Insights into the Black Box of Intra-Amniotic Infection and Its Impact on the Premature Lung: From Clinical and Preclinical Perspectives
Abstract
1. Introduction
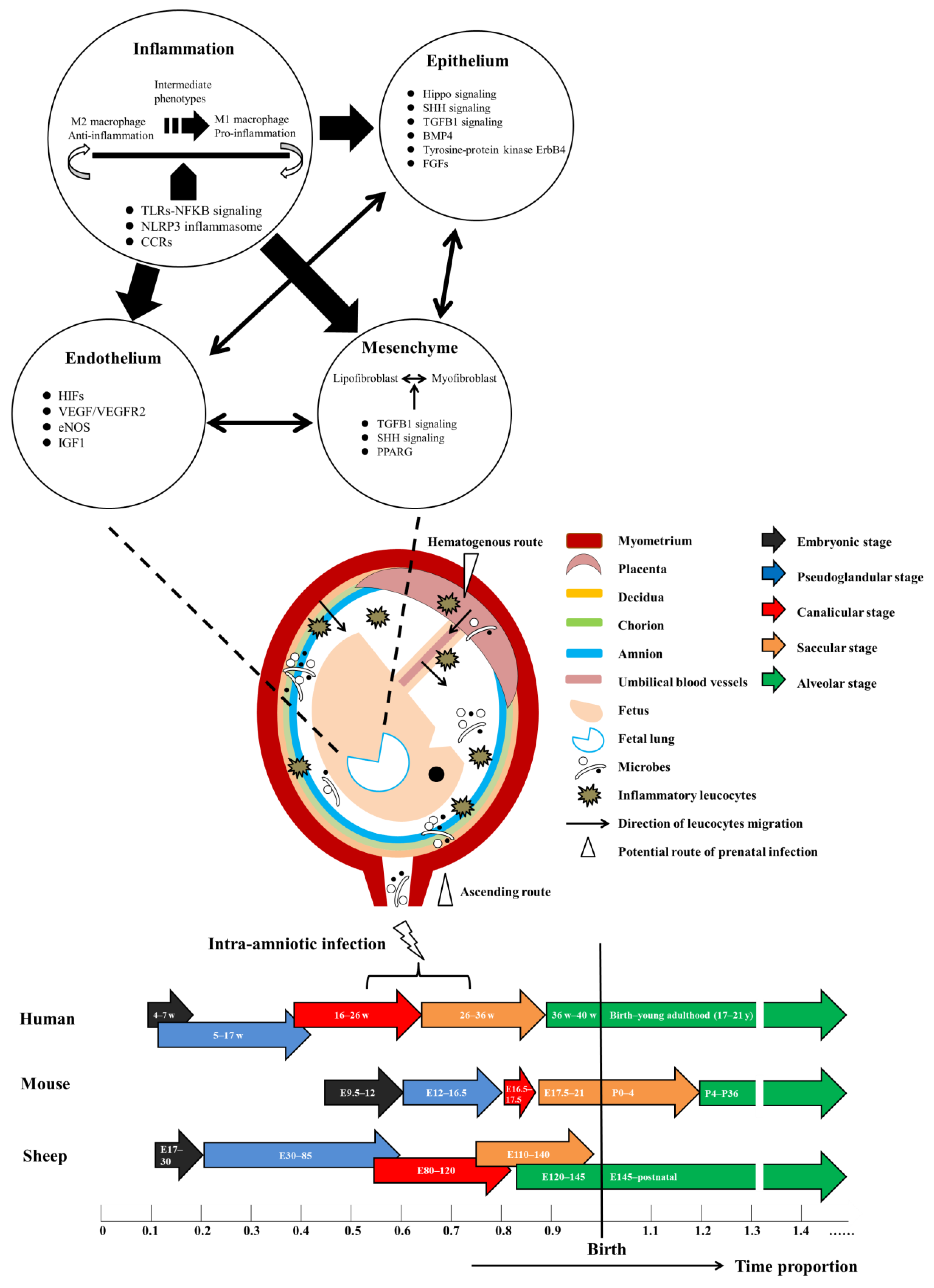
2. Intra-Amniotic Infection: Microbes, Maternal-Fetal Inflammation, Pathological and Clinical Manifestations
3. Intra-Amniotic Infection and BPD: Discrepant Patient-Based Biosample and Clinical Evidence
4. Prenatal Infection and Its Impact on Lung Development: Representative Animal Models
4.1. Rodent Models of Prenatal Infection-Induced Lung Injury
4.1.1. Establishment of Models
4.1.2. Maternal and Fetal Inflammatory Response to Prenatal Infection
4.1.3. Molecular, Morphological and Functional Changes of the Lung
4.2. Sheep Models of IAI-Induced Lung Injury
4.2.1. Establishment of Models
4.2.2. Maternal and Fetal Inflammatory Response to IAI
4.2.3. Molecular, Morphological and Functional Changes of the Lung Post IAI
4.3. Other Animal Models
5. Signaling Networks Underlying IAI-Driven Lung Injury and Potential Targets for Therapeutic Approaches
5.1. Signaling Pathways Involved in the Initial Inflammatory Response
5.2. Downstream Signaling of Epithelium, Endothelium and Mesenchyme in Response to Inflammation
6. Conclusions and Future Clinical and Preclinical Perspectives
- (1)
- From the maternal side, dissection of the microbiome across trimesters under physiological and pathological conditions, being integrated with maternal metabolome, proteome, and immunome, provides the most advanced tools to identify patients at risk of or developing IAI [139,140]. Less invasive or non-invasive approaches are highly desired for this particular population. One pioneer preclinical study using a sheep model has demonstrated that the volatile organic compound profile in exhaled breath could successfully differentiate ewes with UP-induced IAI from non-infected ones with an AUC of 0.93 [141]. Timely detection and appropriate surveillance of high-risk pregnancies is the cornerstone of an optimized healthcare concept starting from the antenatal period, where preventive measures and treatment of prematurity-related morbidities are ideally initiated.
- (2)
- From the neonatal side, minimally invasive exposome analyses of biosamples (e.g., cord blood, oral and laryngotracheal secretion, urine) at birth and longitudinally in the postnatal period are valuable for retrospectively analyzing prenatal events and predicting neonatal morbidities [142,143], yet the roles of many identified biomarkers remain to be further clarified. Cutting-edge single-cell technology and systems biology combining multiple omics will greatly contribute to the exploration and delineation of cell-specific interactions and molecular pathways underlying lung development and injury [38,113,144]. Future neonatal management concept needs to focus on a more personalized approach that accounts for perinatal exposure (e.g., microbial type, duration, and severity of IAI), GA, and multi-omic profiling.
