TGF-β Signalling Mediates the Anti-Inflammatory Activity of Enamel Matrix Derivative In Vitro
Abstract
:1. Introduction
2. Results
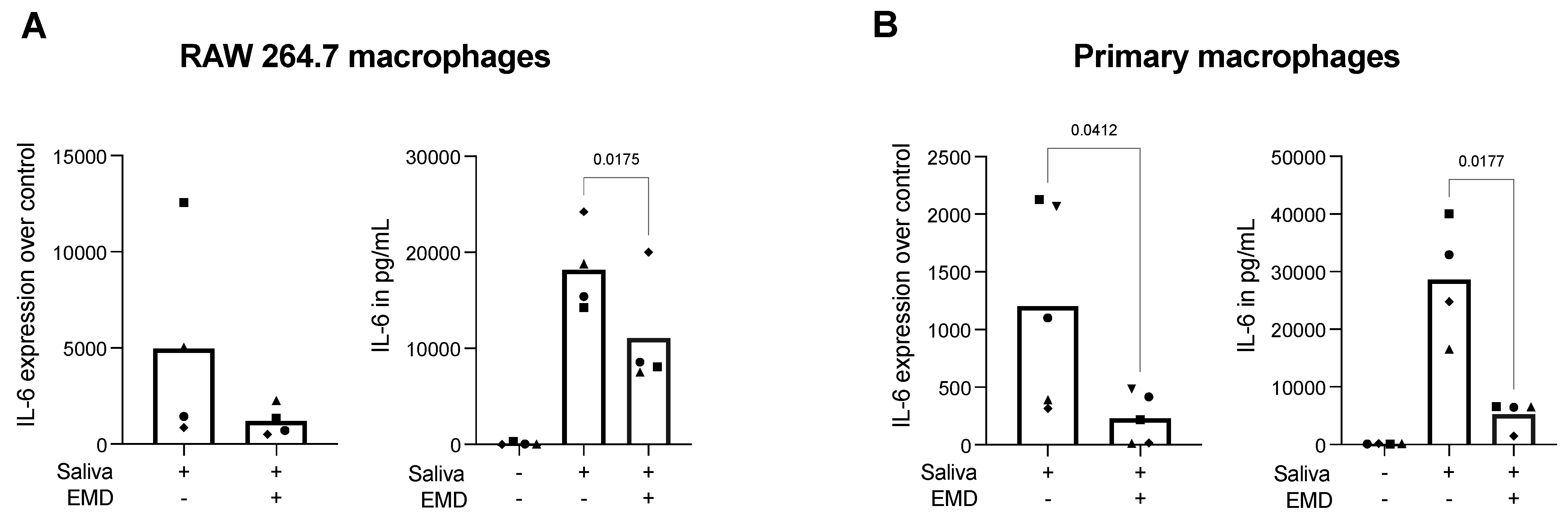
2.1. EMD Reduces Saliva-Induced IL-6 Expression and Protein Release in RAW 264.7 Cells and Primary Macrophages
2.2. RAW 264.7 Cells Treated with EMD or rTGF-β1 Contains TGF-β in Its Supernatants
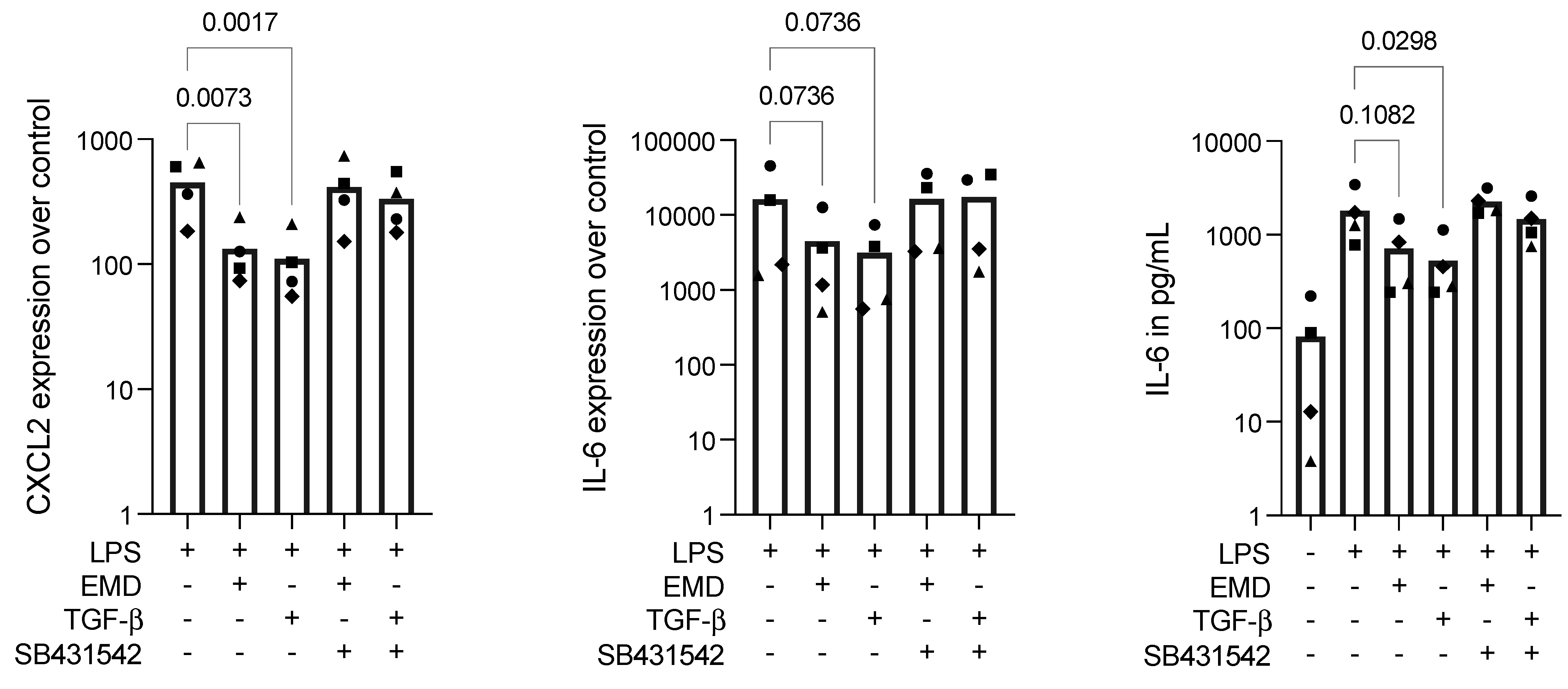
2.3. EMD Reduces Inflammation in LPS-Stimulated RAW 264.7 Cells, Which Is Reversed by SB431542
2.4. EMD Attenuates the Nuclear Translocation of Smad2/3 in RAW 264.7 Macrophages
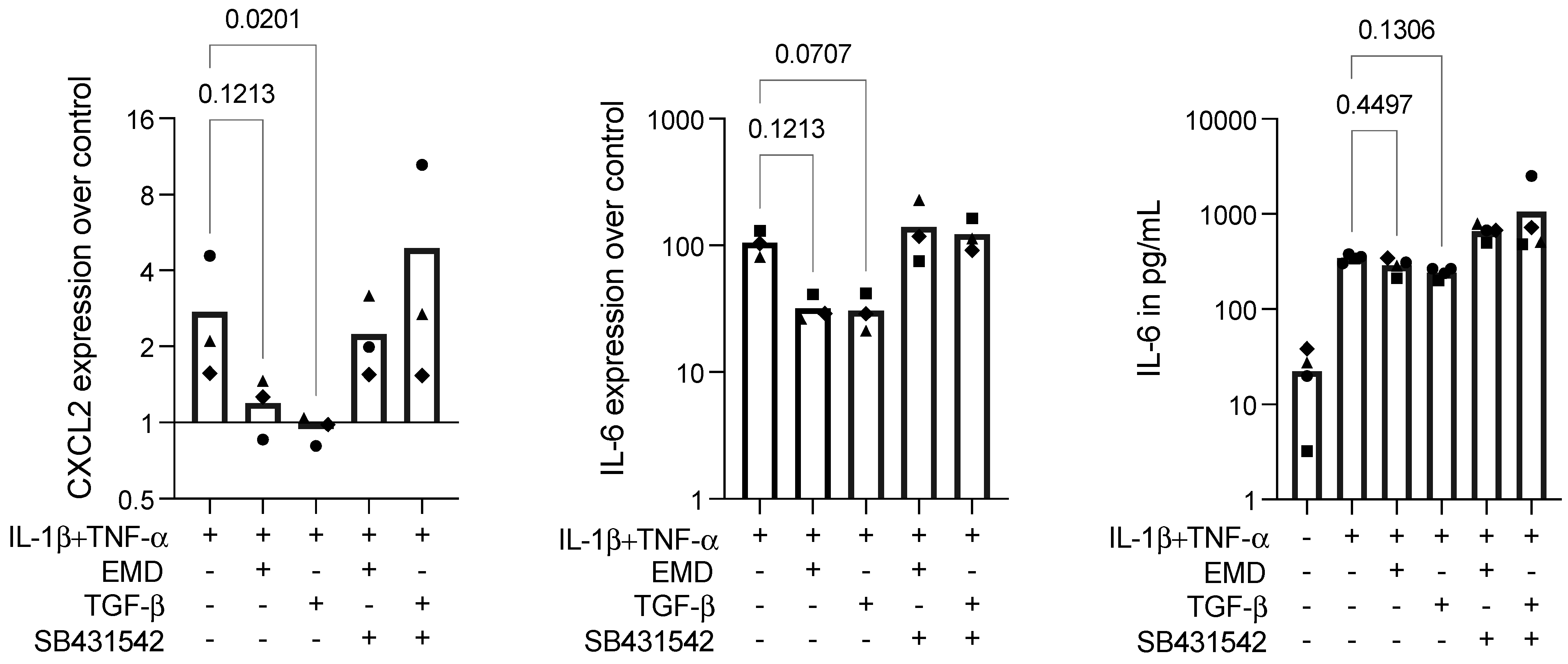
2.5. EMD Shows a Trend in Diminishing Inflammation in IL-1β+TNF-α-Stimulated ST2 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lineages
4.2. Cell Stimulation
4.3. Real-Time Polymerase Chain Reaction (RT-PCR) and Immunoassay
4.4. Immunofluorescence Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89 (Suppl. S1), 267–290. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Kajiya, M.; Kurihara, H. Molecular Mechanisms of Periodontal Disease. Int. J. Mol. Sci. 2021, 22, 930. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Papanikolaou, N.; Coulthard, P.; Worthington, H.V. Enamel matrix derivative (Emdogain) for periodontal tissue regeneration in intrabony defects. A Cochrane systematic review. Eur. J. Oral Implantol. 2009, 2, 247–266. [Google Scholar]
- Graziani, F.; Gennai, S.; Petrini, M.; Bettini, L.; Tonetti, M. Enamel matrix derivative stabilizes blood clot and improves clinical healing in deep pockets after flapless periodontal therapy: A Randomized Clinical Trial. J. Clin. Periodontol. 2019, 46, 231–240. [Google Scholar] [CrossRef]
- Graziani, F.; Peric, M.; Marhl, U.; Petrini, M.; Bettini, L.; Tonetti, M.; Gennai, S. Local application of enamel matrix derivative prevents acute systemic inflammation after periodontal regenerative surgery: A randomized controlled clinical trial. J. Clin. Periodontol. 2020, 47, 747–755. [Google Scholar] [CrossRef]
