Template Imprinting Versus Porogen Imprinting of Small Molecules: A Review of Molecularly Imprinted Polymers in Gas Sensing
Abstract
:1. Introduction
2. Molecular Imprinting of Volatile Molecules
3. Porogen Imprinting
3.1. Selectivity of MIPs Produced by Porogen Imprinting
3.2. Sensitivity of MIPs Produced by Porogen Imprinting
3.3. Small Molecule Porogen Imprinting
3.4. Conclusions from Porogen Imprinting
4. Template Imprinting
4.1. Selectivity of MIPs Produced by Template Imprinting
4.2. Sensitivity of MIPs Produced by Template Imprinting
4.3. Small Molecule Template Imprinting
4.4. Conclusions from Template Imprinting
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mitsubayashi, K.; Toma, K.; Iitani, K.; Arakawa, T. Gas-Phase Biosensors: A Review. Sens. Actuators B Chem. 2022, 367, 132053. [Google Scholar] [CrossRef]
- Gaggiotti, S.; della Pelle, F.; Mascini, M.; Cichelli, A.; Compagnone, D. Peptides, DNA and MIPs in Gas Sensing. From the Realization of the Sensors to Sample Analysis. Sensors 2020, 20, 4433. [Google Scholar] [CrossRef] [PubMed]
- McGinn, C.K.; Lamport, Z.A.; Kymissis, I. Review of Gravimetric Sensing of Volatile Organic Compounds. ACS Sens. 2020, 5, 1514–1534. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Hawari, H.F.; Samsudin, N.M.; Md Shakaff, A.Y.; Ghani, S.A.; Ahmad, M.N.; Wahab, Y.; Hashim, U. Development of Interdigitated Electrode Molecular Imprinted Polymer Sensor for Monitoring Alpha Pinene Emissions from Mango Fruit. Procedia Eng. 2013, 53, 197–202. [Google Scholar] [CrossRef]
- Cengiz, N.; Guclu, G.; Kelebek, H.; Capanoglu, E.; Selli, S. Application of Molecularly Imprinted Polymers for the Detection of Volatile and Off-Odor Compounds in Food Matrices. ACS Omega 2022, 7, 15258–15266. [Google Scholar] [CrossRef] [PubMed]
- Köse, K.; Yalçın Kehribar, D.; Uzun, L. Molecularly Imprinted Polymers in Toxicology: A Literature Survey for the Last 5 Years. Environ. Sci. Pollut. Res. 2021, 28, 35437–35471. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Ahmadifard, M. Novel Molecularly-Imprinted Solid-Phase Microextraction Fiber Coupled with Gas Chromatography for Analysis of Furan. Talanta 2016, 150, 148–154. [Google Scholar] [CrossRef]
- Bunte, G.; Hürttlen, J.; Pontius, H.; Hartlieb, K.; Krause, H. Gas Phase Detection of Explosives Such as 2,4,6-Trinitrotoluene by Molecularly Imprinted Polymers. Anal. Chim. Acta 2007, 591, 49–56. [Google Scholar] [CrossRef]
- Walker, N.R.; Linman, M.J.; Timmers, M.M.; Dean, S.L.; Burkett, C.M.; Lloyd, J.A.; Keelor, J.D.; Baughman, B.M.; Edmiston, P.L. Selective Detection of Gas-Phase TNT by Integrated Optical Waveguide Spectrometry Using Molecularly Imprinted Sol–Gel Sensing Films. Anal. Chim. Acta 2007, 593, 82–91. [Google Scholar] [CrossRef]
- Koudehi, M.F.; Pourmortazavi, S.M.; Zibaseresht, R.; Mirsadeghi, S. MEMS-Based PVA/PPy/MIP Polymeric-Nanofiber Sensor Fabricated by LIFT-OFF Process for Detection 2,4-Dinitrotoluene Vapor. IEEE Sens. J. 2021, 21, 9492–9499. [Google Scholar] [CrossRef]
- Emam, S.; Nasrollahpour, M.; Colarusso, B.; Cai, X.; Grant, S.; Kulkarni, P.; Ekenseair, A.; Gharagouzloo, C.; Ferris, C.F.; Sun, N.-X. Detection of Presymptomatic Alzheimer’s Disease through Breath Biomarkers. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12088. [Google Scholar] [CrossRef]
- Hamidi, S.; Alipour-Ghorbani, N.; Hamidi, A. Solid Phase Microextraction Techniques in Determination of Biomarkers. Crit. Rev. Anal. Chem. 2018, 48, 239–251. [Google Scholar] [CrossRef]
- Hallil, H.; Aouled, N.O.; Plano, B.; Delepee, R.; Agrofoglio, L.; Dejous, C.; Rebiere, D. SH-SAW Sensing System Based on Thin Film Molecularly Imprinted Polymer: Study of Volatile Organic Compounds Adsorption. In Proceedings of the 28th Symposium on Microelectronics Technology and Devices (SBMicro 2013), Curitiba, Brazil, 2–6 September 2013; pp. 1–4. [Google Scholar]
- Lieberzeit, P.A.; Dickert, F.L. Sensor Technology and Its Application in Environmental Analysis. Anal. Bioanal. Chem. 2007, 387, 237–247. [Google Scholar] [CrossRef]
- Chul Yang, J.; Won Hong, S.; Jeon, S.; Ik Park, W.; Byun, M.; Park, J. Molecular Imprinting of Hemispherical Pore-Structured Thin Films via Colloidal Lithography for Gaseous Formaldehyde Gravimetric Sensing. Appl. Surf. Sci. 2021, 570, 151161. [Google Scholar] [CrossRef]
- Debliquy, M.; Krumpmann, A.; Lahem, D.; Tang, X.; Raskin, J.-P. Chemical Sensors for VOC Detection in Indoor Air: Focus on Formaldehyde. In NATO Advanced Research Workshop on Nanoscale Materials for Warfare Agent Detection; Springer: Dordrecht, The Netherlands, 2019; pp. 47–70. [Google Scholar]
- Hiller, T.; Li, L.L.; Holthoff, E.L.; Bamieh, B.; Turner, K.L. System Identification, Design, and Implementation of Amplitude Feedback Control on a Nonlinear Parametric MEM Resonator for Trace Nerve Agent Sensing. J. Microelectromech. Syst. 2015, 24, 1275–1284. [Google Scholar] [CrossRef]
- Edmiston, P.L.; Campbell, D.P.; Gottfried, D.S.; Baughman, J.; Timmers, M.M. Detection of Vapor Phase Trinitrotoluene in the Parts-per-Trillion Range Using Waveguide Interferometry. Sens. Actuators B Chem. 2010, 143, 574–582. [Google Scholar] [CrossRef]
- Adams, J.D.; Emam, S.; Sun, N.; Ma, Y.; Wang, Q.; Shashidhar, R.; Sun, N.-X. A Molecularly Imprinted Polymer-Graphene Sensor Antenna Hybrid for Ultra Sensitive Chemical Detection. IEEE Sens. J. 2019, 19, 6571–6577. [Google Scholar] [CrossRef]
- Hussain, M.; Kotova, K.; Lieberzeit, P. Molecularly Imprinted Polymer Nanoparticles for Formaldehyde Sensing with QCM. Sensors 2016, 16, 1011. [Google Scholar] [CrossRef]
- Rong, Q.; Zhang, Y.; Wang, C.; Zhu, Z.; Zhang, J.; Liu, Q. A High Selective Methanol Gas Sensor Based on Molecular Imprinted Ag-LaFeO3 Fibers. Sci. Rep. 2017, 7, 12110. [Google Scholar] [CrossRef] [Green Version]
- Imahashi, M.; Hayashi, K. Concentrating Materials Covered by Molecular Imprinted Nanofiltration Layer with Reconfigurability Prepared by a Surface Sol–Gel Process for Gas-Selective Detection. J. Colloid Interface Sci. 2013, 406, 186–195. [Google Scholar] [CrossRef]
- Yoshizako, K.; Hosoya, K.; Iwakoshi, Y.; Kimata, K.; Tanaka, N. Porogen Imprinting Effects. Anal. Chem. 1998, 70, 386–389. [Google Scholar] [CrossRef]
- Tsuchiizu, A.; Hasegawa, T.; Katsumoto, Y. Water Sorption on a Thin Film of Stereocontrolled Poly(N-Ethylacrylamide) and Poly(N,N-Diethylacrylamide). MATEC Web Conf. 2013, 4, 03001. [Google Scholar] [CrossRef]
- Tominaga, Y.; Kubo, T.; Yasuda, K.; Kato, K.; Hosoya, K. Development of Molecularly Imprinted Porous Polymers for Selective Adsorption of Gaseous Compounds. Microporous Mesoporous Mater. 2012, 156, 161–165. [Google Scholar] [CrossRef]
- Chaterjee, S.; Krupadam, R.J. Amino Acid-Imprinted Polymers as Highly Selective CO2 Capture Materials. Environ. Chem. Lett. 2019, 17, 465–472. [Google Scholar] [CrossRef]
- He, H.; Zhuang, L.; Chen, S.; Liu, H. Solid Amine Adsorbent Prepared by Molecular Imprinting and Its Carbon Dioxide Adsorption Properties. Chem.—Asian J. 2016, 11, 3055–3061. [Google Scholar] [CrossRef]
