Mapping QTL for Adult-Plant Resistance to Stripe Rust in a Chinese Wheat Landrace
Abstract
:1. Introduction
2. Results
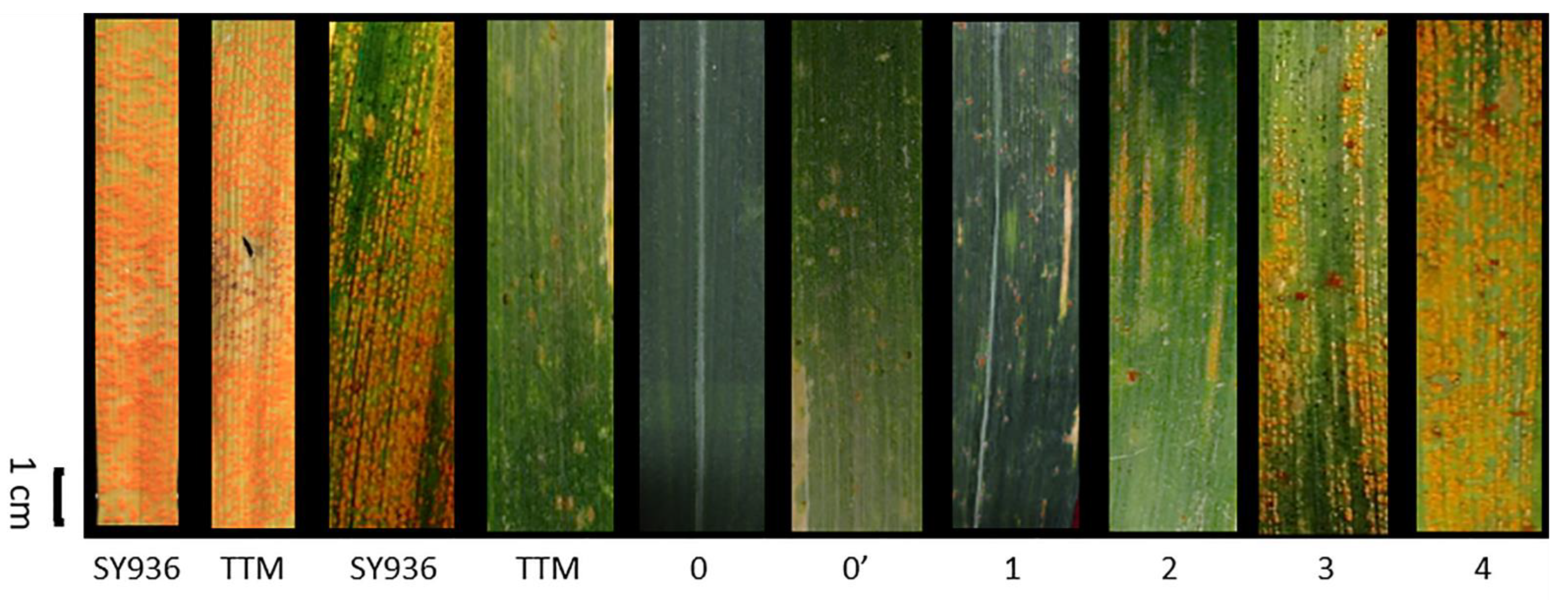
2.1. Phenotypic Variation and Correlation
2.2. QTL Mapping
2.3. Additive Effect of Identified QTLs
2.4. RNA-Seq Analysis of Two Parents
2.5. Enhancing Marker Density in QYr.sdau-1BL Linkage Map
2.6. DEGs within the QYr.sdau-1BL Region
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Seedling Stage YR Resistance Evaluation
4.3. Evaluation of Adult-Stage YR Resistance
4.4. Statistical Analysis
4.5. Linkage Map Construction and QTL Mapping
4.6. RNA-Seq
4.7. KASP Assays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wellings, C.R. Global status of stripe rust: A review of historical and current threats. Euphytica 2011, 179, 129–141. [Google Scholar] [CrossRef]
- Bariana, H.; Forrest, K.; Qureshi, N.; Miah, H.; Hayden, M.; Bansal, U. Adult plant stripe rust resistance gene Yr71 maps close to Lr24 in chromosome 3D of common wheat. Mol. Breed. 2016, 36, 98. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Jiang, Y.Y.; Feng, X.D.; Xia, B.; Zeng, J.; Liu, Y. Epidemic characteristics of wheat stripe rust in China in 2009 and its control strategies. China Plant Prot. 2009, 29, 4. [Google Scholar]
- Feng, J.; Wang, M.; See, D.R.; Chao, S.; Zheng, Y.; Chen, X. Characterization of Novel Gene Yr79 and Four Additional Quantitative Trait Loci for All-Stage and High-Temperature Adult-Plant Resistance to Stripe Rust in Spring Wheat PI 182103. Phytopathology 2018, 108, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Line, R.F. Stripe rust of wheat and barley in North America: A retrospective historical review. Annu. Rev. Phytopathol. 2002, 40, 75–118. [Google Scholar] [CrossRef]
- Ali, S.; Gladieux, P.; Leconte, M.; Gautier, A.; Justesen, A.F.; Hovmøller, M.S.; Enjalbert, J.; de Vallavieille-Pope, C. Origin, migration routes and worldwide population genetic structure of the wheat yellow rust pathogen Puccinia striiformis f.sp. tritici. PLoS Pathog. 2014, 10, e1003903. [Google Scholar] [CrossRef]
- Maree, G.J.; Prins, R.; Bender, C.M.; Boshoff, W.H.P.; Negussie, T.G.; Pretorius, Z.A. Phenotyping Kariega × Avocet S doubled haploid lines containing individual and combined adult plant stripe rust resistance loci. PLoS Pathog. 2019, 68, 659–668. [Google Scholar] [CrossRef]
- Lowe, I.; Jankuloski, L.; Chao, S.; Chen, X.; See, D.; Dubcovsky, J. Mapping and validation of QTL which confer partial resistance to broadly virulent post-2000 North American races of stripe rust in hexaploid wheat. Theor. Appl. Genet. 2011, 123, 143–157. [Google Scholar] [CrossRef]
- Wang, H.; Zou, S.; Li, Y.; Lin, F.; Tang, D. An ankyrin-repeat and WRKY-domain-containing immune receptor confers stripe rust resistance in wheat. Nat. Commun. 2020, 11, 1353. [Google Scholar] [CrossRef]
