Targeted Mitochondrial Epigenetics: A New Direction in Alzheimer’s Disease Treatment
Abstract
:1. Introduction
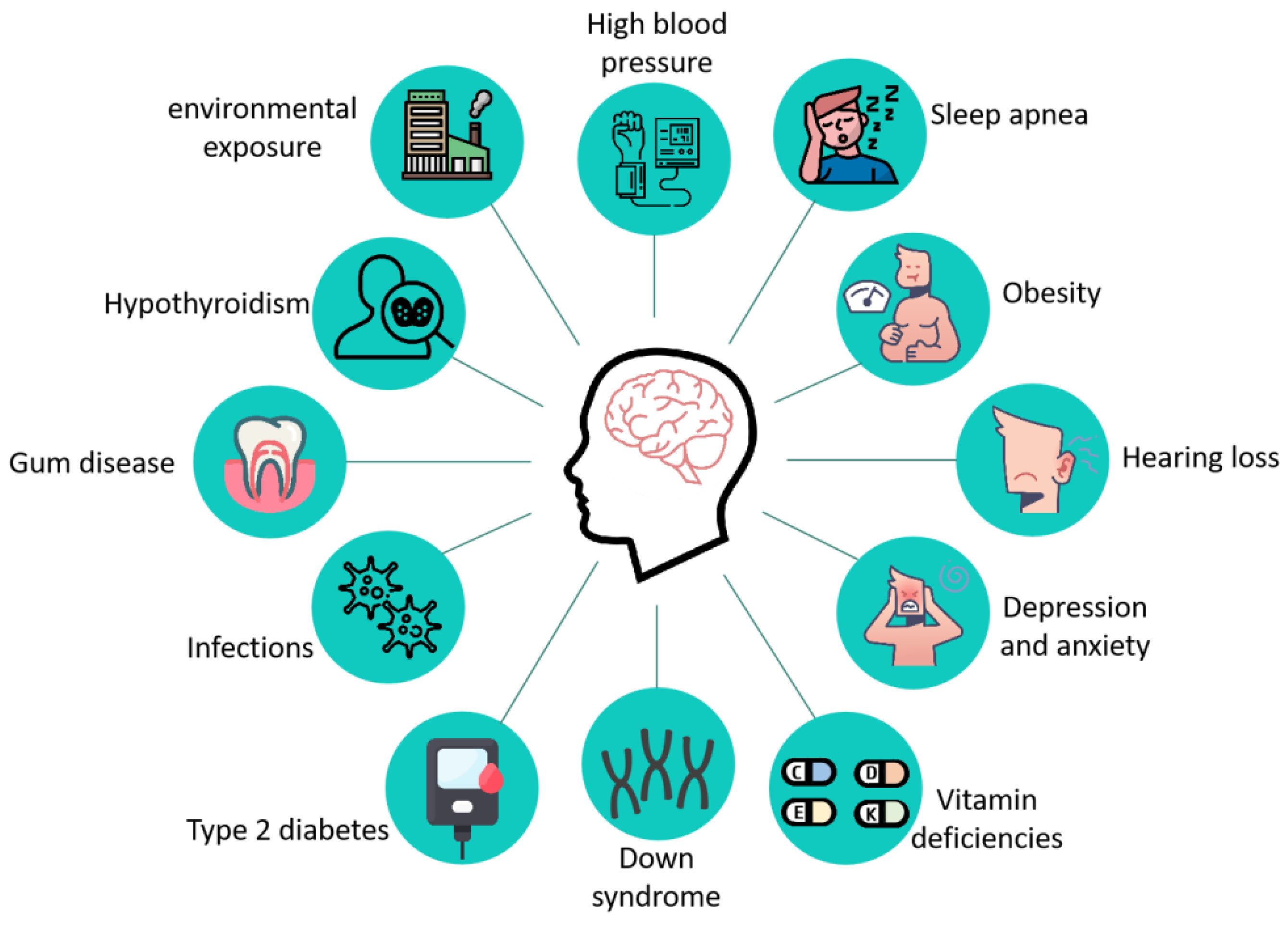
2. Pathological Features of AD
3. Mitochondrial Epigenetic Changes in AD
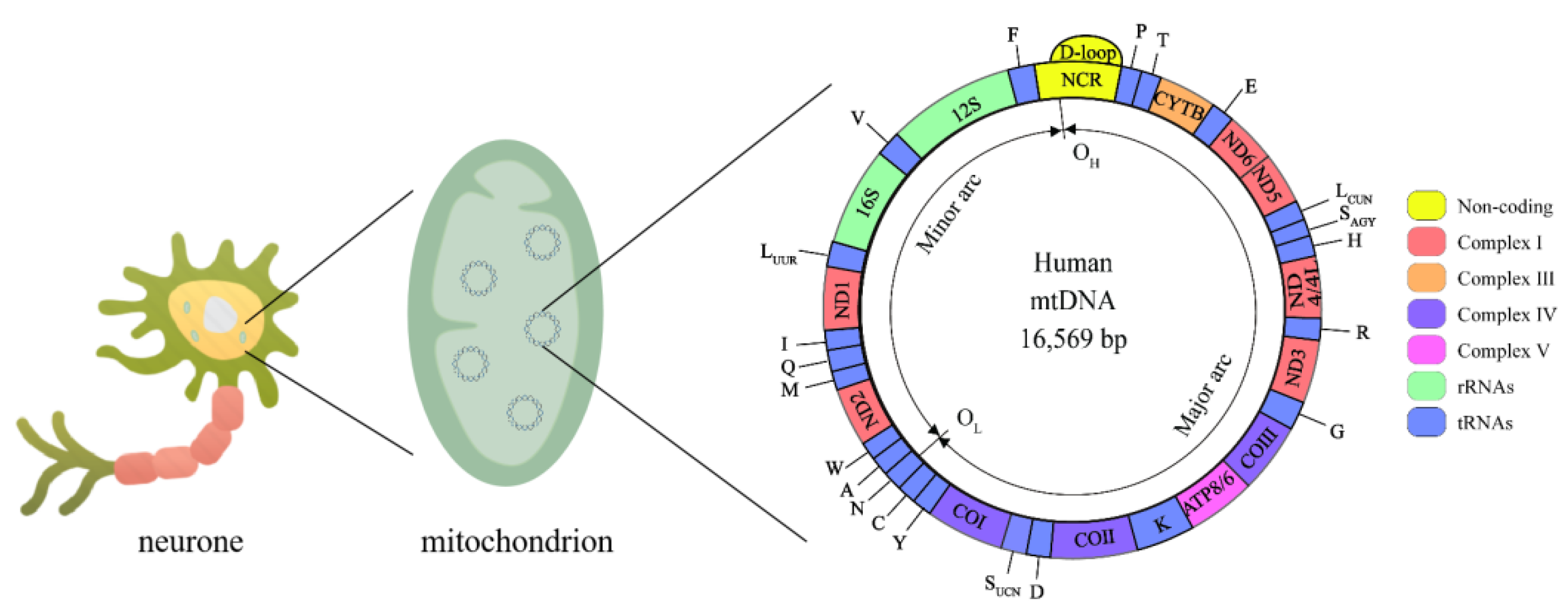
3.1. Mitochondrial Genome and Mitochondrial Epigenetics
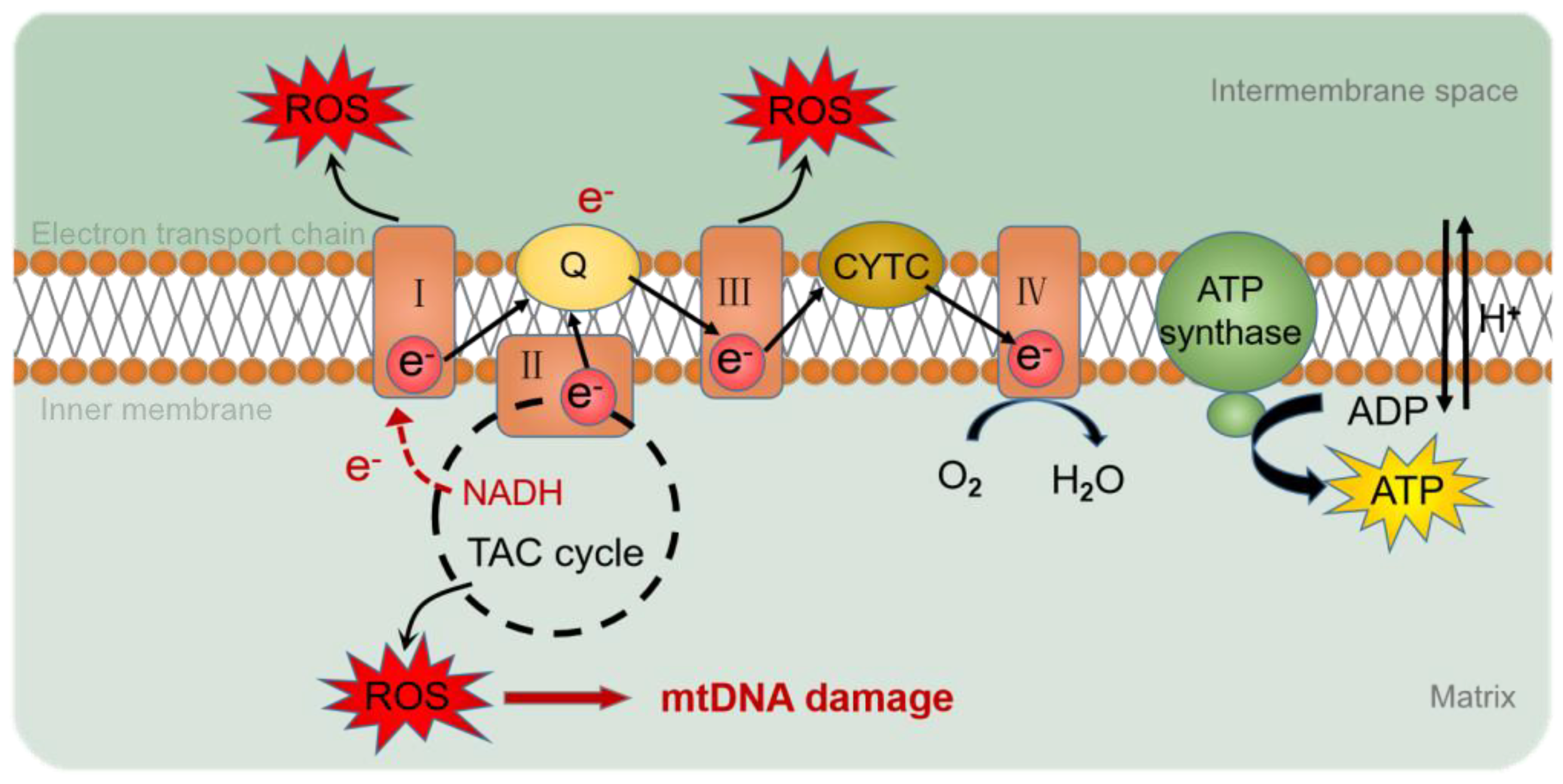
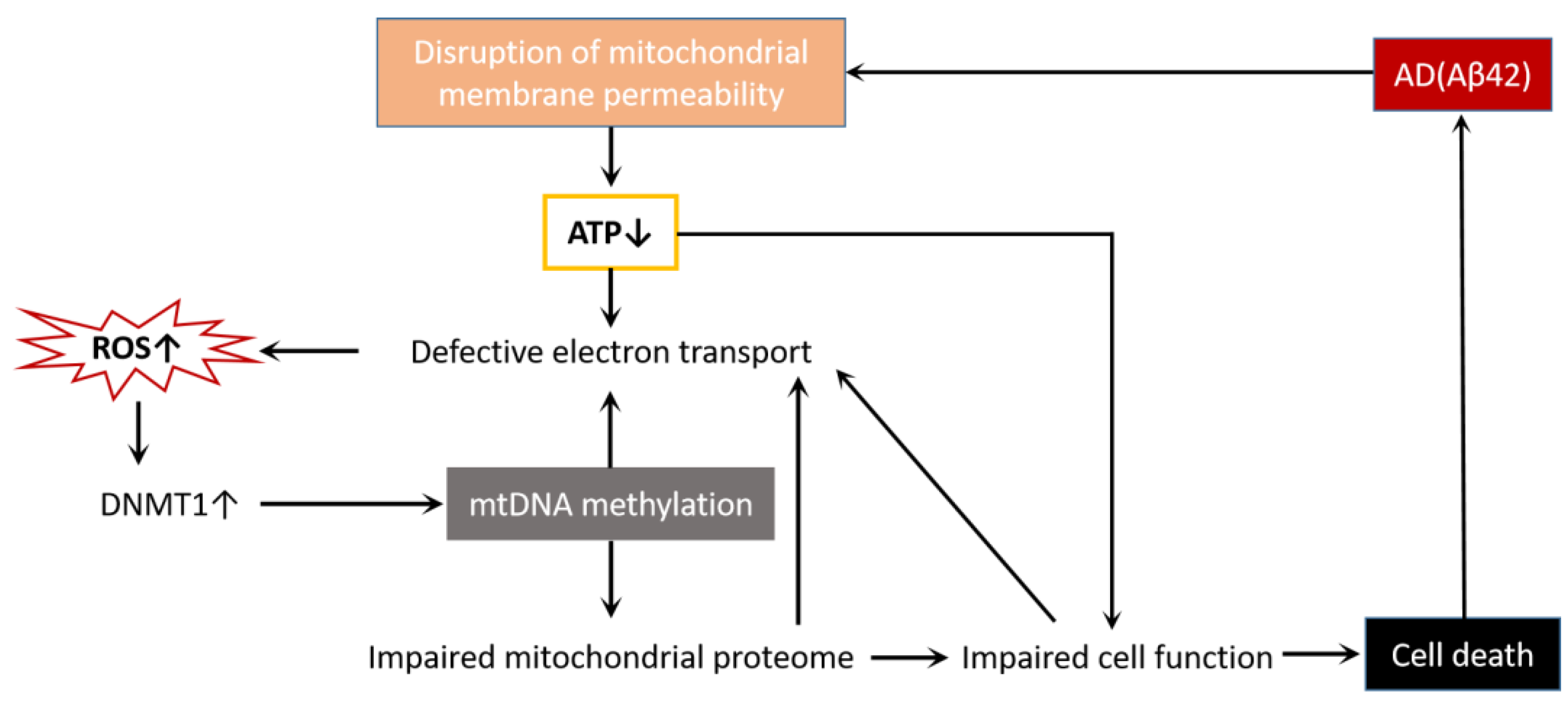
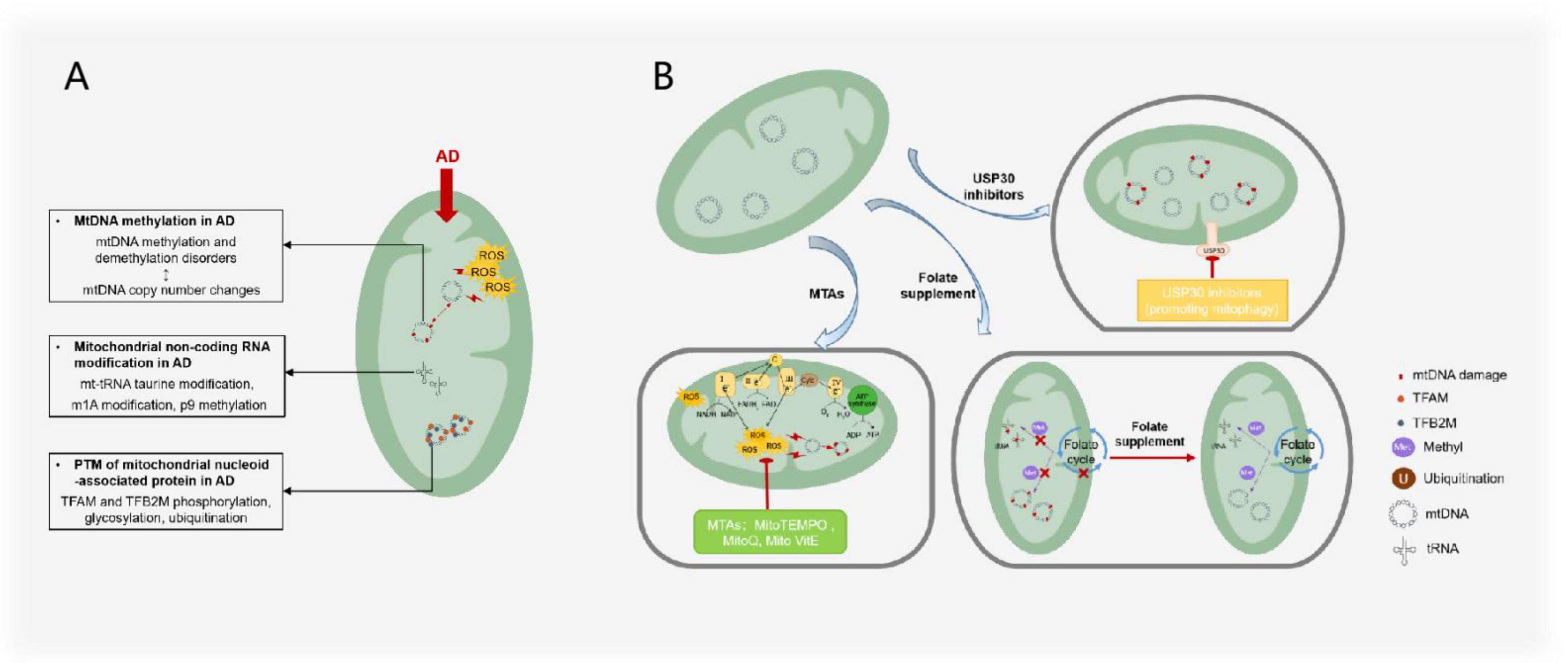
3.2. mtDNA Methylation in AD
3.3. Mitochondrial Noncoding RNA Modification in AD
3.4. Post-Translational Modification of Mitochondrial Nucleoid-Associated Protein in AD
4. AD Treatments by Mitochondrial Epigenetics
4.1. Folate Supplements
4.2. Mitochondria-Targeted Antioxidants
4.3. Targeted Ubiquitin-Specific Proteases
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Disease International. Available online: https://www.alzint.org/ (accessed on 10 July 2021).
- Magierski, R.; Sobow, T. Serotonergic drugs for the treatment of neuropsychiatric symptoms in dementia. Expert Rev. Neurother. 2016, 16, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Soria Lopez, J.A.; Gonzalez, H.M.; Leger, G.C. Alzheimer’s disease. Handb. Clin. Neurol. 2019, 167, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Bastrup, J.; Kastaniegaard, K.; Asuni, A.A.; Volbracht, C.; Stensballe, A. Proteomic and Unbiased Post-Translational Modification Profiling of Amyloid Plaques and Surrounding Tissue in a Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 73, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Schwartz, G.G.; Nicholls, S.J.; Khan, A.; Halliday, C.; Toth, P.P.; Sweeney, M.; Johansson, J.O.; Wong, N.C.W.; Kulikowski, E.; et al. Cognitive Effects of the BET Protein Inhibitor Apabetalone: A Prespecified Montreal Cognitive Assessment Analysis Nested in the BETonMACE Randomized Controlled Trial. J. Alzheimer’s Dis. 2021, 83, 1703–1715. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Y.; Jing, H.; Meng, Q.; Yang, J. Novel DNA methylation loci and genes showing pleiotropic association with Alzheimer’s dementia: A network Mendelian randomization analysis. Epigenetics 2021, 17, 746–758. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, L.; Zeng, Y. DNA methylation in Alzheimer’s disease: In brain and peripheral blood. Mech. Ageing Dev. 2020, 191, 111319. [Google Scholar] [CrossRef]
- Yu, T.W.; Lane, H.Y.; Lin, C.H. Novel Therapeutic Approaches for Alzheimer’s Disease: An Updated Review. Int. J. Mol. Sci. 2021, 22, 8208. [Google Scholar] [CrossRef]
- Abeysinghe, A.; Deshapriya, R.; Udawatte, C. Alzheimer’s disease: A review of the pathophysiological basis and therapeutic interventions. Life Sci. 2020, 256, 117996. [Google Scholar] [CrossRef]
- Moran, C. Author response: Type 2 diabetes mellitus, brain atrophy, and cognitive decline. Neurology 2020, 94, 49. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, B.; Wang, X.; Zhang, X.; Yang, Q.X.; Qing, Z.; Zhang, W.; Zhu, D.; Bi, Y. Olfactory Dysfunction Mediates Adiposity in Cognitive Impairment of Type 2 Diabetes: Insights from Clinical and Functional Neuroimaging Studies. Diabetes Care 2019, 42, 1274–1283. [Google Scholar] [CrossRef]
- Nasrallah, I.M.; Gaussoin, S.A.; Pomponio, R.; Dolui, S.; Erus, G.; Wright, C.B.; Launer, L.J.; Detre, J.A.; Wolk, D.A.; Davatzikos, C.; et al. Association of Intensive vs. Standard Blood Pressure Control with Magnetic Resonance Imaging Biomarkers of Alzheimer Disease: Secondary Analysis of the SPRINT MIND Randomized Trial. JAMA Neurol. 2021, 78, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Rouch, L.; Rolland, Y.; Hanon, O.; Vidal, J.S.; Cestac, P.; Sallerin, B.; Andrieu, S.; Vellas, B.; Barreto, P.S.; Group, M.D. Blood pressure, antihypertensive drugs, and incident frailty: The Multidomain Alzheimer Preventive Trial (MAPT). Maturitas 2022, 162, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Espeland, M.A.; Carmichael, O.; Yasar, S.; Hugenschmidt, C.; Hazzard, W.; Hayden, K.M.; Rapp, S.R.; Neiberg, R.; Johnson, K.C.; Hoscheidt, S.; et al. Sex-related differences in the prevalence of cognitive impairment among overweight and obese adults with type 2 diabetes. Alzheimer’s Dement. 2018, 14, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Ptomey, L.T.; Szabo, A.N.; Willis, E.A.; Gorczyca, A.M.; Greene, J.L.; Danon, J.C.; Donnelly, J.E. Changes in cognitive function after a 12-week exercise intervention in adults with Down syndrome. Disabil. Health J. 2018, 11, 486–490. [Google Scholar] [CrossRef]