- (3)
- In translational preclinical studies, more sophisticated multiple-hit mice models are warranted to expand the current understanding of the pathogenesis of IAI-induced lung injury at tissue, cellular, and molecular levels, given the advantages of transgenic mice. To truly reflect clinically relevant scenarios, future research should include Gram-positive bacteria and fully consider the polymicrobial and sequential nature of IAI. Technical advances in small animal imaging [61,107], lineage labeling [122,127], and spatial multi-omics [144] potentiate the generation of integrative high-resolution atlas of lung development, homeostasis, and disease, thereby revolutionizing the search for therapeutic candidates.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTA2 | actin alpha 2 |
| ANG1 | angiopoietin 1 |
| AT2 | alveolar type 2 |
| AUC | area under curve |
| BMP4 | bone morphogenetic protein 4 |
| BPD | bronchopulmonary dysplasia |
| CCL2 | C-C motif ligand 2 |
| CCR | C-C motif receptor |
| CTGF | connective tissue growth factor |
| CXCL2 | C-X-C motif ligand 2 |
| eNOS | endothelial nitric oxide synthase |
| EPIs | extremely preterm infants |
| FGF | fibroblast growth factor |
| GA | gestational age |
| GBS | group B Streptococcus |
| GLP1R | glucagon-like peptide-1 receptor |
| HIF1 | hypoxia-inducible factor 1 |
| HMOX1 | heme oxygenase 1 |
| IAI | intra-amniotic infection |
| i.a. | intra-amniotic |
| IGF1 | insulin-like growth factor-1 |
| IL | interleukin |
| IL1RA | interleukin 1 receptor antagonist |
| i.p. | intraperitoneal |
| i.u. | intrauterine |
| LPS | lipopolysaccharide |
| miRs | microRNAs |
| MSCs | mesenchymal stromal cells |
| NFKB | nuclear factor kappa B |
| NLRP3 | NLR family pyrin domain-containing protein 3 |
| PAMPs | pathogen associated molecular patterns |
| PPARG | peroxisome proliferator activated receptors gamma |
| PRRs | pattern recognition receptors |
| RIP3 | receptor-interacting kinase 3 |
| RVH | Right ventricular hypertrophy |
| SHH | sonic hedgehog |
| SPs | surfactant proteins |
| sFLT1 | soluble FMS-like tyrosine kinase 1 |
| TGFB1 | transforming growth factor beta 1 |
| TIE2 | tunica interna endothelial cell kinase 2 |
| TLR | toll-like receptor |
| TNF | tumor necrosis factor |
| UP | Ureaplasma parvum |
| VEGF | vascular endothelial growth factor |
| VEGFR2 | vascular endothelial growth factor receptor 2 |
References
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef]
- Jain, V.G.; Willis, K.A.; Jobe, A.; Ambalavanan, N. Chorioamnionitis and neonatal outcomes. Pediatr. Res. 2022, 91, 289–296. [Google Scholar] [CrossRef]
- Schittny, J.C. Development of the lung. Cell Tissue Res. 2017, 367, 427–444. [Google Scholar] [CrossRef]
- Villamor-Martinez, E.; Álvarez-Fuente, M.; Ghazi, A.M.T.; Degraeuwe, P.; Zimmermann, L.J.I.; Kramer, B.W.; Villamor, E. Association of Chorioamnionitis With Bronchopulmonary Dysplasia Among Preterm Infants: A systematic review, meta-analysis, and metaregression. JAMA Netw. Open 2019, 2, e1914611. [Google Scholar] [CrossRef]
- Hartling, L.; Liang, Y.; Lacaze-Masmonteil, T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F8–F17. [Google Scholar] [CrossRef]
- Holzfurtner, L.; Shahzad, T.; Dong, Y.; Rekers, L.; Selting, A.; Staude, B.; Lauer, T.; Schmidt, A.; Rivetti, S.; Zimmer, K.-P.; et al. When inflammation meets lung development—An update on the pathogenesis of bronchopulmonary dysplasia. Mol. Cell. Pediatr. 2022, 9, 7. [Google Scholar] [CrossRef]
- Kotecha, S.J.; Gibbons, J.T.D.; Course, C.W.; Evans, E.E.; Simpson, S.J.; Watkins, W.J.; Kotecha, S. Geographical differences and temporal improvements in forced expiratory volume in 1 second of preterm-born children: A systematic review and meta-analysis. JAMA Pediatr. 2022, e221990. [Google Scholar] [CrossRef]
- Roberts, D.; Brown, J.; Medley, N.; Dalziel, S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2017, 3, CD004454. [Google Scholar] [CrossRef]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Pas, A.T.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Caffeine for Apnea of Prematuri-ty Trial Group Caffeine Therapy for Apnea of Prematurity. N. Engl. J. Med. 2006, 354, 2112–2121. [Google Scholar] [CrossRef]
- Darlow, B.A.; Graham, P.J.; Rojas-Reyes, M.X. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants. Cochrane Database Syst. Rev. 2016, 2016, CD000501. [Google Scholar] [CrossRef] [PubMed]
- Thiess, T.; Lauer, T.; Woesler, A.; Neusius, J.; Stehle, S.; Zimmer, K.-P.; Eckert, G.P.; Ehrhardt, H. Correlation of Early Nutritional Supply and Development of Bronchopulmonary Dysplasia in Preterm Infants < 1000 g. Front. Pediatr. 2021, 9, 741365. [Google Scholar] [CrossRef] [PubMed]
- Franck, L.S.; Waddington, C.; O’Brien, K. Family Integrated Care for Preterm Infants. Crit. Care Nurs. Clin. N. Am. 2020, 32, 149–165. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.H.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, H.; Desplanches, T.; van Heijst, A.F.; Toome, L.; Fenton, A.; Torchin, H.; Nuytten, A.; Mazela, J.; Zeitlin, J.; Maier, R.F.; et al. Mode of Delivery and Incidence of Bronchopulmonary Dysplasia: Results from the Population-Based EPICE Cohort. Neonatology 2022, 119, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Lui, K.; Lee, S.K.; Kusuda, S.; Adams, M.; Vento, M.; Reichman, B.; Darlow, B.A.; Lehtonen, L.; Modi, N.; Norman, M.; et al. Trends in Outcomes for Neonates Born Very Preterm and Very Low Birth Weight in 11 High-Income Countries. J. Pediatr. 2019, 215, 32–40.e14. [Google Scholar] [CrossRef]
