Glutamate Neurotoxicity and Destruction of the Blood–Brain Barrier: Key Pathways for the Development of Neuropsychiatric Consequences of TBI and Their Potential Treatment Strategies
Abstract
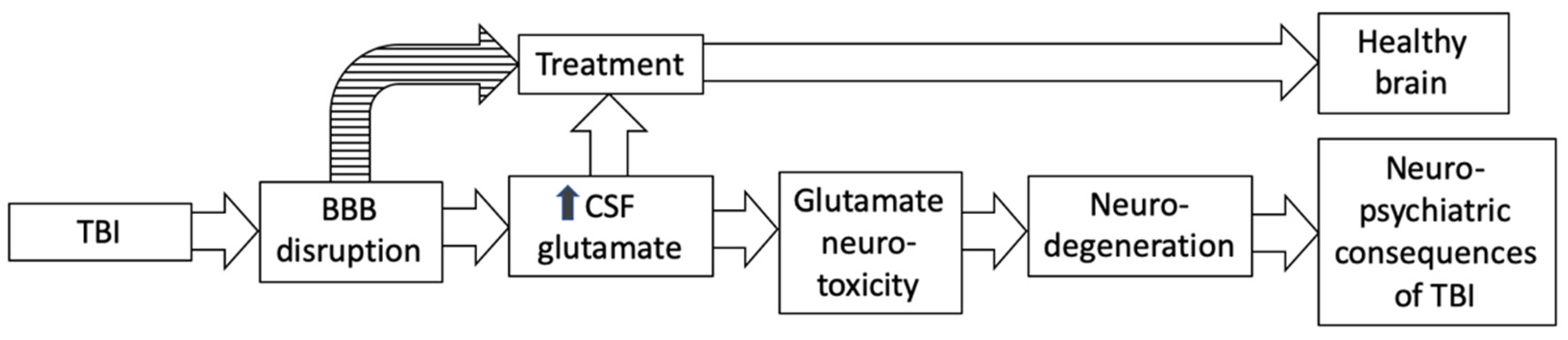
:1. Introduction
2. Pharmacological Basis of Dementia, Anxiety, and Mood Disorders: The Role of Glutamate
3. TBI and Glutamate Dysregulation
3.1. The Mechanisms of Increase in Brain Glutamate Is Associated with Neuronal Death
3.2. The Mechanism of Increase in Brain Glutamate Is Associated with Inflammation [42]
3.3. The Mechanism of Increases in Brain Glutamate Is Associated with Impaired Glutamatergic Recycling and Signaling
3.4. The Mechanism of Increase in Brain Glutamate Is Associated with Prolonged Stress
3.5. The Mechanism of Increase in Brain Glutamate Is Associated with Astrocytic Release of ATP [44]
3.6. The Mechanism of Increase in Brain Glutamate Is Associated with Other Sources of Elevated Intraparenchymal Glutamate
4. Disruption of the BBB
5. Glutamate Neurotoxicity and Its Association with Neurodegeneration
6. New Treatment Strategies for Neuropsychiatric Consequences of TBI Associated with the Hypothesis of Impaired BBB Permeability and Brain–Blood Glutamate Equilibrium
6.1. Treatment Aimed at Recovery of the Integrity of the BBB [95,96]
6.2. Reducing Post-Injury Glutamate Excess Based on Manipulation of Brain–Blood Glutamate Equilibrium
| Intervention | Description |
|---|---|
| Targeting paracellular permeability | Targeting junction molecules (adherens junctions, tight junctions), or their regulators (microRNA, transcription factor) in order to limit or reverse paracellular permeability [95,122]. Examples include chelerythrine chloride [123]. |
| Targeting transcellular permeability | Inhibition of transcytosis in brain endothelial cells, important to maintain neurological function and BBB integrity [95,124,125]. |
| Restoring efflux transporter activity | Restoring efflux transporter activity, such as ATP-binding cassette (ABC) transporters [95,126], important for clearing neurotoxins from the brain. |
| Repair of the neurovascular unit | Reestablishing normal function of the neurovascular unit (neurons, astrocytes, endothelial cells, pericytes, and the basal lamina), by restoring microvascular bed cerebral blood flow, limiting neuronal death, and promoting neurogenesis and angiogenesis [127]. Examples include bone-marrow-derived mesenchymal stem cells (MSCs) [128], pericytes [129,130], endothelial progenitor cells (EPCs) [131], neural and vascular progenitor cells [132,133], bone-marrow-derived macrophages [134], and vascular endothelial growth factor (VEGF) [135]. |
| Targeting inflammation | Targeting inflammation and downstream sequalae to restore the BBB. Examples include COX-2 inhibition [136], AQP4 inhibition [123], docosahexaenoic acid (DHA) [137], inhibition of Na-K-Cl cotransporter [138], and bone marrow mononuclear cells (MNCs) [139,140]. |
| Matrix metalloproteinases (MMP) | Limiting pathologically elevated MMP expression elevated after brain insult [127]. Examples include progesterone [141], TGF- β1 [142], exendin-4 [143], melatonin [144], regulatory T cells [145], EP1 antagonists [146], and minocycline [147]. |
7. Other Factors That Play a Role in the Pathophysiology of TBI
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jorge, R.E.; Arciniegas, D.B. Mood disorders after TBI. Psychiatr. Clin. N. Am. 2014, 37, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Rauen, K.; Reichelt, L.; Probst, P.; Schapers, B.; Muller, F.; Jahn, K.; Plesnila, N. Quality of life up to 10 years after traumatic brain injury: A cross-sectional analysis. Health Qual. Life Outcomes 2020, 18, 166. [Google Scholar] [CrossRef] [PubMed]
- Tateno, A.; Jorge, R.E.; Robinson, R.G. Clinical correlates of aggressive behavior after traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2003, 15, 155–160. [Google Scholar] [CrossRef]
- Hoofien, D.; Gilboa, A.; Vakil, E.; Donovick, P.J. Traumatic brain injury (TBI) 10–20 years later: A comprehensive outcome study of psychiatric symptomatology, cognitive abilities and psychosocial functioning. Brain Inj. 2001, 15, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Koponen, S.; Taiminen, T.; Portin, R.; Himanen, L.; Isoniemi, H.; Heinonen, H.; Hinkka, S.; Tenovuo, O. Axis I and II psychiatric disorders after traumatic brain injury: A 30-year follow-up study. Am. J. Psychiatry 2002, 159, 1315–1321. [Google Scholar] [CrossRef]
- Rivara, F.P.; Koepsell, T.D.; Wang, J.; Temkin, N.; Dorsch, A.; Vavilala, M.S.; Durbin, D.; Jaffe, K.M. Disability 3, 12, and 24 months after traumatic brain injury among children and adolescents. Pediatrics 2011, 128, e1129–e1138. [Google Scholar] [CrossRef]
