Abstract
Tumor recurrence after liver transplantation has been linked to multiple factors, including the recipient’s tumor burden, donor factors, and ischemia-reperfusion injury (IRI). The increasing number of livers accepted from extended criteria donors has forced the transplant community to push the development of dynamic perfusion strategies. The reason behind this progress is the urgent need to reduce the clinical consequences of IRI. Two concepts appear most beneficial and include either the avoidance of ischemia, e.g., the replacement of cold storage by machine perfusion, or secondly, an endischemic organ improvement through perfusion in the recipient center prior to implantation. While several concepts, including normothermic perfusion, were found to reduce recipient transaminase levels and early allograft dysfunction, hypothermic oxygenated perfusion also reduced IRI-associated post-transplant complications and costs. With the impact on mitochondrial injury and subsequent less IRI-inflammation, this endischemic perfusion was also found to reduce the recurrence of hepatocellular carcinoma after liver transplantation. Firstly, this article highlights the contributing factors to tumor recurrence, including the surgical and medical tissue trauma and underlying mechanisms of IRI-associated inflammation. Secondly, it focuses on the role of mitochondria and associated interventions to reduce cancer recurrence. Finally, the role of machine perfusion technology as a delivery tool and as an individual treatment is discussed together with the currently available clinical studies.
1. Introduction
In the United States (US), an increase in newly diagnosed liver cancers of 41‘260 and an increase in related deaths rate of 30‘520 are expected in 2022 [1]. Hepatocellular carcinoma (HCC) is the most common primary liver cancer. Liver transplantation (LT) offers an opportunity to cure, treating both the tumor and the underlying chronic liver disease. With the development of current HCC classifications and tailored listing concepts, the overall results after LT are satisfactory. In addition, LT plays an increasing role in the treatment of other primary liver cancers, including cholangiocarcinoma and colorectal and neuroendocrine liver metastases [2,3,4,5]. However, the disparity between organ utilization and demand limits the access to LT for many of those patients. To overcome such challenges, livers from extended criteria donors (ECDs), including donation after circulatory death (DCD), are increasingly utilized. Based on an evolving experience, risk-adapted organ selection is common practice to avoid well-known complications, including primary non-function (PNF), early allograft dysfunction (EAD), and ischemic cholangiopathy (IC) [6,7]. To limit the overall risk further, ECD livers are often allocated to recipients with liver tumors and preserved liver function, which may better tolerate the inflammation related with post-transplant ischemia-reperfusion injury (IRI). A thorough donor and recipient selection was suggested by several authors of retrospective cohort studies to achieve acceptable tumor recurrence rates with standard cold storage (SCS) liver preservation. However, a significant number of DCD grafts remain unutilized with this concept and novel preservation techniques being currently evaluated [8].
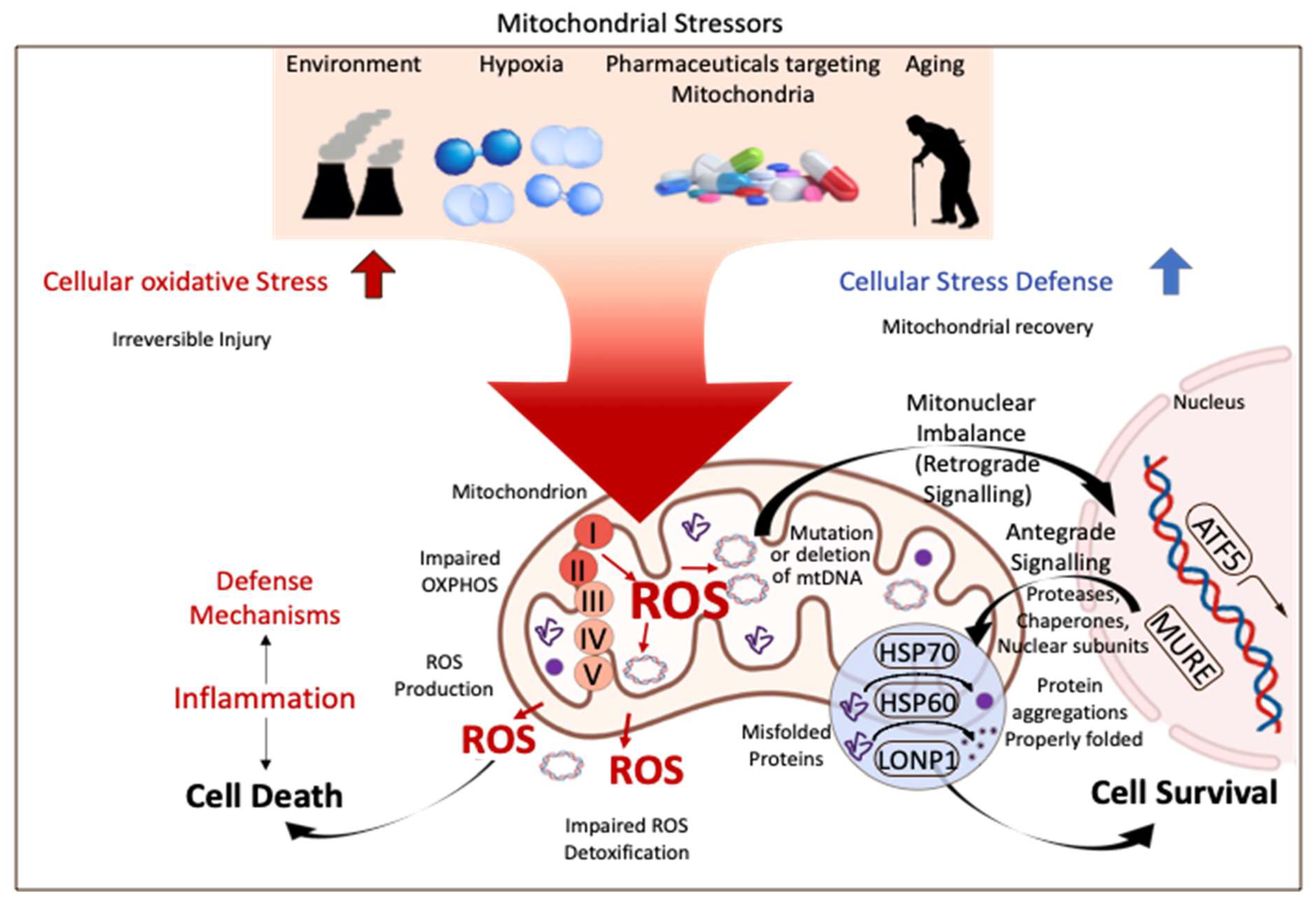
To provide uniform guidelines for donor and recipient selection and tailored preservation, a general understanding of cellular mechanisms of reperfusion injury after SCS and the role of machine perfusion is essential. Mitochondria are increasingly described as key structures in mammalian cells to cope with any type of injury. Environmental factors, drugs, hypoxia, cancer development, and ageing are some well-known features, increasingly linked to mitochondria by many. With their role to provide energy for the entire cellular metabolism, mitochondria are also known as “power houses” and are key to every subcellular function and decisive for the survival of an organism after a specific injury (Figure 1).
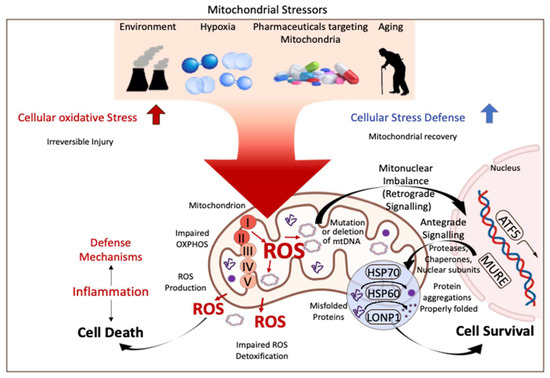
Figure 1.
Mitochondrial stress response and signaling in injury and disease. Mitochondria are key compounds in all cells and balance injury with resilience to defend cells from stress induced by the environment, related to ageing, or from hypoxia. Mitochondria are therefore crucial for cells and organs to survive the process of donation, preservation, and transplantation. ROS: reactive oxygen species; ATF 5: activating transcription factor; HSP 60/70: heparan sulfate 60/70; LONP1: Lon peptidase 1. This figure was designed with the support from biorender.com (accessed on 15 June 2022).
When a donor is available, a certain level of tissue hypoxia always occurs and triggers an overall impaired metabolism. The mitochondrial metabolism is always impaired to a certain extent and prompts the well-known cascade of ischemia-reperfusion injury (IRI) when oxygen becomes available again (reperfusion). The main feature is a pro-inflammatory cascade, which leads to more or less clinical post-transplant complications based on the overall organ quality and function. One direct consequence after reperfusion is the development of a pro-tumor tissue milieu, which enables the migration and regrowth of circulating tumor cells (CTCs) with subsequent cancer recurrence. Machine perfusion (MP) with the overall aim to reduce IRI-features is currently tested as a promising tool to protect recipients from various post-reperfusion and IRI-associated complications. The evaluation of a potential impact on liver cancer recurrence appears as logical consequence. This review article therefore provides mechanistic insights into the IRI cascade and the role of mitochondria and their link to cancer recurrence. An additional focus is on current interventions targeting mitochondria, including machine perfusion, to reduce tumor recurrence and the development of metastases after liver transplantation.
2. Mechanisms of Ischemia-Reperfusion Injury
Organ injury starts already in the donor with episodes of hypoxia and hypoperfusion during rescue treatment to save the donor, e.g., during prolonged intensive care unit admissions, when most brain functions are lost. The better the tissue can cope with such effects, the lower the later inflammatory injury after reoxygenation will be. During and after procurement, livers are exposed to additional periods of warm and cold ischemia.
The ischemic injury starts in the donor when oxygenated blood flow ceases, which corresponds to the time of cold organ flush in brain-dead donors (DBD) and to the time introduced with relevant hypotension and hypoxia during DCD donation (e.g., donor warm ischemia time) [9]. Cellular hypoxia with inhibition of electron flow through the respiratory chain and subsequent ATP-loss appears as immediate consequence together with an accumulation of toxic metabolites and ions, including Na+, K+, and Ca++, due to the failure of ATP-dependent ion channels. Particularly, a prolonged ischemia causes cellular edema, which activates proteases and initiates the cascade of apoptosis [10]. Of great importance is the accumulation of Krebs-cell metabolites, including succinate during hypoxia [11], which trigger the immediate release of reactive oxygen species (ROS) from complex I, when oxygen is reintroduced [11,12]. With the ROS-release in the first few minutes after reperfusion, further pro-inflammatory molecules, including Danger-associated molecular patters (Damps) [13], are also released from severely injured cells, including hepatocytes, and are recognized by activated Kupffer and sinusoidal endothelial cells (SECs). Damps molecules trigger a sterile inflammation through further secretion of pro-inflammatory cytokines (e.g., TNF-α, IL-1b, IL-18) [14]. Damps together with ROS release can activate two pathways: first, the transcription and release of increased levels of pro-cytokines from the nucleus (e.g., IL-1β), and second, the assembling of the inflammasome (NLRP-3) and capsase-1 cleavage to finally activate and cleave the pro-cytokines [14]. Damps receptors are toll-like receptors, which can contribute to trigger the activation of the transcription molecules AP-1, NFκB, and IRF3. Such factors promote the maturation of antigen presenting cells (APCs), including dendritic cells, which present antigens and express co-stimulatory molecules. The direct consequence is the increased inflammatory tissue response with the activation of T cells and other immune cells, leading to additional ROS release with downstream injury of initially healthy cells [14].
This acute and ongoing inflammation also involves further cellular and subcellular compounds including complement proteins (e.g., C3a and C5a), which become activated and recruit circulating recipient neutrophils, which, in turn, migrate directly into the newly implanted liver, amplifying the downstream activation of the innate immune system with further impaired cellular functions and death [15]. Another element is the activation of platelets with the formation of micro-thrombi inside the hepatic sinusoids, which impair the liver microcirculation further, thereby aggravating hypoxia and hypoperfusion [16].
Nowadays, there is mounting evidence demonstrating that mitochondria play an additional key role in oncogenesis. In fact, the ROS produced by mitochondrial metabolism can damage cells and mitochondrial DNA, which can result in new mutations, and upregulate proto-oncogenes together with a downregulation of tumor-suppressor genes [17]. However, the link between regional liver-IRI and systemic effects creating the perfect milieu for existing cancer cells to resettle and replicate is of increasing interest.