- Llano, D.A.; Issa, L.K.; Devanarayan, P.; Devanarayan, V.; Alzheimer’s Disease Neuroimaging Initiative (ADNI). Hearing Loss in Alzheimer’s Disease Is Associated with Altered Serum Lipidomic Biomarker Profiles. Cells 2020, 9, 2556. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Ge, B.; Zhou, D.; Li, M.; Li, W.; Ma, F.; Liu, Z.; Ji, Y.; Huang, G. Effects of Folic Acid and Vitamin B12 Supplementation on Cognitive Impairment and Inflammation in Patients with Alzheimer’s Disease: A Randomized, Single-Blinded, Placebo-Controlled Trial. J. Prev. Alzheimer’s Dis. 2021, 8, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Vyas, C.M.; Okereke, O.I.; Ogata, S.; Albert, M.; Lee, I.M.; D’Agostino, D.; Buring, J.E.; Cook, N.R.; Grodstein, F.; et al. Effect of vitamin D on cognitive decline: Results from two ancillary studies of the VITAL randomized trial. Sci. Rep. 2021, 11, 23253. [Google Scholar] [CrossRef] [PubMed]
- Caselli, R.J.; Knopman, D.S.; Bu, G. An agnostic reevaluation of the amyloid cascade hypothesis of Alzheimer’s disease pathogenesis: The role of APP homeostasis. Alzheimer’s Dement. 2020, 16, 1582–1590. [Google Scholar] [CrossRef]
- Ghosh, S.; Jana, K.; Wakchaure, P.D.; Ganguly, B. Revealing the cholinergic inhibition mechanism of Alzheimer’s by galantamine: A metadynamics simulation study. J. Biomol. Struct. Dyn. 2022, 40, 5100–5111. [Google Scholar] [CrossRef]
- Megur, A.; Baltriukiene, D.; Bukelskiene, V.; Burokas, A. The Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients 2021, 13, 37. [Google Scholar] [CrossRef]
- Sanchez-Sarasua, S.; Fernandez-Perez, I.; Espinosa-Fernandez, V.; Sanchez-Perez, A.M.; Ledesma, J.C. Can We Treat Neuroinflammation in Alzheimer’s Disease? Int. J. Mol. Sci. 2020, 21, 8751. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Ali, S.A.; Singh, R.K. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110112. [Google Scholar] [CrossRef] [PubMed]
- Mejido, D.C.P.; Peny, J.A.; Vieira, M.N.N.; Ferreira, S.T.; De Felice, F.G. Insulin and leptin as potential cognitive enhancers in metabolic disorders and Alzheimer’s disease. Neuropharmacology 2020, 171, 108115. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Maillard, P.; Harvey, D.; Fletcher, E.; Martinez, O.; Johnson, D.K.; Olichney, J.M.; Farias, S.T.; Villeneuve, S.; Jagust, W.; et al. Association of vascular brain injury, neurodegeneration, amyloid, and cognitive trajectory. Neurology 2020, 95, e2622–e2634. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Khachaturian, A.S.; Vergallo, A.; Farlow, M.R.; Snyder, P.J.; Giacobini, E.; Khachaturian, Z.S. Revisiting the Cholinergic Hypothesis in Alzheimer’s Disease: Emerging Evidence from Translational and Clinical Research. J. Prev. Alzheimer’s Dis. 2019, 6, 2–15. [Google Scholar] [CrossRef]
- Mullane, K.; Williams, M. Alzheimer’s disease (AD) therapeutics—1: Repeated clinical failures continue to question the amyloid hypothesis of AD and the current understanding of AD causality. Biochem. Pharmacol. 2018, 158, 359–375. [Google Scholar] [CrossRef]
- Lee, J.H.; Yang, D.S.; Goulbourne, C.N.; Im, E.; Stavrides, P.; Pensalfini, A.; Chan, H.; Bouchet-Marquis, C.; Bleiwas, C.; Berg, M.J.; et al. Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Abeta in neurons, yielding senile plaques. Nat. Neurosci. 2022, 25, 688–701. [Google Scholar] [CrossRef]
- Sharma, N.; Pasala, M.S.; Prakash, A. Mitochondrial DNA: Epigenetics and environment. Environ. Mol. Mutagen. 2019, 60, 668–682. [Google Scholar] [CrossRef]
- Sharma, A.; Schaefer, S.T.; Sae-Lee, C.; Byun, H.M.; Wullner, U. Elevated serum mitochondrial DNA in females and lack of altered platelet mitochondrial methylation in patients with Parkinson’s disease. Int. J. Neurosci. 2021, 131, 279–282. [Google Scholar] [CrossRef]