- Fehily, B.; Bartlett, C.A.; Lydiard, S.; Archer, M.; Milbourn, H.; Majimbi, M.; Hemmi, J.M.; Dunlop, S.A.; Yates, N.J.; Fitzgerald, M. Differential responses to increasing numbers of mild traumatic brain injury in a rodent closed-head injury model. J. Neurochem. 2019, 149, 660–678. [Google Scholar] [CrossRef]
- Orlovska, S.; Pedersen, M.S.; Benros, M.E.; Mortensen, P.B.; Agerbo, E.; Nordentoft, M. Head injury as risk factor for psychiatric disorders: A nationwide register-based follow-up study of 113,906 persons with head injury. Am. J. Psychiatry 2014, 171, 463–469. [Google Scholar] [CrossRef]
- Jorge, R.E.; Robinson, R.G.; Starkstein, S.E.; Arndt, S.V.; Forrester, A.W.; Geisler, F.H. Secondary mania following traumatic brain injury. Am. J. Psychiatry 1993, 150, 916–921. [Google Scholar] [CrossRef]
- McAllister, T.W. Neurobehavioral sequelae of traumatic brain injury: Evaluation and management. World Psychiatry 2008, 7, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Hicks, A.J.; Clay, F.J.; Hopwood, M.; James, A.C.; Jayaram, M.; Perry, L.A.; Batty, R.; Ponsford, J.L. The Efficacy and Harms of Pharmacological Interventions for Aggression after Traumatic Brain Injury—Systematic Review. Front. Neurol. 2019, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Gruenbaum, B.F.; Zlotnik, A.; Frenkel, A.; Fleidervish, I.; Boyko, M. Glutamate Efflux across the Blood–Brain Barrier: New Perspectives on the Relationship between Depression and the Glutamatergic System. Metabolites 2022, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.; Trujillo, D.; Levine, J.; Dwyer, J.B.; Rupp, Z.W.; Bloch, M.H. Glutamate systems in DSM-5 anxiety disorders: Their role and a review of glutamate and GABA psychopharmacology. Front. Psychiatry 2020, 11, 548505. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Alberici, A.; Buratti, E.; Ghidoni, R.; Gardoni, F.; Di Luca, M.; Padovani, A.; Borroni, B. Toward a glutamate hypothesis of frontotemporal dementia. Front. Neurosci. 2019, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Domitrovic Spudic, S.; Nikolac Perkovic, M.; Uzun, S.; Nedic Erjavec, G.; Kozumplik, O.; Svob Strac, D.; Mimica, N.; Pivac, N. Reduced plasma BDNF concentration and cognitive decline in veterans with PTSD. Psychiatry Res. 2022, 316, 114772. [Google Scholar] [CrossRef]
- Spinhoven, P.; Penninx, B.W.; van Hemert, A.M.; de Rooij, M.; Elzinga, B.M. Comorbidity of PTSD in anxiety and depressive disorders: Prevalence and shared risk factors. Child Abuse Negl. 2014, 38, 1320–1330. [Google Scholar] [CrossRef]
- Edition, F. Diagnostic and statistical manual of mental disorders. Am. Psychiatr. Assoc. 2013, 21, 591–643. [Google Scholar]
- Marks, D.M.; Park, M.H.; Ham, B.J.; Han, C.; Patkar, A.A.; Masand, P.S.; Pae, C.U. Paroxetine: Safety and tolerability issues. Expert Opin. Drug Saf. 2008, 7, 783–794. [Google Scholar] [CrossRef]
- Chouinard, G.; Annable, L.; Fontaine, R.; Solyom, L. Alprazolam in the treatment of generalized anxiety and panic disorders: A double-blind placebo-controlled study. Psychopharmacology 1982, 77, 229–233. [Google Scholar] [CrossRef]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef]
- Amiel, J.M.; Mathew, S.J. Glutamate and anxiety disorders. Curr. Psychiatry Rep. 2007, 9, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2011, 13, 22–37. [Google Scholar] [CrossRef]
- Sanacora, G.; Zarate, C.A.; Krystal, J.H.; Manji, H.K. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 2008, 7, 426–437. [Google Scholar] [CrossRef]
- Zoicas, I.; Kornhuber, J. The role of the N-methyl-D-aspartate receptors in social behavior in rodents. Int. J. Mol. Sci. 2019, 20, 5599. [Google Scholar] [CrossRef] [PubMed]
- Bergink, V.; Van Megen, H.J.; Westenberg, H.G. Glutamate and anxiety. Eur. Neuropsychopharmacol. 2004, 14, 175–183. [Google Scholar] [CrossRef]
- Cortese, B.M.; Phan, K.L. The role of glutamate in anxiety and related disorders. CNS Spectr. 2005, 10, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Maren, S. Synapse-Specific Encoding of Fear Memory in the Amygdala. Neuron 2017, 95, 988–990. [Google Scholar] [CrossRef]
- Pare, D. Presynaptic induction and expression of NMDA-dependent LTP. Trends Neurosci. 2004, 27, 440–441. [Google Scholar] [CrossRef]
- McGrath, T.; Baskerville, R.; Rogero, M.; Castell, L. Emerging Evidence for the Widespread Role of Glutamatergic Dysfunction in Neuropsychiatric Diseases. Nutrients 2022, 14, 917. [Google Scholar] [CrossRef]
- Shutter, L.; Tong, K.A.; Holshouser, B.A. Proton MRS in acute traumatic brain injury: Role for glutamate/glutamine and choline for outcome prediction. J. Neurotrauma 2004, 21, 1693–1705. [Google Scholar] [CrossRef]
- Mao, Y.; Zhuang, Z.; Chen, Y.; Zhang, X.; Shen, Y.; Lin, G.; Wu, R. Imaging of glutamate in acute traumatic brain injury using chemical exchange saturation transfer. Quant. Imaging Med. Surg. 2019, 9, 1652–1663. [Google Scholar] [CrossRef] [PubMed]