- McDonald, B.A.; Linde, C. The population genetics of plant pathogens and breeding strategies for durable resistance. Euphytica 2002, 124, 163–180. [Google Scholar] [CrossRef]
- Lu, Y.; Lan, C.; Liang, S.; Zhou, X.; Liu, D.; Zhou, G.; Lu, Q.; Jing, J.; Wang, M.; Xia, X.; et al. QTL mapping for adult-plant resistance to stripe rust in Italian common wheat cultivars Libellula and Strampelli. Theor. Appl. Genet. 2009, 119, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Poland, J.A.; Brown, P.J.; Sorrells, M.E.; Jannink, J.L. Development of high-density genetic maps for barley and wheat using a novel two-enzyme genotyping-by-sequencing approach. PLoS ONE 2012, 7, e32253. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wong, D.; Forrest, K.; Allen, A.; Chao, S.; Huang, B.E.; Maccaferri, M.; Salvi, S.; Milner, S.G.; Cattivelli, L.; et al. Characterization of polyploid wheat genomic diversity using a high-density 90,000 single nucleotide polymorphism array. Plant Biotechnol. J. 2014, 12, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, C.R.; Chao, S.; Wang, S.; Huang, B.E.; Stephen, S.; Kiani, S.; Forrest, K.; Saintenac, C.; Brown-Guedira, G.L.; Akhunova, A.; et al. Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc. Natl. Acad. Sci. USA 2013, 110, 8057–8062. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.M.; Chen, X.; Tabima, J.F.; See, D.R.; Vining, K.J.; Zemetra, R.S. QTL Analysis of Adult Plant Resistance to Stripe Rust in a Winter Wheat Recombinant Inbred Population. Plants 2021, 10, 572. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, T.; Li, X.; Yu, M.; Li, Y.; Yang, S.; Huang, K.; Han, D.; Kang, Z. Genome-wide mapping of adult plant stripe rust resistance in wheat cultivar Toni. Theor. Appl. Genet. 2019, 132, 1693–1704. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Gebrewahid, T.-W.; Liu, H.; Xia, X.; He, Z.; Li, Z.; Liu, D. QTL Mapping of Adult-Plant Resistance to Leaf and Stripe Rust in Wheat Cross SW 8588/Thatcher using the Wheat 55K SNP Array. Plant Dis. 2019, 103, 3041–3049. [Google Scholar] [CrossRef]
- Nsabiyera, V.; Bariana, H.S.; Qureshi, N.; Wong, D.; Hayden, M.J.; Bansal, U.K. Characterisation and mapping of adult plant stripe rust resistance in wheat accession Aus27284. Theor. Appl. Genet. 2018, 131, 1459–1467. [Google Scholar] [CrossRef]
- Voss-Fels, K.P.; Stahl, A.; Hickey, L.T. Q & A: Modern crop breeding for future food security. BMC Biol. 2019, 17, 18. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, T.; Cheng, Y.-K.; Ye, X.-L.; Li, W.; Pu, Z.-E.; Jiang, Q.-T.; Liu, Y.-X.; Wei, Y.-M.; Deng, M.; et al. Molecular mapping of a stripe rust resistance gene in Chinese wheat landrace “Hejiangyizai” using SSR, RGAP, TRAP, and SRAP markers. Crop Prot. 2017, 94, 178–184. [Google Scholar] [CrossRef]