- Abele, A.N.; Taglauer, E.S.; Almeda, M.; Wilson, N.; Abikoye, A.; Seedorf, G.J.; Mitsialis, S.A.; Kourembanas, S.; Abman, S.H. Antenatal mesenchymal stromal cell extracellular vesicle treatment preserves lung development in a model of bronchopulmonary dysplasia due to chorioamnionitis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 322, L179–L190. [Google Scholar] [CrossRef]
- Thébaud, B.; Goss, K.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Prim. 2019, 5, 78. [Google Scholar] [CrossRef]
- Sweeney, E.L.; Kallapur, S.G.; Gisslen, T.; Lambers, D.S.; Chougnet, C.A.; Stephenson, S.-A.; Jobe, A.H.; Knox, C.L. Placental Infection with Ureaplasma species Is Associated with Histologic Chorioamnionitis and Adverse Outcomes in Moderately Preterm and Late-Preterm Infants. J. Infect. Dis. 2016, 213, 1340–1347. [Google Scholar] [CrossRef]
- Prince, A.L.; Ma, J.; Kannan, P.S.; Alvarez, M.; Gisslen, T.; Harris, R.A.; Sweeney, E.L.; Knox, C.L.; Lambers, D.S.; Jobe, A.H.; et al. The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am. J. Obstet. Gynecol. 2016, 214, 627.e1–627.e16. [Google Scholar] [CrossRef]
- Romero, R.; Gomez-Lopez, N.; Winters, A.D.; Jung, E.; Shaman, M.; Bieda, J.; Panaitescu, B.; Pacora, P.; Erez, O.; Greenberg, J.M.; et al. Evidence that intra-amniotic infections are often the result of an ascending invasion—A molecular microbiological study. J. Périnat. Med. 2019, 47, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.; Hallingström, M.; Barman, M.; Viklund, F.; Keelan, J.; Kacerovsky, M.; Payne, M.; Jacobsson, B. Comparison of Bacterial DNA Profiles in Mid-Trimester Amniotic Fluid Samples from Preterm and Term Deliveries. Front. Microbiol. 2020, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Hockney, R.; Waring, G.J.; Taylor, G.; Cummings, S.P.; Robson, S.C.; Orr, C.H.; Nelson, A. Fetal membrane bacterial load is increased in histologically confirmed inflammatory chorioamnionitis: A retrospective cohort study. Placenta 2020, 91, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.B.; Al-Nasiry, S.; Kramer, B.W.; Mueller, M. Understanding Host-Pathogen Interactions in Acute Chorioamnionitis Through the Use of Animal Models. Front. Cell. Infect. Microbiol. 2021, 11, 709309. [Google Scholar] [CrossRef]
- Staude, B.; Oehmke, F.; Lauer, T.; Behnke, J.; Göpel, W.; Schloter, M.; Schulz, H.; Krauss-Etschmann, S.; Ehrhardt, H. The Microbiome and Preterm Birth: A Change in Paradigm with Profound Implications for Pathophysiologic Concepts and Novel Therapeutic Strategies. BioMed Res. Int. 2018, 2018, 7218187. [Google Scholar] [CrossRef]
- Redline, R.W.; Ravishankar, S.; Bagby, C.M.; Saab, S.T.; Zarei, S. Four major patterns of placental injury: A stepwise guide for understanding and implementing the 2016 Amsterdam consensus. Mod. Pathol. 2021, 34, 1074–1092. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Romero, R.; Xu, Y.; Leng, Y.; Garcia-Flores, V.; Miller, D.; Jacques, S.M.; Hassan, S.S.; Faro, J.; Alsamsam, A.; et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am. J. Obstet. Gynecol. 2017, 217, 693.e1–693.e16. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213 (Suppl. 4), S29–S52. [Google Scholar] [CrossRef]
- Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Docheva, N.; Dong, Z.; Kim, C.J.; Kim, Y.M.; Kim, J.-S.; Qureshi, F.; Jacques, S.M.; et al. CXCL10 and IL-6: Markers of two different forms of intra-amniotic inflammation in preterm labor. Am. J. Reprod. Immunol. 2017, 78, e12685. [Google Scholar] [CrossRef]
- Cobo, T.; Vives, I.; Rodríguez-Trujillo, A.; Murillo, C.; Ángeles, M.A.; Bosch, J.; Vergara, A.; Gratacós, E.; Palacio, M. Impact of microbial invasion of amniotic cavity and the type of microorganisms on short-term neonatal outcome in women with preterm labor and intact membranes. Acta Obstet. Gynecol. Scand. 2017, 96, 570–579. [Google Scholar] [CrossRef]
- Romero, R.; Miranda, J.; Chaemsaithong, P.; Chaiworapongsa, T.; Kusanovic, J.P.; Dong, Z.; Ahmed, A.I.; Shaman, M.; Lannaman, K.; Yoon, B.H.; et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J. Matern. Fetal Neonatal Med. 2015, 28, 1394–1409. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.J.; Kim, S.M.; Hong, J.-S.; Maymon, E.; Erez, O.; Panaitescu, B.; Gomez-Lopez, N.; Romero, R.; Yoon, B.H. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am. J. Obstet. Gynecol. 2017, 216, 604.e1–604.e11. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.D.; Saade, G.; Polin, R.A.; Grobman, W.A.; Buhimschi, I.A.; Watterberg, K.; Silver, R.M.; Raju, T.N.; Chorioamnionitis Workshop Participants. Evaluation and Management of Women and Newborns with a Maternal Diagnosis of Chorioamnionitis: Summary of a Workshop. Obstet. Gynecol. 2016, 127, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Du, H.; Cao, Y.; Zhang, Y.; Zhang, J.; Zhang, L.; Li, Z.; Xu, Y.; Zou, H.; Sun, B. Association of histological and clinical chorioamnionitis with perinatal and neonatal outcome. J. Matern. Fetal Neonatal Med. 2021, 34, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Committee on Obstetric Practice Committee Opinion. No. 712: Intrapartum Management of Intraamniotic Infection. Obstet. Gynecol. 2017, 130, e95–e101. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Bellos, I.; Antsaklis, A.; Loutradis, D.; Daskalakis, G. Presence of amniotic fluid sludge and pregnancy outcomes: A systematic review. Acta Obstet. Gynecol. Scand. 2020, 99, 1434–1443. [Google Scholar] [CrossRef]