- Stoccoro, A.; Mosca, L.; Carnicelli, V.; Cavallari, U.; Lunetta, C.; Marocchi, A.; Migliore, L.; Coppede, F. Mitochondrial DNA copy number and D-loop region methylation in carriers of amyotrophic lateral sclerosis gene mutations. Epigenomics 2018, 10, 1431–1443. [Google Scholar] [CrossRef]
- Stoccoro, A.; Smith, A.R.; Mosca, L.; Marocchi, A.; Gerardi, F.; Lunetta, C.; Cereda, C.; Gagliardi, S.; Lunnon, K.; Migliore, L.; et al. Reduced mitochondrial D-loop methylation levels in sporadic amyotrophic lateral sclerosis. Clin. Epigenet. 2020, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Radhakrishnan, R.; Kowluru, R.A. Epigenetic Modifications Compromise Mitochondrial DNA Quality Control in the Development of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3943–3951. [Google Scholar] [CrossRef]
- Shi, F.F.; Qiu, J.Y.; Zhang, S.Z.; Zhao, X.; Feng, D.F.; Feng, X.Z. Exogenous melatonin protects preimplantation embryo development from decabromodiphenyl ethane-induced circadian rhythm disorder and endogenous melatonin reduction. Environ. Pollut. 2022, 292, 118445. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Johnson, J.; St John, J.C. Global DNA methylation synergistically regulates the nuclear and mitochondrial genomes in glioblastoma cells. Nucleic Acids Res. 2018, 46, 5977–5995. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.L.; Yang, M.S.Z.; Wu, G.Y.; Mo, S.; Wu, X.G.; Zhang, S.X.; Yu, R.Y.; Hu, Y.M.; Xu, Y.R.; Li, Z.W.; et al. Epigenetic Induction of Mitochondrial Fission Is Required for Maintenance of Liver Cancer-Initiating Cells. Cancer Res. 2021, 81, 3835–3848. [Google Scholar] [CrossRef]
- Sas, K.; Szabo, E.; Vecsei, L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules 2018, 23, 191. [Google Scholar] [CrossRef]
- Liu, B.J.; Du, Q.Q.; Chen, L.; Fu, G.P.; Li, S.J.; Fu, L.H.; Zhang, X.J.; Ma, C.L.; Cong, B. CpG methylation patterns of human mitochondrial DNA. Sci. Rep. 2016, 6, 23421. [Google Scholar] [CrossRef]
- Stoccoro, A.; Coppede, F. Mitochondrial DNA Methylation and Human Diseases. Int. J. Mol. Sci. 2021, 22, 4594. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, C.; Zhang, J.; Lai, R.; Zhang, X.; Guo, Z. Mitochondrial Displacement Loop Region SNPs Modify Sjogren’s Syndrome Development by Regulating Cytokines Expression in Female Patients. Front. Genet. 2022, 13, 847521. [Google Scholar] [CrossRef]
- Hernandez-Romero, I.A.; Guerra-Calderas, L.; Salgado-Albarran, M.; Maldonado-Huerta, T.; Soto-Reyes, E. The Regulatory Roles of Non-coding RNAs in Angiogenesis and Neovascularization from an Epigenetic Perspective. Front. Oncol. 2019, 9, 1091. [Google Scholar] [CrossRef]
- Gambardella, S.; Limanaqi, F.; Ferese, R.; Biagioni, F.; Campopiano, R.; Centonze, D.; Fornai, F. ccf-mtDNA as a Potential Link Between the Brain and Immune System in Neuro-Immunological Disorders. Front. Immunol. 2019, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.M.; Panni, T.; Motta, V.; Hou, L.; Nordio, F.; Apostoli, P.; Bertazzi, P.A.; Baccarelli, A.A. Effects of airborne pollutants on mitochondrial DNA methylation. Part. Fibre Toxicol. 2013, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Goodrich, J.M.; Strakovsky, R.S. Mitochondrial Epigenetics and Environmental Health: Making a Case for Endocrine Disrupting Chemicals. Toxicol. Sci. 2020, 178, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Zhu, J.J.; Yu Tian, X.; Liu, H.; Zhang, T.; Zhang, Y.P.; Xie, S.A.; Zheng, M.; Kong, W.; Yao, W.J.; et al. Hypermethylation of mitochondrial DNA in vascular smooth muscle cells impairs cell contractility. Cell Death Dis. 2020, 11, 35. [Google Scholar] [CrossRef]
- Wang, W.L.; Liu, X.X.; Wang, X.D. How to breakthrough mitochondrial DNA methylation-associated networks. Cell Biol. Toxicol. 2020, 36, 195–198. [Google Scholar] [CrossRef]
- Hroudova, J.; Singh, N.; Fisar, Z. Mitochondrial dysfunctions in neurodegenerative diseases: Relevance to Alzheimer’s disease. Biomed. Res. Int. 2014, 2014, 175062. [Google Scholar] [CrossRef]