- Gessese, M.; Bariana, H.; Wong, D.; Hayden, M.; Bansal, U. Molecular Mapping of Stripe Rust Resistance Gene Yr81 in a Common Wheat Landrace Aus27430. Plant Dis. 2019, 103, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Mirza, J.I.; Hussain, S.; Qureshi, N.; Forrest, K.; Bariana, H.; Bansal, U. Molecular mapping of all stage stripe rust resistance gene YrPak in wheat landrace PI388231. Euphytica 2021, 217, 121. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Yao, F.; Long, L.; Li, J.; Li, H.; Pu, Z.; Li, W.; Jiang, Q.; Wang, J.; et al. Molecular Mapping of a Novel Quantitative Trait Locus Conferring Adult Plant Resistance to Stripe Rust in Chinese Wheat Landrace Guangtoumai. Plant Dis. 2021, 105, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.-P.; Zeng, Q.-D.; Wu, J.-H.; Wang, Q.-L.; Yang, Z.-J.; Liang, B.-P.; Kang, Z.-S.; Chen, X.-H.; Han, D.-J. QTL Mapping and Validation of Adult Plant Resistance to Stripe Rust in Chinese Wheat Landrace Humai 15. Front. Plant Sci. 2018, 9, 986. [Google Scholar] [CrossRef]
- Rosewarne, G.M.; Herrera-Foessel, S.A.; Singh, R.P.; Huerta-Espino, J.; Lan, C.X.; He, Z.H. Quantitative trait loci of stripe rust resistance in wheat. Theor. Appl. Genet. 2013, 126, 2427–2449. [Google Scholar] [CrossRef]
- Cobo, N.; Pflüger, L.; Chen, X.; Dubcovsky, J. Mapping QTL for Resistance to New Virulent Races of Wheat Stripe Rust from Two Argentinean Wheat Cultivars. Crop Sci. 2018, 58, 2470–2483. [Google Scholar] [CrossRef]
- Yuan, C.; Singh, R.P.; Liu, D.; Randhawa, M.S.; Huerta-Espino, J.; Lan, C. Genome-Wide Mapping of Adult Plant Resistance to Leaf Rust and Stripe Rust in CIMMYT Wheat Line Arableu#1. Plant Dis. 2020, 104, 1455–1464. [Google Scholar] [CrossRef]
- William, M.; Singh, R.P.; Huerta-Espino, J.; Islas, S.O.; Hoisington, D. Molecular Marker Mapping of Leaf Rust Resistance Gene Lr46 and Its Association with Stripe Rust Resistance Gene Yr29 in Wheat. Phytopathology 2003, 93, 153–159. [Google Scholar] [CrossRef]
- Cobo, N.; Wanjugi, H.; Lagudah, E.; Dubcovsky, J. A High-Resolution Map of Wheat QYr.ucw-1BL, an Adult Plant Stripe Rust Resistance Locus in the Same Chromosomal Region as Yr29. Plant Genome 2019, 12, 180055. [Google Scholar] [CrossRef]
- William, H.M.; Singh, R.P.; Huerta-Espino, J.; Palacios, G.; Suenaga, K. Characterization of genetic loci conferring adult plant resistance to leaf rust and stripe rust in spring wheat. Genome 2006, 49, 977–990. [Google Scholar] [CrossRef]