- Villa, O.; Wohlfahrt, J.C.; Koldsland, O.C.; Brookes, S.J.; Lyngstadaas, S.P.; Aass, A.M.; Reseland, J.E. EMD in periodontal regenerative surgery modulates cytokine profiles: A randomised controlled clinical trial. Sci. Rep. 2016, 6, 23060. [Google Scholar] [CrossRef]
- Sato, S.; Kitagawa, M.; Sakamoto, K.; Iizuka, S.; Kudo, Y.; Ogawa, I.; Miyauchi, M.; Chu, E.Y.; Foster, B.L.; Somerman, M.J.; et al. Enamel matrix derivative exhibits anti-inflammatory properties in monocytes. J. Periodontol. 2008, 79, 535–540. [Google Scholar] [CrossRef]
- Myhre, A.E.; Lyngstadaas, S.P.; Dahle, M.K.; Stuestol, J.F.; Foster, S.J.; Thiemermann, C.; Lilleaasen, P.; Wang, J.E.; Aasen, A.O. Anti-inflammatory properties of enamel matrix derivative in human blood. J. Periodontal Res. 2006, 41, 208–213. [Google Scholar] [CrossRef]
- Ramenzoni, L.L.; Annasohn, L.; Miron, R.J.; Attin, T.; Schmidlin, P.R. Combination of enamel matrix derivative and hyaluronic acid inhibits lipopolysaccharide-induced inflammatory response on human epithelial and bone cells. Clin. Oral Investig. 2021, 26, 1773–1783. [Google Scholar] [CrossRef]
- Almqvist, S.; Werthén, M.; Lyngstadaas, S.P.; Gretzer, C.; Thomsen, P. Amelogenins modulate cytokine expression in LPS-challenged cultured human macrophages. Cytokine 2012, 58, 274–279. [Google Scholar] [CrossRef]
- Stout, B.M.; Alent, B.J.; Pedalino, P.; Holbrook, R.; Gluhak-Heinrich, J.; Cui, Y.; Harris, M.A.; Gemperli, A.C.; Cochran, D.L.; Deas, D.E.; et al. Enamel Matrix Derivative: Protein Components and Osteoinductive Properties. J. Periodontol. 2014, 85, e9–e17. [Google Scholar] [CrossRef]
- Gruber, R.; Bosshardt, D.D.; Miron, R.J.; Gemperli, A.C.; Buser, D.; Sculean, A. Enamel matrix derivative inhibits adipocyte differentiation of 3T3-L1 cells via activation of TGF-βRI kinase activity. PLoS ONE 2013, 8, e71046. [Google Scholar]
- Gruber, R.; Stähli, A.; Miron, R.J.; Bosshardt, D.D.; Sculean, A. Common target genes of palatal and gingival fibroblasts for EMD: The microarray approach. J. Periodontal Res. 2015, 50, 103–112. [Google Scholar] [CrossRef]