3. Specific Mechanisms of Cancer Recurrence after Liver Transplantation
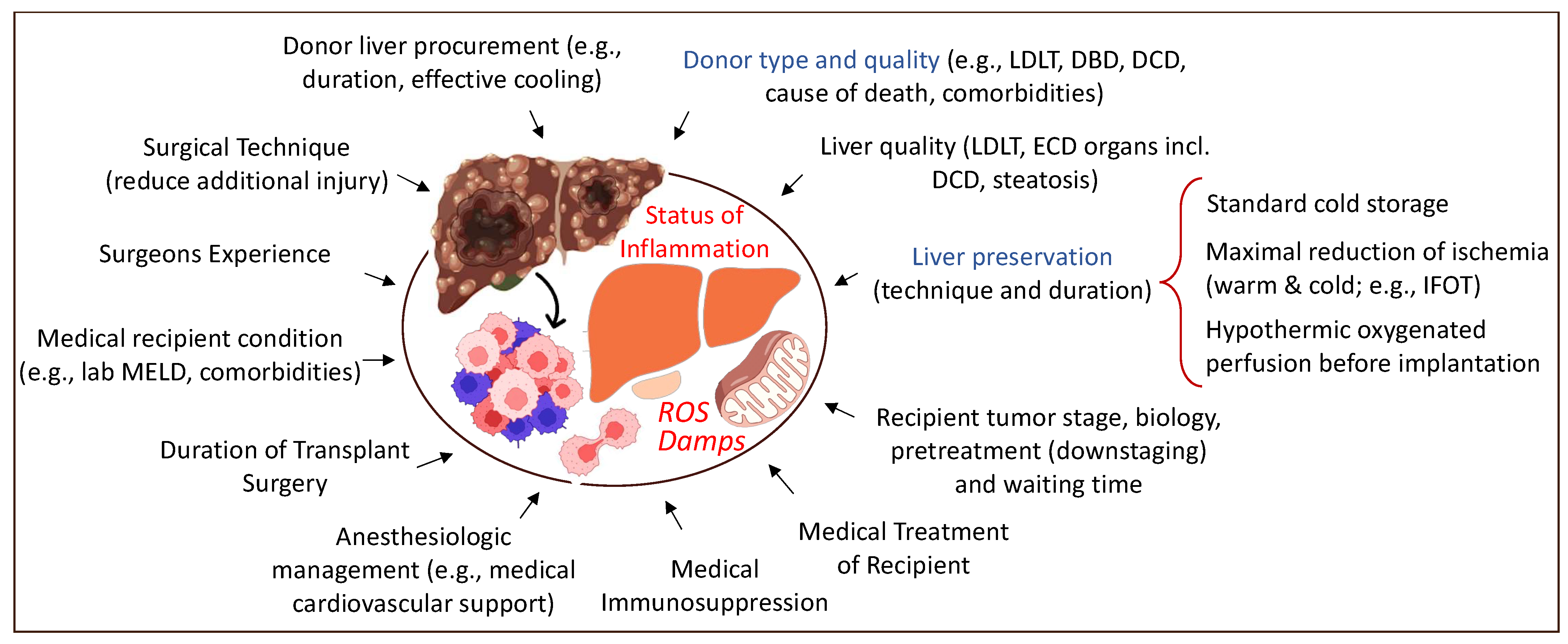
Any intervention with subsequent tissue trauma, including surgery, may trigger inflammation and hypoxia. Organ procurement and transplantation is no exception. The overall aim should, therefore, be considered as the limitation of injury, both surgical and medical (Figure 2). The increasing body of literature demonstrating the link between the level of injury (e.g., organ quality and level of recipient disease), the mitochondrial response during reperfusion, and further downstream effects with organ dysfunction and complications leads the way to understanding how to prevent this cascade. The physiological stress related to the surgery itself also plays an important role [18].
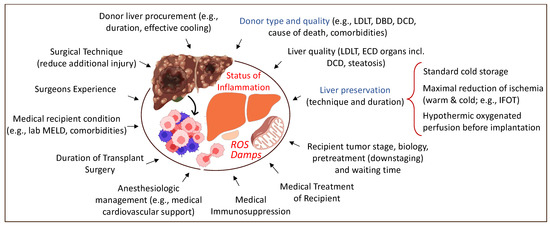
Figure 2.
Overview of tributary factors to tumor recurrence in the context of liver transplantation. Various surgical and medical elements contribute to a higher local and systemic inflammation with frequent tumor recurrence. LDLT: living-donor liver transplantation; DBD: donation after brain death; DCD: donation after circulatory death; ECD: extended criteria donor; MELD: model of end-stage liver disease; IFOT: ischemia-free organ transplantation. This figure was designed with the support from biorender.com (accessed on 15 June 2022).
With an experienced donor and recipient surgical team, the trauma can be reduced, further supported by an efficient anesthesiologic and the medical management of both donor and recipient [19,20]. Donor type, graft quality, and preservation contribute significantly to the overall IRI-level after reperfusion. Livers with prolonged warm and cold ischemia, combined with other risk factors, including macrosteatosis, are at particular risk. Such combinations induce the highest levels of ROS, Damps, and cytokines with the subsequent activation of the innate immune system with a general inflammation and downstream complications after liver transplantation [21].
Additional downstream consequences become visible within the micro-environment of the newly implanted liver, and also systemically. With the size of an adult liver, a large number of molecules are released into the recipient’s circulation after reperfusion, leading to a systemic inflammatory response. Straightforward transplant procedures (donor and recipient surgery) of low or standard liver grafts, which become implanted in low lab MELD candidates with medical stability, will have less inflammatory features with proper immediate multi-organ function, when compared to constellations with a higher risk.
3.1. The Contribution of Mitochondria
Mitochondria were identified as key contributors, which trigger overall tissue inflammation and thereby create an attractive environment for CTCs to resettle and grow. The ROS–Damps–cytokine cascade instigates further downstream effects in the liver micro-environment, enabling CTC migration through the SEC barrier. This cascade is of high relevance in transplantation with higher donor and recipient risk and in the context of the additional risk factors described before.
Mitochondria further contribute to cancer recurrence and new development through various other pathways, for example, with mitochondrial DNA mutations [22]. ROS molecules are also known to be involved in cancer progression, and high ROS levels are often found in cancer cells [23]. Mitochondria further regulate cell death and apoptosis, control the metabolic reprogramming through genetic mutations, encode tricarboxylic acid cycle (TCA) metabolites, and sustain cellular proliferation. Such features are generally linked to tumor generation, progression, and metastases [24].
Another key role of mitochondria is described for the process of metastatic dissemination [25]. The first step is the epithelial–mesenchymal transition (EMT), promoted by the mitochondrial biogenesis and oxidative phosphorylation (OXPHOS) [26]. The downregulation of OXPHOS was found in several tumor types, and is based on mutations seen in the mitochondrial DNA. At the same time, the glycolytic pathway was frequently upregulated [27]. Tumor energy is mainly derived from glycolysis; however, in cancer cells, the TCA cycle is often maintained in an active state to guarantee the availability of anabolic substrates and oncometabolites, which support neoplastic proliferation. Another important mitochondria-related pathway, with a link to cancer, is the biosynthesis of heme. Targeting OXPHOS, heme metabolism in mitochondria can serve as a compelling object for novel therapies to treat cancer [28]. Mitochondrial biogenesis and turnover is another potential target for new anti-cancer interventions [28].
3.2. The Complex Interplay between Liver Micro-Environment and Immune System
Related with numerous pathways present during early IRI, a variety of immune cells play a central role in cancer development and progression. The link between inflammation and cancer succession is well-established and mediators released by cells in the tumor micro-environment (TME) contribute significantly [14,29]. Understanding the TME is, therefore, of importance to identify strategies to reduce the HCC recurrence after LT. IRI-based inflammation triggers local changes with the development of a favorable micro-environment for tumor cells to invade, migrate, and grow [30,31].
Hepatic sinusoidal endothelial cells (SEC) play a key role here, with their direct response to IRI and the swelling and subsequent loss of integrity with increased micro-vascular permeability, which facilitates tumor cell permeation. In fact, hypoxia stimulates the secretion of hypoxia-inducible factor (HIF-1α), which promotes tumor cell proliferation, migration, and angiogenesis, with the secretion of vascular endothelial growth factors (VEGF) [31,32,33,34].
Several other pathways are also linked to IRI and tumor recurrence. The release of ROS and Damps triggers a high concentration of inflammatory cytokines in the circulation. Such molecules, including TNF-α and IL-1, play an important role for tumor progression because their upregulation triggers the expression of adhesion molecules (e.g., e-selectin and vascular cell adhesion molecule-1) on endothelial cells, which act as mediators for tumor growth [33,34].
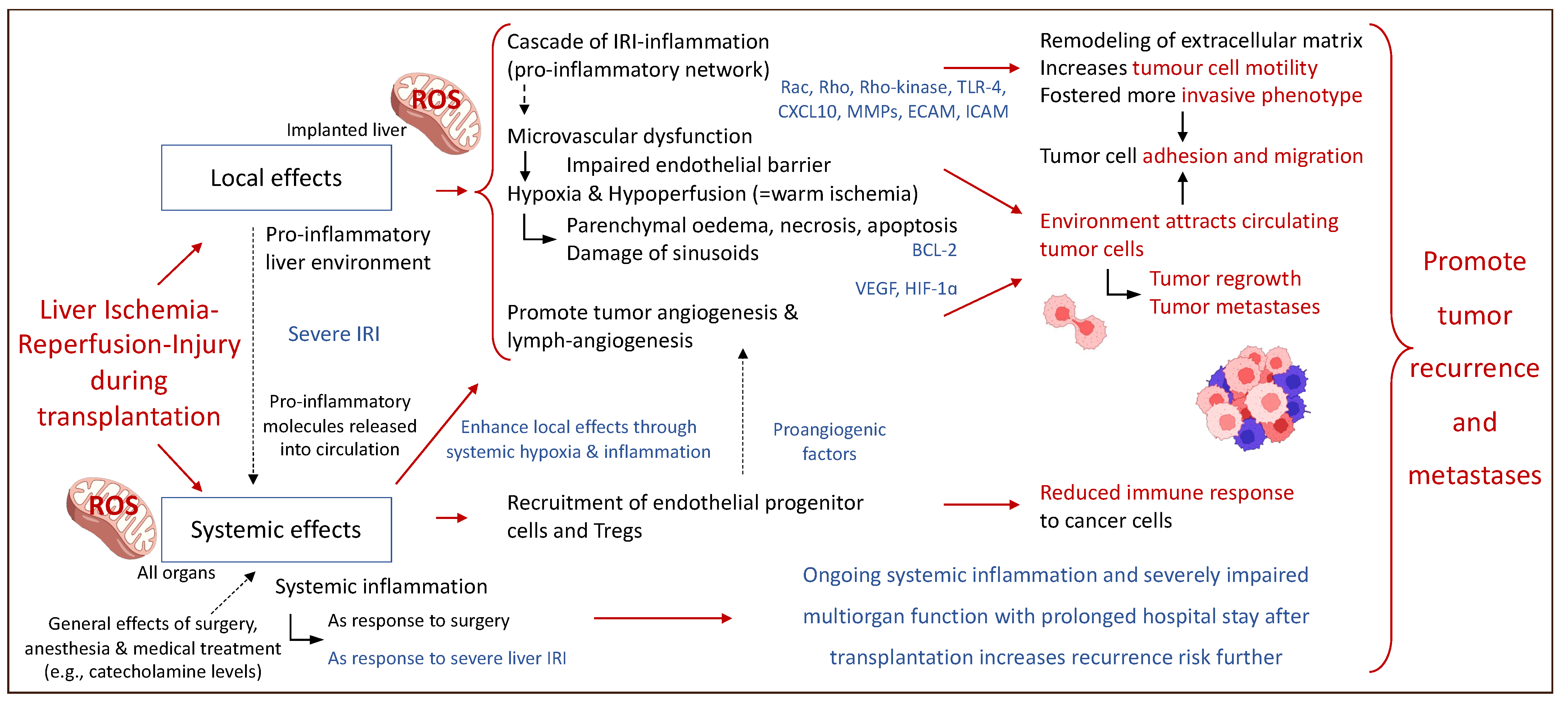
Figure 3 describes some of the local and systemic effects of IRI-associated effects on the micro-environment after liver transplantation and subsequent HCC recurrence.