- Dedryver, F.; Paillard, S.; Mallard, S.; Robert, O.; Trottet, M.; Nègre, S.; Verplancke, G.; Jahier, J. Characterization of Genetic Components Involved in Durable Resistance to Stripe Rust in the Bread Wheat ‘Renan’. Phytopathology 2009, 99, 968–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Chen, G.; Wei, Y.; Liu, Y.; Jiang, Q.; Li, W.; Pu, Z.; Lan, X.; Dai, S.; Zhang, M.; et al. Identification and mapping stripe rust resistance gene YrLM168a using extreme individuals and recessive phenotype class in a complicate genetic background. Mol. Genet. Genomics 2015, 290, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Chen, X.; Wang, M.; See, D.R.; Chao, S.; Bulli, P.; Jing, J. Mapping a Large Number of QTL for Durable Resistance to Stripe Rust in Winter Wheat Druchamp Using SSR and SNP Markers. PLoS ONE 2015, 10, e0126794. [Google Scholar] [CrossRef] [PubMed]
- Maccaferri, M.; Zhang, J.; Bulli, P.; Abate, Z.; Chao, S.; Cantu, D.; Bossolini, E.; Chen, X.; Pumphrey, M.; Dubcovsky, J. A Genome-Wide Association Study of Resistance to Stripe Rust (Puccinia striiformis f. sp. tritici) in a Worldwide Collection of Hexaploid Spring Wheat (Triticum aestivum L.). G3-Genes Genomes Genet. 2015, 5, 449–465. [Google Scholar] [CrossRef]
- Wu, J.; Liu, S.; Wang, Q.; Zeng, Q.; Mu, J.; Huang, S.; Yu, S.; Han, D.; Kang, Z. Rapid identification of an adult plant stripe rust resistance gene in hexaploid wheat by high-throughput SNP array genotyping of pooled extremes. Theor. Appl. Genet. 2018, 131, 43–58. [Google Scholar] [CrossRef]
- Mu, J.; Wu, J.; Liu, S.; Dai, M.; Sun, D.; Huang, S.; Wang, Q.; Zeng, Q.; Yu, S.; Chen, L.; et al. Genome-Wide Linkage Mapping Reveals Stripe Rust Resistance in Common Wheat (Triticum aestivum) Xinong1376. Plant Dis. 2019, 103, 2742–2750. [Google Scholar] [CrossRef]
- Luo, J.-T.; Zheng, J.-M.; Wan, H.-S.; Yang, W.-Y.; Li, S.-Z.; Pu, Z.-J. Identification of QTL for adult plant resistance to stripe rust in bread wheat line C33. J. Integr. Agric. 2020, 19, 624–631. [Google Scholar] [CrossRef]
- Long, L.; Yao, F.; Guan, F.; Cheng, Y.; Duan, L.; Zhao, X.; Li, H.; Pu, Z.; Li, W.; Jiang, Q.; et al. A Stable Quantitative Trait Locus on Chromosome 5BL Combined with Yr18 Conferring High-Level Adult Plant Resistance to Stripe Rust in Chinese Wheat Landrace Anyuehong. Phytopathology 2021, 111, 1594–1601. [Google Scholar] [CrossRef]
- Deng, M.; Long, L.; Cheng, Y.; Yao, F.; Guan, F.; Wang, Y.; Li, H.; Pu, Z.; Li, W.; Jiang, Q.; et al. Mapping a stable adult-plant stripe rust resistance QTL on chromosome 6AL in Chinese wheat landrace Yibinzhuermai. Crop J. 2022, 63, 256–279. [Google Scholar] [CrossRef]
- He, K.; Wu, Y. Receptor-Like Kinases and Regulation of Plant Innate Immunity. Enzymes 2016, 40, 105–142. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Y.; Ren, H.; Li, X.; Zhang, X.; Zhang, Z.; Zhang, C.; Liu, S.; Wang, X.; Zeng, Q.; et al. Epistatic interaction effect between chromosome 1BL (Yr29) and a novel locus on 2AL facilitating resistance to stripe rust in Chinese wheat Changwu 357-9. Theor. Appl. Genet. 2022, 135, 2501–2513. [Google Scholar] [CrossRef] [PubMed]