- Yager, J.Y. Animal Models of Neurodevelopmental Disorders; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Muir, K.W. Glutamate-based therapeutic approaches: Clinical trials with NMDA antagonists. Curr. Opin. Pharmacol. 2006, 6, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; Viña, J.R. How glutamate is managed by the blood–brain barrier. Biology 2016, 5, 37. [Google Scholar] [CrossRef]
- Bai, W.; Zhou, Y.G. Homeostasis of the Intraparenchymal-Blood Glutamate Concentration Gradient: Maintenance, Imbalance, and Regulation. Front. Mol. Neurosci. 2017, 10, 400. [Google Scholar] [CrossRef]
- Teichberg, V.I.; Cohen-Kashi-Malina, K.; Cooper, I.; Zlotnik, A. Homeostasis of glutamate in brain fluids: An accelerated brain-to-blood efflux of excess glutamate is produced by blood glutamate scavenging and offers protection from neuropathologies. Neuroscience 2009, 158, 301–308. [Google Scholar] [CrossRef]
- Haroon, E.; Miller, A.H. Inflammation effects on brain glutamate in depression: Mechanistic considerations and treatment implications. In Inflammation-Associated Depression: Evidence, Mechanisms and Implications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 173–198. [Google Scholar]
- Haroon, E.; Chen, X.; Li, Z.; Patel, T.; Woolwine, B.J.; Hu, X.P.; Felger, J.C.; Miller, A.H. Increased inflammation and brain glutamate define a subtype of depression with decreased regional homogeneity, impaired network integrity, and anhedonia. Transl. Psychiatry 2018, 8, 189. [Google Scholar] [CrossRef]
- King, S.; Jelen, L.A.; Horne, C.M.; Cleare, A.; Pariante, C.M.; Young, A.H.; Stone, J.M. Inflammation, Glutamate, and Cognition in Bipolar Disorder Type II: A Proof of Concept Study. Front. Psychiatry 2019, 10, 66. [Google Scholar] [CrossRef]
- Ho, T.C.; Teresi, G.I.; Segarra, J.R.; Ojha, A.; Walker, J.C.; Gu, M.; Spielman, D.M.; Sacchet, M.D.; Jiang, F.; Rosenberg-Hasson, Y.; et al. Higher Levels of Pro-inflammatory Cytokines Are Associated with Higher Levels of Glutamate in the Anterior Cingulate Cortex in Depressed Adolescents. Front. Psychiatry 2021, 12, 642976. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, J.; Dong, J.-f.; Shi, F.-D. Dissemination of brain inflammation in traumatic brain injury. Cell. Mol. Immunol. 2019, 16, 523–530. [Google Scholar] [CrossRef]
- Gasiorowska, A.; Wydrych, M.; Drapich, P.; Zadrozny, M.; Steczkowska, M.; Niewiadomski, W.; Niewiadomska, G. The biology and pathobiology of glutamatergic, cholinergic, and dopaminergic signaling in the aging brain. Front. Aging Neurosci. 2021, 391. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, F.; De Koninck, Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013, 2013, 429815. [Google Scholar] [CrossRef] [PubMed]
- Sasaki-Hamada, S.; Sanai, E.; Kanemaru, M.; Kamanaka, G.; Oka, J.-I. Long-term exposure to high glucose induces changes in the expression of AMPA receptor subunits and glutamate transmission in primary cultured cortical neurons. Biochem. Biophys. Res. Commun. 2022, 589, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Gruenbaum, B.F.; Shelef, I.; Severynovska, O.; Gal, R.; Dubilet, M.; Zlotnik, A.; Kofman, O.; Boyko, M. Blood glutamate scavenging with pyruvate as a novel preventative and therapeutic approach for depressive-like behavior following traumatic brain injury in a rat model. Front. Neurosci. 2022, 16, 83247821. [Google Scholar] [CrossRef] [PubMed]
- Folkersma, H.; Foster Dingley, J.C.; van Berckel, B.N.; Rozemuller, A.; Boellaard, R.; Huisman, M.C.; Lammertsma, A.A.; Vandertop, W.P.; Molthoff, C.F. Increased cerebral (R)-[11C] PK11195 uptake and glutamate release in a rat model of traumatic brain injury: A longitudinal pilot study. J. Neuroinflamm. 2011, 8, 67. [Google Scholar] [CrossRef]
- Stefani, M.A.; Modkovski, R.; Hansel, G.; Zimmer, E.R.; Kopczynski, A.; Muller, A.P.; Strogulski, N.R.; Rodolphi, M.S.; Carteri, R.K.; Schmidt, A.P. Elevated glutamate and lactate predict brain death after severe head trauma. Ann. Clin. Transl. Neurol. 2017, 4, 392–402. [Google Scholar] [CrossRef]
- Zhang, D.; Xiao, M.; Wang, L.; Jia, W. Blood-based glutamate scavengers reverse traumatic brain injury-induced synaptic plasticity disruption by decreasing glutamate level in hippocampus interstitial fluid, but not cerebral spinal fluid, in vivo. Neurotox. Res. 2019, 35, 360–372. [Google Scholar] [CrossRef]
- Dai, S.-S.; Zhou, Y.-G.; Li, W.; An, J.-H.; Li, P.; Yang, N.; Chen, X.-Y.; Xiong, R.-P.; Liu, P.; Zhao, Y. Local glutamate level dictates adenosine A2A receptor regulation of neuroinflammation and traumatic brain injury. J. Neurosci. 2010, 30, 5802–5810. [Google Scholar] [CrossRef]
- McConeghy, K.W.; Hatton, J.; Hughes, L.; Cook, A.M. A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury. CNS Drugs 2012, 26, 613–636. [Google Scholar] [CrossRef]
- Krishna, G.; Bromberg, C.; Connell, E.C.; Mian, E.; Hu, C.; Lifshitz, J.; Adelson, P.D.; Thomas, T.C. Traumatic Brain Injury-Induced Sex-Dependent Changes in Late-Onset Sensory Hypersensitivity and Glutamate Neurotransmission. Front. Neurol. 2020, 11, 749. [Google Scholar] [CrossRef]