- Shock, L.S.; Thakkar, P.V.; Peterson, E.J.; Moran, R.G.; Taylor, S.M. DNA methyltransferase 1, cytosine methylation, and cytosine hydroxymethylation in mammalian mitochondria. Proc. Natl. Acad. Sci. USA 2011, 108, 3630–3635. [Google Scholar] [CrossRef]
- Xu, Y.; Cheng, L.; Sun, J.; Li, F.; Liu, X.; Wei, Y.; Han, M.; Zhu, Z.; Bi, J.; Lai, C.; et al. Hypermethylation of Mitochondrial Cytochrome b and Cytochrome c Oxidase II Genes with Decreased Mitochondrial DNA Copy Numbers in the APP/PS1 Transgenic Mouse Model of Alzheimer’s Disease. Neurochem. Res. 2021, 46, 564–572. [Google Scholar] [CrossRef]
- Breton, C.V.; Song, A.Y.; Xiao, J.; Kim, S.J.; Mehta, H.H.; Wan, J.; Yen, K.; Sioutas, C.; Lurmann, F.; Xue, S.; et al. Effects of air pollution on mitochondrial function, mitochondrial DNA methylation, and mitochondrial peptide expression. Mitochondrion 2019, 46, 22–29. [Google Scholar] [CrossRef]
- Liu, Q.S.J.; Li, H.Y.; Guo, L.Q.; Chen, Q.; Gao, X.; Li, P.H.; Tang, N.J.; Guo, X.B.A.; Deng, F.R.; Wu, S.W. Effects of short-term personal exposure to air pollution on platelet mitochondrial DNA methylation levels and the potential mitigation by L-arginine supplementation. J. Hazard. Mater. 2021, 417, 125963. [Google Scholar] [CrossRef]
- Mishra, M.; Kowluru, R.A. DNA Methylation-a Potential Source of Mitochondria DNA Base Mismatch in the Development of Diabetic Retinopathy. Mol. Neurobiol. 2019, 56, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, A.A.; Byun, H.M. Platelet mitochondrial DNA methylation: A potential new marker of cardiovascular disease. Clin. Epigenet. 2015, 7, 44. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, S.Y.; Kim, S.A. Mitochondrial DNA Methylation Is Higher in Acute Coronary Syndrome Than in Stable Coronary Artery Disease. In Vivo 2021, 35, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Xiong, L.L.; Ji, Z.N.; Cheng, W.; Yang, H.J. Correlation between increased ND2 expression and demethylated displacement loop of mtDNA in colorectal cancer. Mol. Med. Rep. 2012, 6, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Yang, X.; Pan, Y.F. Can Mitochondria DNA Provide a Novel Biomarker for Evaluating the Risk and Prognosis of Colorectal Cancer? Dis. Markers 2017, 2017, 5189803. [Google Scholar] [CrossRef]
- Zhang, J.W.; Shang, J.K.; Wang, F.Y.; Huo, X.J.; Sun, R.H.; Ren, Z.X.; Wang, W.; Yang, M.M.; Li, G.; Gao, D.D.; et al. Decreased mitochondrial D-loop region methylation mediates an increase in mitochondrial DNA copy number in CADASIL. Clin. Epigenet. 2022, 14, 2. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Interaction of neurons and astrocytes underlies the mechanism of Abeta-induced neurotoxicity. Biochem. Soc. Trans. 2014, 42, 1286–1290. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, L.; Han, M.; Liu, X.; Li, F.; Zhou, X.; Wang, Y.; Bi, J. Altered mitochondrial DNA methylation and mitochondrial DNA copy number in an APP/PS1 transgenic mouse model of Alzheimer disease. Biochem. Biophys. Res. Commun. 2019, 520, 41–46. [Google Scholar] [CrossRef]
- Blanch, M.; Mosquera, J.L.; Ansoleaga, B.; Ferrer, I.; Barrachina, M. Altered Mitochondrial DNA Methylation Pattern in Alzheimer Disease-Related Pathology and in Parkinson Disease. Am. J. Pathol. 2016, 186, 385–397. [Google Scholar] [CrossRef]
- Stoccoro, A.; Tannorella, P.; Migliore, L.; Coppede, F. Polymorphisms of genes required for methionine synthesis and DNA methylation influence mitochondrial DNA methylation. Epigenomics 2020, 12, 1003–1012. [Google Scholar] [CrossRef]
- Stoccoro, A.; Siciliano, G.; Migliore, L.; Coppede, F. Decreased Methylation of the Mitochondrial D-Loop Region in Late-Onset Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 59, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Stoccoro, A.; Baldacci, F.; Ceravolo, R.; Giampietri, L.; Tognoni, G.; Siciliano, G.; Migliore, L.; Coppede, F. Increase in Mitochondrial D-Loop Region Methylation Levels in Mild Cognitive Impairment Individuals. Int. J. Mol. Sci. 2022, 23, 5393. [Google Scholar] [CrossRef] [PubMed]
