [Google Scholar] [CrossRef]
- Steinman, R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991, 9, 271–296. [Google Scholar] [CrossRef]
- Logeswaran, A.; Contreras-Ruiz, L.; Masli, S. Conjunctival Goblet Cell Responses to TLR5 Engagement Promote Activation of Local Antigen-Presenting Cells. Front. Immunol. 2021, 12, 716939. [Google Scholar] [CrossRef]
- Sun, M.; Moreno, I.Y.; Dang, M.; Coulson-Thomas, V.J. Meibomian Gland Dysfunction: What Have Animal Models Taught Us? Int. J. Mol. Sci. 2020, 21, 8822. [Google Scholar] [CrossRef]
- Nien, C.J.; Paugh, J.R.; Massei, S.; Wahlert, A.J.; Kao, W.W.; Jester, J.V. Age-related changes in the meibomian gland. Exp. Eye Res. 2009, 89, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Zhang, M.; Wu, Y.; Jiang, N.; Guo, Y.; He, X.; He, H.; Jeyalatha, M.V.; Reinach, P.S.; Liu, Z.; et al. High-Fat Diet Induces Inflammation of Meibomian Gland. Investig. Ophthalmol. Vis. Sci. 2021, 62, 13. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Parfitt, G.J.; Brown, D.J. Meibomian gland dysfunction: Hyperkeratinization or atrophy? BMC Ophthalmol. 2015, 15 (Suppl. 1), 156. [Google Scholar] [CrossRef]
- Parfitt, G.J.; Xie, Y.; Geyfman, M.; Brown, D.J.; Jester, J.V. Absence of ductal hyper-keratinization in mouse age-related meibomian gland dysfunction (ARMGD). Aging 2013, 5, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Hasíková, L.; Hampel, U.; Grüneboom, A.; Shan, X.; Herrmann, I.; Garreis, F.; Bock, F.; Knopf, J.; Singh, J.; et al. Aggregated neutrophil extracellular traps occlude Meibomian glands during ocular surface inflammation. Ocul. Surf. 2021, 20, 1–12. [Google Scholar] [CrossRef]
- Jamali, A.; Seyed-Razavi, Y.; Chao, C.; Ortiz, G.; Kenyon, B.; Blanco, T.; Harris, D.L.; Hamrah, P. Intravital Multiphoton Microscopy of the Ocular Surface: Alterations in Conventional Dendritic Cell Morphology and Kinetics in Dry Eye Disease. Front. Immunol. 2020, 11, 742. [Google Scholar] [CrossRef]
- Jiao, H.; Naranjo Golborne, C.; Dando, S.J.; McMenamin, P.G.; Downie, L.E.; Chinnery, H.R. Topographical and Morphological Differences of Corneal Dendritic Cells during Steady State and Inflammation. Ocul. Immunol. Inflamm. 2020, 28, 898–907. [Google Scholar] [CrossRef]
- Hingorani, M.; Metz, D.; Lightman, S.L. Characterisation of the normal conjunctival leukocyte population. Exp. Eye Res. 1997, 64, 905–912. [Google Scholar] [CrossRef]
- Alam, J.; de Paiva, C.S.; Pflugfelder, S.C. Immune—Goblet cell interaction in the conjunctiva. Ocul. Surf. 2020, 18, 326–334. [Google Scholar] [CrossRef]
- Bijnen, M.; Bajénoff, M. Gland Macrophages: Reciprocal Control and Function within Their Niche. Trends Immunol. 2021, 42, 120–136. [Google Scholar] [CrossRef]
- Dawson, C.A.; Pal, B.; Vaillant, F.; Gandolfo, L.C.; Liu, Z.; Bleriot, C.; Ginhoux, F.; Smyth, G.K.; Lindeman, G.J.; Mueller, S.N.; et al. Tissue-resident ductal macrophages survey the mammary epithelium and facilitate tissue remodelling. Nat. Cell Biol. 2020, 22, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Plaks, V.; Boldajipour, B.; Linnemann, J.R.; Nguyen, N.H.; Kersten, K.; Wolf, Y.; Casbon, A.J.; Kong, N.; van den Bijgaart, R.J.; Sheppard, D.; et al. Adaptive Immune Regulation of Mammary Postnatal Organogenesis. Dev. Cell 2015, 34, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Stolp, B.; Thelen, F.; Ficht, X.; Altenburger, L.M.; Ruef, N.; Inavalli, V.; Germann, P.; Page, N.; Moalli, F.; Raimondi, A.; et al. Salivary gland macrophages and tissue-resident CD8(+) T cells cooperate for homeostatic organ surveillance. Sci. Immunol. 2020, 5, eaaz4371. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Lee, P.; Yu, F.S. Intraepithelial dendritic cells and sensory nerves are structurally associated and functional interdependent in the cornea. Sci. Rep. 2016, 6, 36414. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Downie, L.E.; Grover, L.M.; Moakes, R.J.A.; Rauz, S.; Logan, A.; Jiao, H.; Hill, L.J.; Chinnery, H.R. The neuroregenerative effects of topical decorin on the injured mouse cornea. J. Neuroinflamm. 2020, 17, 142. [Google Scholar] [CrossRef]
- Wu, M.; Downie, L.E.; Hill, L.J.; Chinnery, H.R. The effect of topical decorin on temporal changes to corneal immune cells after epithelial abrasion. J. Neuroinflamm. 2022, 19, 90. [Google Scholar] [CrossRef]
- Hamrah, P.; Huq, S.O.; Liu, Y.; Zhang, Q.; Dana, M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 2003, 74, 172–178. [Google Scholar] [CrossRef]