- Al-Nasiry, S.; Ambrosino, E.; Schlaepfer, M.; Morré, S.A.; Wieten, L.; Voncken, J.W.; Spinelli, M.; Mueller, M.; Kramer, B.W. The Interplay Between Reproductive Tract Microbiota and Immunological System in Human Reproduction. Front. Immunol. 2020, 11, 378. [Google Scholar] [CrossRef]
- Honda, A.; Hoeksema, M.A.; Sakai, M.; Lund, S.J.; Lakhdari, O.; Butcher, L.D.; Rambaldo, T.C.; Sekiya, N.M.; Nasamran, C.A.; Fisch, K.M.; et al. The lung microenvironment instructs gene transcription in neonatal and adult alveolar macro-phages. J. Immunol. 2022, 208, 1947–1959. [Google Scholar] [CrossRef]
- Twisselmann, N.; Pagel, J.; Künstner, A.; Weckmann, M.; Hartz, A.; Glaser, K.; Hilgendorff, A.; Göpel, W.; Busch, H.; Herting, E.; et al. Hyperoxia/Hypoxia Exposure Primes a Sustained Pro-Inflammatory Profile of Preterm Infant Macrophages Upon LPS Stimulation. Front. Immunol. 2021, 12, 762789. [Google Scholar] [CrossRef]
- Miller, J.D.; Benjamin, J.T.; Kelly, D.R.; Frank, D.B.; Prince, L.S. Chorioamnionitis stimulates angiogenesis in saccular stage fetal lungs via CC chemokines. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 298, L637–L645. [Google Scholar] [CrossRef]
- Cui, T.X.; Brady, A.E.; Fulton, C.T.; Zhang, Y.-J.; Rosenbloom, L.M.; Goldsmith, A.M.; Moore, B.B.; Popova, A.P. CCR2 Mediates Chronic LPS-Induced Pulmonary Inflammation and Hypoalveolarization in a Murine Model of Bronchopulmonary Dysplasia. Front. Immunol. 2020, 11, 579628. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Kapadia, V.S.; Brown, L.S.; Cheong, N.; Longoria, C.; Mija, D.S.; Ramgopal, M.; Mirpuri, J.; McCurnin, D.C.; Savani, R.C. The NLRP3 inflammasome is critically involved in the development of bronchopulmonary dysplasia. Nat. Commun. 2015, 6, 8977. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, U.A.; Buhimschi, C.S.; Zhao, G.; Buhimschi, I.; Bhandari, V. Components of the antepartum, intrapartum, and postpartum exposome impact on distinct short-term adverse neonatal outcomes of premature infants: A prospective cohort study. PLoS ONE 2018, 13, e0207298. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, X.; Jiang, N.; Li, J.; Shen, L.; Zhang, Y. CCR5 signaling promotes lipopolysaccharide-induced macrophage recruitment and alveolar developmental arrest. Cell Death Dis. 2021, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- D’Angio, C.T.; Ambalavanan, N.; Carlo, W.A.; McDonald, S.A.; Skogstrand, K.; Hougaard, D.M.; Shankaran, S.; Goldberg, R.N.; Ehrenkranz, R.A.; Tyson, J.E.; et al. Blood Cytokine Profiles Associated with Distinct Patterns of Bronchopulmonary Dysplasia among Extremely Low Birth Weight Infants. J. Pediatr. 2016, 174, 45–51.e5. [Google Scholar] [CrossRef]
- Mariduena, J.; Ramagopal, M.; Hiatt, M.; Chandra, S.; Laumbach, R.; Hegyi, T. Vascular endothelial growth factor levels and bronchopulmonary dysplasia in preterm infants. J. Matern. Fetal Neonatal Med. 2022, 35, 1517–1522. [Google Scholar] [CrossRef]
- Benjamin, J.T.; Smith, R.J.; Halloran, B.A.; Day, T.J.; Kelly, D.R.; Prince, L.S. FGF-10 is decreased in bronchopulmonary dysplasia and suppressed by Toll-like receptor activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L550–L558. [Google Scholar] [CrossRef]
- Mir, I.N.; Chalak, L.F.; Brown, L.S.; Johnson-Welch, S.; Heyne, R.; Rosenfeld, C.R.; Kapadia, V.S. Impact of multiple placental pathologies on neonatal death, bronchopulmonary dysplasia, and neurodevelopmental impairment in preterm infants. Pediatr. Res. 2020, 87, 885–891. [Google Scholar] [CrossRef]
- Lahra, M.M.; Beeby, P.J.; Jeffery, H.E. Intrauterine Inflammation, Neonatal Sepsis, and Chronic Lung Disease: A 13-Year Hospital Cohort Study. Pediatrics 2009, 123, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Durrmeyer, X.; Kayem, G.; Sinico, M.; Dassieu, G.; Danan, C.; Decobert, F. Perinatal Risk Factors for Bronchopulmonary Dysplasia in Extremely Low Gestational Age Infants: A Pregnancy Disorder–Based Approach. J. Pediatr. 2012, 160, 578–583.e2. [Google Scholar] [CrossRef]
- Leroy, S.; Caumette, E.; Waddington, C.; Hébert, A.; Brant, R.; Lavoie, P.M. A Time-Based Analysis of Inflammation in Infants at Risk of Bronchopulmonary Dysplasia. J. Pediatr. 2018, 192, 60–65.e1. [Google Scholar] [CrossRef] [PubMed]
- Zaramella, P.; Munari, F.; Stocchero, M.; Molon, B.; Nardo, D.; Priante, E.; Tosato, F.; Bonadies, L.; Viola, A.; Baraldi, E. Innate immunity ascertained from blood and tracheal aspirates of preterm newborn provides new clues for assessing bronchopulmonary dysplasia. PLoS ONE 2019, 14, e0221206. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, H.; Pritzke, T.; Oak, P.; Kossert, M.; Biebach, L.; Förster, K.; Koschlig, M.; Alvira, C.M.; Hilgendorff, A. Absence of TNF-α enhances inflammatory response in the newborn lung undergoing mechanical ventilation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L909–L918. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, T.; Chao, C.-M.; Hadzic, S.; Behnke, J.; Biebach, L.; Böttcher-Friebertshäuser, E.; Wilhelm, J.; Hilgendorff, A.; Zimmer, K.-P.; Morty, R.E.; et al. TRAIL protects the immature lung from hyperoxic injury. Cell Death Dis. 2022, 13, 614. [Google Scholar] [CrossRef]