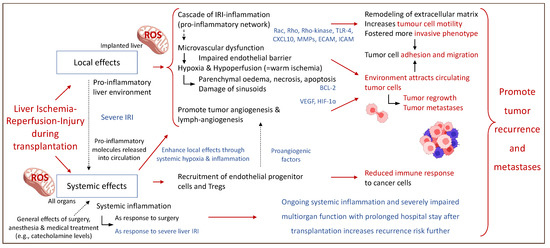
Figure 3.
Perioperative events with an impact on the fate of residual cancer cells after liver transplantation. Local and systemic effects establish a complex interplay and contribute to cancer recurrence involving the entire system of the liver recipient. Rac: Rho family of nucleotide guanosine triphosphate hydrolase enzymes; Rho: Rho family of nucleotide guanosine triphosphate hydrolase enzymes; TLR-4: toll-like receptor-4; CXCL10: C-X-C motif chemokine ligand; MMPs: metalloproteinase; ECAM: epithelial cell adhesion molecule; ICAM: intercellular adhesion molecules; BCL-2: B-cell lymphoma 2 gene; VEGF: vascular endothelial growth fact; HIF-1∝: hypoxia-inducible factor 1∝. This figure was designed with the support from biorender.com (accessed on 15 June 2022).
An activated IRI cascade also promotes tumor cell invasion and migration through an upregulation of multiple Rho-family molecules, which are crucial for cellular motility, proliferation, and apoptosis. Their overexpression is linked to tumor infiltration and metastasis in organs affected by hepatic IRI [35]. Furthermore, IRI promotes the release of CXCL-10, a chemoattractant, which increases the recruitment and differentiation of endothelial progenitor cells (EPC) and macrophages towards the liver. This augments tumor cell invasion and infiltration into blood vessels [36]. The CXCL-10 activation pathway is further associated with neo-angiogenesis and was shown to enhance the tumor cell motility and promote the development of more invasive phenotypes [37,38]. Additional support for this mechanism comes from authors who describe the potential role of circulating EPCs as prognostic marker in HCC patients [39].
Activated through the ROS-Damps axis, APCs including macrophages and dendritic cells, cross-present antigens on major histocompatibility complex (MHC) class-I molecules to CD8+ T cells, using the T-cell receptor (TCR). Subsequently, naïve CD8+ T cells turn into cytotoxic effector T lymphocytes (CTLs). This mechanism is crucial in cancer immunology because CTLs can detect tumor antigens using TCRs, thus destroying tumor cells [40,41]. In HCC, the presence of a high number of CD8+ T cells was associated with a better prognosis [42].
APCs also secrete stimulating factors, including VEGF and matrix metalloproteinases (MMPs), thereby promoting tumor cell growth and dissemination [43]. In addition, endothelial cell activation occurs, which leads to the recruitment of fibroblasts and mesenchymal stem cells, which, in turn, provoke the release of soluble growth factors with subsequent cancer cell progression [44].
Circulating pro-inflammatory mediators, including prostaglandin E2 (PGE2), represent an additional response to the surgical trauma and stimulate cancer-promoting regulatory T cells, the reduction in activated CD8+ T cells, and induce a shift from anti-tumor T helper 1 (TH1) cells towards tumor-promoting TH2 cells [45,46].
Thereafter, tumor-associated macrophages (TAMs) reside in the TME and promote the regional angiogenesis together with tumor progression and metastasis [47]. There is experimental evidence showing that TAMs play an additional role with their direct effect on tumor cells and also through immune system modulation with cytokine secretion (e.g., TNF- α, IL-6, and IL-1β) [48,49].
Further research demonstrated that CXCL-10 interact with CXC-chemokine receptor-3 (CXCR-3), which upregulates the recruitment of regulatory T cells (Tregs) within the liver [50]. Tregs are a subset of CD4+ T cells, characterized by an expression of Foxp3, a protein involved in the immune system response [51]. Their main function is to maintain self-tolerance and to prevent auto-immunity response. Tregs secrete various interleukins (IL-10, IL-35, TGF-β, IDO, VEGF), which induce T-cell suppression. For example, IL-35 facilitates intra-tumoral T-cell exhaustion through the expression of multiple inhibitory receptors (PD-1, TIM-3, and LAG-3, or the BLIMP1-inhibitory receptor axis) in combination with IL-10 [51,52]. Tregs from HCC patients were shown to decrease cellular proliferation, activation, degranulation, and the production of granzyme A, granzyme B, and perforin in CD8+ T cells [53]. Tregs also promote angiogenesis and downregulate the expression of co-stimulatory molecules CD80/CD86 on dendritic cells with subsequent suppression of their inherent cytokine production, including lower TNF-α and IL-12 release [54]. A recent study has linked high levels of Tregs with multiple HCC nodules, poor histological differentiation, higher alpha fetoprotein (AFP) levels, and vascular invasion leading to poorer survival rates [55].
4. Risk Factors for Liver Cancer Recurrence after Transplantation
Although LT offers a curative option for HCC, tumor recurrence remains a concern. The current literature demonstrates a tumor recurrence between 2.8% and 15.3% or even 23.6% when considering different graft types and follow-up durations [8,56,57]. HCC recurrence rates are related to the graft quality and the sub-clinical, unknown extrahepatic spread of the tumor at the time of LT and/or nesting of tumor cells during the manipulation of the liver during transplant surgery. A detailed understanding of tumor characteristics and thorough recipient selection are paramount to obtaining satisfactory results. Although the Milan criteria promote excellent survival rates after LT, they are solely based on the tumor morphology [58]. In the last decades, the understanding of other important biological factors, including total tumor volume and AFP, led to an expansion of the Milan criteria [44,59,60]. Additional parameters beyond standard tumor characteristics, such as donor and graft quality and recipient immunosuppression, were identified as key players. Orci et al. demonstrated that patients who received a liver from elderly donors (>60 y) or with diabetes or a body mass index (BMI) of ≥35 kg/m2 had a higher post-transplant recurrence [61]. Advanced donor age was also found to promote HCC recurrence in other studies, but the results appear controversial [62,63,64].
Based on their significant contribution to the IRI cascade, livers from ECDs were shown to induce higher complication rates after transplantation [65]. Increased risk is also observed with steatotic livers, which accumulate more succinate and NADH during ischemia and subsequently generate more ROS molecules during early reperfusion due to their fat metabolism [66,67]. Similar features were described for DCD livers, where more ROS and other inflammatory molecules are generated [8,68,69,70,71]. Prolonged donor WIT and CIT were both independently associated with a higher risk for HCC recurrence in multiple studies [56,57]. With DCD grafts in particular, it was shown that a prolonged CIT of >8 h is related with poor outcomes [72]. Other studies nominated an advanced donor age of >60 y, prolonged CIT of >6 h or 10 h, and a prolonged graft implantation time of >50 min as key risk factors [8,56,57,61,68,69,70,71,73]. The overall literature is conflicting, with some studies relating DCD grafts with a higher HCC recurrence compared to the use of DBD grafts, while others demonstrate comparable results.
The duration of donor WIT may certainly play a key role. Livers from donors can, however, also entail a higher risk based on an additional prolonged CIT with subsequent higher tumor recurrence rates. Unsurprisingly, elevated peak liver enzymes after LT from ECD donors were associated with increased HCC recurrence risk in candidates within the Milan criteria [73]. The currently available studies focusing on risk factors associated with IRI and HCC recurrence are summarized in Table 1.

Table 1.
Studies evaluating risk factors for IRI associated with HCC recurrence.
5. Strategies with the Potential to Reduce Cancer Recurrence
The option to select and discard certain donors may reduce HCC recurrence, but it will, however, not solve the lack of donor organs for the high number of candidates. The reduction in donor WIT and CIT is routine today, particularly when other risk factors are present. This is of interest as it is a low-cost measure and a widely applicable principle with which to effectively minimize preservation injury. However, multiple other strategies were studied to limit IRI, which include pharmacological interventions, surgical procedures with hepatic inflow modulation, and the introduction of dynamic organ preservation technologies.
5.1. Pharmacological Agents
Anesthetic drugs were previously used to modulate IRI, both in animal models [74] and in randomized controlled trials (RCTs) for patients undergoing liver surgery [75,76]. The results are satisfactory, with a significant reduction in IRI-related features. However, there is a lack of studies regarding the utilization of these agents in the setting of LT. The antibiotic molecule rifaximin was described to modulate the intestinal microbiota, which is thought to contribute to IRI. Authors have demonstrated a rifaximin course of <28 days as an independent risk factor for graft dysfunction, suggesting a therapeutic role of rifaximin [77].
N-acetylcysteine (NAC) was evaluated in an RCT to prevent IRI and was associated with significantly higher concentrations of both anti-inflammatory interleukins before (IL-4, IL-10) and after reperfusion (IL-4), which may have an attenuating IRI-effect [78].
Corticosteroids are common therapeutic measures used to treat inflammation and their role in reducing IRI was evaluated; however, no well-established effects on the liver were reported [79]. Although pharmacological agents seem to reduce IRI, there is no report linking their application with tumor recurrence to date.
5.2. Tailored Immunosuppression
One of the main factors to prevent the progression of cancer cells is immune defense. However, tumors can escape from the immune surveillance by creating an immunosuppressive environment. In this context, medical immunosuppression (IS) and particularly the group of calcineurin inhibitors (CNIs), despite preventing graft rejection, are key targets here. The relation between IS and cancer development is well-established [80]. Looking at HCC recurrence, CNIs were linked to a higher post-transplant tumor recurrence with a dose-dependent association [81,82]. Conversely, other IS regimens, which are based on the mammalian target of rapamycin (mTOR) inhibitors, including sirolimus and everolimus, were found to reduce HCC growth [83]. The association between mTOR inhibition and HCC recurrence after LT was investigated in various studies with controversial results [84,85,86].
There is currently no standardized protocol for the management of liver recipients with HCC, although the type and level of immunosuppressive drugs play a key role [81]. Based on the long-term side effects of IS, minimization strategies were explored and justified by intrinsic liver tolerance, which allows IS tapering without directly compromising patient and graft survival [87,88]. However, the impact of IS tapering on HCC recurrence was only investigated in a single, retrospective study [89] and, therefore, is still under debate, although a few practical guidelines were developed [90]. An Italian working group suggested pathways for the post-transplant immunosuppression, which consider the primary liver disease, the medical recipient status, early post-operative events, and expected complications, including nephrotoxicity and de novo malignancies. Regarding the management of patients undergoing LT for HCC, the authors recommended steroid-free immunosuppression and the reduction in CNI exposure by introducing mTOR inhibitors, given their anti-tumor potential [84,85,86,90].
5.3. Surgical Interventions
The impact of surgical interventions was explored in the context of IRI after transplantation. The Pringle Maneuver is the most common mechanical procedure to occlude liver inflow for a short period followed by repeat reperfusion (“occlusion-release-cycles”). This technique is also called ischemic preconditioning (IP) and was shown to have beneficial effects, with a reduction in IRI-associated inflammation and HCC progression. Indeed, some authors demonstrated that a few short IP cycles reduced the overall tumor burden in ischemic steatotic livers to the level of healthy steatotic controls [91]. Some human studies have demonstrated the beneficial effect of IP in donor livers before transplantation and demonstrate lower peak transaminases and a lower PNF incidence [92,93]. Such findings were confirmed in a meta-analysis in which lower PNF- and better patient survival rates were observed in livers with IP before transplantation [94]. A certain number of short cycles with in situ ischemia (e.g., IP) with subsequent “normothermic reperfusion” seem to upregulate inherent cellular defense mechanisms, such as the HIF-1alpha pathway, with subsequent liver protection after subsequent implantation [95]. Additional protection was seen with the induction of limited IRI-associated inflammation with combined ischemic pre- and post-conditioning [57,96].
Another technique was described with remote ischemic preconditioning (RIPC), where the intermittent pneumatic peripheral compression of a limb was found to upregulate the defense mechanisms related to platelets and the serotonin–VEGF–MMP8 axis [97]. The majority of these techniques require further studies with long-term follow-up to link the known protection from IRI with a lower tumor recurrence rate.