- Stephanopoulos, G.; Garefalaki, M.E.; Lyroudia, K. Genes and Related Proteins Involved in Amelogenesis Imperfecta. J. Dent. Res. 2005, 84, 1117–1126. [Google Scholar]
- Hu, J.C.; Sun, X.; Zhang, C.; Simmer, J.P. A comparison of enamelin and amelogenin expression in developing mouse molars. Eur. J. Oral Sci. 2001, 109, 125–132. [Google Scholar]
- Fong, C.D.; Hammarström, L. Expression of amelin and amelogenin in epithelial root sheath remnants of fully formed rat molars. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000, 90, 218–223. [Google Scholar] [CrossRef]
- Yamamichi, K.; Fukuda, T.; Sanui, T.; Toyoda, K.; Tanaka, U.; Nakao, Y.; Yotsumoto, K.; Yamato, H.; Taketomi, T.; Uchiumi, T.; et al. Amelogenin induces M2 macrophage polarisation via PGE2/cAMP signalling pathway. Arch. Oral Biol. 2017, 83, 241–251. [Google Scholar] [CrossRef]
- Stähli, A.; Bosshardt, D.; Sculean, A.; Gruber, R. Emdogain-regulated gene expression in palatal fibroblasts requires TGF-βRI kinase signaling. PLoS ONE 2014, 9, e105672. [Google Scholar]
- Gruber, R.; Roos, G.; Caballé-Serrano, J.; Miron, R.; Bosshardt, D.D.; Sculean, A. TGF-βRI kinase activity mediates Emdogain-stimulated in vitro osteoclastogenesis. Clin. Oral Investig. 2014, 18, 1639–1646. [Google Scholar]
- Hausmann, E.H.; Hao, S.Y.; Pace, J.L.; Parmely, M.J. Transforming growth factor beta 1 and gamma interferon provide opposing signals to lipopolysaccharide-activated mouse macrophages. Infect. Immun. 1994, 62, 3625–3632. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.Q.; Freire-de-Lima, C.G.; Janssen, W.J.; Morimoto, K.; Lyu, D.; Bratton, D.L.; Henson, P.M. Oxidants selectively reverse TGF-beta suppression of proinflammatory mediator production. J. Immunol. 2006, 176, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Takaki, H.; Minoda, Y.; Koga, K.; Takaesu, G.; Yoshimura, A.; Kobayashi, T. TGF-beta1 suppresses IFN-gamma-induced NO production in macrophages by suppressing STAT1 activation and accelerating iNOS protein degradation. Genes Cells 2006, 11, 871–882. [Google Scholar] [CrossRef]