- Nabavi, S.A.; Vladisavljević, G.T.; Wicaksono, A.; Georgiadou, S.; Manović, V. Production of Molecularly Imprinted Polymer Particles with Amide-Decorated Cavities for CO2 Capture Using Membrane Emulsification/Suspension Polymerisation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 521, 231–238. [Google Scholar] [CrossRef]
- Nabavi, S.A.; Vladisavljević, G.T.; Eguagie, E.M.; Li, B.; Georgiadou, S.; Manović, V. Production of Spherical Mesoporous Molecularly Imprinted Polymer Particles Containing Tunable Amine Decorated Nanocavities with CO2 Molecule Recognition Properties. Chem. Eng. J. 2016, 306, 214–225. [Google Scholar] [CrossRef]
- Nabavi, S.A.; Vladisavljević, G.T.; Zhu, Y.; Manović, V. Synthesis of Size-Tunable CO2-Philic Imprinted Polymeric Particles (MIPs) for Low-Pressure CO2 Capture Using Oil-in-Oil Suspension Polymerization. Environ. Sci. Technol. 2017, 51, 11476–11483. [Google Scholar] [CrossRef]
- Liu, F.; Kuang, Y.; Wang, S.; Chen, S.; Fu, W. Preparation and Characterization of Molecularly Imprinted Solid Amine Adsorbent for CO2 Adsorption. New J. Chem. 2018, 42, 10016–10023. [Google Scholar] [CrossRef]
- Park, J.; Cho, S.Y.; Jung, M.; Lee, K.; Nah, Y.-C.; Attia, N.F.; Oh, H. Efficient Synthetic Approach for Nanoporous Adsorbents Capable of Pre- and Post-Combustion CO2 Capture and Selective Gas Separation. J. CO2 Util. 2021, 45, 101404. [Google Scholar] [CrossRef]
- Li, C.; Lv, M.; Zuo, J.; Huang, X. SnO2 Highly Sensitive CO Gas Sensor Based on Quasi-Molecular-Imprinting Mechanism Design. Sensors 2015, 15, 3789–3800. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H.; Hao, S. Synthesis of Molecularly Imprinted Polymers and Adsorption of NO2 in Flue Gas. Ind. Eng. Chem. Res. 2017, 56, 9116–9123. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, R. Highly Selective Separation of H2S and CO2 Using a H2S-Imprinted Polymers Loaded on a Polyoxometalate@Zr-Based Metal–Organic Framework with a Core–Shell Structure at Ambient Temperature. J. Mater. Chem. A 2019, 7, 12105–12114. [Google Scholar] [CrossRef]
- Ge, L.; Ye, X.; Yu, Z.; Chen, B.; Liu, C.; Guo, H.; Zhang, S.; Sassa, F.; Hayashi, K. A Fully Inkjet-Printed Disposable Gas Sensor Matrix with Molecularly Imprinted Gas-Selective Materials. Npj Flex. Electron. 2022, 6, 40. [Google Scholar] [CrossRef]
- Jha, S.K.; Liu, C.; Hayashi, K. Molecular Imprinted Polyacrylic Acids Based QCM Sensor Array for Recognition of Organic Acids in Body Odor. Sens. Actuators B Chem. 2014, 204, 74–87. [Google Scholar] [CrossRef]
- Han, Z.; Xu, Y.; Tian, H.; Liang, J.; Sun, D. Enhanced Ammonia Adsorption and Separation by a Molecularly Imprinted Polymer after Acid Hydrolysis of Its Ester Crosslinker. J. Hazard. Mater. 2021, 412, 125145. [Google Scholar] [CrossRef]
- Jahangiri-Manesh, A.; Mousazadeh, M.; Nikkhah, M. Fabrication of Chemiresistive Nanosensor Using Molecularly Imprinted Polymers for Acetone Detection in Gaseous State. Iran. Polym. J. 2022, 31, 883–891. [Google Scholar] [CrossRef]
- Janfaza, S.; Kim, E.; O’Brien, A.; Najjaran, H.; Nikkhah, M.; Alizadeh, T.; Hoorfar, M. A Nanostructured Microfluidic Artificial Olfaction for Organic Vapors Recognition. Sci. Rep. 2019, 9, 19051. [Google Scholar] [CrossRef]
- González Vila, Á.; Debliquy, M.; Lahem, D.; Mégret, P.; Caucheteur, C. Formaldehyde Sensing with Plasmonic Near-Infrared Optical Fiber Grating Sensors. In Optical Sensing and Detection IV; Berghmans, F., Mignani, A.G., Eds.; SPIE: Brussels, Belgium, 2016; p. 989917. [Google Scholar]
- Feng, L.; Liu, Y.; Zhou, X.; Hu, J. The Fabrication and Characterization of a Formaldehyde Odor Sensor Using Molecularly Imprinted Polymers. J. Colloid Interface Sci. 2005, 284, 378–382. [Google Scholar] [CrossRef]
- Afzal, A.; Feroz, S.; Iqbal, N.; Mujahid, A.; Rehman, A. A Collaborative Effect of Imprinted Polymers and Au Nanoparticles on Bioanalogous Detection of Organic Vapors. Sens. Actuators B Chem. 2016, 231, 431–439. [Google Scholar] [CrossRef]
- Antwi-Boampong, S.; Peng, J.S.; Carlan, J.; BelBruno, J.J. A Molecularly Imprinted Fluoral-P/Polyaniline Double Layer Sensor System for Selective Sensing of Formaldehyde. IEEE Sens. J. 2014, 14, 1490–1498. [Google Scholar] [CrossRef]
- González-Vila, Á.; Debliquy, M.; Lahem, D.; Zhang, C.; Mégret, P.; Caucheteur, C. Molecularly Imprinted Electropolymerization on a Metal-Coated Optical Fiber for Gas Sensing Applications. Sens. Actuators B Chem. 2017, 244, 1145–1151. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Zhu, Z.; Liu, Q. A Sensor Device with Specific Recognition Sites for Formaldehyde Based on Molecular Imprinting Technique. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012116. [Google Scholar] [CrossRef]
- Debliquy, M.; Dony, N.; Lahem, D.; Tang, X.; Zhang, C.; Raskin, J.-P.; Olivier, M.-G. Acetaldehyde Chemical Sensor Based on Molecularly Imprinted Polypyrrole. Procedia Eng. 2016, 168, 569–573. [Google Scholar] [CrossRef]
- Mirmohseni, A.; Rastgouy-Houjaghan, M. Application of Nanobalance Technique and Principal Component Analysis for Detection of the Soil Fumigant Telone Residues in the Air. J. Environ. Sci. Health Part B 2012, 47, 677–686. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, Y.M.; Zhang, J.; Zhu, Z.Q.; Liu, Q.J. A New and High Response Gas Sensor for Methanol Using Molecularly Imprinted Technique. Sens. Actuators B Chem. 2015, 207, 398–403. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Q.; Zhang, Y.; Zhu, Z.; Liu, Q. Methanol Gas-Sensing Properties of SWCNT-MIP Composites. Nanoscale Res. Lett. 2016, 11, 522. [Google Scholar] [CrossRef]
- Huang, Z.; Ying, P.; Huang, L.; Xu, Q.; Hu, X.-Y. Molecularly Imprinted Polymer Functionalized Reduced Graphene Oxide: A New Platform for the Detection of Hydroxyl Radicals in the Atmosphere. Anal. Methods 2019, 11, 5126–5133. [Google Scholar] [CrossRef]
- Nickel, A.-M.L.; Seker, F.; Ziemer, B.P.; Ellis, A.B. Imprinted Poly(Acrylic Acid) Films on Cadmium Selenide. A Composite Sensor Structure That Couples Selective Amine Binding with Semiconductor Substrate Photoluminescence. Chem. Mater. 2001, 13, 1391–1397. [Google Scholar] [CrossRef]
- Han, Z.; Xu, Y.; Wang, H.; Tian, H.; Qiu, B.; Sun, D. Synthesis of Ammonia Molecularly Imprinted Adsorbents and Ammonia Adsorption Separation during Sludge Aerobic Composting. Bioresour. Technol. 2020, 300, 122670. [Google Scholar] [CrossRef]
- Aznar-Gadea, E.; Sanchez-Alarcon, I.; Soosaimanickam, A.; Rodriguez-Canto, P.J.; Perez-Pla, F.; Martínez-Pastor, J.P.; Abargues, R. Molecularly Imprinted Nanocomposites of CsPbBr3 Nanocrystals: An Approach towards Fast and Selective Gas Sensing of Explosive Taggants. J. Mater. Chem. C 2022, 10, 1754–1766. [Google Scholar] [CrossRef]
- Sakale, G.; Knite, M.; Teteris, V.; Tupureina, V.; Stepina, S.; Liepa, E. The Investigation of Sensing Mechanism of Ethanol Vapour in Polymer-Nanostructured Carbon Composite. Open Phys. 2011, 9, 307–312. [Google Scholar] [CrossRef]