- Zhivov, A.; Stave, J.; Vollmar, B.; Guthoff, R. In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium. Graefe’s Arch. Clin. Exp. Ophthalmol. 2005, 243, 1056–1061. [Google Scholar] [CrossRef]
- Pult, H.; Tosatti, S.G.; Spencer, N.D.; Asfour, J.M.; Ebenhoch, M.; Murphy, P.J. Spontaneous Blinking from a Tribological Viewpoint. Ocul. Surf. 2015, 13, 236–249. [Google Scholar] [CrossRef]
- Knop, N.; Knop, E. The crypt system of the human conjunctiva. Adv. Exp. Med. Biol. 2002, 506, 867–872. [Google Scholar] [CrossRef]
- Knop, N.; Knop, E. Conjunctiva-Associated Lymphoid Tissue in the Human Eye. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1270–1279. [Google Scholar]
- Reinoso, R.; Martín-Sanz, R.; Martino, M.; Mateo, M.E.; Blanco-Salado, R.; Calonge, M.; Corell, A. Topographical distribution and characterization of epithelial cells and intraepithelial lymphocytes in the human ocular mucosa. Mucosal Immunol. 2012, 5, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Welss, J.; Punchago, N.; Feldt, J.; Paulsen, F. The distribution of conjunctival goblet cells in mice. Ann. Anat. 2021, 234, 151664. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Xiao, Y.; Barbosa, F.L.; de Souza, R.G.; Hernandez, H.; Yu, Z.; Pflugfelder, S.C.; de Paiva, C.S. Age-associated antigen-presenting cell alterations promote dry-eye inducing Th1 cells. Mucosal Immunol. 2019, 12, 897–908. [Google Scholar] [CrossRef]
- Steuhl, K.P.; Sitz, U.; Knorr, M.; Thanos, S.; Thiel, H.J. Abstract: Age-dependent distribution of Langerhans cells within human conjunctival epithelium. Der Ophthalmologe 1995, 92, 21–25. [Google Scholar]
- Schaumberg, D.A.; Nichols, J.J.; Papas, E.B.; Tong, L.; Uchino, M.; Nichols, K.K. The international workshop on meibomian gland dysfunction: Report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1994–2005. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Medina-Contreras, O.; Geem, D.; Laur, O.; Williams, I.R.; Lira, S.A.; Nusrat, A.; Parkos, C.A.; Denning, T.L. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J. Clin. Investig. 2011, 121, 4787–4795. [Google Scholar] [CrossRef]
- Niess, J.H.; Brand, S.; Gu, X.; Landsman, L.; Jung, S.; McCormick, B.A.; Vyas, J.M.; Boes, M.; Ploegh, H.L.; Fox, J.G.; et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 2005, 307, 254–258. [Google Scholar] [CrossRef]
- Magny, R.; Kessal, K.; Regazzetti, A.; Ben Yedder, A.; Baudouin, C.; Mélik Parsadaniantz, S.; Brignole-Baudouin, F.; Laprévote, O.; Auzeil, N. Lipidomic analysis of epithelial corneal cells following hyperosmolarity and benzalkonium chloride exposure: New insights in dry eye disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158728. [Google Scholar] [CrossRef]
- Ayaki, M.; Iwasawa, A. Cytotoxicity of prostaglandin analog eye drops preserved with benzalkonium chloride in multiple corneoconjunctival cell lines. Clin. Ophthalmol. 2010, 4, 919–924. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kahook, M.Y.; Noecker, R.J. Comparison of corneal and conjunctival changes after dosing of travoprost preserved with sofZia, latanoprost with 0.02% benzalkonium chloride, and preservative-free artificial tears. Cornea 2008, 27, 339–343. [Google Scholar] [CrossRef]
- Baudouin, C.; Pisella, P.J.; Fillacier, K.; Goldschild, M.; Becquet, F.; De Saint Jean, M.; Béchetoille, A. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: Human and animal studies. Ophthalmology 1999, 106, 556–563. [Google Scholar] [CrossRef]
- Uusitalo, H.; Egorov, E.; Kaarniranta, K.; Astakhov, Y.; Ropo, A. Benefits of switching from latanoprost to preservative-free tafluprost eye drops: A meta-analysis of two Phase IIIb clinical trials. Clin. Ophthalmol. 2016, 10, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.P.; Azar, D.T.; Baudouin, C.; Efron, N.; Hirayama, M.; Horwath-Winter, J.; Kim, T.; Mehta, J.S.; Messmer, E.M.; Pepose, J.S.; et al. TFOS DEWS II iatrogenic report. Ocul. Surf. 2017, 15, 511–538. [Google Scholar] [CrossRef]
- Zhivov, A.; Kraak, R.; Bergter, H.; Kundt, G.; Beck, R.; Guthoff, R.F. Influence of benzalkonium chloride on langerhans cells in corneal epithelium and development of dry eye in healthy volunteers. Curr. Eye Res. 2010, 35, 762–769. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Harrison, D. Life Span as a Biomarker. Available online: https://www.jax.org/research-and-faculty/research-labs/the-harrison-lab/gerontology/life-span-as-a-biomarker (accessed on 6 May 2022).