- Selwyn, R.; Hockenbury, N.; Jaiswal, S.; Mathur, S.; Armstrong, R.C.; Byrnes, K.R. Mild traumatic brain injury results in depressed cerebral glucose uptake: An (18)FDG PET study. J. Neurotrauma 2013, 30, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Dorsett, C.R.; McGuire, J.L.; Niedzielko, T.L.; DePasquale, E.A.; Meller, J.; Floyd, C.L.; McCullumsmith, R.E. Traumatic Brain Injury Induces Alterations in Cortical Glutamate Uptake without a Reduction in Glutamate Transporter-1 Protein Expression. J. Neurotrauma 2017, 34, 220–234. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, D.A.; Nicholas, M.A.; Lajud, N.; Kline, A.E.; Bondi, C.O. Preclinical Models of Traumatic Brain Injury: Emerging Role of Glutamate in the Pathophysiology of Depression. Front. Pharmacol. 2018, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Vornov, J.J.; Wozniak, K.; Lu, M.; Jackson, P.; Tsukamoto, T.; Wang, E.; Slusher, B. Blockade of NAALADase: A novel neuroprotective strategy based on limiting glutamate and elevating NAAG. Ann. N. Y. Acad. Sci. 1999, 890, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Chung, S.-Y.; Lin, M.-C.; Cheng, F.-C. Effects of magnesium sulfate on energy metabolites and glutamate in the cortex during focal cerebral ischemia and reperfusion in the gerbil monitored by a dual-probe microdialysis technique. Life Sci. 2002, 71, 803–811. [Google Scholar] [CrossRef]
- Han, J.-l.; Blank, T.; Schwab, S.; Kollmar, R. Corrigendum to “Inhibited glutamate release by granulocyte-colony stimulating factor after experimental stroke”[Neurosci. Lett. 432 (2008) 167–169]. Neurosci. Lett. 2009, 3, 258. [Google Scholar] [CrossRef]
- Hurtado, O.; De Cristobal, J.; Sanchez, V.; Lizasoain, I.; Cardenas, A.; Pereira, M.P.; Colado, M.I.; Leza, J.C.; Lorenzo, P.; Moro, M.A. Inhibition of glutamate release by delaying ATP fall accounts for neuroprotective effects of antioxidants in experimental stroke. FASEB J. 2003, 17, 2082–2084. [Google Scholar] [CrossRef]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef]
- Hodgson, C.L.; Higgins, A.M.; Bailey, M.; Barrett, J.; Bellomo, R.; Cooper, D.J.; Gabbe, B.J.; Iwashyna, T.; Linke, N.; Myles, P.S.; et al. Comparison of 6-month outcomes of sepsis versus non-sepsis critically ill patients receiving mechanical ventilation. Crit. Care 2022, 26, 174. [Google Scholar] [CrossRef]
- Quinn, C.M.; Kasibante, J.; Namudde, A.; Bangdiwala, A.S.; Kabahubya, M.; Nakasujja, N.; Lofgren, S.; Elliott, A.; Boulware, D.R.; Meya, D.B.; et al. Neurocognitive outcomes of tuberculous meningitis in a primarily HIV-positive Ugandan cohort. Wellcome Open Res. 2021, 6, 208. [Google Scholar] [CrossRef]
- Andersen, J.V.; Markussen, K.H.; Jakobsen, E.; Schousboe, A.; Waagepetersen, H.S.; Rosenberg, P.A.; Aldana, B.I. Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration. Neuropharmacology 2021, 196, 108719. [Google Scholar] [CrossRef]
- Zhong, C.; Talmage, D.A.; Role, L.W. Nicotine elicits prolonged calcium signaling along ventral hippocampal axons. PLoS ONE 2013, 8, e82719. [Google Scholar] [CrossRef] [PubMed]
- Zwanzger, P.; Zavorotnyy, M.; Gencheva, E.; Diemer, J.; Kugel, H.; Heindel, W.; Ruland, T.; Ohrmann, P.; Arolt, V.; Domschke, K.; et al. Acute shift in glutamate concentrations following experimentally induced panic with cholecystokinin tetrapeptide—A 3T-MRS study in healthy subjects. Neuropsychopharmacology 2013, 38, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Houtepen, L.C.; Schur, R.R.; Wijnen, J.P.; Boer, V.O.; Boks, M.P.; Kahn, R.S.; Joels, M.; Klomp, D.W.; Vinkers, C.H. Acute stress effects on GABA and glutamate levels in the prefrontal cortex: A 7T (1)H magnetic resonance spectroscopy study. Neuroimage Clin. 2017, 14, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Hasler, G.; van der Veen, J.W.; Grillon, C.; Drevets, W.C.; Shen, J. Effect of acute psychological stress on prefrontal GABA concentration determined by proton magnetic resonance spectroscopy. Am. J. Psychiatry 2010, 167, 1226–1231. [Google Scholar] [CrossRef]
- Zhang, J.M.; Wang, H.K.; Ye, C.Q.; Ge, W.; Chen, Y.; Jiang, Z.L.; Wu, C.P.; Poo, M.M.; Duan, S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 2003, 40, 971–982. [Google Scholar] [CrossRef]
- Chodobski, A.; Zink, B.J.; Szmydynger-Chodobska, J. Blood–brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res. 2011, 2, 492–516. [Google Scholar] [CrossRef]
- Toyota, S.; Graf, R.; Valentino, M.; Yoshimine, T.; Heiss, W.D. Malignant infarction in cats after prolonged middle cerebral artery occlusion: Glutamate elevation related to decrease of cerebral perfusion pressure. Stroke 2002, 33, 1383–1391. [Google Scholar] [CrossRef]