- Delbarba, A.; Abate, G.; Prandelli, C.; Marziano, M.; Buizza, L.; Arce Varas, N.; Novelli, A.; Cuetos, F.; Martinez, C.; Lanni, C.; et al. Mitochondrial Alterations in Peripheral Mononuclear Blood Cells from Alzheimer’s Disease and Mild Cognitive Impairment Patients. Oxid. Med. Cell. Longev. 2016, 2016, 5923938. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Lee, S.Y.; Park, M.; Kim, S.Y.; Kim, J.W.; Kim, S.A.; Kim, B.N. Peripheral Mitochondrial DNA Copy Number is Increased in Korean Attention-Deficit Hyperactivity Disorder Patients. Front. Psychiatry 2019, 10, 506. [Google Scholar] [CrossRef]
- Sanyal, T.; Bhattacharjee, P.; Bhattacharjee, S.; Bhattacharjee, P. Hypomethylation of mitochondrial D-loop and ND6 with increased mitochondrial DNA copy number in the arsenic-exposed population. Toxicology 2018, 408, 54–61. [Google Scholar] [CrossRef]
- Chen, H.; Shi, Z.; Guo, J.; Chang, K.J.; Chen, Q.; Yao, C.H.; Haigis, M.C.; Shi, Y. The human mitochondrial 12S rRNA m(4)C methyltransferase METTL15 is required for mitochondrial function. J. Biol. Chem. 2020, 295, 8505–8513. [Google Scholar] [CrossRef]
- Chakraborty, A.; Viswanathan, P. Methylation-Demethylation Dynamics: Implications of Changes in Acute Kidney Injury. Anal. Cell. Pathol. 2018, 2018, 8764384. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Cavalcante, G.C.; Brito, L.M.; Schaan, A.P.; Ribeiro-Dos-Santos, A.; de Araujo, G.S.; On Behalf of Alzheimer’s Disease Neuroimaging, I. Mitochondrial Genetics Reinforces Multiple Layers of Interaction in Alzheimer’s Disease. Biomedicines 2022, 10, 880. [Google Scholar] [CrossRef]
- Janssen, B.G.; Byun, H.M.; Gyselaers, W.; Lefebvre, W.; Baccarelli, A.A.; Nawrot, T.S. Placental mitochondrial methylation and exposure to airborne particulate matter in the early life environment: An ENVIRONAGE birth cohort study. Epigenetics 2015, 10, 536–544. [Google Scholar] [CrossRef] [Green Version]
- Devall, M.; Mill, J.; Lunnon, K. The mitochondrial epigenome: A role in Alzheimer’s disease? Epigenomics 2014, 6, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Nikolac Perkovic, M.; Videtic Paska, A.; Konjevod, M.; Kouter, K.; Svob Strac, D.; Nedic Erjavec, G.; Pivac, N. Epigenetics of Alzheimer’s Disease. Biomolecules 2021, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Nagao, A.; Suzuki, T. Human mitochondrial diseases caused by lack of taurine modification in mitochondrial tRNAs. Wiley Interdiscip. Rev. RNA 2011, 2, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Shafik, A.M.; Zhou, H.; Lim, J.; Dickinson, B.; Jin, P. Dysregulated mitochondrial and cytosolic tRNA m1A methylation in Alzheimer’s disease. Hum. Mol. Genet. 2022, 31, 1673–1680. [Google Scholar] [CrossRef]
- Silzer, T.K.; Pathak, G.A.; Phillips, N.R. Mitochondrial tRNA methylation in Alzheimer’s disease and progressive supranuclear palsy. BMC Med. Genom. 2020, 13, 71. [Google Scholar] [CrossRef]
- Idaghdour, Y.; Hodgkinson, A. Integrated genomic analysis of mitochondrial RNA processing in human cancers. Genome Med. 2017, 9, 36. [Google Scholar] [CrossRef]
- Sharma, J.; Kumari, R.; Bhargava, A.; Tiwari, R.; Mishra, P.K. Mitochondrial-induced Epigenetic Modifications: From Biology to Clinical Translation. Curr. Pharm. Des. 2021, 27, 159–176. [Google Scholar] [CrossRef]
- Hensen, F.; Cansiz, S.; Gerhold, J.M.; Spelbrink, J.N. To be or not to be a nucleoid protein: A comparison of mass-spectrometry based approaches in the identification of potential mtDNA-nucleoid associated proteins. Biochimie 2014, 100, 219–226. [Google Scholar] [CrossRef]
- Ramachandran, A.; Basu, U.; Sultana, S.; Nandakumar, D.; Patel, S.S. Human mitochondrial transcription factors TFAM and TFB2M work synergistically in promoter melting during transcription initiation. Nucleic Acids Res. 2017, 45, 861–874. [Google Scholar] [CrossRef]
- Lin, M.M.; Liu, N.; Qin, Z.H.; Wang, Y. Mitochondrial-derived damage-associated molecular patterns amplify neuroinflammation in neurodegenerative diseases. Acta Pharmacol. Sin. 2022. [Google Scholar] [CrossRef]