- Alizadeh, T.; Rezaloo, F. A New Chemiresistor Sensor Based on a Blend of Carbon Nanotube, Nano-Sized Molecularly Imprinted Polymer and Poly Methyl Methacrylate for the Selective and Sensitive Determination of Ethanol Vapor. Sens. Actuators B Chem. 2013, 176, 28–37. [Google Scholar] [CrossRef]
- Shevchenko, N.; Pankova, G.; Laishevkina, S.; Iakobson, O.; Koshkin, A.; Shabsels, B. Core-Shell Polymer Particles Containing Derivatives of 1,3-Diphenyl-β-Diketonate Boron Difluoride: Synthesis and Spectroscopic Investigation of Toluene Vapor Sorption. Colloids Surf. A Physicochem. Eng. Asp. 2019, 562, 310–320. [Google Scholar] [CrossRef]
- Moskalenko, Y.E.; Shevchenko, N.N.; Mokeev, M.V.; Men’shikova, A.Y.; Yakimanskii, A.V.; Gribanov, A.V. Solid-State 13C NMR Spectroscopic Examination of Lower Alcohol Vapor Sorption by Cross-Linked Poly(Methyl Methacrylate) Particles. Rus. J. Appl. Chem. 2010, 83, 400–405. [Google Scholar] [CrossRef]
- Men’shikova, A.Y.; Shevchenko, N.N.; Evseeva, T.G.; Koshkin, A.V.; Pankova, G.A.; Shabsel’s, B.M.; Faraonova, V.V.; Goikhman, M.Y.; Yakimanskii, A.V.; Sazhnikov, V.A.; et al. Crosslinked Monodisperse Particles Containing Luminophore Groups in Shells for Molecular Recognition of Lower Alcohols. Polym. Sci. Ser. B 2012, 54, 21–29. [Google Scholar] [CrossRef]
- Guney-Altay, O.; Pestov, D.; Tepper, G. Organic Zeolites from a Diolefinic Monomer. J. Am. Chem. Soc. 2007, 129, 13957–13962. [Google Scholar] [CrossRef]
- Dickert, F.L.; Forth, P.; Lieberzeit, P.; Tortschanoff, M. Molecular Imprinting in Chemical Sensing—Detection of Aromatic and Halogenated Hydrocarbons as Well as Polar Solvent Vapors. Fresenius’ J. Anal. Chem. 1998, 360, 759–762. [Google Scholar] [CrossRef]
- Dickert, F.L.; Greibl, W.; Sikorski, R.; Tortschanoff, M.; Weber, K.; Bulst, W.E.; Fischerauer, G. Chemical Microsensors with Molecularly Imprinted Sensitive Layers. In Chemical Microsensors and Applications; Buettgenbach, S., Ed.; SPIE: Boston, MA, USA, 1998; pp. 114–122. [Google Scholar]
- Dickert, F.L.; Thierer, S. Molecularly Imprinted Polymers for Optochemical Sensors. Adv. Mater. 1996, 8, 987–990. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Du, T.; Zhu, Z.; Zhang, J.; Liu, Q. A Gas Sensor Array for the Simultaneous Detection of Multiple VOCs. Sci. Rep. 2017, 7, 1960. [Google Scholar] [CrossRef] [PubMed]
- Rong, Q.; Zhang, Y.; Lv, T.; Shen, K.; Zi, B.; Zhu, Z.; Zhang, J.; Liu, Q. Highly Selective and Sensitive Methanol Gas Sensor Based on Molecular Imprinted Silver-Doped LaFeO3 Core–Shell and Cage Structures. Nanotechnology 2018, 29, 145503. [Google Scholar] [CrossRef] [PubMed]
- Rong, Q.; Zhang, Y.; Hu, J.; Li, K.; Wang, H.; Chen, M.; Lv, T.; Zhu, Z.; Zhang, J.; Liu, Q. Design of Ultrasensitive Ag-LaFeO3 Methanol Gas Sensor Based on Quasi Molecular Imprinting Technology. Sci. Rep. 2018, 8, 14220. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Raskin, J.-P.; Lahem, D.; Krumpmann, A.; Decroly, A.; Debliquy, M. A Formaldehyde Sensor Based on Molecularly-Imprinted Polymer on a TiO2 Nanotube Array. Sensors 2017, 17, 675. [Google Scholar] [CrossRef]
- Rong, Q.; Li, K.; Wang, C.; Zhang, Y.; Chen, M.; Zhu, Z.; Zhang, J.; Liu, Q. Enhanced Performance of an Acetone Gas Sensor Based on Ag-LaFeO3 Molecular Imprinted Polymers and Carbon Nanotubes Composite. Nanotechnology 2020, 31, 405701. [Google Scholar] [CrossRef]
- Azenha, M.; Schillinger, E.; Sanmartin, E.; Regueiras, M.T.; Silva, F.; Sellergren, B. Vapor-Phase Testing of the Memory-Effects in Benzene- and Toluene-Imprinted Polymers Conditioned at Elevated Temperature. Anal. Chim. Acta 2013, 802, 40–45. [Google Scholar] [CrossRef]
- Dickert, F.L.; Lieberzeit, P.A.; Achatz, P.; Palfinger, C.; Fassnauer, M.; Schmid, E.; Werther, W.; Horner, G. QCM Array for On-Line-Monitoring of Composting Procedures. Analyst 2004, 129, 432. [Google Scholar] [CrossRef]
- Lieberzeit, P.A.; Rehman, A.; Najafi, B.; Dickert, F.L. Real-Life Application of a QCM-Based e-Nose: Quantitative Characterization of Different Plant-Degradation Processes. Anal. Bioanal. Chem. 2008, 391, 2897–2903. [Google Scholar] [CrossRef]
- Dickert, F.; Lieberzeit, P.; Hayden, O.; Gazda-Miarecka, S.; Halikias, K.; Mann, K.; Palfinger, C. Chemical Sensors—From Molecules, Complex Mixtures to Cells—Supramolecular Imprinting Strategies. Sensors 2003, 3, 381–392. [Google Scholar] [CrossRef]
- Janfaza, S.; Banan Nojavani, M.; Nikkhah, M.; Alizadeh, T.; Esfandiar, A.; Ganjali, M.R. A Selective Chemiresistive Sensor for the Cancer-Related Volatile Organic Compound Hexanal by Using Molecularly Imprinted Polymers and Multiwalled Carbon Nanotubes. Microchim. Acta 2019, 186, 137. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Jahangiri-Manesh, A.; Nikkhah, M.; Abbasian, S.; Moshaii, A.; Masroor, M.J.; Norouzi, P. Detection of Hexanal Gas as a Volatile Organic Compound Cancer Biomarker Using a Nanocomposite of Gold Nanoparticles and Selective Polymers. J. Electroanal. Chem. 2022, 905, 115962. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Gu, S.; Wang, J. Detection of Hexanal in Humid Circumstances Using Hydrophobic Molecularly Imprinted Polymers Composite. Sens. Actuators B Chem. 2019, 291, 141–147. [Google Scholar] [CrossRef]
- Liu, C.; Wyszynski, B.; Yatabe, R.; Hayashi, K.; Toko, K. Molecularly Imprinted Sol-Gel-Based QCM Sensor Arrays for the Detection and Recognition of Volatile Aldehydes. Sensors 2017, 17, 382. [Google Scholar] [CrossRef]
- Jha, S.K.; Hayashi, K. A Quick Responding Quartz Crystal Microbalance Sensor Array Based on Molecular Imprinted Polyacrylic Acids Coating for Selective Identification of Aldehydes in Body Odor. Talanta 2015, 134, 105–119. [Google Scholar] [CrossRef]
- Jha, S.K.; Hayashi, K. Polyacrylic Acid Polymer and Aldehydes Template Molecule Based MIPs Coated QCM Sensors for Detection of Pattern Aldehydes in Body Odor. Sens. Actuators B Chem. 2015, 206, 471–487. [Google Scholar] [CrossRef]
- Guo, W.; Hu, F.; Liu, W.; Jiang, M.; Chen, Z.; Zhang, X.; Zhang, L.; Lin, S.; Wang, Y. Molecular Imprinted Polymer Modified Terahertz Metamaterial Sensor for Specific Detection of Gaseous Hexanal. Mater. Lett. 2022, 322, 132468. [Google Scholar] [CrossRef]
- Imahashi, M.; Watanabe, M.; Jha, S.; Hayashi, K. Olfaction-Inspired Sensing Using a Sensor System with Molecular Recognition and Optimal Classification Ability for Comprehensive Detection of Gases. Sensors 2014, 14, 5221–5238. [Google Scholar] [CrossRef]
- Shinohara, S.; Sassa, F.; Hayashi, K. Gas Selective Chemiresistor Composed of Molecularly Imprinted Polymer Composit Ink. In Proceedings of the 2016 IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar]
- Nakanishi, K.; Sassa, F.; Hayashi, K. Photo-Tunable Molecular Recognizing Smart Material for Gas Sensing. In Proceedings of the 2017 19th International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Kaohsiung, Taiwan, 18–22 June 2017; pp. 1376–1379. [Google Scholar]