- Tripathi, B.J.; Tripathi, R.C.; Kolli, S.P. Cytotoxicity of ophthalmic preservatives on human corneal epithelium. Lens Eye Toxic. Res. 1992, 9, 361–375. [Google Scholar]
- Lindquist, R.L.; Shakhar, G.; Dudziak, D.; Wardemann, H.; Eisenreich, T.; Dustin, M.L.; Nussenzweig, M.C. Visualizing dendritic cell networks in vivo. Nat. Immunol. 2004, 5, 1243–1250. [Google Scholar] [CrossRef]
- Karlmark, K.R.; Zimmermann, H.W.; Roderburg, C.; Gassler, N.; Wasmuth, H.E.; Luedde, T.; Trautwein, C.; Tacke, F. The fractalkine receptor CX3CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology 2010, 52, 1769–1782. [Google Scholar] [CrossRef]
- Landsman, L.; Bar-On, L.; Zernecke, A.; Kim, K.W.; Krauthgamer, R.; Shagdarsuren, E.; Lira, S.A.; Weissman, I.L.; Weber, C.; Jung, S. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 2009, 113, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Łyszkiewicz, M.; Witzlau, K.; Pommerencke, J.; Krueger, A. Chemokine receptor CX3CR1 promotes dendritic cell development under steady-state conditions. Eur. J. Immunol. 2011, 41, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Aliberti, J.; Graemmel, P.; Sunshine, M.J.; Kreutzberg, G.W.; Sher, A.; Littman, D.R. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 2000, 20, 4106–4114. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, J.; Blomster, L.V.; Chinnery, H.R.; Weninger, W.; Jung, S.; McMenamin, P.G.; Ruitenberg, M.J. Bone marrow chimeric mice reveal a role for CX3CR1 in maintenance of the monocyte-derived cell population in the olfactory neuroepithelium. J. Leukoc. Biol. 2010, 88, 645–654. [Google Scholar] [CrossRef]
- Guzmán, M.; Miglio, M.; Keitelman, I.; Shiromizu, C.M.; Sabbione, F.; Fuentes, F.; Trevani, A.S.; Giordano, M.N.; Galletti, J.G. Transient tear hyperosmolarity disrupts the neuroimmune homeostasis of the ocular surface and facilitates dry eye onset. Immunology 2020, 161, 148–161. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.Y.; Wu, M.; Huang, X.; Gu, B.J.; Maldonado-Codina, C.; Morgan, P.B.; Downie, L.E.; Chinnery, H.R. Topographical Distribution and Phenotype of Resident Meibomian Gland Orifice Immune Cells (MOICs) in Mice and the Effects of Topical Benzalkonium Chloride (BAK). Int. J. Mol. Sci. 2022, 23, 9589. https://doi.org/10.3390/ijms23179589
Wu CY, Wu M, Huang X, Gu BJ, Maldonado-Codina C, Morgan PB, Downie LE, Chinnery HR. Topographical Distribution and Phenotype of Resident Meibomian Gland Orifice Immune Cells (MOICs) in Mice and the Effects of Topical Benzalkonium Chloride (BAK). International Journal of Molecular Sciences. 2022; 23(17):9589. https://doi.org/10.3390/ijms23179589
Chicago/Turabian StyleWu, Ching Yi, Mengliang Wu, Xin Huang, Ben J. Gu, Carole Maldonado-Codina, Philip B. Morgan, Laura E. Downie, and Holly R. Chinnery. 2022. "Topographical Distribution and Phenotype of Resident Meibomian Gland Orifice Immune Cells (MOICs) in Mice and the Effects of Topical Benzalkonium Chloride (BAK)" International Journal of Molecular Sciences 23, no. 17: 9589. https://doi.org/10.3390/ijms23179589
APA StyleWu, C. Y., Wu, M., Huang, X., Gu, B. J., Maldonado-Codina, C., Morgan, P. B., Downie, L. E., & Chinnery, H. R. (2022). Topographical Distribution and Phenotype of Resident Meibomian Gland Orifice Immune Cells (MOICs) in Mice and the Effects of Topical Benzalkonium Chloride (BAK). International Journal of Molecular Sciences, 23(17), 9589. https://doi.org/10.3390/ijms23179589