- Fang, Y.; Akimoto, M.; Mayanagi, K.; Hatano, A.; Matsumoto, M.; Matsuda, S.; Yasukawa, T.; Kang, D. Chemical acetylation of mitochondrial transcription factor A occurs on specific lysine residues and affects its ability to change global DNA topology. Mitochondrion 2020, 53, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lee, J.; Nie, X.; Li, M.; Morozov, Y.I.; Venkatesh, S.; Bogenhagen, D.F.; Temiakov, D.; Suzuki, C.K. Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA+ Lon protease. Mol. Cell 2013, 49, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Suarez, J.; Hu, Y.; Makino, A.; Fricovsky, E.; Wang, H.; Dillmann, W.H. Alterations in mitochondrial function and cytosolic calcium induced by hyperglycemia are restored by mitochondrial transcription factor A in cardiomyocytes. Am. J. Physiol.-Cell Physiol. 2008, 295, C1561–C1568. [Google Scholar] [CrossRef] [PubMed]
- Garedew, A.; Andreassi, C.; Moncada, S. Mitochondrial dynamics, biogenesis, and function are coordinated with the cell cycle by APC/C CDH1. Cell Metab. 2012, 15, 466–479. [Google Scholar] [CrossRef]
- Reardon, S.D.; Mishanina, T.V. Phosphorylation and acetylation of mitochondrial transcription factor A promote transcription processivity without compromising initiation or DNA compaction. J. Biol. Chem. 2022, 298, 101815. [Google Scholar] [CrossRef]
- King, G.A.; Hashemi Shabestari, M.; Taris, K.H.; Pandey, A.K.; Venkatesh, S.; Thilagavathi, J.; Singh, K.; Krishna Koppisetti, R.; Temiakov, D.; Roos, W.H.; et al. Acetylation and phosphorylation of human TFAM regulate TFAM-DNA interactions via contrasting mechanisms. Nucleic Acids Res. 2018, 46, 3633–3642. [Google Scholar] [CrossRef]
- Wang, K.Z.; Zhu, J.; Dagda, R.K.; Uechi, G.; Cherra, S.J., 3rd; Gusdon, A.M.; Balasubramani, M.; Chu, C.T. ERK-mediated phosphorylation of TFAM downregulates mitochondrial transcription: Implications for Parkinson’s disease. Mitochondrion 2014, 17, 132–140. [Google Scholar] [CrossRef]
- Santos, J.M.; Mishra, M.; Kowluru, R.A. Posttranslational modification of mitochondrial transcription factor A in impaired mitochondria biogenesis: Implications in diabetic retinopathy and metabolic memory phenomenon. Exp. Eye Res. 2014, 121, 168–177. [Google Scholar] [CrossRef]
- Esselun, C.; Theyssen, E.; Eckert, G.P. Effects of Urolithin A on Mitochondrial Parameters in a Cellular Model of Early Alzheimer Disease. Int. J. Mol. Sci. 2021, 22, 8333. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Xu, L.L.; Cao, D.Y.; Song, C.Y.; Wang, Y.Z.; Li, M.Y.; Yan, J.K.; Xie, Z.H. Effect of orexin-A on mitochondrial biogenesis, mitophagy and structure in HEK293-APPSWE cell model of Alzheimer’s disease. Clin. Exp. Pharmacol. Physiol. 2021, 48, 355–360. [Google Scholar] [CrossRef]
- Posse, V.; Gustafsson, C.M. Human Mitochondrial Transcription Factor B2 Is Required for Promoter Melting during Initiation of Transcription. J. Biol. Chem. 2017, 292, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Bostwick, A.M.; Moya, G.E.; Senti, M.L.; Basu, U.; Shen, J.Y.; Patel, S.S.; Dittenhafer-Reed, K.E. Phosphorylation of mitochondrial transcription factor B2 controls mitochondrial DNA binding and transcription. Biochem. Biophys. Res. Commun. 2020, 528, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Hinterberger, M.; Fischer, P. Folate and Alzheimer: When time matters. J. Neural Transm. 2013, 120, 211–224. [Google Scholar] [CrossRef]
- An, Y.; Feng, L.; Zhang, X.; Wang, Y.; Wang, Y.; Tao, L.; Qin, Z.; Xiao, R. Dietary intakes and biomarker patterns of folate, vitamin B6, and vitamin B12 can be associated with cognitive impairment by hypermethylation of redox-related genes NUDT15 and TXNRD1. Clin. Epigenet. 2019, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Morscher, R.J.; Ducker, G.S.; Li, S.H.J.; Mayer, J.A.; Gitai, Z.; Sperl, W.; Rabinowitz, J.D. Mitochondrial translation requires folate-dependent tRNA methylation. Nature 2018, 554, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Hama, Y.; Hamano, T.; Shirafuji, N.; Hayashi, K.; Ueno, A.; Enomoto, S.; Nagata, M.; Kimura, H.; Matsunaga, A.; Ikawa, M.; et al. Influences of Folate Supplementation on Homocysteine and Cognition in Patients with Folate Deficiency and Cognitive Impairment. Nutrients 2020, 12, 3138. [Google Scholar] [CrossRef] [PubMed]