5.4. The Role of Organ Preservation Strategies
With the given donor and recipient risk parameters, organ preservation seems the most attractive approach to improve results after LT and is, therefore, currently a hot topic in our field. Machine perfusion offers the opportunity to improve organ function and test viability before transplantation [98]. Two main concepts are explored today. First, in situ perfusion where, for example, organs in the abdominal compartment undergo normothermic regional perfusion (NRP) immediately after a period of donor WIT [99]. The results from non-randomized trials appear encouraging, with satisfactory short-term outcomes after transplantation of DCD livers procured with NRP [99,100,101,102,103]. This technique is routinely used to procure DCD livers in Spain, France, Italy, and in some regions in the United Kingdom (UK) [104]. Another concept emerges with the ex-situ perfusion of routinely procured organs, either starting at the donor site and throughout transport or in the recipient center following a period of SCS.
While all techniques are performed under various conditions, including different perfusion timings (e.g., at donor hospital, during organ transport, and/or pre-implantation) and temperatures, the common goal is to reintroduce oxygen into hypoxic tissues as soon as possible [105,106]. Such ex-situ techniques are either performed under normothermic conditions with the aim to achieve a near-physiological environment using blood-based perfusate or under hypothermic conditions. The cold technique was first used in clinical practice in 2010 by Guarrera et al. [107]. The key is a high oxygen concentration of >60 kPa in the perfusate to trigger the typical reprogramming and repair of mitochondria before rewarming. Such hypothermic oxygenated perfusion (HOPE) re-establishes a forward electron flow with the functioning aerobic metabolisms and subsequent ATP recharging, and a reduction in previously accumulated toxic metabolites, including NADH and succinate [12]. At subsequent normothermic reperfusion, ROS release is significantly lower compared to direct normothermic reperfusion, with lower IRI-features and complications. Due to this mitochondrial protection, downstream inflammation is reduced and other cellular structures are protected, including the nucleus [108]. In addition to the protection of hepatocytes, the activation of other liver cells is reduced, including Kupffer and endothelial cells. Importantly, the micro-environment of the liver appears less inflamed and is, therefore, less attractive for CTCs to resettle and regrow. Livers after HOPE treatment induce a significantly reduced immune response [109]. Of note, this was also seen in kidneys [110]. Related to this reduced inflammation, the HOPE procedure further diminishes later biliary complications based on a lower release of profibrotic molecules from stellate cells and myofibroblasts after LT [111,112]. Whilst these findings were mainly explored in experimental and clinical studies with DCD livers, there is also some evidence in DBD grafts from elderly donors and in fatty livers, where HOPE was found to reduce IRI with subsequent improved early function and graft survival [67,113,114].
The impact of HOPE on clinical results was investigated in many studies, which consistently show the protective effects, with reduced IRI, less bile duct injury, and better graft function, with a follow-up of up to 5 years [107,114,115,116,117,118]. Additional evidence comes from three RCTs, which confirmed the HOPE effect, with protection from post-transplant biliary complications, EAD, high peak transaminases, and better graft survival rates in various graft types, including DCD livers [119,120,121]. While further studies on the optimal perfusion timing and duration are ongoing, recent evidence from Europe demonstrates the safe facilitation of transplantation logistics and a prolonged preservation period with cold perfusion [122].
In addition, the analysis of HOPE perfusates also allows for the assessment of liver viability, because when oxygen is reintroduced after ischemia, various molecules are released from mitochondrial complex proteins, including flavin mononucleotide (FMN), a marker with great potential to predict liver function during machine perfusion [12,123]. Perfusate FMN levels were associated with later graft survival and complication after LT.
In contrast, ex situ normothermic machine perfusion (NMP) techniques are routinely performed with oxygenated blood at 37 °C [124,125,126]. During NMP, the liver is metabolically active, which may offer another opportunity to assess viability, for example, through biochemical perfusate and bile analyses, including transaminases, glucose, lactate, bile volume, and pH [127,128,129]. Two RCTs have explored the role of NMP in clinical practice and demonstrated the effective reduction in post-transplant recipient transaminases with subsequently lower EAD rates [130,131]. When performed instead of cold storage during transport, NMP reduces the release of pro-inflammatory molecules and upregulates genes of tissue regeneration and platelet function [132].
Of note, the most efficient NMP technique is the labor-intensive version of “ischemia-free” organ transplantation (IFOT), which is currently being explored by colleagues from China. The perfusion device is connected to the liver in the donor and entirely bridges the time between donor and recipient, where the organ is disconnected and implanted with overall minimal warm ischemia and the avoidance of cold flush and cold storage [106]. In contrast, the use of NMP after relevant SCS leads to a comparable inflammation as that seen with cold storage preservation alone, including similar biliary complication rates with the use of DCD livers [129,133]. The overall landscape of studies with machine perfusion confirms the underlying mechanisms of IRI induction when oxygen is reintroduced under normothermic conditions. This perfusion technique may, however, work well in cases with limited injury, such as short warm or cold ischemia times [130,133,134]. This was recently confirmed with the achievement of a prolonged 3-day NMP of a low-risk human liver with subsequent implantation [135].
6. The Impact of Machine Liver Perfusion on Tumor Recurrence
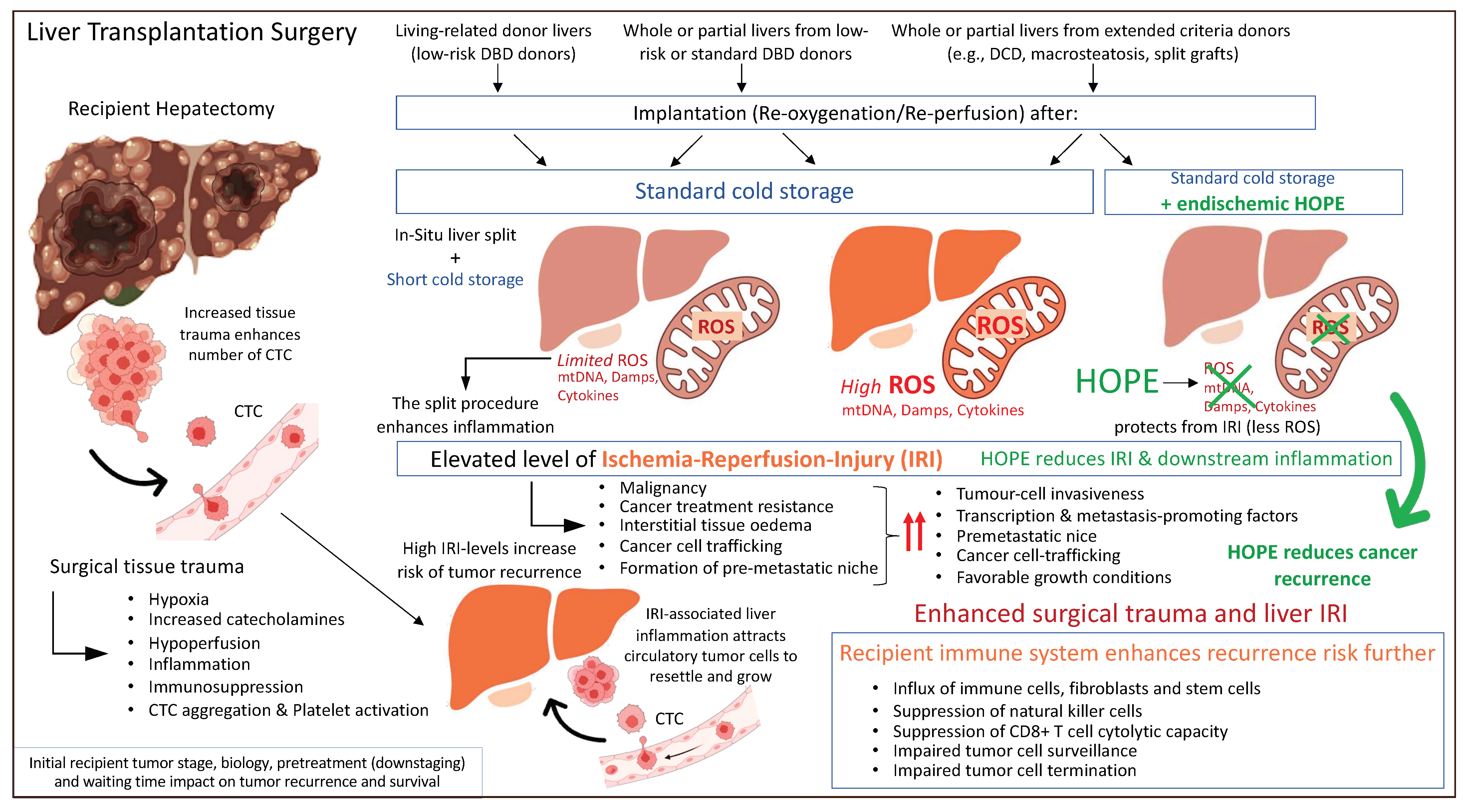
Based on previously described concepts of IRI-related inflammation with an impact on the liver micro-environment and post-transplant complications including cancer recurrence, machine perfusion appears to be attractive tool to improve current results. The protective effect of HOPE was also recently demonstrated in the context of liver cancer. A retrospective matched cohort study from two centers in the UK and Switzerland assessed the outcome after transplantation of candidates with HCC. Recipients of cold-stored DBD livers experienced 4-times higher HCC recurrences rates compared to high-risk DCD liver recipients that underwent endischemic HOPE treatment prior to implantation [136]. Of note, the recipient tumor burden was relatively high with 20–30% of candidates outside the standard HCC criteria, including Milan, UCSF, and Metroticket 2.0 [136]. These results demonstrate that a short mitochondrial treatment with HOPE before normothermic reperfusion is key to limit the inflammatory response and provide an anti-cancer effect (Figure 4).
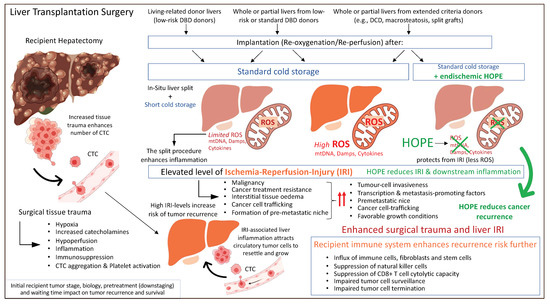
Figure 4.
Peri-transplant events with an impact on residual cancer cells and tumor recurrence after liver transplantation. CTC: circulating tumor cells; Damps: danger associated molecular pattern; DBD: donation after brain death; DCD: donation after circulatory death; HOPE: hypothermic oxygenated perfusion; ROS: reactive oxygen species. This figure was designed with the support from biorender.com (accessed on 15 June 2022).
In contrast, the IFOT approach, could be a feasible technique in organs from extended DBD donors. Interestingly, one study demonstrated the protective effect of IFOT in recipients with HCC. Lower recurrence rates were seen through an IRI reduction [137]. In this propensity score matched analysis, the authors illustrated a better recurrence-free survival after IFOT (HR 3.728, 95% CI 1.172–11.861, p = 0.026), when compared to SCS alone [137]. The authors showed recurrence-free survival rates at 1 and 3 years after LT in recipients with HCC in the IFOT group of 92% and 87%, respectively, which were significantly higher than those (73.0% and 46.3%) seen in the cold storage group [137]. Looking at the HCC burden, in the entire cohort, the pre-transplant AFP level was higher in the non-IFOT group compared to the IFOT group (p = 0.016). The percentage of LT recipients within the Milan criteria and micro-vascular invasion were also higher in the non-IFOT group than in the IFOT group (p = 0.032 and p = 0.042, respectively). There were no differences in the size of the largest HCC lesion, the number of lesions, tumor differentiation, or the type of previous treatment between the two groups [137]. The two clinical studies with the impact of HOPE and IFOT are summarized in Table 2.

Table 2.
Studies evaluating the impact of MP on IRI-associated HCC recurrence and recipient survival.
Although there is some evidence to demonstrate that the underlying liver disease could play a role in the development of post-LT HCC recurrence, especially a hepatitis B and C infection [138,139], the effect of novel perfusion technologies in this context remains unclear due to a lack of available data. Further studies to identify the subset of grafts and recipients who would benefit most from MP in the context of the underlying liver disease are required.