- Hone, E.; Hu, H.; Sprowls, S.; Farooqi, I.; Grasmick, K.; Lockman, P.; Simpkins, J.; Ren, X. Biphasic blood-brain barrier openings after stroke. Neurol. Disord. Stroke Int. 2018, 11. [Google Scholar] [CrossRef]
- Başkaya, M.K.; Rao, A.M.; Doğan, A.; Donaldson, D.; Dempsey, R.J. The biphasic opening of the blood–brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci. Lett. 1997, 226, 33–36. [Google Scholar] [CrossRef]
- Chamoun, R.; Suki, D.; Gopinath, S.P.; Goodman, J.C.; Robertson, C. Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J. Neurosurg. 2010, 113, 564–570. [Google Scholar] [CrossRef]
- Bullock, R.; Zauner, A.; Woodward, J.J.; Myseros, J.; Choi, S.C.; Ward, J.D.; Marmarou, A.; Young, H.F. Factors affecting excitatory amino acid release following severe human head injury. J. Neurosurg. 1998, 89, 507–518. [Google Scholar] [CrossRef]
- Vespa, P.; Prins, M.; Ronne-Engstrom, E.; Caron, M.; Shalmon, E.; Hovda, D.A.; Martin, N.A.; Becker, D.P. Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: A microdialysis study. J. Neurosurg. 1998, 89, 971–982. [Google Scholar] [CrossRef]
- Yu, M.; Wang, M.; Yang, D.; Wei, X.; Li, W. Dynamics of blood brain barrier permeability and tissue microstructure following controlled cortical impact injury in rat: A dynamic contrast-enhanced magnetic resonance imaging and diffusion kurtosis imaging study. Magn. Reson. Imaging 2019, 62, 1–9. [Google Scholar] [CrossRef]
- van Vliet, E.A.; Ndode-Ekane, X.E.; Lehto, L.J.; Gorter, J.A.; Andrade, P.; Aronica, E.; Grohn, O.; Pitkanen, A. Long-lasting blood-brain barrier dysfunction and neuroinflammation after traumatic brain injury. Neurobiol. Dis. 2020, 145, 105080. [Google Scholar] [CrossRef]
- Hay, J.R.; Johnson, V.E.; Young, A.M.; Smith, D.H.; Stewart, W. Blood-Brain Barrier Disruption Is an Early Event that May Persist for Many Years after Traumatic Brain Injury in Humans. J. Neuropathol. Exp. Neurol. 2015, 74, 1147–1157. [Google Scholar] [CrossRef]
- Cash, A.; Theus, M.H. Mechanisms of blood–brain barrier dysfunction in traumatic brain injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, H.; Guo, X.; Pluimer, B.; Zhao, Z. Blood–brain barrier dysfunction in mild traumatic brain injury: Evidence from preclinical murine models. Front. Physiol. 2020, 11, 1030. [Google Scholar] [CrossRef]
- Hawkins, R.A. The blood-brain barrier and glutamate. Am. J. Clin. Nutr. 2009, 90, 867S–874S. [Google Scholar] [CrossRef]
- Kobeissy, F.; Dixon, C.E.; Hayes, R.L.; Mondello, S. Injury Models of the Central Nervous System; Springer: Berlin/Heidelberg, Germany, 1940. [Google Scholar]
- Okada, T.; Suzuki, H.; Travis, Z.D.; Zhang, J.H. The stroke-induced blood-brain barrier disruption: Current progress of inspection technique, mechanism, and therapeutic target. Curr. Neuropharmacol. 2020, 18, 1187–1212. [Google Scholar]
- Lau, A.; Tymianski, M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Arch.-Eur. J. Physiol. 2010, 460, 525–542. [Google Scholar] [CrossRef]
- Al-Nasser, M.N.; Mellor, I.R.; Carter, W.G. Is L-Glutamate Toxic to Neurons and Thereby Contributes to Neuronal Loss and Neurodegeneration? A Systematic Review. Brain Sci. 2022, 12, 577. [Google Scholar] [CrossRef]
- Borbely, E.; Simon, M.; Fuchs, E.; Wiborg, O.; Czeh, B.; Helyes, Z. Novel drug developmental strategies for treatment-resistant depression. Br. J. Pharmacol. 2022, 179, 1146–1186. [Google Scholar] [CrossRef]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- Blaylock, R.L.; Faria, M. New concepts in the development of schizophrenia, autism spectrum disorders, and degenerative brain diseases based on chronic inflammation: A working hypothesis from continued advances in neuroscience research. Surg. Neurol. Int. 2021, 12, 556. [Google Scholar]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells. Front. Cell. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef]
- Simões, A.P.; Silva, C.G.; Marques, J.M.; Pochmann, D.; Porciúncula, L.O.; Ferreira, S.; Oses, J.P.; Beleza, R.O.; Real, J.I.; Köfalvi, A. Glutamate-induced and NMDA receptor-mediated neurodegeneration entails P2Y1 receptor activation. Cell Death Dis. 2018, 9, 297. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.L.; Babu, G.N. Cell death mechanisms in the early stages of acute glutamate neurotoxicity. Neurosci. Res. 2010, 66, 271–278. [Google Scholar] [CrossRef]
- Lewerenz, J.; Maher, P. Chronic glutamate toxicity in neurodegenerative diseases—What is the evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef]
- Meldrum, B.S. The role of glutamate in epilepsy and other CNS disorders. Neurology 1994, 44, S14–S23. [Google Scholar]
- Meldrum, B.; Garthwaite, J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol. Sci. 1990, 11, 379–387. [Google Scholar] [CrossRef]
- Li, J.; Zheng, M.; Shimoni, O.; Banks, W.A.; Bush, A.I.; Gamble, J.R.; Shi, B. Development of novel therapeutics targeting the blood–brain barrier: From barrier to carrier. Adv. Sci. 2021, 8, 2101090. [Google Scholar] [CrossRef]