- Park, C.-W.; Park, J.S.; Jun, J.K.; Yoon, B.H. Mild to Moderate, but Not Minimal or Severe, Acute Histologic Chorioamnionitis or Intra-Amniotic Inflammation Is Associated with a Decrease in Respiratory Distress Syndrome of Preterm Newborns without Fetal Growth Restriction. Neonatology 2015, 108, 115–123. [Google Scholar] [CrossRef]
- Sarno, L.; Della Corte, L.; Saccone, G.; Sirico, A.; Raimondi, F.; Zullo, F.; Guida, M.; Martinelli, P.; Maruotti, G.M. Histological chorioamnionitis and risk of pulmonary complications in preterm births: A systematic review and Meta-analysis. J. Matern. Fetal Neonatal Med. 2021, 34, 3803–3812. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Ito, M.; Oshima, T.; Kojima, S.; Ohno, K. Hydrogen-rich water ameliorates bronchopulmonary dysplasia (BPD) in newborn rats. Pediatr. Pulmonol. 2016, 51, 928–935. [Google Scholar] [CrossRef]
- Tang, J.-R.; Seedorf, G.J.; Muehlethaler, V.; Walker, D.L.; Markham, N.E.; Balasubramaniam, V.; Abman, S.H. Moderate postnatal hyperoxia accelerates lung growth and attenuates pulmonary hypertension in infant rats after exposure to intra-amniotic endotoxin. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 299, L735–L748. [Google Scholar] [CrossRef]
- Tang, J.-R.; Michaelis, K.A.; Nozik-Grayck, E.; Seedorf, G.J.; Hartman-Filson, M.; Abman, S.H.; Wright, C.J. The NF-κB Inhibitory Proteins IκBα and IκBβ Mediate Disparate Responses to Inflammation in Fetal Pulmonary Endothelial Cells. J. Immunol. 2013, 190, 2913–2923. [Google Scholar] [CrossRef]
- Garcia-Flores, V.; Romero, R.; Miller, D.; Xu, Y.; Done, B.; Veerapaneni, C.; Leng, Y.; Arenas-Hernández, M.; Khan, N.; Panaitescu, B.; et al. Inflammation-Induced Adverse Pregnancy and Neonatal Outcomes Can Be Improved by the Immunomodulatory Peptide Exendin-4. Front. Immunol. 2018, 9, 1291. [Google Scholar] [CrossRef]
- Velten, M.; Britt, R.D., Jr.; Heyob, K.M.; Welty, S.E.; Eiberger, B.; Tipple, T.E.; Rogers, L.K. Prenatal inflammation exacerbates hyperoxia-induced functional and structural changes in adult mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R279–R290. [Google Scholar] [CrossRef] [PubMed]
- Velten, M.; Heyob, K.M.; Rogers, L.K.; Welty, S.E. Deficits in lung alveolarization and function after systemic maternal inflammation and neonatal hyperoxia exposure. J. Appl. Physiol. 2010, 108, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Hudalla, H.; Karenberg, K.; Kuon, R.-J.; Pöschl, J.; Tschada, R.; Frommhold, D. LPS-induced maternal inflammation promotes fetal leukocyte recruitment and prenatal organ infiltration in mice. Pediatr. Res. 2018, 84, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Faksh, A.; Britt, R.D., Jr.; Vogel, E.R.; Kuipers, I.; Thompson, M.A.; Sieck, G.; Pabelick, C.M.; Martin, R.J.; Prakash, Y.S. Effects of antenatal lipopolysaccharide and postnatal hyperoxia on airway reactivity and remodeling in a neonatal mouse model. Pediatr. Res. 2016, 79, 391–400. [Google Scholar] [CrossRef]
- Kuper-Sassé, M.E.; MacFarlane, P.M.; Mayer, C.A.; Martin, R.J.; Prakash, Y.S.; Pabelick, C.M.; Raffay, T.M. Prenatal Maternal Lipopolysaccharide and Mild Newborn Hyperoxia Increase Intrapulmonary Airway but Not Vessel Reactivity in a Mouse Model. Children 2021, 8, 195. [Google Scholar] [CrossRef]
- Nold, M.F.; Mangan, N.E.; Rudloff, I.; Cho, S.X.; Shariatian, N.; Samarasinghe, T.D.; Skuza, E.M.; Pedersen, J.; Veldman, A.; Berger, P.J.; et al. Interleukin-1 receptor antagonist prevents murine bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Proc. Natl. Acad. Sci. USA 2013, 110, 14384–14389. [Google Scholar] [CrossRef]
- Prince, L.S.; Okoh, V.O.; Moninger, T.O.; Matalon, S. Lipopolysaccharide increases alveolar type II cell number in fetal mouse lungs through Toll-like receptor 4 and NF-κB. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L999–L1006. [Google Scholar] [CrossRef]
- Wallace, B.; Peisl, A.; Seedorf, G.; Nowlin, T.; Kim, C.; Bosco, J.; Kenniston, J.; Keefe, D.; Abman, S.H. Anti–sFlt-1 Therapy Preserves Lung Alveolar and Vascular Growth in Antenatal Models of Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2018, 197, 776–787. [Google Scholar] [CrossRef]
- Hirsch, K.; Taglauer, E.; Seedorf, G.; Callahan, C.; Mandell, E.; White, C.W.; Kourembanas, S.; Abman, S.H. Perinatal Hypoxia-Inducible Factor Stabilization Preserves Lung Alveolar and Vascular Growth in Experimental Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2020, 202, 1146–1158. [Google Scholar] [CrossRef]
- Salminen, A.; Paananen, R.; Vuolteenaho, R.; Metsola, J.; Ojaniemi, M.; Autio-Harmainen, H.; Hallman, M. Maternal Endotoxin-Induced Preterm Birth in Mice: Fetal Responses in Toll-Like Receptors, Collectins, and Cytokines. Pediatr. Res. 2008, 63, 280–286. [Google Scholar] [CrossRef]