- Reddy, S.T.; Gilbert, R.S.; Xie, W.; Luner, S.; Herschman, H.R. TGF-beta 1 inhibits both endotoxin-induced prostaglandin synthesis and expression of the TIS10/prostaglandin synthase 2 gene in murine macrophages. J. Leukoc Biol. 1994, 55, 192–200. [Google Scholar] [CrossRef]
- Nasirzade, J.; Kargarpour, Z.; Hasannia, S.; Strauss, F.J.; Gruber, R. Platelet-rich fibrin elicits an anti-inflammatory response in macrophages in vitro. J. Periodontol. 2020, 91, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Pourgonabadi, S.; Müller, H.D.; Mendes, J.R.; Gruber, R. Saliva initiates the formation of pro-inflammatory macrophages in vitro. Arch. Oral Biol. 2017, 73, 295–301. [Google Scholar] [CrossRef]
- Müller, H.D.H.D.; Cvikl, B.B.; Lussi, A.A.; Gruber, R.R. Salivary pellets induce a pro-inflammatory response involving the TLR4-NF-kB pathway in gingival fibroblasts. BMC Oral Health 2016, 17, 15. [Google Scholar] [CrossRef]
- van Caam, A.; Madej, W.; Garcia de Vinuesa, A.; Goumans, M.J.; Ten Dijke, P.; Blaney Davidson, E.; van der Kraan, P. TGFβ1-induced SMAD2/3 and SMAD1/5 phosphorylation are both ALK5-kinase-dependent in primary chondrocytes and mediated by TAK1 kinase activity. Arthritis Res. Ther. 2017, 19, 112. [Google Scholar] [CrossRef]
- Hammarström, L.; Heijl, L.; Gestrelius, S. Periodontal regeneration in a buccal dehiscence model in monkeys after application of enamel matrix proteins. J. Clin. Periodontol. 1997, 24, 669–677. [Google Scholar] [CrossRef]
- Heng, N.H.M.; N’Guessan, P.D.; Kleber, B.M.; Bernimoulin, J.P.; Pischon, N. Enamel matrix derivative induces connective tissue growth factor expression in human osteoblastic cells. J. Periodontol. 2007, 78, 2369–2379. [Google Scholar] [CrossRef]
- Haruyama, N.; Hatakeyama, J.; Moriyama, K.; Kulkarni, A.B. Amelogenins: Multi-Functional Enamel Matrix Proteins and Their Binding Partners. J. Oral Biosci. 2011, 53, 257–266. [Google Scholar] [CrossRef]