- Shuanghong, W.; Sassa, F.; Hayashi, K. An Odor Visualization Film Based on Multi Colors Fluorescent Microbeads and Single Color Fluorescent Multi Microbeads. In Proceedings of the 2018 IEEE Sensors, New Delhi, India, 28–31 October 2018; pp. 1–4. [Google Scholar]
- Ge, L.; Chen, B.; Kawano, H.; Sassa, F.; Hayashi, K. Inkjet-Printed Gas Sensor Matrix with Molecularly Imprinted Gas Selective Materials. In Proceedings of the 2019 IEEE Sensors, Montreal, QC, Canada, 27–30 October 2019; pp. 1–3. [Google Scholar]
- Ge, L.; Sassa, F.; Hayashi, K. Flexible Gas Sensor Array Based on Matrix of Molecularly Imprinted Materials and Full Printing Process. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (Transducers & Eurosensors XXXIII), Berlin, Germany, 23–27 June 2019; pp. 765–768. [Google Scholar]
- Ying, X.; He, J.; Li, X. Molecularly Imprinted Electrospun Fiber Membrane for Colorimetric Detection of Hexanoic Acid. e-Polymers 2021, 21, 500–510. [Google Scholar] [CrossRef]
- Shang, L.; Liu, C.; Watanabe, M.; Chen, B.; Hayashi, K. LSPR Sensor Array Based on Molecularly Imprinted Sol-Gels for Pattern Recognition of Volatile Organic Acids. Sens. Actuators B Chem. 2017, 249, 14–21. [Google Scholar] [CrossRef]
- Imahashi, M.; Nakano, K.; Hayashi, K. Odor Sensor System Using Molecular Imprinting Filter. In Proceedings of the 2012 IEEE Sensors, Taipei, Taiwan, 28–31 October 2012; pp. 1–4. [Google Scholar]
- Imahashi, M.; Chiyomaru, Y.; Hayashi, K. Ultrathin Reconfigurable Molecular Filter for Gas-Selective Sensing. In Proceedings of the 2013 IEEE Sensors, Baltimore, MD, USA, 3–6 November 2013; pp. 1–4. [Google Scholar]
- Hwang, M.-J.; Shim, W.-G.; Yoon, S.-D.; Moon, H. Adsorption of Toxic Gases on Molecularly Imprinted Polymer Coated QCM: Measurements and Modeling for Partial Pressure in Gas Mixture. Adsorption 2019, 25, 825–832. [Google Scholar] [CrossRef]
- Zhu, B.; Guo, X.-Y.; Wang, X.-Y.; Lian, L.-L.; Zhang, H.; Gao, W.-X.; Tian, Y.-Y.; Zhang, X.-Y.; Lou, D.-W. Development of A Fiber-Packed In-Tube Extraction Device and Its Application in BTEX Analysis. Chin. J. Anal. Chem. 2019, 47, e19053–e19058. [Google Scholar] [CrossRef]
- Fu, Y.; Finklea, H.O. Quartz Crystal Microbalance Sensor for Organic Vapor Detection Based on Molecularly Imprinted Polymers. Anal. Chem. 2003, 75, 5387–5393. [Google Scholar] [CrossRef]
- Gavrila, A.M.; Iordache, T.V.; Lazau, C.; Rotariu, T.; Cernica, I.; Stroescu, H.; Stoica, M.; Orha, C.; Bandas, C.E.; Sarbu, A. Biomimetic Sensitive Elements for 2,4,6-Trinitrotoluene Tested on Multi-Layered Sensors. Coatings 2020, 10, 273. [Google Scholar] [CrossRef]
- Holthoff, E.L.; Stratis-Cullum, D.N.; Hankus, M.E. A Nanosensor for TNT Detection Based on Molecularly Imprinted Polymers and Surface Enhanced Raman Scattering. Sensors 2011, 11, 2700–2714. [Google Scholar] [CrossRef]
- Li, L.L.; Holthoff, E.L.; Shaw, L.A.; Burgner, C.B.; Turner, K.L. Noise Squeezing Controlled Parametric Bifurcation Tracking of MIP-Coated Microbeam MEMS Sensor for TNT Explosive Gas Sensing. J. Microelectromech. Syst. 2014, 23, 1228–1236. [Google Scholar] [CrossRef]
- Niu, Q.; Gao, K.; Lin, Z.; Wu, W. Surface Molecular-Imprinting Engineering of Novel Cellulose Nanofibril/Conjugated Polymer Film Sensors towards Highly Selective Recognition and Responsiveness of Nitroaromatic Vapors. Chem. Commun. 2013, 49, 9137. [Google Scholar] [CrossRef]
- Losev, V.V.; Medved’, A.V.; Roshchin, A.V.; Kryshtal’, R.G.; Zapadinskii, B.I.; Epinat’ev, I.D.; Kumpanenko, I.V. An Acoustic Study of the Selective Absorption of Vapors by Microporous Polymer Films. Rus. J. Phys. Chem. B 2009, 3, 990–1003. [Google Scholar] [CrossRef]
- Bunte, G.; Heil, M.; Röseling, D.; Hürttlen, J.; Pontius, H.; Krause, H. Trace Detection of Explosives Vapours by Molecularly Imprinted Polymers for Security Measures. Propellants Explos. Pyrotech. 2009, 34, 245–251. [Google Scholar] [CrossRef]
- Bunte, G.; Hürttlen, J.; Krause, H.; Pontius, H.; Schreiter, M.; Weber, J. Mip-Based Low-Cost Sensor for Short-Range Detection of Explosives. In Stand-Off Detection of Suicide Bombers and Mobile Subjects; Springer: Dordrecht, The Netherlands, 2006; pp. 17–27. [Google Scholar]
- Zhu, W.; Wang, C.; Wang, H.; Li, G. Theory and Simulation of Diffusion–Adsorption into a Molecularly Imprinted Mesoporous Film and Its Nanostructured Counterparts. Experimental Application for Trace Explosive Detection. RSC Adv. 2014, 4, 40676–40685. [Google Scholar] [CrossRef]
- Holthoff, E.L.; Li, L.; Hiller, T.; Turner, K.L. A Molecularly Imprinted Polymer (MIP)-Coated Microbeam MEMS Sensor for Chemical Detection. In Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XVI; Fountain, A.W., Ed.; SPIE: Baltimore, MD, USA, 2015; p. 94550W. [Google Scholar]
- Bloshenko, A.V.; Roshchin, A.V.; Kumpanenko, I.V.; Ivanova, N.A. An Analysis of Absorption-Desorption of Volatile Organic Compounds by Molecularly Imprinted Polymer Films. Rus. J. Phys. Chem. B 2011, 5, 332–344. [Google Scholar] [CrossRef]
- Losev, V.V.; Roshchin, A.V.; Epinat’ev, I.D.; Ivanova, N.A.; Kumpanenko, I.V. The Effect of Selective Absorption Sites in the Diffusion Processes in Polymer Films. Polym. Sci. Ser. A 2010, 52, 308–316. [Google Scholar] [CrossRef]
- Medina-Castillo, A.L.; Fernandez-Sanchez, J.F.; Segura-Carretero, A.; Fernandez-Gutierrez, A. A Semi-Empirical Model to Simplify the Synthesis of Homogeneous and Transparent Cross-Linked Polymers and Their Application in the Preparation of Optical Sensing Films. Biosens. Bioelectron. 2009, 25, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Dickert, F.L.; Lieberzeit, P.; Gazda-Miarecka, S.; Halikias, K.; Mann, K.-J. Modifying Polymers by Self-Organisation for the Mass-Sensitive Detection of Environmental and Biogeneous Analytes. Sens. Actuators B Chem. 2004, 100, 112–116. [Google Scholar] [CrossRef]
- Koudehi, M.F.; Pourmortazavi, S.M. Polyvinyl Alcohol/Polypyrrole/Molecularly Imprinted Polymer Nanocomposite as Highly Selective Chemiresistor Sensor for 2,4-DNT Vapor Recognition. Electroanalysis 2018, 30, 2302–2310. [Google Scholar] [CrossRef]
- Turner, N.W.; Holdsworth, C.I.; McCluskey, A.; Bowyer, M.C. N-2-Propenyl-(5-Dimethylamino)-1-Naphthalene Sulfonamide, a Novel Fluorescent Monomer for the Molecularly Imprinted Polymer-Based Detection of 2,4-Dinitrotoluene in the Gas Phase. Aust. J. Chem. 2012, 65, 1405. [Google Scholar] [CrossRef]
- Turner, N.W.; Holmes, N.; Brisbane, C.; McGeachie, A.B.; Bowyer, M.C.; McCluskey, A.; Holdsworth, C.I. Effect of Template on the Formation of Phase-Inversed Molecularly Imprinted Polymer Thin Films: An Assessment. Soft Matter 2009, 5, 3663. [Google Scholar] [CrossRef]
- Chegel, V.I.; Lopatynskyi, A.M.; Lytvyn, V.K.; Demydov, P.V.; Martínez-Pastor, J.P.; Abargues, R.; Gadea, E.A.; Piletsky, S.A. Localized Surface Plasmon Resonance Nanochips with Molecularly Imprinted Polymer Coating for Explosives Sensing. Semicond. Phys. Quantum Electron. Optoelectron. 2020, 23, 431–436. [Google Scholar] [CrossRef]