7. What Is the Potential Impact of IRI on the Recurrence of Other Liver Tumors and Metastases?
In the last decade, LT was explored as a therapeutic option for other types of primary and secondary liver tumors, including cholangiocarcinoma (CCA), neuroendocrine (NETs), and colorectal liver metastasis (CLRM) [5,140]. It is known that the immune system plays a key role in the context of the progression and recurrence of non-HCC liver tumors. Indeed, a suppressive immunologic TME plays a tumor-promoting role in CRLM, which is related to TAMs and Tregs. However, TAMs maintain the immunosuppressive environment by expressing checkpoint-ligand-programmed-death-ligand-1 (PDL1), PDL2, and other inhibitory receptors and activate Tregs by secreting IL-10 and tumor growing factor (TGF)-β. TAMs also release multiple other molecules, which remodel the extracellular matrix (ECM), including the plasminogen activation system, MMPs, and kallikrein-related peptidases. Such factors augment the migration of tumor cells. In addition, when targeting the CCL2/CCR2 chemokine axis, TAMs infiltration at the metastatic site is reduced and metastatic colorectal cancer cells are sensitized to tumor T cells [141].
Patients affected by CCA often present with cholestasis, which has been linked to increased IRI and associated damage [142]. In the development of CCA, several molecular and genetic pathways within the hepatic TME have been demonstrated. Importantly, necroptosis, a recently discovered process of regulated cell death, was found to contribute to CCA growth [143]. Damps molecules, released by necroptotic hepatocytes, can activate the immune response with the induction of a pro-inflammatory environment, determining the outgrowth of CCA from transformed hepatocytes. Epigenome and transcriptome profiling of mouse HCC and ICC singled out Tbx3 and Prdm5 as the main micro-environment-dependent and epigenetically regulated lineage-commitment factors, a function that is conserved in humans [143]. Several other described mechanisms, including the HIF-1α-related pathways, promote CCA progression and the development of metastases [144]. Such molecular mechanisms are potentially targeted by HOPE and IFOT through the reduction in mitochondria-associated IRI features and the subsequent limited activation of the innate immune system. With more evidence in the future, LT could be a well-defined approach to treat CCA, metastatic NETs, and CRLM.
In this setting, the modulation of inflammation and the subsequent reduction in tumor recurrence appears to be of great importance. Future studies should, therefore, also assess the role of MP on outcomes after transplantation in recipients with non-HCC liver tumors and metastases.
8. Summary and Future Perspective
Based on the underlying mechanisms of cancer recurrence, the importance of the TME, and the role of mitochondria, it appears that one effective strategy to prevent HCC recurrence after LT is the reduction in IRI. Nowadays, ECDs are being utilized more frequently and their vulnerability to elevated IRI warrants careful decision making when accepting such organs. In this context, dynamic perfusion strategies, which improve mitochondrial metabolism and reduce IRI, are of great interest. Further studies, including large prospective trials, are needed to confirm these results and thus change the current standard preservation techniques and improve future outcomes. With the increasing acceptance of candidates with liver metastases and liver cancer types, other than HCCs, frequently transplanted with marginal grafts, the concept of IRI reduction deserves recognition and should be further explored in the future. Equally, the impact of early liver function, inflammation, and recipient recovery together with the maintenance of mitochondrial health may play a key role in the development of secondary cancers after liver transplantation. More experimental and large clinical cohort studies with long-term follow-ups are required to provide further details regarding the potential role of new preservation strategies in the context of tumor recurrence and secondary cancer development.
Author Contributions
Conceptualization, A.P. and A.S.; methodology, A.P. and A.S.; resources, all authors; writing—original draft preparation, A.P. and A.S.; writing—review and editing, A.P., M.F.C., J.E., P.D. and A.S.; visualization Table: A.P. and A.S., Figures: M.F.C., J.E. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were not applicable for this study not involving humans or animals.
Informed Consent Statement
Patient consent was not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Some of the figures were developed with the use of biorender.com (accessed on).
Conflicts of Interest
The authors declare no conflict of interest related to this article.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Alejandro, R.; Ruffolo, L.I.; Sasaki, K.; Tomiyama, K.; Orloff, M.S.; Pineda-Solis, K.; Nair, A.; Errigo, J.; Dokus, M.K.; Cattral, M.; et al. Recipient and Donor Outcomes After Living-Donor Liver Transplant for Unresectable Colorectal Liver Metastases. JAMA Surg. 2022, 157, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.; Ivanics, T.; Heimbach, J. Liver Transplantation for Intrahepatic Cholangiocarcinoma: Ready for Prime Time? Hepatology 2022, 75, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Hibi, T.; Rela, M.; Eason, J.D.; Line, P.-D.; Fung, J.; Sakamoto, S.; Selzner, N.; Man, N.K.; Ghobrial, R.M.; Sapisochin, G. Liver Transplantation for Colorectal and Neuroendocrine Liver Metastases and Hepatoblastoma. Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1131–1135. [Google Scholar] [CrossRef]
- Sapisochin, G.; Javle, M.; Lerut, J.; Ohtsuka, M.; Ghobrial, M.; Hibi, T.; Kwan, N.M.; Heimbach, J. Liver Transplantation for Cholangiocarcinoma and Mixed Hepatocellular Cholangiocarcinoma: Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1125–1130. [Google Scholar] [CrossRef]
- Abt, P.L.; Desai, N.M.; Crawford, M.D.; Forman, L.M.; Markmann, J.W.; Olthoff, K.M.; Markmann, J.F. Survival Following Liver Transplantation From Non-Heart-Beating Donors. Ann. Surg. 2004, 239, 87–92. [Google Scholar] [CrossRef]
- Jiménez-Romero, C.; Maestro, O.C.; Molero, F.C.; Alonso, I.J.; Torrado, C.A.; Municio, A.M.; Pulido, J.C.; Segurola, C.L.; González, E.M. Using old liver grafts for liver transplantation: Where are the limits? World J. Gastroenterol. 2014, 20, 10691–10702. [Google Scholar] [CrossRef]
- Silverstein, J.; Roll, G.; Dodge, J.L.; Grab, J.D.; Yao, F.Y.; Mehta, N. Donation After Circulatory Death Is Associated With Similar Posttransplant Survival in All but the Highest-Risk Hepatocellular Carcinoma Patients. Liver Transplant. 2020, 26, 1100–1111. [Google Scholar] [CrossRef]
- Panconesi, R.; Carvalho, M.F.; Muiesan, P.; Dutkowski, P.; Schlegel, A. Liver perfusion strategies: What is best and do ischemia times still matter? Curr. Opin. Organ. Transplant. 2022, 27, 285–299. [Google Scholar] [CrossRef]
- Abu-Amara, M.; Yang, S.Y.; Tapuria, N.; Fuller, B.; Davidson, B.; Seifalian, A. Liver ischemia/reperfusion injury: Processes in inflammatory networks-A review. Liver Transplant. 2010, 16, 1016–1032. [Google Scholar] [CrossRef]
- Martin, J.L.; Costa, A.S.H.; Gruszczyk, A.V.; Beach, T.E.; Allen, F.M.; Prag, H.A.; Hinchy, E.C.; Mahbubani, K.; Hamed, M.; Tronci, L.; et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat. Metab. 2019, 1, 966–974. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.-A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. Ebiomedicine 2020, 60, 103014. [Google Scholar] [CrossRef]
- Nardo, B.; Caraceni, P.; Pasini, P.; Domenicali, M.; Catena, F.; Cavallari, G.; Santoni, B.; Maiolini, E.; Grattagliano, I.; Vendemiale, G.; et al. Increased Generation of Reactive Oxygen Species in Isolated Rat Fatty Liver during Postischemic Reoxygenation1. Transplantation 2001, 71, 1816–1820. [Google Scholar] [CrossRef]
- Panconesi, R.; Carvalho, M.F.; Dondossola, D.; Muiesan, P.; Dutkowski, P.; Schlegel, A. Impact of Machine Perfusion on the Immune Response After Liver Transplantation—A Primary Treatment or Just a Delivery Tool. Front. Immunol. 2022, 13, 855263. [Google Scholar] [CrossRef]
- Diepenhorst, G.M.P.; van Gulik, T.M.; Hack, C.E. Complement-Mediated Ischemia-Reperfusion Injury: Lessons Learned From Animal and Clinical Studies. Ann. Surg. 2009, 249, 889–899. [Google Scholar] [CrossRef]
- Peralta, C.; Jiménez-Castro, M.B.; Gracia-Sancho, J. Hepatic ischemia and reperfusion injury: Effects on the liver sinusoidal milieu. J. Hepatol. 2013, 59, 1094–1106. [Google Scholar] [CrossRef]
- Lu, J.; Sharma, L.K.; Bai, Y. Implications of mitochondrial DNA mutations and mitochondrial dysfunction in tumorigenesis. Cell Res. 2009, 19, 802–815. [Google Scholar] [CrossRef]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E.K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2018, 15, 205–218. [Google Scholar] [CrossRef]
- Buchholz, B.M.; Gerlach, U.A.; Chandrabalan, V.V.; Hodson, J.; Gunson, B.K.; Mergental, H.; Muiesan, P.; Isaac, J.R.; Roberts, K.J.; Mirza, D.F.; et al. Revascularization Time in Liver Transplantation: Independent Prediction of Inferior Short- and Long-term Outcomes by Prolonged Graft Implantation. Transplantation 2018, 102, 2038–2055. [Google Scholar] [CrossRef]
- Kalisvaart, M.; Schlegel, A.; Umbro, I.; de Haan, J.E.; Scalera, I.; Polak, W.G.; IJzermans, J.N.M.; Mirza, D.F.; Perera, M.T.P.R.; Isaac, J.I.; et al. The Impact of Combined Warm Ischemia Time on Development of Acute Kid-ney Injury in Donation after Circulatory Death Liver Transplantation: Stay within the Golden Hour. Transplantation 2018, 102, 783–793. [Google Scholar] [CrossRef]
- Saeb-Parsy, K.; Martin, J.L.; Summers, D.M.; Watson, C.J.; Krieg, T.; Murphy, M.P. Mitochondria as Therapeutic Targets in Transplantation. Trends Mol. Med. 2021, 27, 185–198. [Google Scholar] [CrossRef]
- Nomoto, S.; Yamashita, K.; Koshikawa, K.; Nakao, A.; Sidransky, D. Mitochondrial D-loop mutations as clonal markers in multicentric hepatocellular carcinoma and plasma. Clin. Cancer Res. 2000, 8, 481–487. [Google Scholar]
- Kumari, S.; Badana, A.K.; Murali, M.G.; Shailender, G.; Malla, R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef]
- Grasso, D.; Zampieri, L.X.; Capelôa, T.; Van De Velde, J.A.; Sonveaux, P. Mitochondria in cancer. Cell Stress 2020, 4, 114–146. [Google Scholar] [CrossRef]