- Taylor, A.L.; Haze-Filderman, A.; Blumenfeld, A.; Shay, B.; Dafni, L.; Rosenfeld, E.; YoavLeiser, Y.; Fermon, E.; Gruenbaum-Cohen, Y.; Deutsch, D. High yield of biologically active recombinant human amelogenin using the baculovirus expression system. Protein Expr. Purif. 2006, 45, 43–53. Available online: https://pubmed.ncbi.nlm.nih.gov/16055347/ (accessed on 28 July 2022). [CrossRef] [PubMed]
- Kawase, T.; Okuda, K.; Momose, M.; Kato, Y.; Yoshie, H.; Burns, D.M. Enamel matrix derivative (EMDOGAIN) rapidly stimulates phosphorylation of the MAP kinase family and nuclear accumulation of smad2 in both oral epithelial and fibroblastic human cells. J. Periodontal Res. 2001, 36, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Bosshardt, D.D.; Zhang, Y.; Buser, D.; Sculean, A. Gene array of primary human osteoblasts exposed to enamel matrix derivative in combination with a natural bone mineral. Clin. Oral Investig. 2013, 17, 405–410. [Google Scholar] [CrossRef]
- Kawase, T.; Okuda, K.; Yoshie, H.; Burns, D.M. Anti-TGF-beta antibody blocks enamel matrix derivative-induced upregulation of p21WAF1/cip1 and prevents its inhibition of human oral epithelial cell proliferation. J. Periodontal Res. 2002, 37, 255–262. [Google Scholar] [CrossRef]
- Okubo, K.; Kobayashi, M.; Takiguchi, T.; Takada, T.; Ohazama, A.; Okamatsu, Y.; Hasegawa, K. Participation of endogenous IGF-I and TGF-beta 1 with enamel matrix derivative-stimulated cell growth in human periodontal ligament cells. J. Periodontal Res. 2003, 38, 1–9. [Google Scholar] [CrossRef]
- Panahipour, L.; Kochergina, E.; Kreissl, A.; Haiden, N.; Gruber, R. Milk modulates macrophage polarization in vitro. Cytokine X 2019, 1, 100009. [Google Scholar] [CrossRef]
- Panahipour, L.; Tabatabaei, A.A.; Gruber, R. Hypoallergenic infant formula lacks transforming growth factor beta activity and has a lower anti-inflammatory activity than regular infant formula. J. Dairy Sci. 2020, 103, 6771–6781. [Google Scholar] [CrossRef]