- Patil, G.; Chaudhary, J.; Vuong, T.D.; Jenkins, B.; Qiu, D.; Kadam, S.; Shannon, G.J.; Nguyen, H.T. Development of SNP Genotyping Assays for Seed Composition Traits in Soybean. Int. J. Plant Genom. 2017, 2017, 6572969. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Cai, S.; Wang, S.; Liu, S.; Zhang, G.; Bai, G. Genotyping-by-sequencing (GBS) identified SNP tightly linked to QTL for pre-harvest sprouting resistance. Theor. Appl. Genet. 2015, 128, 1385–1395. [Google Scholar] [CrossRef]
- Line, R.F.; Qayoum, A. Virulence, aggressiveness, evolution and distribution of races of Puccinia striiformis (the cause of stripe rust of wheat) in North America, 1968–1987. US Dep. Agric. Tech. Bull. 1992, 1788, 44. [Google Scholar]
- Zadoks, J.C. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zeng, S.M. Wheat Rust in China; China Agriculture Press: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Shaner, G.E. The Effect of Nitrogen Fertilization on the Expression of Slow-Mildewing Resistance in Knox Wheat. Phytopathology 1977, 77, 1051. [Google Scholar] [CrossRef]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]
- Liu, S.; Bai, G.; Cai, S.; Chen, C. Dissection of genetic components of preharvest sprouting resistance in white wheat. Mol. Breed. 2011, 27, 511–523. [Google Scholar] [CrossRef]
- Silva Lda, C.; Wang, S.; Zeng, Z.B. Composite interval mapping and multiple interval mapping: Procedures and guidelines for using Windows QTL Cartographer. Methods Mol. Biol. 2012, 871, 75–119. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- IWGSC. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Putri, G.H.; Anders, S.; Pyl, P.T.; Pimanda, J.E.; Zanini, F. Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics 2022, 38, 2943–2945. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [Green Version]





| Trait | Factor | Df | SS | MS | F-Value | p-Value | Heritability |
|---|---|---|---|---|---|---|---|
| IT | Genotype | 145 | 947.0 | 6.5 | 22.6 | 2.9 × 10−138 | 0.83 |
| Environment | 3 | 136.3 | 45.4 | 157.5 | 4.3 × 10−69 | ||
| G × E interaction | 435 | 365.3 | 0.8 | 2.9 | 6.8 × 10−28 | ||
| Replication/Env | 3 | 3.8 | 1.3 | 4.4 | 0.0044 | ||
| Residuals | 435 | 125.5 | 0.3 | ||||
| MDS | Genotype | 145 | 42,4371.7 | 2926.7 | 28.2 | 3.9 × 10−156 | 0.80 |
| Environment | 3 | 102,679.6 | 34,226.5 | 329.9 | 1.2 × 10−111 | ||
| G × E interaction | 435 | 195,868.6 | 450.3 | 4.3 | 4.0 × 10−149 | ||
| Replication/Env | 3 | 3215.9 | 1072.0 | 10.3 | 1.4 × 10−6 | ||
| Residuals | 435 | 45,129.7 | 103.7 | ||||
| AUDPC | Genotype | 145 | 25,294,308.9 | 174,443.5 | 36.2 | 4.8 × 10−177 | 0.83 |
| Environment | 3 | 4,829,528.6 | 1,609,842.9 | 334.3 | 1.7 × 10−112 | ||
| G × E interaction | 435 | 9,435,447.8 | 21,690.7 | 4.5 | 2.5 × 10−51 | ||
| Replication/Env | 3 | 347,671.7 | 115,890.6 | 24.1 | 2.0 × 10−14 | ||