- Dayal, H.; Ng, W.Y.; Lin, X.H.; Li, S.F.Y. Development of a Hydrophilic Molecularly Imprinted Polymer for the Detection of Hydrophilic Targets Using Quartz Crystal Microbalance. Sens. Actuators B Chem. 2019, 300, 127044. [Google Scholar] [CrossRef]
- Alizadeh, T.; Akhoundian, M. Graphene/Nano-Sized Imprinted Polymer/Poly(Methyl Methacrylate) Nanocomposite as a New Gas Sensor for the Determination of Nitrobenzene Vapors. Anal. Bioanal. Electrochem. 2017, 9, 1070–1079. [Google Scholar]
- Pan, Y.; Guo, T.; Zhang, G.; Yang, J.; Yang, L.; Cao, B. Detection of Organophosphorus Compounds Using a Surface Acoustic Wave Array Sensor Based on Supramolecular Self-Assembling Imprinted Films. Anal. Methods 2020, 12, 2206–2214. [Google Scholar] [CrossRef]
- Findeisen, A.; Wackerlig, J.; Samardzic, R.; Pitkänen, J.; Anttalainen, O.; Dickert, F.L.; Lieberzeit, P.A. Artificial Receptor Layers for Detecting Chemical and Biological Agent Mimics. Sens. Actuators B Chem. 2012, 170, 196–200. [Google Scholar] [CrossRef]
- Dickert, F.L.; Hayden, O.; Halikias, K.P. Synthetic Receptors as Sensor Coatings for Molecules and Living Cells. Analyst 2001, 126, 766–771. [Google Scholar] [CrossRef]
- Araki, S.; Watanabe, M.; Sassa, F.; Hayashi, K. Raman Enhanced Structure with Reconfigured Molecularly-Imprinted-Polymer for Gas Deteciton. In Proceedings of the 2016 IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar]
- Palaprat, G.; Mingotaud, A.-F.; Langevin, D.; Mauzac, M.; Marty, J.-D. Molecularly Imprinted Cholesteric Materials for Enhanced Enantiomeric Separation. Polymer 2022, 243, 124654. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, H.; Li, H.; Nie, L.; Yao, S. Stereoselective Histidine Sensor Based on Molecularly Imprinted Sol-Gel Films. Anal. Biochem. 2005, 336, 108–116. [Google Scholar] [CrossRef]
- Banerjee, M.B.; Tudu, B.; Ghosh, M.; Bandyopadhyay, R.; Pramanik, P.; Roy, R.B. Detection of Carvacrol Content in Oregano Essential Oil by Molecularly Imprinted Polymer Coated Quartz Crystal Microbalance Sensor. IEEE Sens. J. 2022, 22, 7692–7699. [Google Scholar] [CrossRef]
- Bhattacharyya Banerjee, M.; Pradhan, S.; Banerjee Roy, R.; Tudu, B.; Das, D.K.; Bandyopadhyay, R.; Pramanik, P. Detection of Benzene and Volatile Aromatic Compounds by Molecularly Imprinted Polymer-Coated Quartz Crystal Microbalance Sensor. IEEE Sens. J. 2019, 19, 885–892. [Google Scholar] [CrossRef]
- Liang, R.; Chen, L.; Qin, W. Potentiometric Detection of Chemical Vapors Using Molecularly Imprinted Polymers as Receptors. Sci. Rep. 2015, 5, 12462. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Uno, T. Molecular Imprinting Strategy for Solvent Molecules and Its Application for QCM-Based VOC Vapor Sensing. Sens. Actuators B Chem. 2006, 113, 94–99. [Google Scholar] [CrossRef]
- Alizadeh, T.; Rezaloo, F. Toluene Chemiresistor Sensor Based on Nano-Porous Toluene-Imprinted Polymer. Int. J. Environ. Anal. Chem. 2013, 93, 919–934. [Google Scholar] [CrossRef]
- Shim, D.-Y.; Chang, S.-M.; Kim, J.M. Development of Fast Resettable Gravimetric Aromatic Gas Sensors Using Quartz Crystal Microbalance. Sens. Actuators B Chem. 2021, 329, 129143. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Li, H.; Gong, M. Preparation of Toluene-Imprinted Homogeneous Microspheres and Determination of Their Molecular Recognition Toward Template Vapor by Inverse Gas Chromatography. Chromatographia 2017, 80, 453–461. [Google Scholar] [CrossRef]
- Lou, D.; Chen, H.; Wang, X.; Lian, L.; Zhu, B.; Yang, Q.; Guo, T.; Li, Q.; Wang, R.; Guo, X. Preparation and Application of a Coated-Fiber Needle Extraction Device. J. Sep. Sci. 2016, 39, 3769–3774. [Google Scholar] [CrossRef]
- Liu, C.; Shang, L.; Yoshioka, H.-T.; Chen, B.; Hayashi, K. Preparation of Molecularly Imprinted Polymer Nanobeads for Selective Sensing of Carboxylic Acid Vapors. Anal. Chim. Acta 2018, 1010, 1–10. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Zhang, D.; Zheng, F.; Huang, M.; Sun, J.; Sun, X.; Li, H.; Wang, J.; Sun, B. A Fluorescent Nanoprobe for 4-Ethylguaiacol Based on the Use of a Molecularly Imprinted Polymer Doped with a Covalent Organic Framework Grafted onto Carbon Nanodots. Microchim. Acta 2019, 186, 182. [Google Scholar] [CrossRef]
- Domínguez-Renedo, O.; Navarro-Cuñado, A.M.; Arnáiz-Lozano, V.; Alonso-Lomillo, M.A. Molecularly Imprinted Polypyrrole Based Electrochemical Sensor for Selective Determination of 4-Ethylphenol. Talanta 2020, 207, 120351. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, N.-Y.; Tsow, F.; Xian, X.; Forzani, E.S. Adsorption Thermodynamic Analysis of a Quartz Tuning Fork Based Sensor for Volatile Organic Compounds Detection. ACS Sens. 2017, 2, 1662–1668. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, C.; Qin, X.; Xian, X.; Alford, T.L.; Choi, H.W.; Tsow, F.; Forzani, E.S. Aging Effect of a Molecularly Imprinted Polymer on a Quartz Tuning Fork Sensor for Detection of Volatile Organic Compounds. Sens. Actuators B Chem. 2015, 211, 25–32. [Google Scholar] [CrossRef]
- Tsow, F.; Forzani, E.; Rai, A.; Wang, R.; Tsui, R.; Mastroianni, S.; Knobbe, C.; Gandolfi, A.J.; Tao, N.J. A Wearable and Wireless Sensor System for Real-Time Monitoring of Toxic Environmental Volatile Organic Compounds. IEEE Sens. J. 2009, 9, 1734–1740. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Lv, T.; Li, K.; Zhu, Z.; Zhang, J.; Zhang, R.; Liu, Q. Ag-LaFeO3 Nanoparticles Using Molecular Imprinting Technique for Selective Detection of Xylene. Mater. Res. Bull. 2018, 107, 271–279. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Zhang, J.; Li, K.; Lv, T.; Shen, K.; Zhu, Z.; Liu, Q. Facile Lotus-Leaf-Templated Synthesis and Enhanced Xylene Gas Sensing Properties of Ag-LaFeO3 Nanoparticles. J. Mater. Chem. C 2018, 6, 6138–6145. [Google Scholar] [CrossRef]
- Chen, C.; Tsow, F.; Campbell, K.D.; Iglesias, R.; Forzani, E.; Tao, N. A Wireless Hybrid Chemical Sensor for Detection of Environmental Volatile Organic Compounds. IEEE Sens. J. 2013, 13, 1748–1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickert, F.L.; Forth, P.; Tortschanoff, M.; Bulst, W.E.; Fischerauer, G.; Knauer, U. SAW and QMB for Chemical Sensing. In Proceedings of the International Frequency Control Symposium, IEEE, Orlando, FL, USA, 30 May 1997; pp. 120–123. [Google Scholar]
- Bender, S.; Dickert, F.L.; Mokwa, W.; Pachatz, P. Investigations on Temperature Controlled Monolithic Integrated Surface Acoustic Wave (SAW) Gas Sensors. Sens. Actuators B Chem. 2003, 93, 164–168. [Google Scholar] [CrossRef]
- Mustafa, G.; Lieberzeit, P.A. Molecularly Imprinted Polymer–Ag2S Nanoparticle Composites for Sensing Volatile Organics. RSC Adv. 2014, 4, 12723–12728. [Google Scholar] [CrossRef]
- Sierra-Res, A.; Robles-Her, B.; Bernad, M.J.; Día, R.; Peñ, S.I.; Vargas-Est, D.; Gracia-Mor, J. Designing and Preclinical Evaluation of a Molecular Imprint Polymer-Based Cocaine Odor Mimic for Conditioning Detection Dogs. Int. J. Pharmacol. 2022, 18, 171–181. [Google Scholar] [CrossRef]