- Schmiedl, A.; Bokel, K.; Huhn, V.; Ionescu, L.; Zscheppang, K.; Dammann, C.E. Bone marrow stem cells accelerate lung maturation and prevent the LPS-induced delay of morphological and functional fetal lung development in the presence of ErbB4. Cell Tissue Res. 2020, 380, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Schmiedl, A.; Behrens, J.; Zscheppang, K.; Purevdorj, E.; Von Mayersbach, D.; Liese, A.; Dammann, C.E.L. Lipopolysaccharide-induced injury is more pronounced in fetal transgenic ErbB4-deleted lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L490–L499. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ueda, K.; Cho, K.; Matsuda, T.; Okajima, S.; Uchida, M.; Kobayashi, Y.; Minakami, H.; Kobayashi, K. A Rat Model for Arrest of Alveolarization Induced by Antenatal Endotoxin Administration. Pediatr. Res. 2006, 59, 396–400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Köroğlu, A.; Macfarlane, P.M.; Balan, K.V.; Zenebe, W.J.; Jafri, A.; Martin, R.J.; Kc, P.; Prabha, K. Anti-Inflammatory Effect of Caffeine Is Associated with Improved Lung Function after Lipopolysaccharide-Induced Amnionitis. Neonatology 2014, 106, 235–240. [Google Scholar] [CrossRef]
- Lee, J.Y.; Na, Q.; Shin, N.E.; Shin, H.E.; Kang, Y.; Chudnovets, A.; Lei, J.; Song, H.; Burd, I. Melatonin for prevention of fetal lung injury associated with intrauterine inflammation and for improvement of lung maturation. J. Pineal Res. 2020, 69, e12687. [Google Scholar] [CrossRef] [PubMed]
- Dedja, A.; Gucciardi, A.; Giordano, G.; Di Gangi, I.M.; Porzionato, A.; Navaglia, F.; Baraldi, E.; Grisafi, D.; Zaramella, P. Lipopolysaccharide-induced chorioamnionitis and postnatal lung injury: The beneficial effects of L-citrulline in newborn rats. Exp. Lung Res. 2018, 44, 226–240. [Google Scholar] [CrossRef]
- Seedorf, G.; Kim, C.; Wallace, B.; Mandell, E.W.; Nowlin, T.; Shepherd, D.; Abman, S.H. rhIGF-1/BP3 Preserves Lung Growth and Prevents Pulmonary Hypertension in Experimental Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2020, 201, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chen, F.; Ni, W.; Li, J.; Zhang, Y. Theophylline improves lipopolysaccharide-induced alveolarization arrest through inflammatory regulation. Mol. Med. Rep. 2014, 10, 269–275. [Google Scholar] [CrossRef]
- Normann, E.; Lacaze-Masmonteil, T.; Eaton, F.; Schwendimann, L.; Gressens, P.; Thébaud, B. A Novel Mouse Model of Ureaplasma-Induced Perinatal Inflammation: Effects on Lung and Brain Injury. Pediatr. Res. 2009, 65, 430–436. [Google Scholar] [CrossRef]
- Andrade, E.B.; Magalhães, A.I.; Puga, A.; Costa, M.; Bravo, J.; Portugal, C.; Ribeiro, A.; Correia-Neves, M.; Faustino, A.; Firon, A.; et al. A mouse model reproducing the pathophysiology of neonatal group B streptococcal infection. Nat. Commun. 2018, 9, 3138. [Google Scholar] [CrossRef]
- Stranik, J.; Kacerovsky, M.; Vescicik, P.; Faist, T.; Jacobsson, B.; Musilova, I. A rodent model of intra-amniotic inflammation/infection, induced by the administration of inflammatory agent in a gestational sac, associated with preterm delivery: A systematic review. J. Matern. Fetal Neonatal Med. 2022, 35, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.; Brace, R.A. Amniotic Fluid Volume and Composition in Mouse Pregnancy. J. Soc. Gynecol. Investig. 2005, 12, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.F.; Catalano, R.D.; Wade, J.; Rossi, A.G.; Norman, J. Decidual Neutrophil Infiltration Is Not Required for Preterm Birth in a Mouse Model of Infection-Induced Preterm Labor. J. Immunol. 2014, 192, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Faro, J.; Romero, R.; Schwenkel, G.; Garcia-Flores, V.; Arenas-Hernandez, M.; Leng, Y.; Xu, Y.; Miller, D.; Hassan, S.S.; Gomez-Lopez, N. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasome†. Biol. Reprod. 2019, 100, 1290–1305. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.F.; Makieva, S.; Frew, L.; Wade, J.; Thomson, A.J.; Moran, C.M.; Norman, J.E.; Stock, S.J. Ultrasound-Guided Intrauterine Injection of Lipopolysaccharide as a Novel Model of Preterm Birth in the Mouse. Am. J. Pathol. 2015, 185, 1201–1206. [Google Scholar] [CrossRef]
- Widowski, H.; Ophelders, D.R.M.G.; van Leeuwen, A.J.C.N.; Nikkels, P.G.J.; Severens-Rijvers, C.A.H.; LaPointe, V.L.S.; Cleutjens, J.P.M.; Hütten, M.C.; Kemp, M.W.; Payne, M.S.; et al. Chorioamnionitis induces changes in ovine pulmonary endogenous epithelial stem/progenitor cells in utero. Pediatr. Res. 2021, 90, 549–558. [Google Scholar] [CrossRef]
- Widowski, H.; Reynaert, N.L.; Ophelders, D.R.M.G.; Hütten, M.C.; Nikkels, P.G.J.; Severens-Rijvers, C.A.H.; Cleutjens, J.P.M.; Kemp, M.W.; Newnham, J.P.; Saito, M.; et al. Sequential Exposure to Antenatal Microbial Triggers Attenuates Alveolar Growth and Pulmonary Vascular Development and Impacts Pulmonary Epithelial Stem/Progenitor Cells. Front. Med. 2021, 8, 614239. [Google Scholar] [CrossRef]
- Kunzmann, S.; Hütten, M.; Ottensmeier, B.; Kramer, B.W.; Fehrholz, M. A20 Is Increased in Fetal Lung in a Sheep LPS Model of Chorioamnionitis. Oxidative Med. Cell. Longev. 2022, 2022, 6421419. [Google Scholar] [CrossRef]
- Kunzmann, S.; Collins, J.J.P.; Yang, Y.; Uhlig, S.; Kallapur, S.G.; Speer, C.P.; Jobe, A.H.; Kramer, B.W. Antenatal Inflammation Reduces Expression of Caveolin-1 and Influences Multiple Signaling Pathways in Preterm Fetal Lungs. Am. J. Respir. Cell Mol. Biol. 2011, 45, 969–976. [Google Scholar] [CrossRef]