- Scheid, A.D.; Beadnell, T.C.; Welch, D.R. Roles of mitochondria in the hallmarks of metastasis. Br. J. Cancer 2021, 124, 124–135. [Google Scholar] [CrossRef]
- Porporato, P.E.; Payen, V.L.; Pérez-Escuredo, J.; De Saedeleer, C.J.; Danhier, P.; Copetti, T.; Dhup, S.; Tardy, M.; Vazeille, T.; Bouzin, C.; et al. A Mitochondrial Switch Promotes Tumor Metastasis. Cell Rep. 2014, 8, 754–766. [Google Scholar] [CrossRef]
- Ashton, T.M.; McKenna, W.G.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin. Cancer Res. 2018, 24, 2482–2490. [Google Scholar] [CrossRef]
- Frattaruolo, L.; Brindisi, M.; Curcio, R.; Marra, F.; Dolce, V.; Cappello, A.R. Targeting the Mitochondrial Metabolic Network: A Promising Strategy in Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 6014. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-X.; Man, K.; Lo, C.-M. The Impact of Liver Graft Injury on Cancer Recurrence Posttransplantation. Transplantation 2017, 101, 2665–2670. [Google Scholar] [CrossRef] [PubMed]
- van der Bilt, J.D.; Kranenburg, O.; Nijkamp, M.W.; Smakman, N.; Veenendaal, L.M.; Te Velde, E.A.; Voest, E.E.; van Diest, P.J.; Borel Rinkes, I.H. Ischemia/reperfusion accelerates the outgrowth of hepatic micrometastases in a highly standardized murine model. Hepatology. 2005, 42, 165–175. [Google Scholar] [CrossRef]
- Carmeliet, P.; Dor, Y.; Herbert, J.M.; Fukumura, D.; Brusselmans, K.; Dewerchin, M.; Neeman, M.; Bono, F.; Abramovitch, R.; Maxwell, P.; et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998, 30, 485–490. [Google Scholar] [CrossRef]
- Brodt, P.; Fallavollita, L.; Bresalier, R.S.; Meterissian, S.; Norton, C.R.; Wolitzky, B.A. Liver endothelial E-selectin mediates carcinoma cell adhesion and promotes liver metastasis. Int. J. Cancer 1997, 71, 612–619. [Google Scholar] [CrossRef]
- Higashiyama, A.; Watanabe, H.; Okumura, K.; Yagita, H. Involvement of tumor necrosis factor α and very late activation antigen 4/vascular cell adhesion molecule 1 interaction in surgical-stress-enhanced experimental metastasis. Cancer Immunol. Immunother. 1996, 181, 411–415. [Google Scholar] [CrossRef]
- Man, K.; Ng, K.T.; Lo, C.M.; Ho, J.W.; Sun, B.S.; Sun, C.K.; Lee, T.K.; Poon, R.T.P.; Fan, S.T. Ischemia-reperfusion of small liver remnant promotes liver tumor growth and metastases—Activation of cell invasion and migration pathways. Liver Transplant. 2007, 13, 1669–1677. [Google Scholar] [CrossRef]
- Boteon, Y.; Carvalho, M.A.F.; Panconesi, R.; Muiesan, P.; Schlegel, A. Preventing Tumour Recurrence after Liver Transplantation: The Role of Machine Perfusion. Int. J. Mol. Sci. 2020, 21, 5791. [Google Scholar] [CrossRef]
- Man, K.; Shih, K.C.; Ng, K.T.P.; Xiao, J.W.; Guo, D.Y.; Sun, C.K.W.; Lim, Z.X.H.; Cheng, Q.; Liu, Y.; Fan, S.T.; et al. Molecular Signature Linked to Acute Phase Injury and Tumor Invasiveness in Small-for-Size Liver Grafts. Ann. Surg. 2010, 251, 1154–1161. [Google Scholar] [CrossRef]
- Ling, C.-C.; Ng, K.T.-P.; Shao, Y.; Geng, W.; Xiao, J.-W.; Liu, H.; Li, C.-X.; Liu, X.-B.; Ma, Y.-Y.; Yeung, W.-H.; et al. Post-transplant endothelial progenitor cell mobilization via CXCL10/CXCR3 signaling promotes liver tumor growth. J. Hepatol. 2014, 60, 103–109. [Google Scholar] [CrossRef]
- Ho, J.W.Y.; Pang, R.W.C.; Lau, C.; Sun, C.K.; Yu, W.C.; Fan, S.T.; Poon, R.T.P. Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. Hepatology 2006, 44, 836–843. [Google Scholar] [CrossRef]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.M.; Kastenmüller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef]
- Xu, X.; Tan, Y.; Qian, Y.; Xue, W.; Wang, Y.; Du, J.; Jin, L.; Ding, W. Clinicopathologic and prognostic significance of tumor-infiltrating CD8+ T cells in patients with hepatocellular carcinoma: A meta-analysis. Medicine 2019, 98, e13923. [Google Scholar] [CrossRef]
- Murdoch, C.; Muthana, M.; Coffelt, S.B.; Lewis, C.E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer 2008, 8, 618–631. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. Eicosanoids and cancer. Nat. Rev. Cancer 2010, 10, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; So, S.-P. Prostaglandin E2 produced by inducible COX-2 and mPGES-1 promoting cancer cell proliferation in vitro and in vivo. Life Sci. 2014, 116, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Pollard, J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Salcedo, R.; Mivechi, N.F.; Trinchieri, G.; Horuzsko, A. The Proinflammatory Myeloid Cell Receptor TREM-1 Controls Kupffer Cell Activation and Development of Hepatocellular Carcinoma. Cancer Res. 2012, 72, 3977–3986. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, X.-D.; Sun, H.-C.; Xiong, Y.-Q.; Zhuang, P.-Y.; Xu, H.-X.; Kong, L.-Q.; Wang, L.; Wu, W.-Z.; Tang, Z.-Y. Depletion of Tumor-Associated Macrophages Enhances the Effect of Sorafenib in Metastatic Liver Cancer Models by Antimetastatic and Antiangiogenic Effects. Clin. Cancer Res. 2010, 16, 3420–3430. [Google Scholar] [CrossRef]
- Li, C.X.; Ling, C.C.; Shao, Y.; Xu, A.; Li, X.C.; Ng, K.T.-P.; Liu, X.B.; Ma, Y.Y.; Qi, X.; Liu, H.; et al. CXCL10/CXCR3 signaling mobilized-regulatory T cells promote liver tumor recurrence after transplantation. J. Hepatol. 2016, 65, 944–952. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Turnis, M.E.; Sawant, D.V.; Szymczak-Workman, A.L.; Andrews, L.P.; Delgoffe, G.M.; Yano, H.; Beres, A.J.; Vogel, P.; Workman, C.J.; Vignali, D.A. Interleukin-35 Limits Anti-Tumor Immunity. Immunity 2016, 44, 316–329. [Google Scholar] [CrossRef]
- Fu, J.; Xu, D.; Liu, Z.; Shi, M.; Zhao, P.; Fu, B.; Zhang, Z.; Yang, H.; Zhang, H.; Zhou, C.; et al. Increased Regulatory T Cells Correlate With CD8 T-Cell Impairment and Poor Survival in Hepatocellular Carcinoma Patients. Gastroenterology 2007, 132, 2328–2339. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Huang, Z. CD4+CD25+ Treg derived from hepatocellular carcinoma mice inhibits tumor immunity. Immunol. Lett. 2012, 148, 83–89. [Google Scholar] [CrossRef]
- Sun, L.; Xu, G.; Liao, W.; Yang, H.; Xu, H.; Du, S.; Zhao, H.; Lu, X.; Sang, X.; Mao, Y. Clinicopathologic and prognostic significance of regulatory T cells in patients with hepatocellular carcinoma: A meta-analysis. Oncotarget 2017, 8, 39658–39672. [Google Scholar] [CrossRef]
- Nagai, S.; Yoshida, A.; Facciuto, M.; Moonka, D.; Abouljoud, M.S.; Schwartz, M.E.; Florman, S.S. Ischemia time impacts recurrence of hepatocellular carcinoma after liver transplantation. Hepatology 2015, 61, 895–904. [Google Scholar] [CrossRef]
- Kornberg, A.; Witt, U.; Kornberg, J.; Friess, H.; Thrum, K. Extended Ischemia Times Promote Risk of HCC Recurrence in Liver Transplant Patients. Dig. Dis. Sci. 2015, 60, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.M.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–700. [Google Scholar] [CrossRef]
- Duvoux, C.; Roudot-Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver Transplantation French Study Group. Liver transplantation for hepatocellular carcinoma: A model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012, 143, 986–994. [Google Scholar] [CrossRef]
- Toso, C.; Meeberg, G.; Hernandez-Alejandro, R.; Dufour, J.; Marotta, P.; Majno, P.; Kneteman, N.M. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology 2015, 62, 158–165. [Google Scholar] [CrossRef]
- Orci, L.A.; Berney, T.; Majno, P.E.; Lacotte, S.; Oldani, G.; Morel, P.; Mentha, G.; Toso, C. Donor characteristics and risk of hepatocellular carcinoma recurrence after liver transplantation. Br. J. Surg. 2015, 102, 1250–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Welch, K.; Hussain, H.; Pelletier, S.J.; Fontana, R.J.; Marrero, J.; Merion, R.M. Incidence and risk factors of hepatocellular carcinoma recurrence after liver transplantation in the MELD era. Dig. Dis. Sci. 2012, 57, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Vagefi, P.A.; Dodge, J.L.; Yao, F.Y.; Roberts, J.P.; Yao, F.Y. Potential role of the donor in hepatocellular carcinoma recurrence after liver transplantation. Liver Transplant. 2015, 21, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, C.; De Carlis, L.; Centonze, L.; Lesourd, R.; Sandri, G.B.L.; Lauterio, A.; De Carlis, R.; Ferla, F.; Di Sandro, S.; Camus, C.; et al. Advanced donor age does not increase risk of hepatocellular carcinoma recurrence after liver transplantation: A retrospective two-centre analysis using competing risk analysis. Transpl. Int. 2021, 34, 1948–1958. [Google Scholar] [CrossRef]
- Kan, C.; Ungelenk, L.; Lupp, A.; Dirsch, O.; Dahmen, U. Ischemia-Reperfusion Injury in Aged Livers—The Energy Metabolism, Inflammatory Response, and Autophagy. Transplantion 2018, 102, 368–377. [Google Scholar] [CrossRef]
- Baidya, R.; Crawford, D.H.G.; Gautheron, J.; Wang, H.; Bridle, K.R. Necroptosis in Hepatosteatotic Ischaemia-Reperfusion Injury. Int. J. Mol. Sci. 2020, 21, 5931. [Google Scholar] [CrossRef]
- Kron, P.; Schlegel, A.; Mancina, L.; Clavien, P.A.; Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J. Hepatol. 2018, 68, 82–91. [Google Scholar] [CrossRef]
- Croome, K.P.; Wall, W.; Chandok, N.; Beck, G.; Marotta, P.; Hernandez-Alejandro, R. Inferior survival in liver transplant recipients with hepatocellular carcinoma receiving donation after cardiac death liver allografts. Liver Transplant. 2013, 19, 1214–1223. [Google Scholar] [CrossRef]
- Croome, K.P.; Lee, D.D.; Burns, J.M.; Musto, K.; Paz, D.; Nguyen, J.H.; Perry, D.K.; Harnois, D.M.; Taner, C.B. The Use of Donation After Cardiac Death Allografts Does Not Increase Recurrence of Hepatocellular Carcinoma. Am. J. Transplant. 2015, 15, 2704–2711. [Google Scholar] [CrossRef]
- Khorsandi, S.E.; Yip, V.S.; Cortes, M.; Jassem, W.; Quaglia, A.; O’Grady, J.; Heneghan, M.; Aluvihare, V.; Agarwal, K.; Menon, K.; et al. Does Donation After Cardiac Death Utilization Adversely Affect Hepatocellular Cancer Survival? Transplantation 2016, 100, 1916–1924. [Google Scholar] [CrossRef]
- Martinez-Insfran, L.A.; Ramirez, P.; Cascales, P.; Alconchel, F.; Ferreras, D.; Febrero, B.; Martinez, M.; González, M.R.; Sanchez-Bueno, F.; Robles, R.; et al. Early Outcomes of Liver Transplantation Using Donors After Circulatory Death in Patients With Hepatocellular Carcinoma: A Comparative Study. Transplant. Proc. 2019, 51, 359–364. [Google Scholar] [CrossRef]
- Foley, D.P.; Fernandez, L.A.; Leverson, G.; Anderson, M.; Mezrich, J.; Sollinger, H.W.; D’Alessandro, A. Biliary complications after liver transplantation from donation after cardiac death donors: An analysis of risk factors and long-term outcomes from a single center. Ann. Surg. 2011, 253, 817–825. [Google Scholar] [CrossRef]