- Iqbal, N.; Mustafa, G.; Rehman, A.; Biedermann, A.; Najafi, B.; Lieberzeit, P.A.; Dickert, F.L. QCM-Arrays for Sensing Terpenes in Fresh and Dried Herbs via Bio-Mimetic MIP Layers. Sensors 2010, 10, 6361–6376. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Shitang, H.; Shunzhou, L.; Minghua, L.; Yong, P. Enhanced Sensitivity of SAW Gas Sensor Coated Molecularly Imprinted Polymer Incorporating High Frequency Stability Oscillator. Sens. Actuators B Chem. 2007, 125, 422–427. [Google Scholar] [CrossRef]
- Roy, S.; Nag, S.; Bhattacharyya Banerjee, M.; Dasgupta, S.; Pramanik, P.; Bandyopadhyay, R. Detection of Geraniol in Palmarosa Essential Oil Using Silicone Sealant as Molecularly Imprinted Polymer in a QCM Sensor. J. Mater. NanoSci. 2022, 9, 120–124. [Google Scholar]
- Ghatak, B.; Ali, S.B.; Naskar, H.; Tudu, B.; Pramanik, P.; Mukherji, S.; Bandyopadhyay, R. Selective and Sensitive Detection of Limonene in Mango Using Molecularly Imprinted Polymer Based Quartz Crystal Microbalance Sensor. In Proceedings of the 2019 IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Fukuoka, Japan, 26–29 May 2019; pp. 1–3. [Google Scholar]
- Wen, T.; Nie, Q.; Han, L.; Gong, Z.; Li, D.; Ma, Q.; Wang, Z.; He, W.; Wen, L.; Peng, H. Molecularly Imprinted Polymers-Based Piezoelectric Coupling Sensor for the Rapid and Nondestructive Detection of Infested Citrus. Food Chem. 2022, 387, 132905. [Google Scholar] [CrossRef]
- Völkle, J.; Kumpf, K.; Feldner, A.; Lieberzeit, P.; Fruhmann, P. Development of Conductive Molecularly Imprinted Polymers (CMIPs) for Limonene to Improve and Interconnect QCM and Chemiresistor Sensing. Sens. Actuators B Chem. 2022, 356, 131293. [Google Scholar] [CrossRef]
- Hawari, H.F.; Samsudin, N.M.; Ahmad, M.N.; Shakaff, A.Y.M.; Ghani, S.A.; Wahab, Y.; Za’aba, S.K.; Akitsu, T. Array of MIP-Based Sensor for Fruit Maturity Assessment. Procedia Chem. 2012, 6, 100–109. [Google Scholar] [CrossRef]
- Shang, L.; Liu, C.; Chen, B.; Hayashi, K. Plant Biomarker Recognition by Molecular Imprinting Based Localized Surface Plasmon Resonance Sensor Array: Performance Improvement by Enhanced Hotspot of Au Nanostructure. ACS Sens. 2018, 3, 1531–1538. [Google Scholar] [CrossRef]
- Hawari, H.F.; Samsudin, N.M.; Ahmad, M.N.; Shakaff, A.Y.M.; Ghani, S.A.; Wahab, Y.; Hashim, U. Recognition of Limonene Volatile Using Interdigitated Electrode Molecular Imprinted Polymer Sensor. In Proceedings of the 2012 Third International Conference on Intelligent Systems Modelling and Simulation, Kota Kinabalu, Malaysia, 8–10 February 2012; pp. 723–726. [Google Scholar]
- Kikuchi, M.; Tsuru, N.; Shiratori, S. Recognition of Terpenes Using Molecular Imprinted Polymer Coated Quartz Crystal Microbalance in Air Phase. Sci. Technol. Adv. Mater. 2006, 7, 156–161. [Google Scholar] [CrossRef]
- Lee, S.P. Terpene Sensor Array with Bridge-Type Resistors by CMOS Technology. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012065. [Google Scholar] [CrossRef]
- Hardoyono, F.; Windhani, K. Identification and Detection of Bioactive Compounds in Turmeric (Curcuma longa L.) Using a Gas Sensor Array Based on Molecularly Imprinted Polymer Quartz Crystal Microbalance. New J. Chem. 2021, 45, 17930–17940. [Google Scholar] [CrossRef]
- Shang, L.; Liu, C.; Chen, B.; Hayashi, K. Development of Molecular Imprinted Sol-Gel Based LSPR Sensor for Detection of Volatile Cis-Jasmone in Plant. Sens. Actuators B Chem. 2018, 260, 617–626. [Google Scholar] [CrossRef]
- Shang, L.; Liu, C.; Hayashi, K. Localized Surface Plasmon Resonance Modified with Molecularly Imprinted Sol-Gel Sensor for Cis-Jasmone Vapor Detection. In Proceedings of the 2017 IEEE Sensors, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar]
- Shahar, T.; Feldheim, G.; Marx, S.; Mandler, D. Core–Shell Nanoparticles for Gas Phase Detection Based on Silver Nanospheres Coated with a Thin Molecularly Imprinted Polymer Adsorbed on a Chemiresistor. Nanoscale 2018, 10, 17593–17602. [Google Scholar] [CrossRef]
- Hardoyono, F.; Windhani, K.; Sambodo, H.; Pudjianto, H. Identification of Bioactive Compounds in Ginger Based on Molecularly Imprinted Polymer Quartz Crystal Microbalance Gas Sensor. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 032012. [Google Scholar] [CrossRef]
- Jahangiri-Manesh, A.; Mousazadeh, M.; Nikkhah, M.; Abbasian, S.; Moshaii, A.; Masroor, M.J.; Norouzi, P. Molecularly Imprinted Polymer-Based Chemiresistive Sensor for Detection of Nonanal as a Cancer Related Biomarker. Microchem. J. 2022, 173, 106988. [Google Scholar] [CrossRef]
- Debabhuti, N.; Neogi, S.; Mukherjee, S.; Dhar, A.; Sharma, P.; Vekariya, R.L.; Sarkar, M.P.; Tudu, B.; Bhattacharyya, N.; Bandyopadhyay, R.; et al. Development of QCM Sensor to Detect α-Terpinyl Acetate in Cardamom. Sens. Actuators A Phys. 2021, 319, 112521. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.; Gu, S.; Wang, J.; Wang, Y. Discrimination of Wood Borers Infested Platycladus orientalis Trunks Using Quartz Crystal Microbalance Gas Sensor Array. Sens. Actuators B Chem. 2020, 309, 127767. [Google Scholar] [CrossRef]
- Chen, B.; Liu, C.; Hayashi, K. Selective Terpene Vapor Detection Using Molecularly Imprinted Polymer Coated Au Nanoparticle LSPR Sensor. IEEE Sens. J. 2014, 14, 3458–3464. [Google Scholar] [CrossRef]
- Hawari, H.F.; Samsudin, N.M.; Shakaff, A.Y.M.; Wahab, Y.; Hashim, U.; Zakaria, A.; Ghani, S.A.; Ahmad, M.N. Highly Selective Molecular Imprinted Polymer (MIP) Based Sensor Array Using Interdigitated Electrode (IDE) Platform for Detection of Mango Ripeness. Sens. Actuators B Chem. 2013, 187, 434–444. [Google Scholar] [CrossRef]
- Hawari, H.F.; Saad, S.M.; Samsudin, N.M.; Md Shakaff, A.Y.; Wahab, Y.; Hashim, U. Sensory System for Detection of Harumanis Mango Fruit Maturity. In Proceedings of the 2013 IEEE International Conference on Circuits and Systems (ICCAS), Kuala Lumpur, Malaysia, 18–19 September 2013; pp. 146–149. [Google Scholar]
- Chen, B.; Liu, C.; Ge, L.; Hayashi, K. Localized Surface Plasmon Resonance Gas Sensor of Au Nano-Islands Coated with Molecularly Imprinted Polymer: Influence of Polymer Thickness on Sensitivity and Selectivity. Sens. Actuators B Chem. 2016, 231, 787–792. [Google Scholar] [CrossRef]
- Chen, B.; Liu, C.; Xie, Y.; Jia, P.; Hayashi, K. Localized Surface Plasmon Resonance Gas Sensor Based on Molecularly Imprinted Polymer Coated Au Nano-Island Films: Influence of Nanostructure on Sensing Characteristics. IEEE Sens. J. 2016, 16, 3532–3540. [Google Scholar] [CrossRef]
- Chen, B.; Liu, C.; Sun, X.; Hayashi, K. Molecularly Imprinted Polymer Coated Au Nanoparticle Sensor for α-Pinene Vapor Detection. In Proceedings of the 2013 IEEE Sensors, Baltimore, MD, USA, 3–6 November 2013; pp. 1–4. [Google Scholar]
- Emam, S.; Adedoyin, A.; Geng, X.; Zaeimbashi, M.; Adams, J.; Ekenseair, A.; Podlaha-Murphy, E.; Sun, N.X. A Molecularly Imprinted Electrochemical Gas Sensor to Sense Butylated Hydroxytoluene in Air. J. Sens. 2018, 2018, 3437149. [Google Scholar] [CrossRef]