- Willems, M.G.M.; Ophelders, D.; Nikiforou, M.; Jellema, R.; Butz, A.; Delhaas, T.; Kramer, B.W.; Wolfs, T.G.A.M. Systemic interleukin-2 administration improves lung function and modulates chorioamnionitis-induced pulmonary inflammation in the ovine fetus. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L1–L7. [Google Scholar] [CrossRef]
- Kramer, B.W.; Moss, T.J.; Willet, K.E.; Newnham, J.P.; Sly, P.D.; Kallapur, S.G.; Ikegami, M.; Jobe, A.H. Dose and Time Response after Intraamniotic Endotoxin in Preterm Lambs. Am. J. Respir. Crit. Care Med. 2001, 164, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Willems, M.G.M.; Kemp, M.W.; Fast, L.A.; Wagemaker, N.M.M.; Janssen, L.E.W.; Newnham, J.P.; Payne, M.S.; Spiller, B.; Kallapur, S.G.; Jobe, A.H.; et al. Pulmonary vascular changes in extremely preterm sheep after intra-amniotic exposure to Ureaplasma parvum and lipopolysaccharide. PLoS ONE 2017, 12, e0180114. [Google Scholar] [CrossRef]
- Kallapur, S.G.; Kramer, B.W.; Knox, C.L.; Berry, C.A.; Collins, J.J.P.; Kemp, M.W.; Nitsos, I.; Polglase, G.R.; Robinson, J.; Hillman, N.H.; et al. Chronic Fetal Exposure to Ureaplasma parvum Suppresses Innate Immune Responses in Sheep. J. Immunol. 2011, 187, 2688–2695. [Google Scholar] [CrossRef] [PubMed]
- Kallapur, S.G.; Nitsos, I.; Moss, T.; Kramer, B.W.; Newnham, J.; Ikegami, M.; Jobe, A.H. Chronic endotoxin exposure does not cause sustained structural abnormalities in the fetal sheep lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L966–L974. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moss, T.J.M.; Newnham, J.P.; Willett, K.E.; Kramer, B.W.; Jobe, A.H.; Ikegami, M. Early Gestational Intra-Amniotic Endotoxin: Lung func-tion, surfactant, and morphometry. Am. J. Respir. Crit. Care Med. 2002, 165, 805–811. [Google Scholar] [CrossRef]
- Kunzmann, S.; Speer, C.P.; Jobe, A.H.; Kramer, B.W. Antenatal inflammation induced TGF-β1 but suppressed CTGF in preterm lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L223–L231. [Google Scholar] [CrossRef]
- Bachurski, C.J.; Ross, G.F.; Ikegami, M.; Kramer, B.W.; Jobe, A.H. Intra-amniotic endotoxin increases pulmonary surfactant proteins and induces SP-B processing in fetal sheep. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L279–L285. [Google Scholar] [CrossRef]
- Willet, K.E.; Kramer, B.W.; Kallapur, S.G.; Ikegami, M.; Newnham, J.P.; Moss, T.J.; Sly, P.D.; Jobe, A.H. Intra-amniotic injection of IL-1 induces inflammation and maturation in fetal sheep lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L411–L420. [Google Scholar] [CrossRef]
- Collins, J.J.P.; Kuypers, E.; Nitsos, I.; Pillow, J.J.; Polglase, G.R.; Kemp, M.W.; Newnham, J.P.; Cleutjens, J.P.; Frints, S.G.M.; Kallapur, S.G.; et al. LPS-induced chorioamnionitis and antenatal corticosteroids modulate Shh signaling in the ovine fetal lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L778–L787. [Google Scholar] [CrossRef]
- Visconti, K.; Senthamaraikannan, P.; Kemp, M.W.; Saito, M.; Kramer, B.W.; Newnham, J.P.; Jobe, A.H.; Kallapur, S.G. Extremely preterm fetal sheep lung responses to antenatal steroids and inflammation. Am. J. Obstet. Gynecol. 2018, 218, 349.e1–349.e10. [Google Scholar] [CrossRef]
- Guen, C.G.-L.; Denis, C.; Franco-Montoya, M.-L.; Jarry, A.; Delacourt, C.; Potel, G.; Bourbon, J.; Rozé, J.-C.; Jarreau, P.-H. Antenatal infection in the rabbit impairs post-natal growth and lung alveolarisation. Eur. Respir. J. 2008, 32, 1520–1528. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Thymann, T.; Goericke-Pesch, S.K.; Ren, S.; Wei, W.; Skovgaard, K.; Damborg, P.; Brunse, A.; van Gorp, C.; Kramer, B.W.; et al. Prenatal Intra-Amniotic Endotoxin Induces Fetal Gut and Lung Immune Responses and Postnatal Systemic Inflammation in Preterm Pigs. Am. J. Pathol. 2018, 188, 2629–2643. [Google Scholar] [CrossRef] [PubMed]
- Senthamaraikannan, P.; Presicce, P.; Rueda, C.M.; Maneenil, G.; Schmidt, A.F.; Miller, L.A.; Waites, K.B.; Jobe, A.H.; Kallapur, S.G.; Chougnet, C.A. Intra-amniotic Ureaplasma parvum–Induced Maternal and Fetal Inflammation and Immune Responses in Rhesus Macaques. J. Infect. Dis. 2016, 214, 1597–1604. [Google Scholar] [CrossRef]
- Jackson, C.M.; Mukherjee, S.; Wilburn, A.N.; Cates, C.; Lewkowich, I.P.; Deshmukh, H.; Zacharias, W.J.; Chougnet, C.A. Pulmonary Consequences of Prenatal Inflammatory Exposures: Clinical Perspective and Review of Basic Immunological Mechanisms. Front. Immunol. 2020, 11, 1285. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Royce, S.G.; Nold, M.F.; Bui, C.; Donovan, C.; Lam, M.; Lamanna, E.; Rudloff, I.; Bourke, J.E.; Nold-Petry, C.A. Airway Remodeling and Hyperreactivity in a Model of Bronchopulmonary Dysplasia and Their Modulation by IL-1 Receptor Antagonist. Am. J. Respir. Cell Mol. Biol. 2016, 55, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Bui, C.B.; Kolodziej, M.; Lamanna, E.; Elgass, K.; Sehgal, A.; Rudloff, I.; Schwenke, D.O.; Tsuchimochi, H.; Kroon, M.A.G.M.; Cho, S.X.; et al. Interleukin-1 Receptor Antagonist Protects Newborn Mice Against Pulmonary Hypertension. Front. Immunol. 2019, 10, 1480. [Google Scholar] [CrossRef] [PubMed]