- Panahipour, L.; Kochergina, E.; Laggner, M.; Zimmermann, M.; Mildner, M.; Ankersmit, H.J.; Gruber, R. Role for Lipids Secreted by Irradiated Peripheral Blood Mononuclear Cells in Inflammatory Resolution in Vitro. Int. J. Mol. Sci. 2020, 21, 4694. [Google Scholar] [CrossRef]
- Fujishiro, N.; Anan, H.; Hamachi, T.; Maeda, K. The role of macrophages in the periodontal regeneration using Emdogain gel. J. Periodontal Res. 2008, 43, 143–155. [Google Scholar] [CrossRef]
- Okuda, K.; Miyazaki, A.; Momose, M.; Murata, M.; Nomura, T.; Kubota, T.; Wolff, L.F.; Yoshie, H. Levels of tissue inhibitor of metalloproteinases-1 and matrix metalloproteinases-1 and -8 in gingival crevicular fluid following treatment with enamel matrix derivative (EMDOGAIN). J. Periodontal Res. 2001, 36, 309–316. [Google Scholar] [CrossRef] [PubMed]


| LPS | EMD + LPS | SB431542 + EMD + LPS |
|---|---|---|
| 2128 | 54 | 1016 |
| 2067 | 284 | 4753 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panahipour, L.; Sordi, M.B.; Kargarpour, Z.; Gruber, R. TGF-β Signalling Mediates the Anti-Inflammatory Activity of Enamel Matrix Derivative In Vitro. Int. J. Mol. Sci. 2022, 23, 9778. https://doi.org/10.3390/ijms23179778
Panahipour L, Sordi MB, Kargarpour Z, Gruber R. TGF-β Signalling Mediates the Anti-Inflammatory Activity of Enamel Matrix Derivative In Vitro. International Journal of Molecular Sciences. 2022; 23(17):9778. https://doi.org/10.3390/ijms23179778
Chicago/Turabian StylePanahipour, Layla, Mariane Beatriz Sordi, Zahra Kargarpour, and Reinhard Gruber. 2022. "TGF-β Signalling Mediates the Anti-Inflammatory Activity of Enamel Matrix Derivative In Vitro" International Journal of Molecular Sciences 23, no. 17: 9778. https://doi.org/10.3390/ijms23179778
APA StylePanahipour, L., Sordi, M. B., Kargarpour, Z., & Gruber, R. (2022). TGF-β Signalling Mediates the Anti-Inflammatory Activity of Enamel Matrix Derivative In Vitro. International Journal of Molecular Sciences, 23(17), 9778. https://doi.org/10.3390/ijms23179778