- Latif, U.; Rohrer, A.; Lieberzeit, P.A.; Dickert, F.L. QCM Gas Phase Detection with Ceramic Materials—VOCs and Oil Vapors. Anal. Bioanal. Chem. 2011, 400, 2457–2462. [Google Scholar] [CrossRef]
- Huang, X.H.; Song, J.J.; Li, H.; Gong, M.T.; Zhang, Y. Selective Removal of Nicotine from the Main Stream Smoke by Using a Surface-Imprinted Polymer Monolith as Adsorbent. J. Hazard. Mater. 2019, 365, 53–63. [Google Scholar] [CrossRef]
- Aouled, N.O.; Hallil, H.; Plano, B.; Rebiere, D.; Dejous, C.; Delepee, R.; Agrofoglio, L. Love Wave Sensor Based on Thin Film Molecularly Imprinted Polymer: MIP Layer Morphology and Nucleosides Analogs Detection. In Proceedings of the 2013 IEEE Sensors, Baltimore, MD, USA, 3–6 November 2013; pp. 1–4. [Google Scholar]
- Jenkins, A.L.; Ellzy, M.W.; Buettner, L.C. Molecularly Imprinted Polymer Sensors for Detection in the Gas, Liquid, and Vapor Phase. J. Mol. Recognit. 2012, 25, 330–335. [Google Scholar] [CrossRef]
- Marx, S.; Zaltsman, A.; Turyan, I.; Mandler, D. Parathion Sensor Based on Molecularly Imprinted Sol-Gel Films. Anal. Chem. 2004, 76, 120–126. [Google Scholar] [CrossRef]
- Ji, H.-S.; McNiven, S.; Ikebukuro, K.; Karube, I. Selective Piezoelectric Odor Sensors Using Molecularly Imprinted Polymers. Anal. Chim. Acta 1999, 390, 93–100. [Google Scholar] [CrossRef]
- Percival, C.J.; Stanley, S.; Galle, M.; Braithwaite, A.; Newton, M.I.; McHale, G.; Hayes, W. Molecular-Imprinted, Polymer-Coated Quartz Crystal Microbalances for the Detection of Terpenes. Anal. Chem. 2001, 73, 4225–4228. [Google Scholar] [CrossRef] [PubMed]
- Jenik, M.; Seifner, A.; Lieberzeit, P.; Dickert, F.L. Pollen-Imprinted Polyurethanes for QCM Allergen Sensors. Anal. Bioanal. Chem. 2009, 394, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, J.; He, S.; Gong, H.; Chen, C.; Cai, C. A Mild and Safe Gas-Responsive Molecularly Imprinted Sensor for Highly Specific Recognition of Hepatitis B Virus. Sens. Actuators B Chem. 2022, 366, 131990. [Google Scholar] [CrossRef]
- Dery, L.; Shauloff, N.; Turkulets, Y.; Shalish, I.; Jelinek, R.; Mandler, D. Size-Selective Detection of Nanoparticles in Solution and Air by Imprinting. ACS Sens. 2022, 7, 296–303. [Google Scholar] [CrossRef]
- Lieberzeit, P.A.; Rehman, A.; Yaqub, S.; Dickert, F.L. Nanostructured particles and layers for sensing contaminants in air and water. Nano 2008, 3, 205–208. [Google Scholar] [CrossRef]
- Pestov, D.; Levit, N.; Maniscalco, V.; Deveney, B.; Tepper, G. Molecular Imprinting Using Monomers with Solid-State Polymerization. Anal. Chim. Acta 2004, 504, 31–35. [Google Scholar] [CrossRef]
- Rahimpoor, R.; Firoozichahak, A.; Alizadeh, S.; Soleymani-Ghoozhdi, D.; Mehregan, F. Application of a Needle Trap Device Packed with a MIP@MOF Nano-Composite for Efficient Sampling and Determination of Airborne Diazinon Pesticide. RSC Adv. 2022, 12, 16267–16276. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Safitri, N.; Zulfa, A.; Neli, N.; Rahayu, D. Factors Affecting Preparation of Molecularly Imprinted Polymer and Methods on Finding Template-Monomer Interaction as the Key of Selective Properties of the Materials. Molecules 2021, 26, 5612. [Google Scholar] [CrossRef]
- Cowen, T.; Stefanucci, E.; Piletska, E.; Marrazza, G.; Canfarotta, F.; Piletsky, S.A. Synthetic Mechanism of Molecular Imprinting at the Solid Phase. Macromolecules 2020, 53, 1435–1442. [Google Scholar] [CrossRef]
- Pratama, K.F.; Manik, M.E.R.; Rahayu, D.; Hasanah, A.N. Effect of the Molecularly Imprinted Polymer Component Ratio on Analytical Performance. Chem. Pharm. Bull. 2020, 68, 1013–1024. [Google Scholar] [CrossRef]
- Iuga, C.; Ortiz, E.; Noreña, L.E. Interaction between Volatile Organic Compounds and Functional Monomers in Molecularly Imprinted Materials. Health Environ. Res. Online 2011, 3, 777–780. [Google Scholar]
- Wang, W.; He, S.; Pan, Y. Viscoelastic Analysis of a Surface Acoustic Wave Gas Sensor Coated by a New Deposition Technique. Chin. J. Chem. Phys. 2006, 19, 47–53. [Google Scholar] [CrossRef]
- Darby, J.F.; Hopkins, A.P.; Shimizu, S.; Roberts, S.M.; Brannigan, J.A.; Turkenburg, J.P.; Thomas, G.H.; Hubbard, R.E.; Fischer, M. Water Networks Can Determine the Affinity of Ligand Binding to Proteins. J. Am. Chem. Soc. 2019, 141, 15818–15826. [Google Scholar] [CrossRef]
- Ferguson, A.L.; Debenedetti, P.G.; Panagiotopoulos, A.Z. Solubility and Molecular Conformations of N-Alkane Chains in Water. J. Phys. Chem. B 2009, 113, 6405–6414. [Google Scholar] [CrossRef]
- Esfandyari-Manesh, M.; Javanbakht, M.; Atyabi, F.; Badiei, A.; Dinarvand, R. Effect of Porogenic Solvent on the Morphology, Recognition and Release Properties of Carbamazepine-Molecularly Imprinted Polymer Nanospheres. J. Appl. Polym. Sci. 2011, 121, 1118–1126. [Google Scholar] [CrossRef]
- Cormack, P.A.G.; Elorza, A.Z. Molecularly Imprinted Polymers: Synthesis and Characterisation. J. Chromatogr. B 2004, 804, 173–182. [Google Scholar] [CrossRef]
- Cowen, T.; Karim, K.; Piletsky, S.A. Solubility and Size of Polymer Nanoparticles. Polym. Chem. 2018, 9, 4566–4573. [Google Scholar] [CrossRef]
- Schmidt, R.H.; Belmont, A.-S.; Haupt, K. Porogen Formulations for Obtaining Molecularly Imprinted Polymers with Optimized Binding Properties. Anal. Chim. Acta 2005, 542, 118–124. [Google Scholar] [CrossRef]
- Kim, H.; Jin, Y.-J.; Kim, B.S.-I.; Aoki, T.; Kwak, G. Optically Active Conjugated Polymer Nanoparticles from Chiral Solvent Annealing and Nanoprecipitation. Macromolecules 2015, 48, 4754–4757. [Google Scholar] [CrossRef]
- Pardeshi, S.; Dhodapkar, R.; Kumar, A. Influence of Porogens on the Specific Recognition of Molecularly Imprinted Poly(Acrylamide-Co-Ethylene Glycol Dimethacrylate). Compos. Interfaces 2014, 21, 13–30. [Google Scholar] [CrossRef]
- Fischerauer, G.; Dickert, F.; Forth, P.; Knauer, U. Chemical Sensors Based on SAW Resonators Working at up to 1 GHz. In Proceedings of the 1996 IEEE Ultrasonics Symposium, San Antonio, TX, USA, 3–6 November 2016; pp. 439–442. [Google Scholar]
- Dickert, F. Molecular Imprinting in Chemical Sensing. TrAC Trends Anal. Chem. 1999, 18, 192–199. [Google Scholar] [CrossRef]
- Grate, J.W.; Abraham, M.H. Solubility Interactions and the Design of Chemically Selective Sorbent Coatings for Chemical Sensors and Arrays. Sens. Actuators B Chem. 1991, 3, 85–111. [Google Scholar] [CrossRef]
- Bhandaru, N. Solvo-Selective Imprinting of a Thin Polymer Blend Film for Creating Multi-Length Scale Patterns. Bull. Mater. Sci. 2020, 43, 180. [Google Scholar] [CrossRef]
- Joly, C.; le Cerf, D.; Chappey, C.; Langevin, D.; Muller, G. Residual Solvent Effect on the Permeation Properties of Fluorinated Polyimide Films. Sep. Purif. Technol. 1999, 16, 47–54. [Google Scholar] [CrossRef]
- Chiarello, M.; Anfossi, L.; Cavalera, S.; di Nardo, F.; Artusio, F.; Pisano, R.; Baggiani, C. Effect of Polymerization Time on the Binding Properties of Ciprofloxacin-Imprinted NanoMIPs Prepared by Solid-Phase Synthesis. Polymers 2021, 13, 2656. [Google Scholar] [CrossRef]