- Grąt, M.; Krawczyk, M.; Wronka, K.M.; Stypułkowski, J.; Lewandowski, Z.; Wasilewicz, M.; Krawczyk, P.; Grąt, K.; Patkowski, W.; Zieniewicz, K. Ischemia-reperfusion injury and the risk of hepatocellular carcinoma recurrence after deceased donor liver transplantation. Sci. Rep. 2018, 12, 8935. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, J.; Wu, J.; Qi, F.; Wang, H.; Wang, Z.; Wang, Z. The Effects of Two Anesthetics, Propofol and Sevoflurane, on Liver Ischemia/Reperfusion Injury. Cell. Physiol. Biochem. 2016, 38, 1631–1642. [Google Scholar] [CrossRef]
- Beck-Schimmer, B.; Breitenstein, S.; Urech, S.; De Conno, E.; Wittlinger, M.; Puhan, M.; Jochum, W.; Spahn, D.R.; Graf, R.; Clavien, P.-A. A Randomized Controlled Trial on Pharmacological Preconditioning in Liver Surgery Using a Volatile Anesthetic. Ann. Surg. 2008, 248, 909–918. [Google Scholar] [CrossRef]
- Beck-Schimmer, B.; Breitenstein, S.; Bonvini, J.M.; Lesurtel, M.; Ganter, M.; Weber, A.; Puhan, M.A.; Clavien, P.-A. Protection of Pharmacological Postconditioning in Liver Surgery: Results of a prospective randomized controlled trial. Ann. Surg. 2012, 256, 837–845. [Google Scholar] [CrossRef]
- Ito, T.; Nakamura, K.; Kageyama, S.; Korayem, I.M.; Hirao, H.; Kadono, K.; Aziz, J.; Younan, S.; DiNorcia, J.; Agopian, V.G.; et al. Impact of Rifaximin Therapy on Ischemia/Reperfusion Injury in Liver Transplantation: A Propensity Score–Matched Analysis. Liver Transplant. 2019, 25, 1778–1789. [Google Scholar] [CrossRef]
- Santiago, F.; Bueno, P.; Olmedo, C.; Muffak-Granero, K.; Comino, A.; Serradilla, M.; Mansilla, A.; Villar, J.; Garrote, D.; Ferrón, J. Effect of N-Acetylcysteine Administration on Intraoperative Plasma Levels of Interleukin-4 and Interleukin-10 in Liver Transplant Recipients. Transplant. Proc. 2008, 40, 2978–2980. [Google Scholar] [CrossRef]
- Gazia, C.; Lenci, I.; Manzia, T.M.; Martina, M.; Tisone, G.; Angelico, R.; Abenavoli, L.; Grassi, G.; Signorello, A.; Baiocchi, L. Current Strategies to Minimize Ischemia-Reperfusion Injury in Liver Transplantation: A Systematic Review. Rev. Recent Clin. Trials 2021, 16, 372–380. [Google Scholar] [CrossRef]
- Hojo, M.; Morimoto, T.; Maluccio, M.; Asano, T.; Morimoto, K.; Lagman, M.; Shimbo, T.; Suthanthiran, M. Cyclosporine Induces Cancer Progression by a Cell-Autonomous Mechanism. Nature 1999, 397, 530–534. [Google Scholar] [CrossRef]
- Vivarelli, M.; Cucchetti, A.; La Barba, G.; Ravaioli, M.; Del Gaudio, M.; Lauro, A.; Grazi, G.L.; Pinna, A.D. Liver Transplantation for Hepatocellular Carcinoma under Calcineurin Inhibitors: Reassessment of Risk Factors for Tumor Recurrence. Ann. Surg. 2008, 248, 857–862. [Google Scholar] [CrossRef]
- Rodríguez-Perálvarez, M.; Tsochatzis, E.; Naveas, M.C.; Pieri, G.; García-Caparrós, C.; O’Beirne, J.; Poyato-González, A.; Ferrín-Sánchez, G.; Montero-Álvarez, J.L.; Patch, D.; et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J. Hepatol. 2013, 59, 1193–1199. [Google Scholar] [CrossRef]
- Bhat, M.; Sonenberg, N.; Gores, G.J. The mTOR Pathway in Hepatic Malignancies. Hepatology 2013, 58, 810–818. [Google Scholar] [CrossRef]
- Toso, C.; Merani, S.; Bigam, D.L.; Shapiro, A.J.; Kneteman, N.M. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology 2010, 51, 1237–1243. [Google Scholar] [CrossRef]
- Liang, W.; Wang, D.; Ling, X.; Kao, A.A.; Kong, Y.; Shang, Y.; Guo, Z.; He, X. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: A meta-analysis. Liver Transplant. 2012, 18, 62–69. [Google Scholar] [CrossRef]
- Geissler, E.K.; Schnitzbauer, A.A.; Zülke, C.; Lamby, P.E.; Proneth, A.; Duvoux, C.; Burra, P.; Jauch, K.W.; Rentsch, M.; Ganten, T.M.; et al. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation 2016, 100, 116–125. [Google Scholar] [CrossRef]
- Benítez, C.; Londoño, M.-C.; Miquel, R.; Manzia, T.-M.; Abraldes, J.G.; Lozano, J.-J.; Martínez-Llordella, M.; López, M.; Angelico, R.; Bohne, F.; et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology 2013, 58, 1824–1835. [Google Scholar] [CrossRef]
- Lerut, J.P.; Pinheiro, R.S.; Lai, Q.; Stouffs, V.; Orlando, G.; Juri, J.M.R.; Ciccarelli, O.; Sempoux, C.; Roggen, F.M.; De Reyck, C.; et al. Is Minimal, [Almost] Steroid-Free Immunosuppression a Safe Approach in Adult Liver Transplantation? Long-term Outcome of a Prospective, Double Blind, Placebo-Controlled, Randomized, Investigator-Driven Study. Ann. Surg. 2014, 260, 886–892. [Google Scholar] [CrossRef]
- Angelico, R.; Parente, A.; Manzia, T.M. Using a weaning immunosuppression protocol in liver transplantation recipients with hepatocellular carcinoma: A compromise between the risk of recurrence and the risk of rejection? Transl. Gastroenterol. Hepatol. 2017, 2, 74. [Google Scholar] [CrossRef]
- Cillo, U.; De Carlis, L.; Del Gaudio, M.; De Simone, P.; Fagiuoli, S.; Lupo, F.; Tisone, G.; Volpes, R. Immunosuppressive regimens for adult liver transplant recipients in real-life practice: Consensus recommendations from an Italian Working Group. Hepatol. Int. 2020, 14, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Orci, L.A.; Lacotte, S.; Oldani, G.; Slits, F.; De Vito, C.; Crowe, L.A.; Rubbia-Brandt, L.; Vallée, J.; Morel, P.; Toso, C. Effect of ischaemic preconditioning on recurrence of hepatocellular carcinoma in an experimental model of liver steatosis. Br. J. Surg. 2016, 103, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Cescon, M.; Grazi, G.L.; Grassi, A.; Ravaioli, M.; Vetrone, G.; Ercolani, G.; Varotti, G.; D’Errico, A.; Ballardini, G.; Pinna, A.D. Effect of ischemic preconditioning in whole liver transplantation from deceased donors. A pilot study. Liver Transplant. 2006, 12, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Koneru, B.; Shareef, A.; Dikdan, G.; Desai, K.; Klein, K.M.; Peng, B.; Wachsberg, R.H.; De La Torre, A.N.; Debroy, M.; Fisher, A.; et al. The Ischemic Preconditioning Paradox in Deceased Donor Liver Transplantation—Evidence from a Prospective Randomized Single Blind Clinical Trial. Am. J. Transplant. 2007, 7, 2788–2796. [Google Scholar] [CrossRef]
- Robertson, F.P.; Magill, L.J.; Wright, G.P.; Fuller, B.; Davidson, B.R. A systematic review and meta-analysis of donor ischaemic preconditioning in liver transplantation. Transpl. Int. 2016, 29, 1147–1154. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, L.; Zhou, Y.; Sheng, J.; Long, D.; Li, S.; Li, Y. Systematic review with meta-analysis: HIF-1α attenuates liver ischemia–reperfusion injury. Transplant. Rev. 2015, 29, 127–134. [Google Scholar] [CrossRef]
- Song, X.; Zhang, N.; Xu, H.; Cao, L.; Zhang, H. Combined Preconditioning and Postconditioning Provides Synergistic Protection against Liver Ischemic Reperfusion Injury. Int. J. Biol. Sci. 2012, 8, 707–718. [Google Scholar] [CrossRef]
- Oberkofler, C.E.; Limani, P.; Jang, J.-H.; Rickenbacher, A.; Lehmann, K.; Raptis, D.A.; Ungethuem, U.; Tian, Y.; Grabliauskaite, K.; Humar, R.; et al. Systemic protection through remote ischemic preconditioning is spread by platelet-dependent signaling in mice. Hepatology 2014, 60, 1409–1417. [Google Scholar] [CrossRef]
- Dutkowski, P.; Guarrera, J.V.; de Jonge, J.; Martins, P.N.; Porte, R.J.; Clavien, P.-A. Evolving Trends in Machine Perfusion for Liver Transplantation. Gastroenterology 2019, 156, 1542–1547. [Google Scholar] [CrossRef]
- Hessheimer, A.J.; Rosa, G.; Gastaca, M.; Ruíz, P.; Otero, A.; Gómez, M.; Alconchel, F.; Ramírez, P.; Bosca, A.; López-Andújar, R.; et al. Abdominal normothermic regional perfusion in controlled donation after circulatory determination of death liver transplantation: Outcomes and risk factors for graft loss. Am. J. Transplant. 2022, 22, 1169–1181. [Google Scholar] [CrossRef]
- Oniscu, G.C.; Randle, L.V.; Muiesan, P.; Butler, A.J.; Currie, I.S.; Perera, M.T.P.R.; Forsythe, J.L.; Watson, C.J.E. In Situ Normothermic Regional Perfusion for Controlled Donation After Circulatory Death-The United Kingdom Experience. Am. J. Transplant. 2014, 14, 2846–2854. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Hunt, F.; Messer, S.; Currie, I.; Large, S.; Sutherland, A.; Crick, K.; Wigmore, S.J.; Fear, C.; Cornateanu, S.; et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am. J. Transplant. 2019, 19, 1745–1758. [Google Scholar] [CrossRef] [Green Version]
- Hessheimer, A.J.; Coll, E.; Torres, F.; Ruíz, P.; Gastaca, M.; Rivas, J.I.; Gómez, M.; Sánchez, B.; Santoyo, J.; Ramírez, P.; et al. Normothermic regional perfusion vs. super-rapid recovery in controlled donation after circulatory death liver transplantation. J. Hepatol. 2019, 70, 658–665. [Google Scholar] [CrossRef]
- Savier, E.; Lim, C.; Rayar, M.; Orlando, F.; Boudjema, K.; Mohkam, K.; Lesurtel, M.; Mabrut, J.Y.; Pittau, G.; Begdadi, N.; et al. Favorable Outcomes of Liver Transplantation from Controlled Circulatory Death Donors Using Normothermic Regional Perfusion Compared to Brain Death Donors. Transplantation 2020, 104, 1943–1951. [Google Scholar] [CrossRef]
- Schlegel, A.A.; Kalisvaart, M.; Muiesan, P. Machine perfusion in liver transplantation: An essential treatment or just an expensive toy? Minerva Anestesiol. 2018, 84, 236–245. [Google Scholar] [CrossRef]
- de Meijer, V.E.; Fujiyoshi, M.; Porte, R.J. Ex situ machine perfusion strategies in liver transplantation. J. Hepatol. 2019, 70, 203–205. [Google Scholar] [CrossRef]
- He, X.; Guo, Z.; Zhao, Q.; Ju, W.; Wang, D.; Wu, L.; Yang, L.; Ji, F.; Tang, Y.; Zhang, Z.; et al. The first case of ischemia-free organ transplantation in humans: A proof of concept. Am. J. Transplant. 2018, 18, 737–744. [Google Scholar] [CrossRef]
- Guarrera, J.V.; Henry, S.D.; Samstein, B.; Odeh-Ramadan, R.; Kinkhabwala, M.; Goldstein, M.J.; Ratner, L.E.; Renz, J.F.; Lee, H.T.; Brown, J.R.S.; et al. Hypothermic Machine Preservation in Human Liver Transplantation: The First Clinical Series. Am. J. Transplant. 2010, 10, 372–381. [Google Scholar] [CrossRef]
- Schlegel, A.; de Rougemont, O.; Graf, R.; Clavien, P.-A.; Dutkowski, P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J. Hepatol. 2013, 58, 278–286. [Google Scholar] [CrossRef]
- Schlegel, A.; Kron, P.; Graf, R.; Clavien, P.-A.; Dutkowski, P. Hypothermic Oxygenated Perfusion (HOPE) Downregulates the Immune Response in a Rat Model of Liver Transplantation. Ann. Surg. 2014, 260, 931–938. [Google Scholar] [CrossRef]