- Monacelli, F.; Acquarone, E.; Giannotti, C.; Borghi, R.; Nencioni, A. Vitamin C, Aging and Alzheimer’s Disease. Nutrients 2017, 9, 670. [Google Scholar] [CrossRef]
- Yu, J.T.; Xu, W.; Tan, C.C.; Andrieu, S.; Suckling, J.; Evangelou, E.; Pan, A.; Zhang, C.; Jia, J.; Feng, L.; et al. Evidence-based prevention of Alzheimer’s disease: Systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1201–1209. [Google Scholar] [CrossRef]
- Casati, M.; Boccardi, V.; Ferri, E.; Bertagnoli, L.; Bastiani, P.; Ciccone, S.; Mansi, M.; Scamosci, M.; Rossi, P.D.; Mecocci, P.; et al. Vitamin E and Alzheimer’s disease: The mediating role of cellular aging. Aging Clin. Exp. Res. 2020, 32, 459–464. [Google Scholar] [CrossRef]
- McManus, M.J.; Murphy, M.P.; Franklin, J.L. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 15703–15715. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.Y.; Fan, Z.; Zhang, Z.; Fan, J. MitoQ protects against high glucose-induced brain microvascular endothelial cells injury via the Nrf2/HO-1 pathway. J. Pharmacol. Sci. 2021, 145, 105–114. [Google Scholar] [CrossRef]
- McCormick, B.; Lowes, D.A.; Colvin, L.; Torsney, C.; Galley, H.F. MitoVitE, a mitochondria-targeted antioxidant, limits paclitaxel-induced oxidative stress and mitochondrial damage in vitro, and paclitaxel-induced mechanical hypersensitivity in a rat pain model. Br. J. Anaesth. 2016, 117, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Yu, Q.; Yan, S.S. Mitochondrial oxidative stress contributes to the pathological aggregation and accumulation of tau oligomers in Alzheimer’s disease. Hum. Mol. Genet. 2022, 31, 2498–2507. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Li, R.; Sun, Y.; Dou, M.; Yang, W.; He, S.; Zhang, Y. Effect of mito-TEMPO, a mitochondria-targeted antioxidant, in rats with neuropathic pain. Neuroreport 2018, 29, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Perumal, E.; Bi, X.; Wang, Y.; Ding, W. Potential mechanisms of uremic muscle wasting and the protective role of the mitochondria-targeted antioxidant Mito-TEMPO. Int. Urol. Nephrol. 2020, 52, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, Y.; Liu, Y.; Tang, C.; et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017, 11, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.D.; Jasem, S.; Licchesi, J.D.F. The Ubiquitin System in Alzheimer’s Disease. Adv. Exp. Med. Biol. 2020, 1233, 195–221. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.Y.; Song, B.B.; Li, G.L.; Cai, F.; Wu, M.L.; Zhao, Y.J.; Jiang, L.L.; Guo, T.T.; Shen, M.Y.; Hou, H.; et al. USP25 inhibition ameliorates Alzheimer’s pathology through the regulation of APP processing and A beta generation. J. Clin. Investig. 2022, 132, e152170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Li, G.; Wang, S.; Zhou, Y.; Liu, K.; Gao, Y.; Zhou, Y.; Zheng, L.; Zhu, L.; Deng, Q.; et al. Trisomy 21-induced dysregulation of microglial homeostasis in Alzheimer’s brains is mediated by USP25. Sci. Adv. 2021, 7, eabe1340. [Google Scholar] [CrossRef] [PubMed]
- Rusilowicz-Jones, E.V.; Jardine, J.; Kallinos, A.; Pinto-Fernandez, A.; Guenther, F.; Giurrandino, M.; Barone, F.G.; McCarron, K.; Burke, C.J.; Murad, A.; et al. USP30 sets a trigger threshold for PINK1-PARKIN amplification of mitochondrial ubiquitylation. Life Sci. Alliance 2020, 3, e202000768. [Google Scholar] [CrossRef]
- Nakamura, N.; Hirose, S. Regulation of mitochondrial morphology by USP30, a deubiquitinating enzyme present in the mitochondrial outer membrane. Mol. Biol. Cell 2008, 19, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wang, R.; Xu, H.; Tu, L.; Chen, H.; Li, H.; Liu, N.; Wang, J.; Li, S.; Yin, F.; et al. Identification of an autoinhibitory, mitophagy-inducing peptide derived from the transmembrane domain of USP30. Autophagy 2022, 18, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mechta, M.; Ingerslev, L.R.; Barres, R. Methodology for Accurate Detection of Mitochondrial DNA Methylation. J. Vis. Exp. 2018, 135, e57772. [Google Scholar] [CrossRef] [PubMed]