| Residuals | 435 | 2,095,044.3 | 4816.2 |
| QTL | Genetic Distance (cM) | Physical Distance (Mb) | Year-Trait | LOD | PVE (%) | Add |
|---|---|---|---|---|---|---|
| QYr.sdau-1B | 126.3–150.7 | 653.0–682.1 | 16IT | 4.3 | 8.0 | −1.1 |
| 16MDS | 7.5 | 17.7 | −21.3 | |||
| 16AUDPC | 6.8 | 21.2 | −97.5 | |||
| 17IT | 4.2 | 9.7 | −0.5 | |||
| 17MDS | 4.2 | 11.0 | −10.8 | |||
| 17AUDPC | 2.7 | 13.5 | −76.9 | |||
| 18IT | 2.8 | 13.8 | −0.4 | |||
| 18MDS | 2.6 | 10.8 | −8.9 | |||
| 18AUDPC | 2.8 | 9.2 | −46.5 | |||
| QYr.sdau-5B | 32.2–39.4 | 617.7–657.4 | 16IT | 4 | 14.7 | 0.7 |
| 16MDS | 5.9 | 22.7 | 19.5 | |||
| 16AUDPC | 4.6 | 19.2 | 129.3 | |||
| 18IT | 2.6 | 10.1 | 0.5 | |||
| 18MDS | 2.6 | 12.1 | 12.4 | |||
| 18AUDPC | 3.0 | 13.4 | 74.1 | |||
| QYr.sdau-6B | 19.2–27.6 | 500.6–598.5 | 16AUDPC | 2.6 | 12.1 | −87.5 |
| 17IT | 4.5 | 16.5 | −0.5 | |||
| 17MDS | 2.9 | 12.4 | −8.9 | |||
| 18IT | 4.5 | 18.0 | −0.7 | |||
| 18MDS | 3.2 | 13.7 | −14.3 | |||
| 18AUDPC | 3.0 | 11.6 | −69.4 | |||
| 19MDS | 3.2 | 12.1 | −14.7 | |||
| 19AUDPC | 4.4 | 16.4 | −131.5 |
| GeneID | Functional Annotation |
|---|---|
| TraesCS1B01G438800 | Disease-resistance protein (TIR-NBS-LRR class) family |
| TraesCS1B01G439400 | Disease-resistance protein (TIR-NBS-LRR class) family |
| TraesCS1B01G440700 | WRKY transcription factor |
| TraesCS1B01G447400 | Disease-resistance protein RPM1 |
| TraesCS1B01G448700 | Receptor-like protein kinase |
| TraesCS1B01G451600 | Receptor-like protein kinase |
| TraesCS1B01G452600 | Disease-resistance protein (CC-NBS-LRR class) family |
| TraesCS1B01G454600 | Receptor-like protein kinase, putative, expressed |
| TraesCS1B01G456700 | Calcium-dependent protein kinase |
| TraesCS1B01G460100 | Disease-resistance protein (NBS-LRR class) family |
| TraesCS1B01G464400 | Disease-resistance protein RGA2 |
| TraesCS1B01G466400 | Disease-resistance protein |
| TraesCS1B01G466900 | Disease-resistance protein |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.; Liu, C.; Lin, M.; Ni, F.; Li, W.; Cai, J.; Zhang, Z.; Zhu, H.; Liu, J.; Wu, J.; et al. Mapping QTL for Adult-Plant Resistance to Stripe Rust in a Chinese Wheat Landrace. Int. J. Mol. Sci. 2022, 23, 9662. https://doi.org/10.3390/ijms23179662
Pang Y, Liu C, Lin M, Ni F, Li W, Cai J, Zhang Z, Zhu H, Liu J, Wu J, et al. Mapping QTL for Adult-Plant Resistance to Stripe Rust in a Chinese Wheat Landrace. International Journal of Molecular Sciences. 2022; 23(17):9662. https://doi.org/10.3390/ijms23179662
Chicago/Turabian StylePang, Yunlong, Chunxia Liu, Meng Lin, Fei Ni, Wenhui Li, Jin Cai, Ziliang Zhang, Huaqiang Zhu, Jingxian Liu, Jiajie Wu, and et al. 2022. "Mapping QTL for Adult-Plant Resistance to Stripe Rust in a Chinese Wheat Landrace" International Journal of Molecular Sciences 23, no. 17: 9662. https://doi.org/10.3390/ijms23179662