- Barar, J.; Rafi, M.A.; Pourseif, M.M.; Omidi, Y. Blood-brain barrier transport machineries and targeted therapy of brain diseases. BioImpacts BI 2016, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.; Zubko, O.; Bradley, R.; Harper, E.; Pank, L.; O’brien, J.; Fox, C.; Tabet, N.; Livingston, G.; Bentham, P. Minocycline at 2 different dosages vs placebo for patients with mild Alzheimer disease: A randomized clinical trial. JAMA Neurol. 2020, 77, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.P.; Xie, Y.; Meng, X.Y.; Kang, J.S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct Target Ther. 2019, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Loza, M.I.; Mirelman, D.; Brea, J.; Blanco, M.; Sobrino, T.; Campos, F. A novel mechanism of neuroprotection: Blood glutamate grabber. J. Cereb. Blood Flow Metab. 2016, 36, 292–301. [Google Scholar] [CrossRef]
- Cederberg, H.H.; Uhd, N.C.; Brodin, B. Glutamate efflux at the blood-brain barrier: Cellular mechanisms and potential clinical relevance. Arch. Med. Res. 2014, 45, 639–645. [Google Scholar] [CrossRef]
- Li, Y.; Hou, X.; Qi, Q.; Wang, L.; Luo, L.; Yang, S.; Zhang, Y.; Miao, Z.; Zhang, Y.; Wang, F.; et al. Scavenging of blood glutamate for enhancing brain-to-blood glutamate efflux. Mol. Med. Rep. 2014, 9, 305–310. [Google Scholar] [CrossRef]
- Zlotnik, A.; Gurevich, B.; Cherniavsky, E.; Tkachov, S.; Matuzani-Ruban, A.; Leon, A.; Shapira, Y.; Teichberg, V.I. The contribution of the blood glutamate scavenging activity of pyruvate to its neuroprotective properties in a rat model of closed head injury. Neurochem. Res. 2008, 33, 1044–1050. [Google Scholar] [CrossRef]
- Boyko, M.; Melamed, I.; Gruenbaum, B.F.; Gruenbaum, S.E.; Ohayon, S.; Leibowitz, A.; Brotfain, E.; Shapira, Y.; Zlotnik, A. The effect of blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome in a rat model of subarachnoid hemorrhage. Neurotherapeutics 2012, 9, 649–657. [Google Scholar] [CrossRef]
- Perez-Mato, M.; Ramos-Cabrer, P.; Sobrino, T.; Blanco, M.; Ruban, A.; Mirelman, D.; Menendez, P.; Castillo, J.; Campos, F. Human recombinant glutamate oxaloacetate transaminase 1 (GOT1) supplemented with oxaloacetate induces a protective effect after cerebral ischemia. Cell Death Dis. 2014, 5, e992. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.; Sobrino, T.; Ramos-Cabrer, P.; Castellanos, M.; Blanco, M.; Rodriguez-Yanez, M.; Serena, J.; Leira, R.; Castillo, J. High blood glutamate oxaloacetate transaminase levels are associated with good functional outcome in acute ischemic stroke. J. Cereb. Blood Flow Metab. 2011, 31, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Kuts, R.; Tsenter, P.; Gruenbaum, B.F.; Grinshpun, Y.; Zvenigorodsky, V.; Shelef, I.; Natanel, D.; Brotfain, E.; Zlotnik, A. The effect of pyruvate on the development and progression of post-stroke depression: A new therapeutic approach. Neuropharmacology 2019, 155, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Dopico-Lopez, A.; Perez-Mato, M.; da Silva-Candal, A.; Iglesias-Rey, R.; Rabinkov, A.; Bugallo-Casal, A.; Sobrino, T.; Mirelman, D.; Castillo, J.; Campos, F. Inhibition of endogenous blood glutamate oxaloacetate transaminase enhances the ischemic damage. Transl. Res. 2021, 230, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Boyko, M.; Zlotnik, A.; Gruenbaum, B.F.; Gruenbaum, S.E.; Ohayon, S.; Kuts, R.; Melamed, I.; Regev, A.; Shapira, Y.; Teichberg, V.I. Pyruvate’s blood glutamate scavenging activity contributes to the spectrum of its neuroprotective mechanisms in a rat model of stroke. Eur. J. Neurosci. 2011, 34, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Briggs, Z.; Rink, C. Inducible glutamate oxaloacetate transaminase as a therapeutic target against ischemic stroke. Antioxid. Redox Signal. 2015, 22, 175–186. [Google Scholar] [CrossRef]
- Campos, F.; Sobrino, T.; Ramos-Cabrer, P.; Argibay, B.; Agulla, J.; Pérez-Mato, M.; Rodríguez-González, R.; Brea, D.; Castillo, J. Neuroprotection by glutamate oxaloacetate transaminase in ischemic stroke: An experimental study. J. Cereb. Blood Flow Metab. 2011, 31, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.; Lyons, K.; Khosla, S.; Nashatizadeh, M.; Pahwa, R. A pilot Study of oxaloacetate 100 mg capsules in Parkinson’s disease patients. J. Parkinsons Dis. Alzheimers Dis. 2016, 3, 4. [Google Scholar]
- Swerdlow, R.H.; Bothwell, R.; Hutfles, L.; Burns, J.M.; Reed, G.A. Tolerability and pharmacokinetics of oxaloacetate 100 mg capsules in Alzheimer’s subjects. BBA Clin. 2016, 5, 120–123. [Google Scholar] [CrossRef]
- Koh-Banerjee, P.K.; Ferreira, M.P.; Greenwood, M.; Bowden, R.G.; Cowan, P.N.; Almada, A.; Kreider, R.B. Effects of calcium pyruvate supplementation during training on body composition, exercise capacity, and metabolic responses to exercise. Nutrition 2005, 21, 312–319. [Google Scholar] [CrossRef]