- Iacobazzi, V.; Castegna, A.; Infantino, V.; Andria, G. Mitochondrial DNA methylation as a next-generation biomarker and diagnostic tool. Mol. Genet. Metab. 2013, 110, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Ji, L.; Wu, T.; Ji, Y.; Zhou, Y.; Zheng, M.; Zhang, M.; Xu, W.; Huang, G. Folic Acid Supplementation Mitigates Alzheimer’s Disease by Reducing Inflammation: A Randomized Controlled Trial. Mediat. Inflamm. 2016, 2016, 5912146. [Google Scholar] [CrossRef]
- Tsefou, E.; Walker, A.S.; Clark, E.H.; Hicks, A.R.; Luft, C.; Takeda, K.; Watanabe, T.; Ramazio, B.; Staddon, J.M.; Briston, T.; et al. Investigation of USP30 inhibition to enhance Parkin-mediated mitophagy: Tools and approaches. Biochem. J. 2021, 478, 4099–4118. [Google Scholar] [CrossRef]
| Sample | mtDNA Methylation | mtDNA Copy Number | Sequencing Methods | Observation | Reference |
|---|---|---|---|---|---|
| The hippocampi of APP/PS1 transgenic mice | The mtDNA methylations increase in CYTB and COX II | Decreases | Bisulfite pyrosequencing | Hypermethylation of the mitochondrial CYTB and COX II genes reduces mtDNA CN and gene expression in the APP/PS1 mouse model of AD. | [49] |
| The hippocampi of APP/PS1 transgenic mice | The mtDNA methylation reduces in the D-loop and increases in the 12S rRNA gene | Decreases | Bisulfite pyrosequencing | Increased methylation levels of the 12S rRNA gene decreased 12S rRNA gene expression, decreasing mitochondrial biogenesis and mitochondrial dysfunction. | [59] |
| Entorhinal cortex in brain samples with Alzheimer’s disease-related pathology and cerebral cortex of APP/PS1 mice | The mtDNA methylation reduces in the MT-ND1 gene and increases in the D-loop | / | 454 GS FLX Titanium pyrosequencer | An increase in 5 mC in the promoter gene is usually accompanied by 5 mC damage to the coding region. | [60] |
| A previously described dataset of 263 subjects of Caucasian origin | The mtDNA methylation reduces in the D-loop | / | Methylation-sensitive high-resolution melting analysis | Concerning DNMT3A-448A>G polymorphisms, AA genotype carriers were only observed in patients with AD, and the level of D-loop methylation in AA genotype carriers was significantly higher than in GG and GA carriers. | [61] |
| Peripheral blood of LOAD samples | The mtDNA methylation reduces in the D-loop | / | Methylation-sensitive high-resolution melting analysis | The degree of methylation in the D-loop region may characterize different tissue or stages of disease in individuals with AD. | [62] |
| The peripheral blood cells of 14 mild cognitive impairment (MCI) patients, 18 early stage AD patients, 70 advanced-stage AD patients, and 105 healthy control subjects | D-loop methylation decreased in patients with AD in the late stages, while there was no significant change in the early stages, and D-loop methylation increased in patients with MCI in the late stages | / | Methylation-sensitive high-resolution melting analysis | D-loop methylation is inversely correlated with cerebrospinal fluid p-tau levels and age at the time of sampling. Mitochondrial methylation of the D-loop is associated with different stages of AD, and these methylation changes are recognizable in the peripheral blood and therefore may provide biomarkers for the disease. | [63] |
| peripheral blood mononuclear cells | / | Decreases | / | CYT C, CYT B, PGC-1 α, and TFAM were decreased in AD | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Zhu, X.-Y.; Zhang, X.-M.; Xiong, H. Targeted Mitochondrial Epigenetics: A New Direction in Alzheimer’s Disease Treatment. Int. J. Mol. Sci. 2022, 23, 9703. https://doi.org/10.3390/ijms23179703
Song Y, Zhu X-Y, Zhang X-M, Xiong H. Targeted Mitochondrial Epigenetics: A New Direction in Alzheimer’s Disease Treatment. International Journal of Molecular Sciences. 2022; 23(17):9703. https://doi.org/10.3390/ijms23179703
Chicago/Turabian StyleSong, Ying, Xin-Yi Zhu, Xiao-Min Zhang, and He Xiong. 2022. "Targeted Mitochondrial Epigenetics: A New Direction in Alzheimer’s Disease Treatment" International Journal of Molecular Sciences 23, no. 17: 9703. https://doi.org/10.3390/ijms23179703