- Kron, P.; Schlegel, A.; Muller, X.; Gaspert, A.; Clavien, P.-A.; Dutkowski, P. Hypothermic Oxygenated Perfusion: A Simple and Effective Method to Modulate the Immune Response in Kidney Transplantation. Transplantation 2019, 103, e128–e136. [Google Scholar] [CrossRef]
- Schlegel, A.; Graf, R.; Clavien, P.-A.; Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) protects from biliary injury in a rodent model of DCD liver transplantation. J. Hepatol. 2013, 59, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, R.; van Leeuwen, O.; Matton, A.P.M.; Burlage, L.C.; Wiersema-Buist, J.; Heuvel, M.C.V.D.; De Kleine, R.H.J.; De Boer, M.T.; Gouw, A.S.H.; Porte, R.J. Hypothermic oxygenated machine perfusion reduces bile duct reperfusion injury after transplantation of donation after circulatory death livers. Liver Transplant. 2018, 24, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Patrono, D.; Catalano, G.; Rizza, G.; Lavorato, N.; Berchialla, P.; Gambella, A.; Caropreso, P.; Mengozzi, G.; Romagnoli, R. Perfusate Analysis During Dual Hypothermic Oxygenated Machine Perfusion of Liver Grafts. Transplantation 2020, 104, 1929–1942. [Google Scholar] [CrossRef]
- Patrono, D.; Cussa, D.; Sciannameo, V.; Montanari, E.; Panconesi, R.; Berchialla, P.; Lepore, M.; Gambella, A.; Rizza, G.; Catalano, G.; et al. Outcome of liver transplantation with grafts from brain-dead donors treated with dual hypothermic oxygenated machine perfusion, with particular reference to elderly donors. Am. J. Transplant. 2022, 22, 1382–1395. [Google Scholar] [CrossRef]
- Dutkowski, P.; Schlegel, A.; de Oliveira, M.; Müllhaupt, B.; Neff, F.; Clavien, P.-A. HOPE for human liver grafts obtained from donors after cardiac death. J. Hepatol. 2014, 60, 765–772. [Google Scholar] [CrossRef]
- van Rijn, R.; Karimian, N.; Matton, A.P.M.; Burlage, L.C.; Westerkamp, A.C.; Berg, A.P.V.D.; de Kleine, R.H.J.; de Boer, M.T.; Lisman, T.; Porte, R.J. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br. J. Surg. 2017, 104, 907–917. [Google Scholar] [CrossRef]
- Patrono, D.; Surra, A.; Catalano, G.; Rizza, G.; Berchialla, P.; Martini, S.; Tandoi, F.; Lupo, F.; Mirabella, S.; Stratta, C.; et al. Hypothermic Oxygenated Machine Perfusion of Liver Grafts from Brain-Dead Donors. Sci. Rep. 2019, 27, 9337. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Kalisvaart, M.; Muellhaupt, B.; Perera, M.T.P.; Isaac, J.R.; Clavien, P.-A.; Muiesan, P.; Dutkowski, P. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J. Hepatol. 2019, 70, 50–57. [Google Scholar] [CrossRef]
- van Rijn, R.; Schurink, I.J.; de Vries, Y.; Berg, A.P.V.D.; Cerisuelo, M.C.; Murad, S.D.; Erdmann, J.I.; Gilbo, N.; de Haas, R.J.; Heaton, N.; et al. Hypothermic Machine Perfusion in Liver Transplantation—A Randomized Trial. N. Engl. J. Med. 2021, 384, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Czigany, Z.; Pratschke, J.; Froněk, J.; Guba, M.; Schöning, W.; Raptis, D.A.; Andrassy, J.; Kramer, M.; Strnad, P.; Tolba, R.H.; et al. Hypothermic Oxygenated Machine Perfusion Reduces Early Allograft Injury and Improves Post-transplant Outcomes in Extended Criteria Donation Liver Transplantation From Donation After Brain Death: Results From a Multicenter Randomized Controlled Trial (HOPE ECD-DBD). Ann. Surg. 2021, 274, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; Germinario, G.; Dajti, G.; Sessa, M.; Vasuri, F.; Siniscalchi, A.; Morelli, M.C.; Serenari, M.; Del Gaudio, M.; Zanfi, C.; et al. Hypothermic oxygenated perfusion in extended criteria donor liver transplantation—A randomized clinical trial. Am. J. Transplant. 2022. [Google Scholar] [CrossRef]
- Brüggenwirth, I.M.A.; Mueller, M.; Lantinga, V.A.; Camagni, S.; De Carlis, R.; De Carlis, L.; Colledan, M.; Dondossola, D.; Drefs, M.; Eden, J.; et al. Prolonged preservation by hypothermic machine perfusion facilitates logistics in liver transplantation: A European observational cohort study. Am. J. Transplant. 2022, 22, 1842–1851. [Google Scholar] [CrossRef]
- Muller, X.; Schlegel, A.; Kron, P.; Eshmuminov, D.; Würdinger, M.; Meierhofer, D.; Clavien, P.-A.; Dutkowski, P. Novel Real-time Prediction of Liver Graft Function During Hypothermic Oxygenated Machine Perfusion Before Liver Transplantation. Ann. Surg. 2019, 270, 783–790. [Google Scholar] [CrossRef]
- Perera, T.; Mergental, H.; Stephenson, B.; Roll, G.R.; Cilliers, H.; Liang, R.; Angelico, R.; Hubscher, S.; Neil, D.A.; Reynolds, G.; et al. First human liver transplantation using a marginal allograft resuscitated by normothermic machine perfusion. Liver Transplant. 2015, 22, 120–124. [Google Scholar] [CrossRef]
- Ravikumar, R.; Jassem, W.; Mergental, H.; Heaton, N.; Mirza, D.; Perera, M.T.P.R.; Quaglia, A.; Holroyd, D.; Vogel, T.; Coussios, C.C.; et al. Liver Transplantation After Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am. J. Transplant. 2016, 16, 1779–1787. [Google Scholar] [CrossRef]
- Mergental, H.; Perera, M.T.P.R.; Laing, R.W.; Muiesan, P.; Isaac, J.R.; Smith, A.; Stephenson, B.T.F.; Cilliers, H.; Neil, D.A.H.; Hübscher, S.G.; et al. Transplantation of Declined Liver Allografts Following Normothermic Ex-Situ Evaluation. Am. J. Transplant. 2016, 16, 3235–3245. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Kosmoliaptsis, V.; Pley, C.; Randle, L.; Fear, C.; Crick, K.; Gimson, A.E.; Allison, M.; Upponi, S.; Brais, R.; et al. Observations on the ex situ perfusion of livers for transplantation. Am. J. Transplant. 2018, 18, 2005–2020. [Google Scholar] [CrossRef]
- Mergental, H.; Stephenson, B.T.F.; Laing, R.W.; Kirkham, A.J.; Neil, D.A.H.; Wallace, L.L.; Boteon, Y.L.; Widmer, J.; Bhogal, R.H.; Perera, M.T.P.R.; et al. Development of Clinical Criteria for Functional Assessment to Predict Primary Nonfunction of High-Risk Livers Using Normothermic Machine Perfusion. Liver Transplant. 2018, 24, 1453–1469. [Google Scholar] [CrossRef]
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Perera, M.T.P.R.; Boteon, Y.L.; Attard, J.; Barton, D.; Curbishley, S.; Wilkhu, M.; Neil, D.A.H.; et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 2020, 11, 2939. [Google Scholar] [CrossRef]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; García-Valdecasas, J.C.; Heaton, N.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef]
- Ghinolfi, D.; Rreka, E.; De Tata, V.; Franzini, M.; Pezzati, D.; Fierabracci, V.; Masini, M.; Insilla, A.C.; Bindi, M.L.; Marselli, L.; et al. Pilot, Open, Randomized, Prospective Trial for Normothermic Machine Perfusion Evaluation in Liver Transplantation From Older Donors. Liver Transplant. 2019, 25, 436–449. [Google Scholar] [CrossRef]
- Jassem, W.; Xystrakis, E.; Ghnewa, Y.G.; Yuksel, M.; Pop, O.; Martinez-Llordella, M.; Jabri, Y.; Huang, X.; Lozano, J.J.; Quaglia, A.; et al. Normothermic Machine Perfusion (NMP) Inhibits Proinflammatory Responses in the Liver and Promotes Regeneration. Hepatology 2019, 70, 682–695. [Google Scholar] [CrossRef]
- Gaurav, R.; Butler, A.J.; Kosmoliaptsis, V.; Mumford, L.; Fear, C.; Swift, L.; Fedotovs, A.; Upponi, S.; Khwaja, S.; Richards, J.; et al. Liver Transplantation Outcomes From Controlled Circulatory Death Donors: SCS vs in situ NRP vs ex situ NMP. Ann. Surg. 2022, 275, 1156–1164. [Google Scholar] [CrossRef]
- Mohkam, K.; Nasralla, D.; Mergental, H.; Muller, X.; Butler, A.; Jassem, W.; Imber, C.; Monbaliu, D.; Perera, M.T.P.; Laing, R.W.; et al. In-situ normothermic regional perfusion versus ex-situ normothermic machine perfusion in liver transplantation from donation after circulatory death. Liver Transplant. 2022. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Dutkowski, P.; Mueller, M.; Eshmuminov, D.; Bautista Borrego, L.; Weber, A.; Muellhaupt, B.; Sousa Da Silva, R.X.; Burg, B.R.; Rudolf von Rohr, P.; et al. Transplantation of a human liver following 3 days of ex situ normothermic preservation. Nat. Biotechnol. 2022, 1–7. [Google Scholar] [CrossRef]
- Mueller, M.; Kalisvaart, M.; O’Rourke, J.; Shetty, S.; Parente, A.; Muller, X.; Isaac, J.; Muellhaupt, B.; Muiesan, P.; Shah, T.; et al. Hypothermic Oxygenated Liver Perfusion (HOPE) Prevents Tumor Recurrence in Liver Transplantation From Donation After Circulatory Death. Ann. Surg. 2020, 272, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, T.; Ju, W.; Li, F.; Zhang, Q.; Chen, Z.; Gong, J.; Zhao, Q.; Wang, D.; Chen, M.; et al. Ischemic-Free Liver Transplantation Reduces the Recurrence of Hepatocellular Carcinoma After Liver Transplantation. Front. Oncol. 2021, 11, 773535. [Google Scholar] [CrossRef]
- Wu, T.-J.; Chan, K.-M.; Chou, H.-S.; Lee, C.-F.; Wu, T.-H.; Chen, T.-C.; Yeh, C.-T.; Lee, W.-C. Liver Transplantation in Patients with Hepatitis B Virus-Related Hepatocellular Carcinoma: The Influence of Viral Characteristics on Clinical Outcome. Ann. Surg. Oncol. 2013, 20, 3582–3590. [Google Scholar] [CrossRef]
- Yang, J.D.; Aqel, B.A.; Pungpapong, S.; Gores, G.J.; Roberts, L.; Leise, M.D. Direct acting antiviral therapy and tumor recurrence after liver transplantation for hepatitis C-associated hepatocellular carcinoma. J. Hepatol. 2016, 65, 859–860. [Google Scholar] [CrossRef]
- Line, P.-D.; Dueland, S. Liver transplantation for secondary liver tumours: The difficult balance between survival and recurrence. J. Hepatol. 2020, 73, 1557–1562. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Z.; Wang, Y.; Wen, X.; Amador, E.H.; Yuan, L.; Ran, X.; Xiong, L.; Ran, Y.; Chen, W.; et al. Colorectal liver metastasis: Molecular mechanism and interventional therapy. Signal Transduct. Target. Ther. 2022, 4, 70. [Google Scholar] [CrossRef] [PubMed]
- Kloek, J.J.; Maréchal, X.; Roelofsen, J.; Houtkooper, R.H.; van Kuilenburg, A.B.; Kulik, W.; Bezemer, R.; Nevière, R.; van Gulik, T.M.; Heger, M. Cholestasis Is Associated with Hepatic Microvascular Dysfunction and Aberrant Energy Metabolism Before and During Ischemia-Reperfusion. Antioxid. Redox Signal. 2012, 17, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Seehawer, M.; Heinzmann, F.; D’Artista, L.; Harbig, J.; Roux, P.F.; Hoenicke, L.; Dang, H.; Klotz, S.; Robinson, L.; Doré, G.; et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature 2018, 562, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Thongchot, S.; Yongvanit, P.; Loilome, W.; Seubwai, W.; Phunicom, K.; Tassaneeyakul, W.; Pairojkul, C.; Promkotra, W.; Techasen, A.; Namwat, N. High Expression of HIF-1α, BNIP3 and PI3KC3: Hypoxia-Induced Autophagy Predicts Cholangiocarcinoma Survival and Metastasis. Asian Pac. J. Cancer Prev. 2014, 15, 5873–5878. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).