- Stone, M.H.; Sanborn, K.; Smith, L.L.; O’Bryant, H.S.; Hoke, T.; Utter, A.C.; Johnson, R.L.; Boros, R.; Hruby, J.; Pierce, K.C. Effects of in-season (5 weeks) creatine and pyruvate supplementation on anaerobic performance and body composition in American football players. Int. J. Sport Nutr. Exerc. Metab. 1999, 9, 146–165. [Google Scholar] [CrossRef] [PubMed]
- Stanko, R.T.; Reynolds, H.R.; Lonchar, K.D.; Arch, J.E. Plasma lipid concentrations in hyperlipidemic patients consuming a high-fat diet supplemented with pyruvate for 6 wk. Am. J. Clin. Nutr. 1992, 56, 950–954. [Google Scholar] [CrossRef]
- Morrison, M.A.; Spriet, L.L.; Dyck, D.J. Pyruvate ingestion for 7 days does not improve aerobic performance in well-trained individuals. J. Appl. Physiol. 2000, 89, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Stanko, R.T.; Reynolds, H.R.; Hoyson, R.; Janosky, J.E.; Wolf, R. Pyruvate supplementation of a low-cholesterol, low-fat diet: Effects on plasma lipid concentrations and body composition in hyperlipidemic patients. Am. J. Clin. Nutr. 1994, 59, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Ahmetovic, Z. The effect of 4 weeks treatment with a 2-gram daily dose of pyruvate on body composition in healthy trained men. Int. J. Vitam. Nutr. Res. 2009, 79, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Metzger, J.; Lautmann, K.; Shushakov, V.; Purpura, M.; Geiss, K.-R.; Maassen, N. The effects of creatine pyruvate and creatine citrate on performance during high intensity exercise. J. Int. Soc. Sports Nutr. 2008, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Kalman, D.; Colker, C.M.; Wilets, I.; Roufs, J.B.; Antonio, J. The effects of pyruvate supplementation on body composition in overweight individuals. Nutrition 1999, 15, 337–340. [Google Scholar] [CrossRef]
- Tully, L.; Humiston, J.; Cash, A. Oxaloacetate reduces emotional symptoms in premenstrual syndrome (PMS): Results of a placebo-controlled, cross-over clinical trial. Obstet. Gynecol. Sci. 2020, 63, 195–204. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Bhattacharya, M.; Lee, S.-S. MicroRNAs: Possible regulatory molecular switch controlling the BBB microenvironment. Mol. Ther.-Nucleic Acids 2020, 19, 933–936. [Google Scholar] [CrossRef]
- Zhu, M.-x.; Lu, C.; Xia, C.-m.; Qiao, Z.-w.; Zhu, D.-n. Simvastatin pretreatment protects cerebrum from neuronal injury by decreasing the expressions of phosphor-CaMK II and AQP4 in ischemic stroke rats. J. Mol. Neurosci. 2014, 54, 591–601. [Google Scholar] [CrossRef]
- Coureuil, M.; Lécuyer, H.; Bourdoulous, S.; Nassif, X. A journey into the brain: Insight into how bacterial pathogens cross blood–brain barriers. Nat. Rev. Microbiol. 2017, 15, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Kooij, G.; Van Horssen, J.; Bandaru, V.V.R.; Haughey, N.J.; De Vries, H.E. The role of ATP-binding cassette transporters in neuro-inflammation: Relevance for bioactive lipids. Front. Pharmacol. 2012, 3, 74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, L.; An, C.; Wang, R.; Yang, L.; Yu, W.; Li, P.; Gao, Y. The blood brain barrier in cerebral ischemic injury—Disruption and repair. Brain Hemorrhages 2020, 1, 34–53. [Google Scholar] [CrossRef]
- Cui, X.; Chopp, M.; Zacharek, A.; Roberts, C.; Lu, M.; Savant-Bhonsale, S.; Chen, J. Chemokine, vascular and therapeutic effects of combination Simvastatin and BMSC treatment of stroke. Neurobiol. Dis. 2009, 36, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Dore-Duffy, P. Pericytes: Pluripotent cells of the blood brain barrier. Curr. Pharm. Des. 2008, 14, 1581–1593. [Google Scholar] [CrossRef]
- Liu, S.; Agalliu, D.; Yu, C.; Fisher, M. The role of pericytes in blood-brain barrier function and stroke. Curr. Pharm. Des. 2012, 18, 3653–3662. [Google Scholar] [CrossRef]
- Hayakawa, K.; Pham, L.-D.D.; Katusic, Z.S.; Arai, K.; Lo, E.H. Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc. Natl. Acad. Sci. USA 2012, 109, 7505–7510. [Google Scholar] [CrossRef]
- Li, J.; Tang, Y.; Wang, Y.; Tang, R.; Jiang, W.; Yang, G.-Y.; Gao, W.-Q. Neurovascular recovery via cotransplanted neural and vascular progenitors leads to improved functional restoration after ischemic stroke in rats. Stem Cell Rep. 2014, 3, 101–114. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, J.; Lin, X.; Wang, L.; Shao, B.; Jin, K.; Wang, Y.; Yang, G.-Y. Neural stem cell protects aged rat brain from ischemia–reperfusion injury through neurogenesis and angiogenesis. J. Cereb. Blood Flow Metab. 2014, 34, 1138–1147. [Google Scholar] [CrossRef]
- Gliem, M.; Mausberg, A.K.; Lee, J.I.; Simiantonakis, I.; van Rooijen, N.; Hartung, H.P.; Jander, S. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann. Neurol. 2012, 71, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-f.; Sun, Y.-b.; Meng, Q.-h.; Li, S.-r.; Yao, W.-c.; Hu, G.-j.; Li, Z.-j.; Wang, R.-z. Recombinant adeno-associated virus serotype 1-vascular endothelial growth factor promotes neurogenesis and neuromigration in the subventricular zone and rescues neuronal function in ischemic rats. Neurosurgery 2009, 65, 771–779. [Google Scholar] [CrossRef]