- Wei, Y.; Zeng, Q.; Huang, J.; Guo, X.; Wang, L.; Wang, L. Preparation of Gas-Responsive Imprinting Hydrogel and Their Gas-Driven Switchable Affinity for Target Protein Recognition. ACS Appl. Mater. Interfaces 2020, 12, 24363–24369. [Google Scholar] [CrossRef]
- Kitayama, Y.; Isomura, M. Gas-Stimuli-Responsive Molecularly Imprinted Polymer Particles with Switchable Affinity for Target Protein. Chem. Commun. 2018, 54, 2538–2541. [Google Scholar] [CrossRef]
- Kitayama, Y.; Isomura, M. Molecularly Imprinted Polymer Particles with Gas-Stimuli Responsive Affinity toward Target Proteins Prepared Using Switchable Functional Monomer. Polymer 2020, 203, 122781. [Google Scholar] [CrossRef]
- Sharma, P.S.; Garcia-Cruz, A.; Cieplak, M.; Noworyta, K.R.; Kutner, W. ‘Gate Effect’ in Molecularly Imprinted Polymers: The Current State of Understanding. Curr. Opin. Electrochem. 2019, 16, 50–56. [Google Scholar] [CrossRef]
- Cavalera, S.; Chiarello, M.; di Nardo, F.; Anfossi, L.; Baggiani, C. Effect of Experimental Conditions on the Binding Abilities of Ciprofloxacin-Imprinted Nanoparticles Prepared by Solid-Phase Synthesis. React. Funct. Polym. 2021, 163, 104893. [Google Scholar] [CrossRef]
- Alizadeh, T.; Hamedsoltani, L. Graphene/Graphite/Molecularly Imprinted Polymer Nanocomposite as the Highly Selective Gas Sensor for Nitrobenzene Vapor Recognition. J. Environ. Chem. Eng. 2014, 2, 1514–1526. [Google Scholar] [CrossRef]
- Alizadeh, T. Application of Electrochemical Impedance Spectroscopy and Conventional Rebinding Experiments for the Investigation of Recognition Characteristic of Bulky and Nano-Sized Imprinted Polymers. Mater. Chem. Phys. 2012, 135, 1012–1023. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Liu, Q. Gas Sensors Based on Molecular Imprinting Technology. Sensors 2017, 17, 1567. [Google Scholar] [CrossRef] [Green Version]
- Anfossi, L.; Cavalera, S.; di Nardo, F.; Spano, G.; Giovannoli, C.; Baggiani, C. Delayed Addition of Template Molecules Enhances the Binding Properties of Diclofenac-Imprinted Polymers. Polymers 2020, 12, 1178. [Google Scholar] [CrossRef]
- Bossi, A.M.; Bucciarelli, A.; Maniglio, D. Molecularly Imprinted Silk Fibroin Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 31431–31439. [Google Scholar] [CrossRef]
- Bossi, A.M.; Maniglio, D. BioMIPs: Molecularly Imprinted Silk Fibroin Nanoparticles to Recognize the Iron Regulating Hormone Hepcidin. Microchim. Acta 2022, 189, 66. [Google Scholar] [CrossRef]
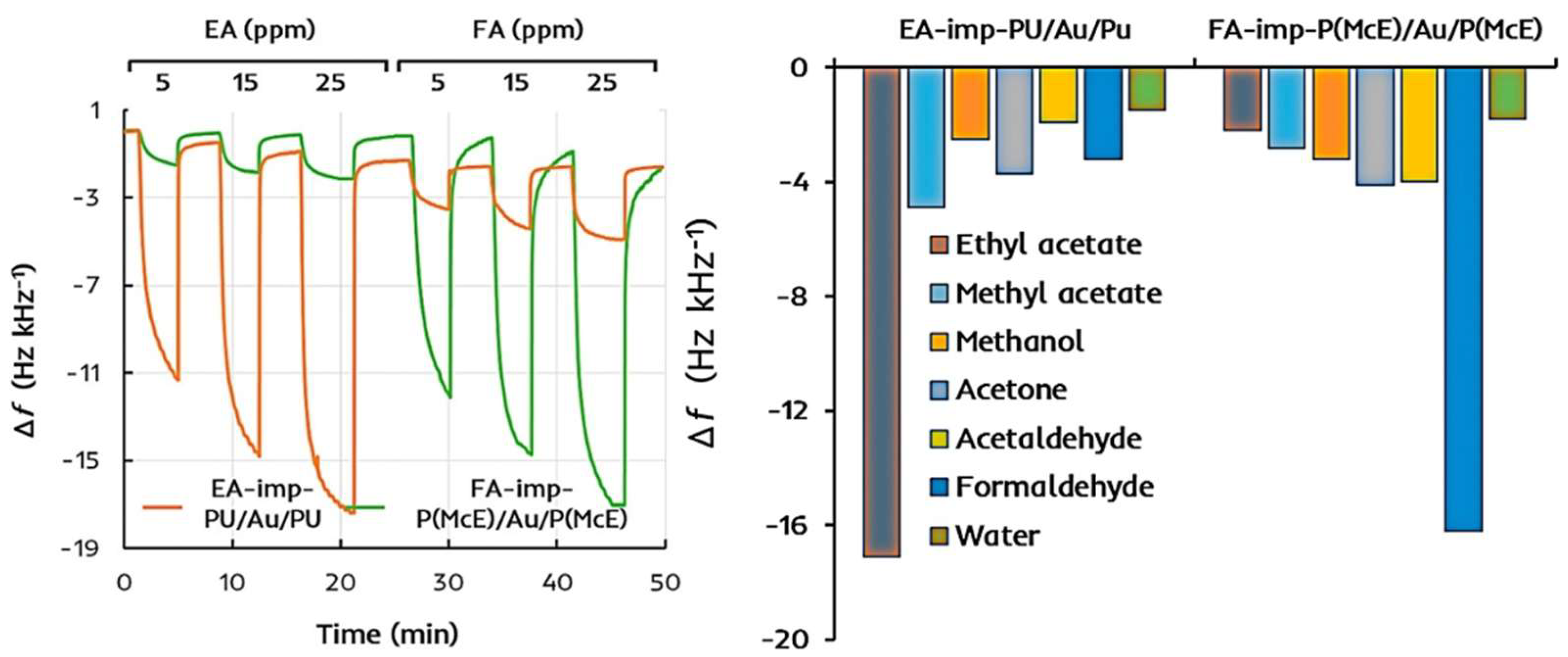
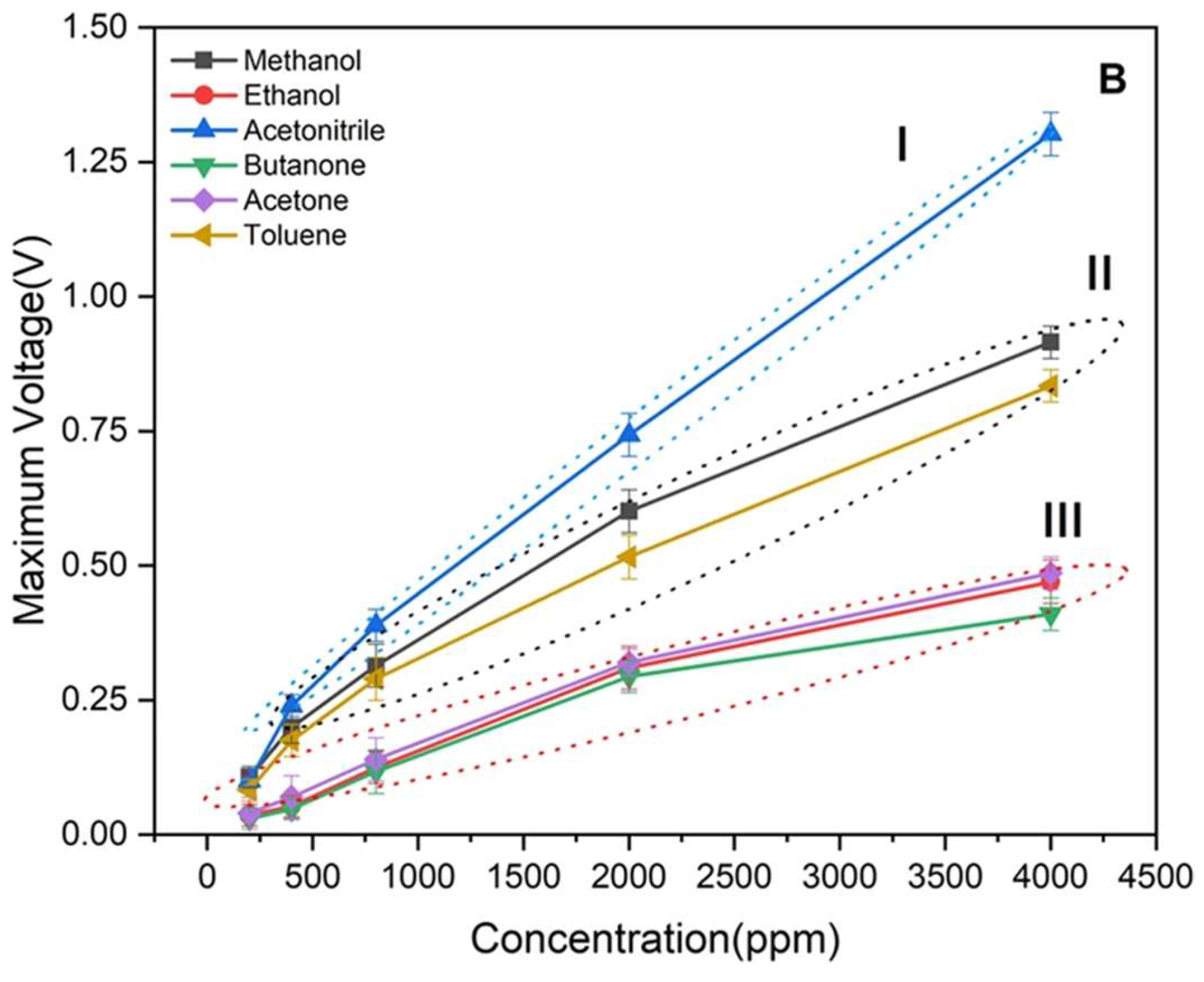

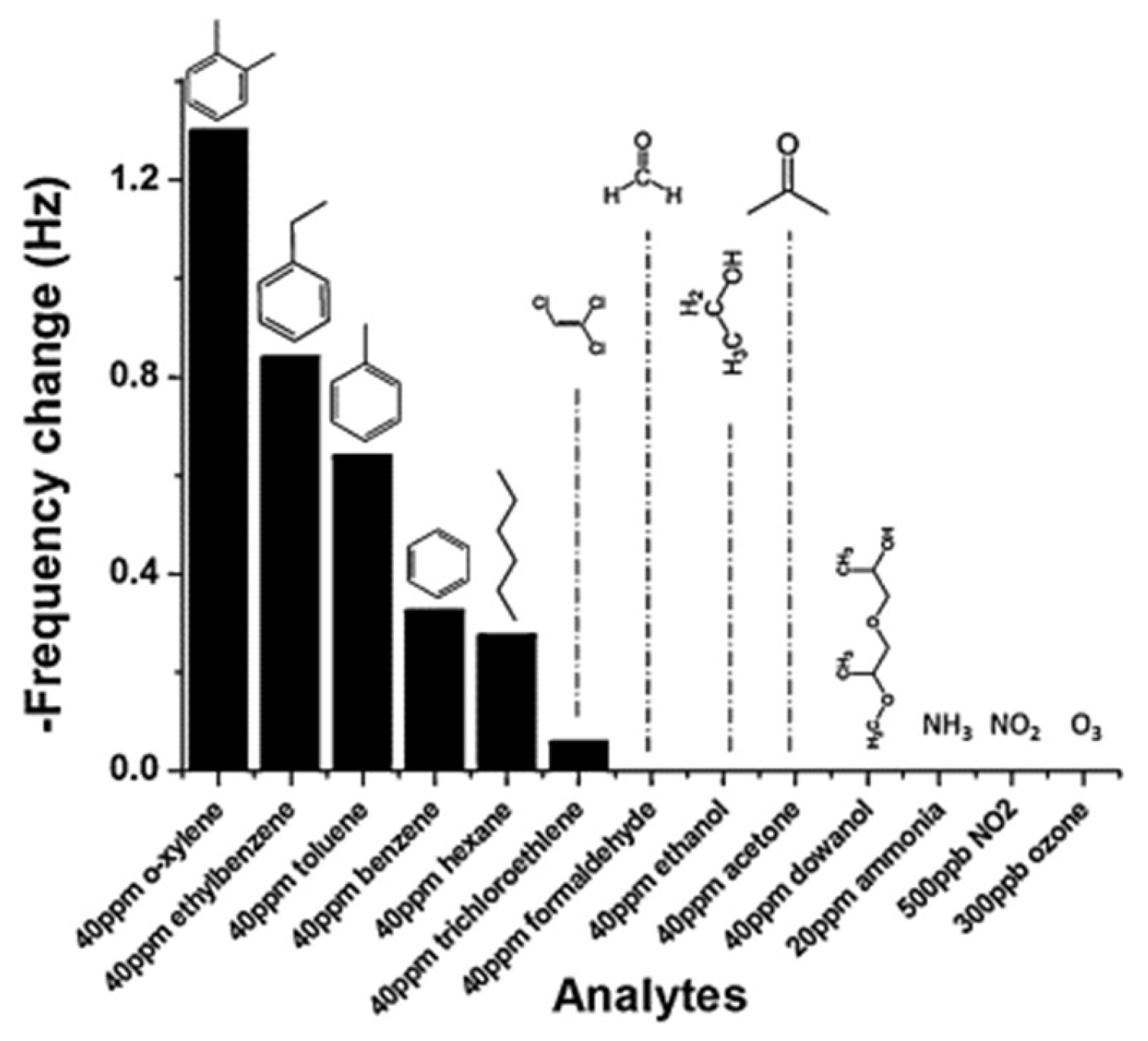

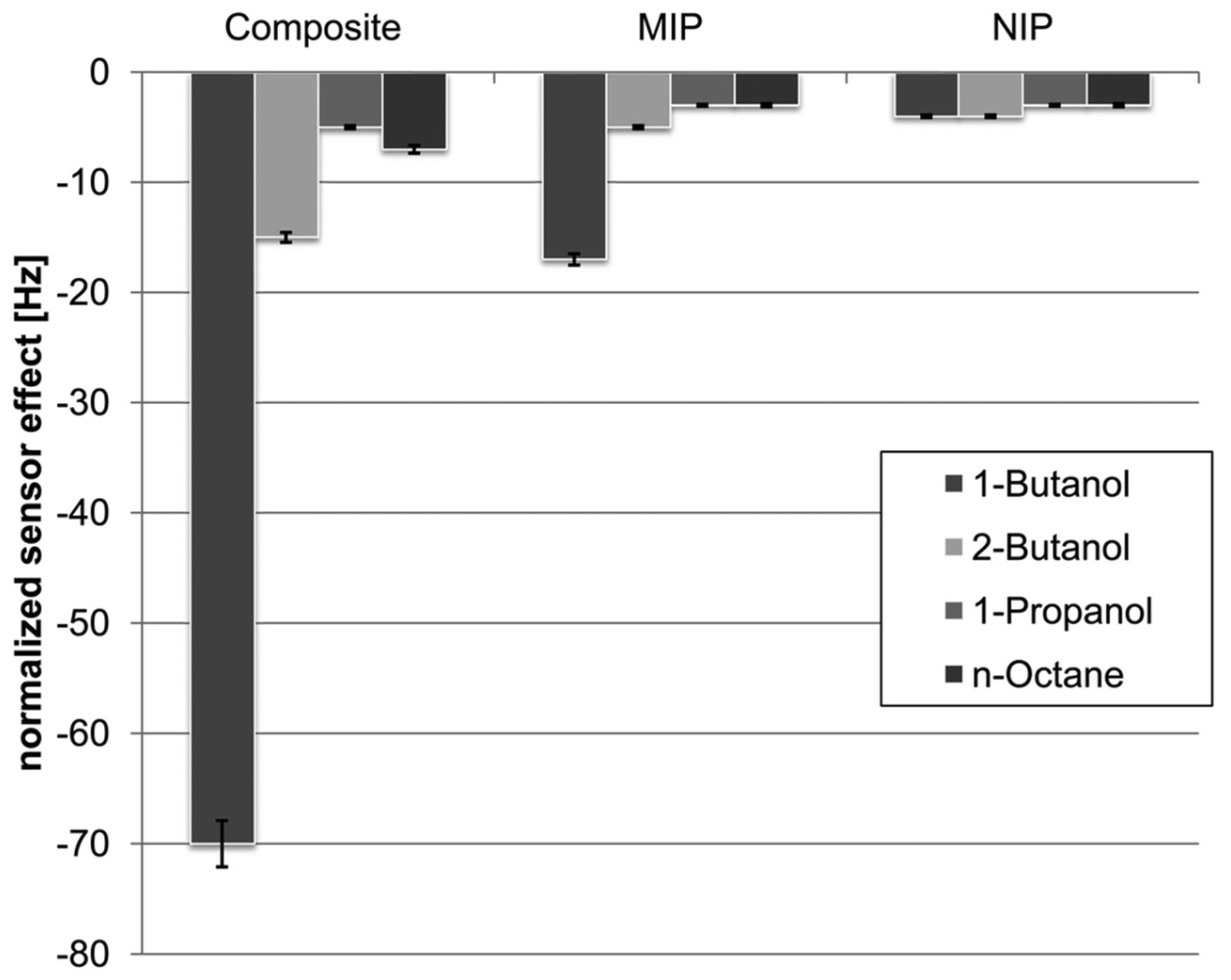

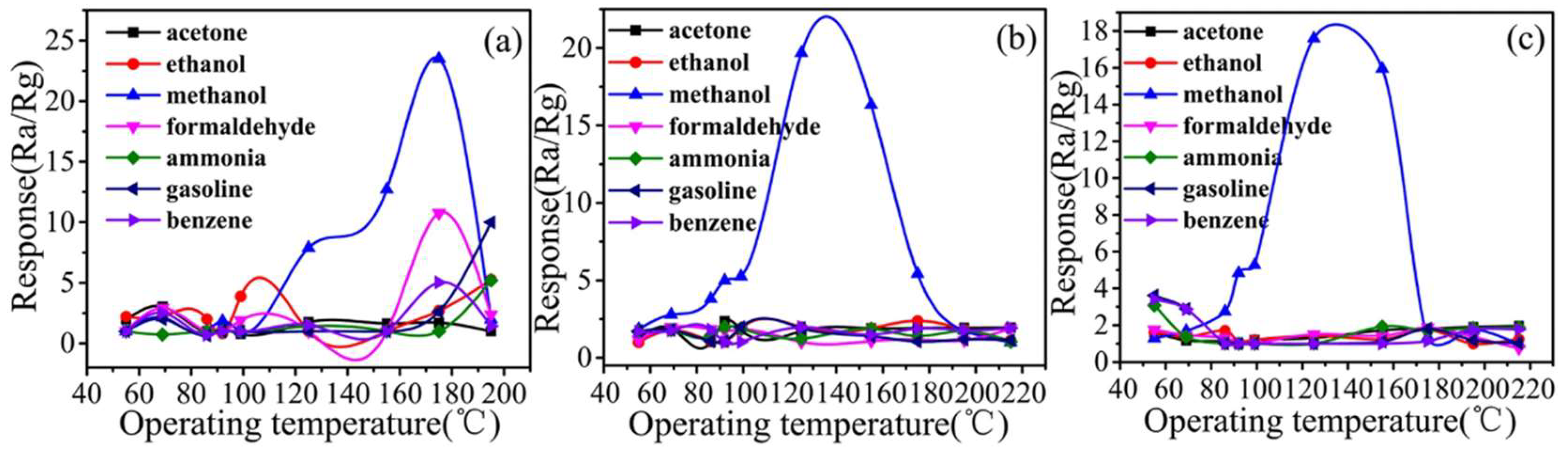
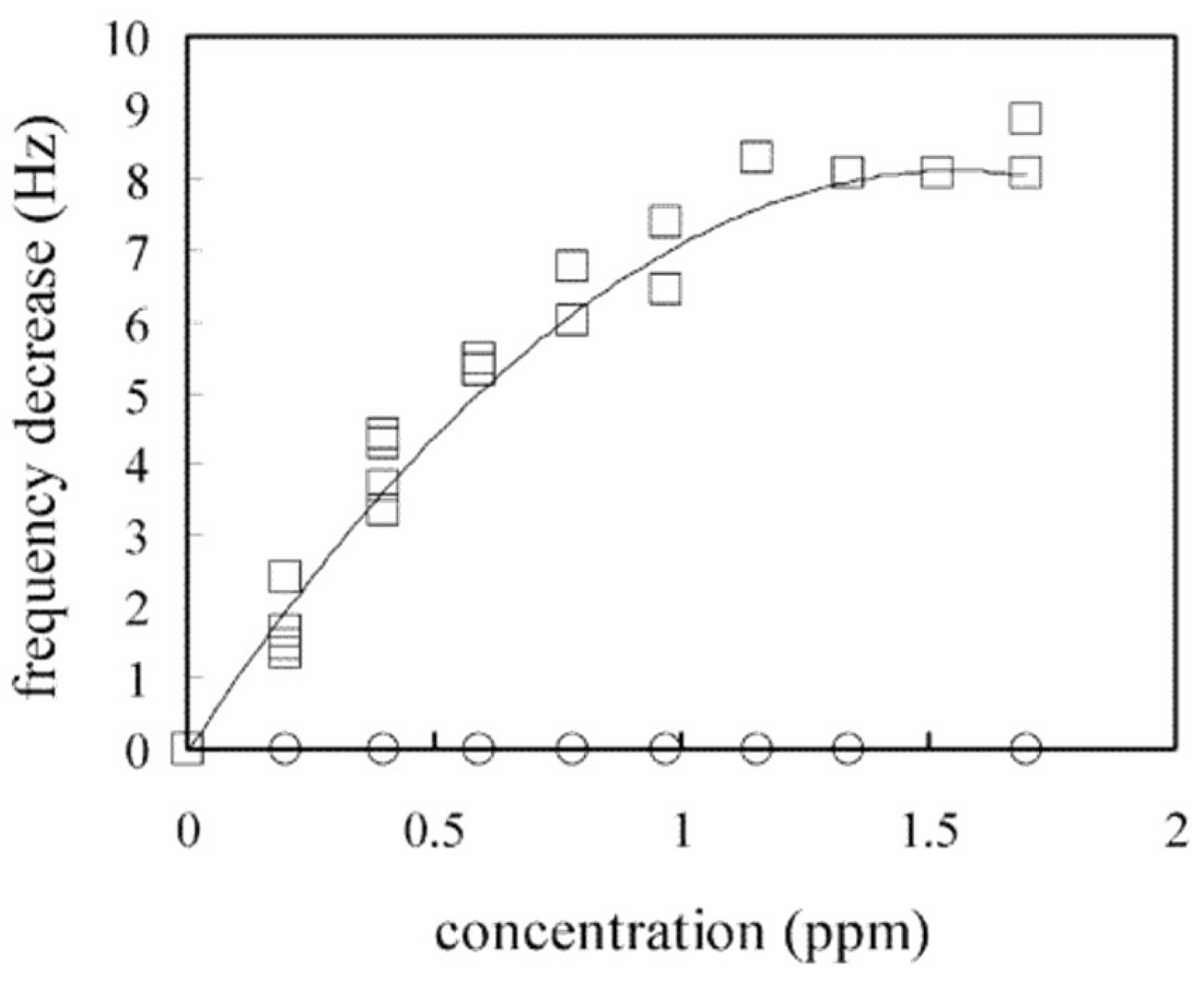
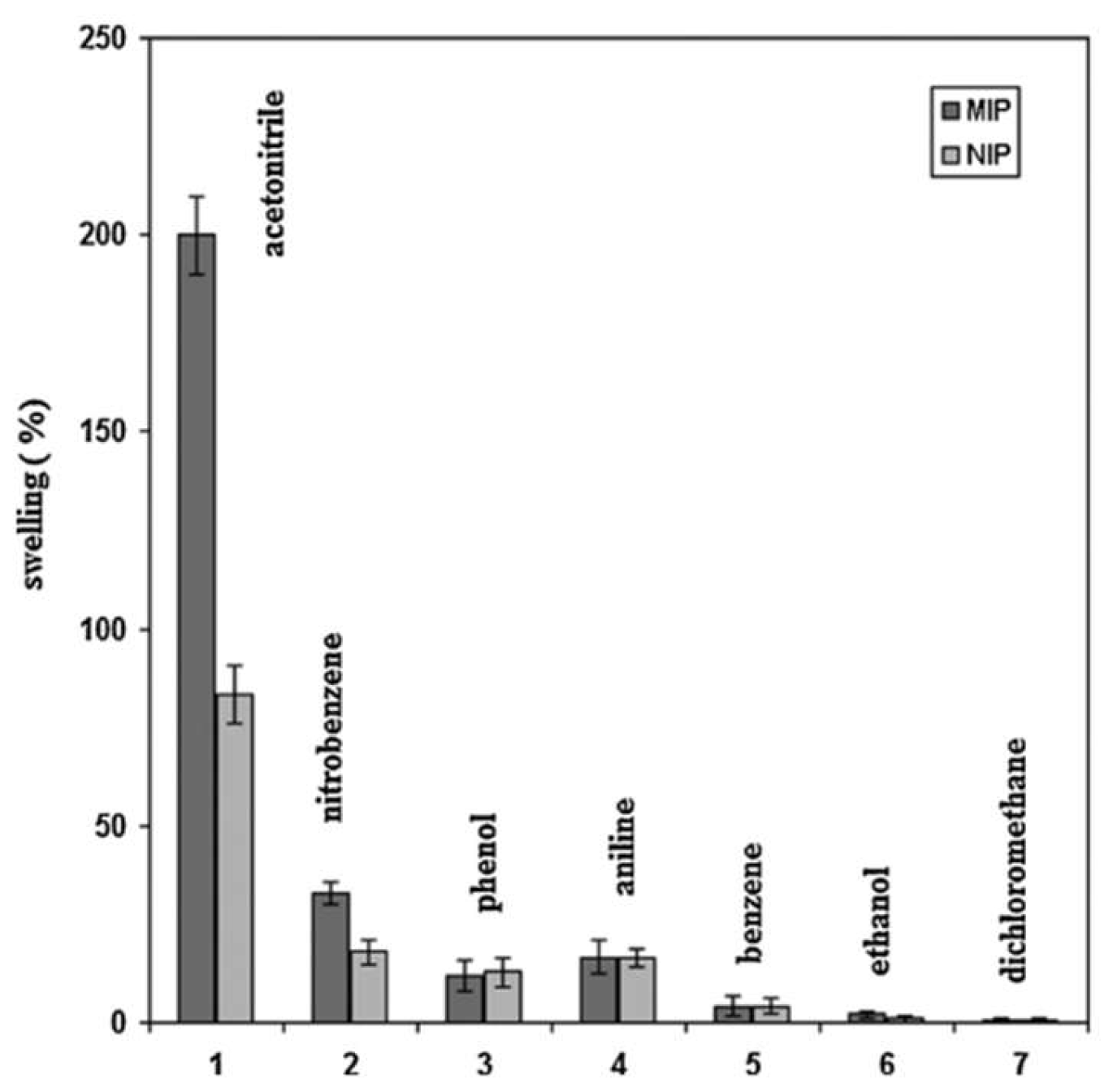

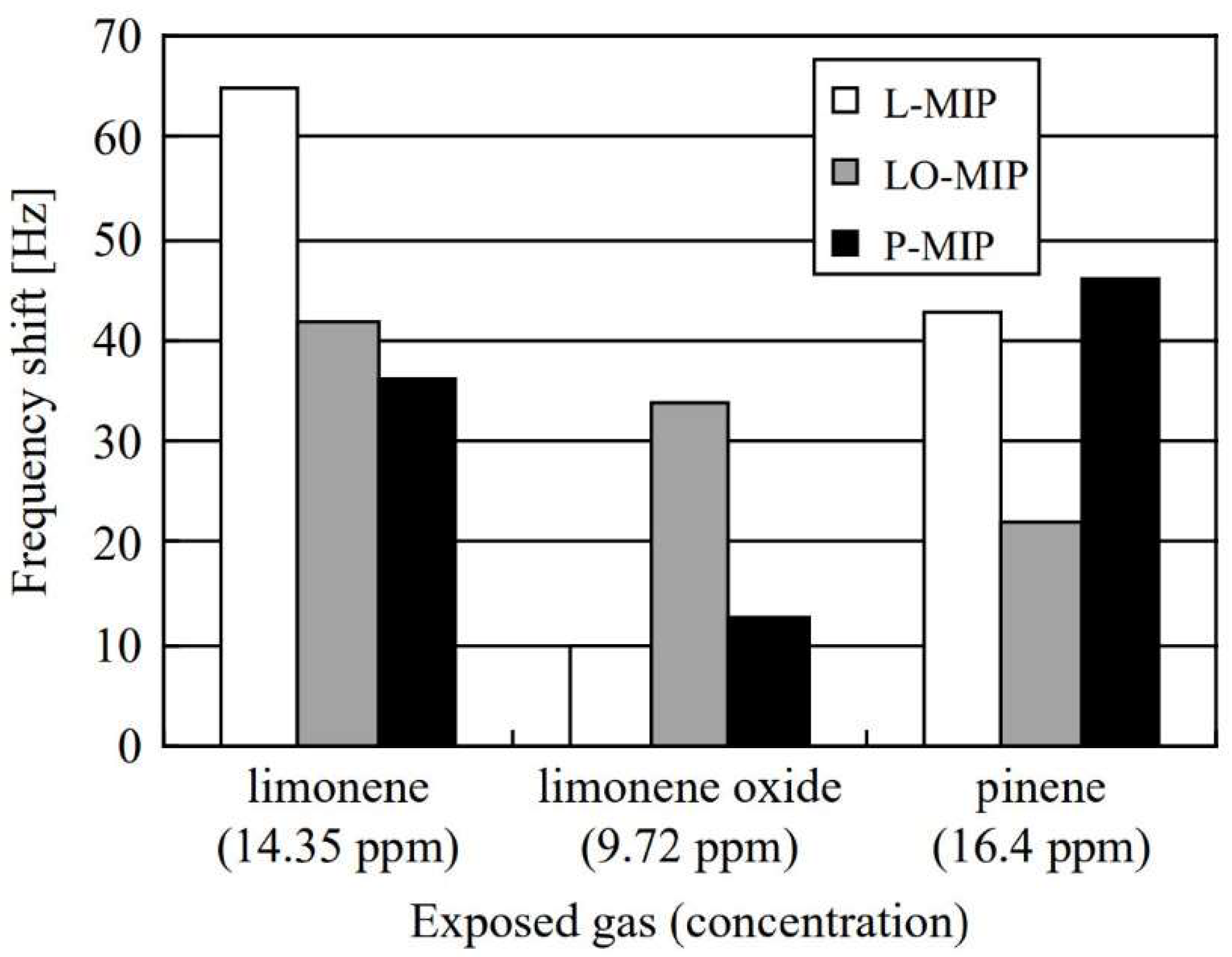

| Size | Template Imprinting | Porogen Imprinting | Structure |
|---|---|---|---|
| Small targets ≤ 10 atoms | Carbon dioxide [27,28,29,30,31,32,33], carbon monoxide [34], nitrogen dioxide [35], hydrogen sulfide [36], propenoic acid [37,38], ammonia [39], acetone [40,41], formaldehyde [16,21,42,43,44,45,46,47], furan [8], acetaldehyde [48], 1,3-dichloropropene [49], methanol [50,51], hydroxyl radical [52], ammonia [53,54] | Nitromethane [55], ethanol [56,57,58,59,60,61,62,63,64], methanol [22,60,65,66,67], formaldehyde [65,68], acetone [65,69], acetonitrile [41,70], water [61,71,72,73], chloroform [26] | Films: 42% Monoliths: 35% Nanoparticles: 24% |
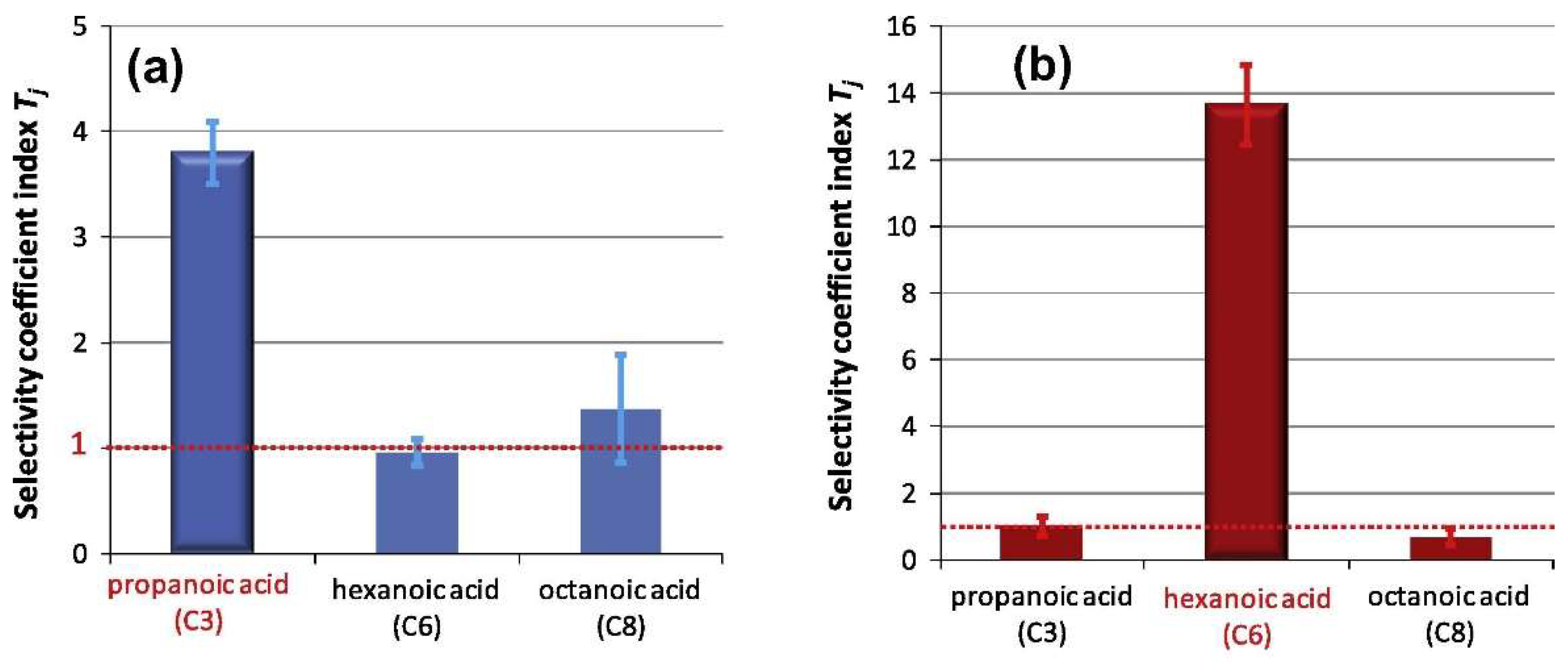
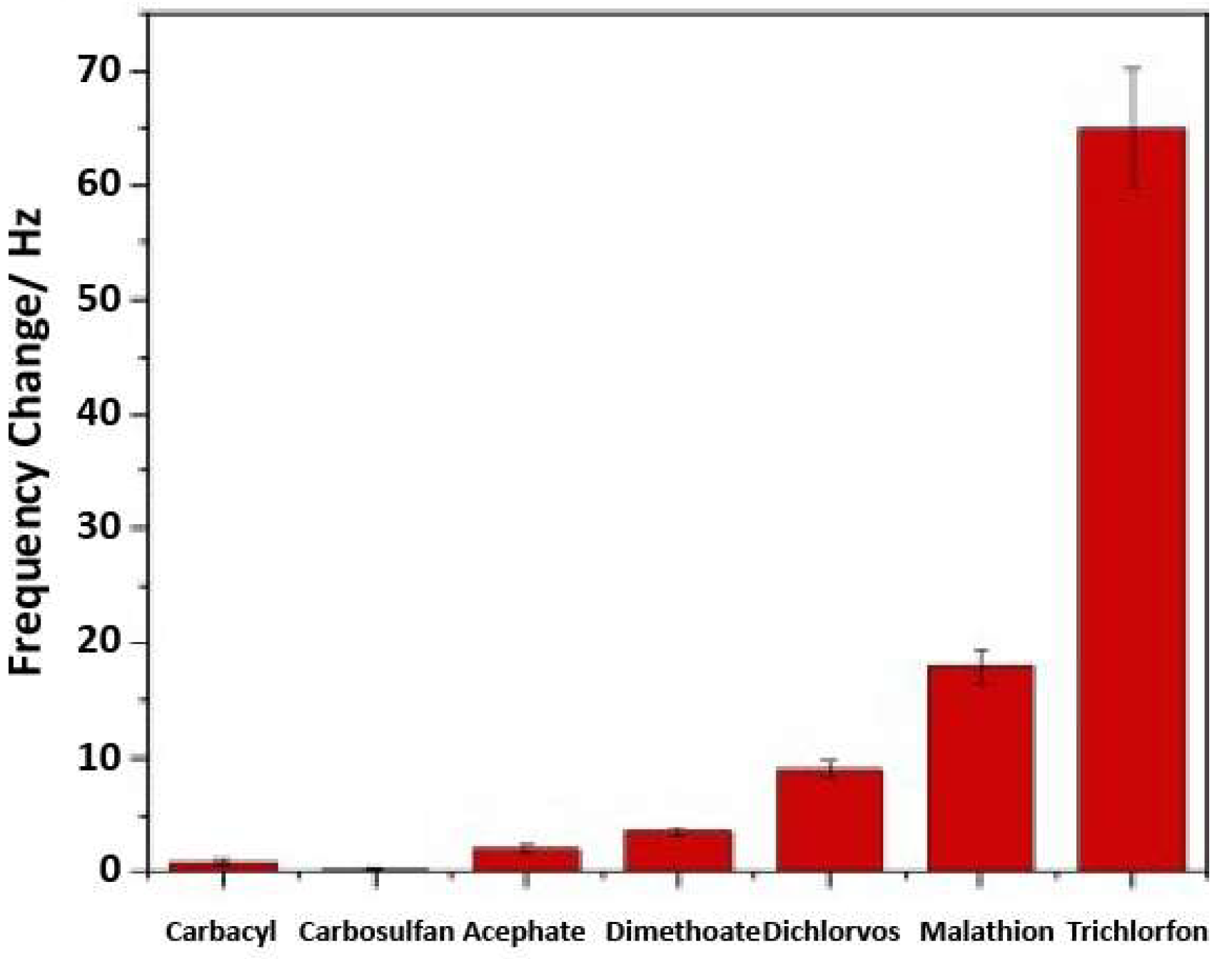
| Medium targets 11-25 atoms | Hexanal [74,75,76,77,78,79,80], hexanoic acid [23,37,81,82,83,84,85,86,87,88], propanoic acid [23,81,85,86,88], hexanone [81], heptanone [81], heptanal [23,37,79,89,90], acetoin [91], phenol [91], multiple aromatics [92,93], benzaldehyde [77], trinitrotoluene [9,10,19,94,95,96,97,98,99,100,101,102], octanone [23,81,89], heptanoic acid [23,85,86,88,90], hydroquinone [103,104], toluene [105,106], benzaldehyde [90], 2,4-dinitrotoluene [11,97,107,108,109], 4-nitrotoluene [110], trichlorfon [111], nitrobenzene [112], dimethyl methylphosphonate [18,113,114], diisopropyl methyl phosphonate [113], pyridine [26], benzene [73,106], naphthalene [115], trimethylamine [53], 2-phenylethanol [116], (R)-α-methylbenzylamine [117], histidine [118], carvacrol [119] | 3-Nitrotoluene [55], toluene [24,26,70,120,121,122,123,124,125,126], phenol [124], propionic acid [127], hexanoic acid [127], 4-ethylguaiacol [128], 4-ethylphenol [129], xylene [24,62,122,130,131,132,133,134,135,136,137], benzene [26,65,70], isopropanol [60], 1-butanol [61,71,72,73,138], ethyl acetate [44,62,71,72,73], 1-propanol [71,72,73], methyl benzoate [139], estragole [140], methyl salicylate [20], dimethyl methylphosphonate [141], tetrahydrofuran [62,64] | Films: 65% Monoliths: 20% Nanoparticles: 15% |
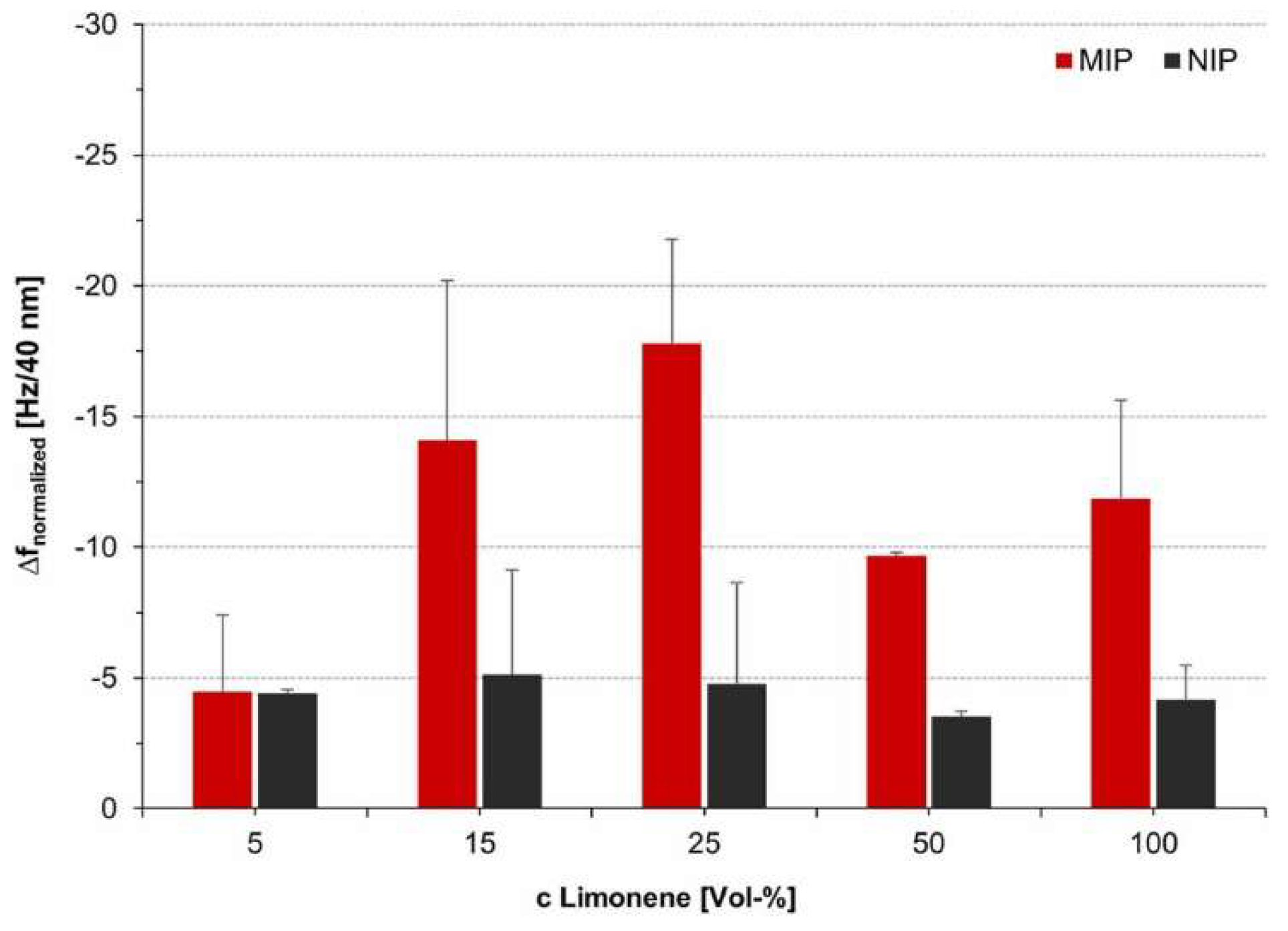
| Large targets >25 atoms | Geraniol [142], octanoic acid [23,37,38,81,85,86,88,89], limonene [143,144,145,146,147,148,149,150], limonene oxide [149], turmerone [151], curlone [151], ethyl-p-methoxycinnamate [151], cis-jasmone [147,152,153], linalool [150,154], geranial [155], neral [155], borneol [155], geraniol [150,155], nonanal [77,78,79,156], α-terpinyl acetate [157], α-pinene [146,147,149,150,158,159,160,161,162,163,164], β-phellandrene [158], 3-carene [158], cis-thujopsene [158], butylated hydroxytoluene [12,165], decanoic acid [166], nonanone [81], γ-terpinene [146,147,160,161], terpinolene [160,161], nicotine [167], adenosine monophosphate [14,168], pinacolyl methylphosphonate [169], parathion [170], 2-methylisoborneol [171], L-menthol [172], macromolecules [73,115,173,174,175,176] | Octanoic acid [127], heptane [61,177], limonene [71,72,73,140], α-pinene [140], β-pinene [140], eucalyptol [140], terpinene [140], diazinon [178] | Films: 88% Monoliths: 6% Nanoparticles: 5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cowen, T.; Cheffena, M. Template Imprinting Versus Porogen Imprinting of Small Molecules: A Review of Molecularly Imprinted Polymers in Gas Sensing. Int. J. Mol. Sci. 2022, 23, 9642. https://doi.org/10.3390/ijms23179642
Cowen T, Cheffena M. Template Imprinting Versus Porogen Imprinting of Small Molecules: A Review of Molecularly Imprinted Polymers in Gas Sensing. International Journal of Molecular Sciences. 2022; 23(17):9642. https://doi.org/10.3390/ijms23179642
Chicago/Turabian StyleCowen, Todd, and Michael Cheffena. 2022. "Template Imprinting Versus Porogen Imprinting of Small Molecules: A Review of Molecularly Imprinted Polymers in Gas Sensing" International Journal of Molecular Sciences 23, no. 17: 9642. https://doi.org/10.3390/ijms23179642
APA StyleCowen, T., & Cheffena, M. (2022). Template Imprinting Versus Porogen Imprinting of Small Molecules: A Review of Molecularly Imprinted Polymers in Gas Sensing. International Journal of Molecular Sciences, 23(17), 9642. https://doi.org/10.3390/ijms23179642