- Candelario-Jalil, E. Nimesulide as a promising neuroprotectant in brain ischemia: New experimental evidences. Pharmacol. Res. 2008, 57, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-H.; Khoutorova, L.; Bazan, N.G.; Belayev, L. Docosahexaenoic acid improves behavior and attenuates blood–brain barrier injury induced by focal cerebral ischemia in rats. Exp. Transl. Stroke Med. 2015, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Dempsey, R.J.; Flemmer, A.; Forbush, B.; Sun, D. Inhibition of Na+–K+–Cl− cotransporter during focal cerebral ischemia decreases edema and neuronal damage. Brain Res. 2003, 961, 22–31. [Google Scholar] [CrossRef]
- Brenneman, M.; Sharma, S.; Harting, M.; Strong, R.; Cox, C.S., Jr.; Aronowski, J.; Grotta, J.C.; Savitz, S.I. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J. Cereb. Blood Flow Metab. 2010, 30, 140–149. [Google Scholar] [CrossRef]
- Friedrich, M.A.; Martins, M.P.; Araújo, M.D.; Klamt, C.; Vedolin, L.; Garicochea, B.; Raupp, E.F.; Ammar, J.S.E.; Machado, D.C.; Da Costa, J.C. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant. 2012, 21, 13–21. [Google Scholar] [CrossRef]
- Won, S.; Lee, J.H.; Wali, B.; Stein, D.G.; Sayeed, I. Progesterone attenuates hemorrhagic transformation after delayed tPA treatment in an experimental model of stroke in rats: Involvement of the VEGF–MMP pathway. J. Cereb. Blood Flow Metab. 2014, 34, 72–80. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, X.; Chen, W.; Wang, Z.; Xu, G.; Zeng, Y.; Ma, Y. TGF-β1 prevents blood–brain barrier damage and hemorrhagic transformation after thrombolysis in rats. Exp. Neurol. 2015, 266, 120–126. [Google Scholar] [CrossRef]
- Kuroki, T.; Tanaka, R.; Shimada, Y.; Yamashiro, K.; Ueno, Y.; Shimura, H.; Urabe, T.; Hattori, N. Exendin-4 inhibits matrix metalloproteinase-9 activation and reduces infarct growth after focal cerebral ischemia in hyperglycemic mice. Stroke 2016, 47, 1328–1335. [Google Scholar] [CrossRef]
- Sarkar, S.; Mukherjee, A.; Das, N.; Swarnakar, S. Protective roles of nanomelatonin in cerebral ischemia-reperfusion of aged brain: Matrixmetalloproteinases as regulators. Exp. Gerontol. 2017, 92, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gan, Y.; Sun, B.L.; Zhang, F.; Lu, B.; Gao, Y.; Liang, W.; Thomson, A.W.; Chen, J.; Hu, X. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann. Neurol. 2013, 74, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Frankowski, J.C.; DeMars, K.M.; Ahmad, A.S.; Hawkins, K.E.; Yang, C.; Leclerc, J.L.; Doré, S.; Candelario-Jalil, E. Detrimental role of the EP1 prostanoid receptor in blood-brain barrier damage following experimental ischemic stroke. Sci. Rep. 2015, 5, 17956. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.S.; Kozak, A.; Ergul, A.; Hess, D.C.; Borlongan, C.V.; Fagan, S.C. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Izzy, S.; Chen, P.M.; Tahir, Z.; Grashow, R.; Radmanesh, F.; Cote, D.J.; Yahya, T.; Dhand, A.; Taylor, H.; Shih, S.L. Association of traumatic brain injury with the risk of developing chronic cardiovascular, endocrine, neurological, and psychiatric disorders. JAMA Netw. Open 2022, 5, e229478. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruenbaum, B.F.; Zlotnik, A.; Fleidervish, I.; Frenkel, A.; Boyko, M. Glutamate Neurotoxicity and Destruction of the Blood–Brain Barrier: Key Pathways for the Development of Neuropsychiatric Consequences of TBI and Their Potential Treatment Strategies. Int. J. Mol. Sci. 2022, 23, 9628. https://doi.org/10.3390/ijms23179628
Gruenbaum BF, Zlotnik A, Fleidervish I, Frenkel A, Boyko M. Glutamate Neurotoxicity and Destruction of the Blood–Brain Barrier: Key Pathways for the Development of Neuropsychiatric Consequences of TBI and Their Potential Treatment Strategies. International Journal of Molecular Sciences. 2022; 23(17):9628. https://doi.org/10.3390/ijms23179628
Chicago/Turabian StyleGruenbaum, Benjamin F., Alexander Zlotnik, Ilya Fleidervish, Amit Frenkel, and Matthew Boyko. 2022. "Glutamate Neurotoxicity and Destruction of the Blood–Brain Barrier: Key Pathways for the Development of Neuropsychiatric Consequences of TBI and Their Potential Treatment Strategies" International Journal of Molecular Sciences 23, no. 17: 9628. https://doi.org/10.3390/ijms23179628
APA StyleGruenbaum, B. F., Zlotnik, A., Fleidervish, I., Frenkel, A., & Boyko, M. (2022). Glutamate Neurotoxicity and Destruction of the Blood–Brain Barrier: Key Pathways for the Development of Neuropsychiatric Consequences of TBI and Their Potential Treatment Strategies. International Journal of Molecular Sciences, 23(17), 9628. https://doi.org/10.3390/ijms23179628