- Kalymbetova, T.V.; Selvakumar, B.; Rodríguez-Castillo, J.A.; Gunjak, M.; Malainou, C.; Heindl, M.R.; Moiseenko, A.; Chao, C.-M.; Vadász, I.; Mayer, K.; et al. Resident alveolar macrophages are master regulators of arrested alveolarization in experimental bronchopulmonary dysplasia. J. Pathol. 2018, 245, 153–159. [Google Scholar] [CrossRef]
- Blackwell, T.S.; Hipps, A.N.; Yamamoto, Y.; Han, W.; Barham, W.J.; Ostrowski, M.C.; Yull, F.E.; Prince, L.S. NF-κB Signaling in Fetal Lung Macrophages Disrupts Airway Morphogenesis. J. Immunol. 2011, 187, 2740–2747. [Google Scholar] [CrossRef]
- Stouch, A.N.; McCoy, A.M.; Greer, R.M.; Lakhdari, O.; Yull, F.E.; Blackwell, T.S.; Hoffman, H.M.; Prince, L.S. IL-1β and Inflammasome Activity Link Inflammation to Abnormal Fetal Airway Development. J. Immunol. 2016, 196, 3411–3420. [Google Scholar] [CrossRef]
- Jones, C.V.; Williams, T.M.; A Walker, K.; Dickinson, H.; Sakkal, S.; A Rumballe, B.; Little, M.H.; Jenkin, G.; Ricardo, S.D. M2 macrophage polarisation is associated with alveolar formation during postnatal lung development. Respir. Res. 2013, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.; Zaramela, L.S.; Hernandez, G.E.; Mai, U.; Taheri, S.; Dang, D.; Stouch, A.N.; Medal, R.M.; McCoy, A.M.; Aschner, J.L.; et al. Transcriptional profiling of lung macrophages identifies a predictive signature for inflammatory lung disease in preterm infants. Commun. Biol. 2020, 3, 259. [Google Scholar] [CrossRef] [PubMed]
- Hurskainen, M.; Mižíková, I.; Cook, D.P.; Andersson, N.; Cyr-Depauw, C.; Lesage, F.; Helle, E.; Renesme, L.; Jankov, R.P.; Heikinheimo, M.; et al. Single cell transcriptomic analysis of murine lung development on hyperoxia-induced damage. Nat. Commun. 2021, 12, 1565. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-M.; Moiseenko, A.; Kosanovic, D.; Rivetti, S.; El Agha, E.; Wilhelm, J.; Kampschulte, M.; Yahya, F.; Ehrhardt, H.; Zimmer, K.-P.; et al. Impact of Fgf10 deficiency on pulmonary vasculature formation in a mouse model of bronchopulmonary dysplasia. Hum. Mol. Genet. 2019, 28, 1429–1444. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, A.; Mammoto, T. Vascular Niche in Lung Alveolar Development, Homeostasis, and Regeneration. Front. Bioeng. Biotechnol. 2019, 7, 318. [Google Scholar] [CrossRef]
- Jones, M.; Dilai, S.; Lingampally, A.; Chao, C.-M.; Danopoulos, S.; Carraro, G.; Mukhametshina, R.; Wilhelm, J.; Baumgart-Vogt, E.; Al Alam, D.; et al. A Comprehensive Analysis of Fibroblast Growth Factor Receptor 2b Signaling on Epithelial Tip Progenitor Cells During Early Mouse Lung Branching Morphogenesis. Front. Genet. 2019, 9, 746. [Google Scholar] [CrossRef]
- Chao, C.-M.; Yahya, F.; Moiseenko, A.; Tiozzo, C.; Shrestha, A.; Ahmadvand, N.; El Agha, E.; Quantius, J.; Dilai, S.; Kheirollahi, V.; et al. Fgf10 deficiency is causative for lethality in a mouse model of bronchopulmonary dysplasia. J. Pathol. 2017, 241, 91–103. [Google Scholar] [CrossRef]
- Noe, N.; Shim, A.; Millette, K.; Luo, Y.; Azhar, M.; Shi, W.; Warburton, D.; Turcatel, G. Mesenchyme-specific deletion of Tgf-β1 in the embryonic lung disrupts branching morphogenesis and induces lung hypoplasia. Lab. Investig. 2019, 99, 1363–1375. [Google Scholar] [CrossRef]
- Taghizadeh, S.; Jones, M.R.; Olmer, R.; Ulrich, S.; Danopoulos, S.; Shen, C.; Chen, C.; Wilhelm, J.; Martin, U.; Chen, C.; et al. Fgf10 Signaling-Based Evidence for the Existence of an Embryonic Stage Distinct from the Pseudoglandular Stage During Mouse Lung Development. Front. Cell Dev. Biol. 2020, 8, 576604. [Google Scholar] [CrossRef]
- Le Cras, T.D.; Hardie, W.D.; Deutsch, G.H.; Albertine, K.; Ikegami, M.; Whitsett, J.A.; Korfhagen, T.R. Transient induction of TGF-α disrupts lung morphogenesis, causing pulmonary disease in adulthood. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L718–L729. [Google Scholar] [CrossRef]
- Benjamin, J.T.; Carver, B.J.; PLoSa, E.J.; Yamamoto, Y.; Miller, J.D.; Liu, J.-H.; van der Meer, R.; Blackwell, T.S.; Prince, L.S. NF-κB Activation Limits Airway Branching through Inhibition of Sp1-Mediated Fibroblast Growth Factor-10 Expression. J. Immunol. 2010, 185, 4896–4903. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, S.; Chao, C.-M.; Guenther, S.; Glaser, L.; Gersmann, L.; Michel, G.; Kraut, S.; Goth, K.; Koepke, J.; Heiner, M.; et al. FGF10 triggers de novo alveologenesis in a BPD model: Impact on the resident mesenchymal niche cells. Stem Cells 2022, 40, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Sveiven, S.N.; Nordgren, T.M. Lung-resident mesenchymal stromal cells are tissue-specific regulators of lung homeostasis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L197–L210. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, S.; Heiner, M.; Vazquez-Armendariz, A.I.; Wilhelm, J.; Herold, S.; Chen, C.; Zhang, J.S.; Bellusci, S. Characterization in Mice of the Resident Mesenchymal Niche Maintaining At2 Stem Cell Proliferation in Homeostasis and Disease. Stem Cells 2021, 39, 1382–1394. [Google Scholar] [CrossRef]
- Vazquez-Armendariz, A.I.; Heiner, M.; El Agha, E.; Salwig, I.; Hoek, A.; Hessler, M.C.; Shalashova, I.; Shrestha, A.; Carraro, G.; Mengel, J.P.; et al. Multilineage murine stem cells generate complex organoids to model distal lung development and disease. EMBO J. 2020, 39, e103476. [Google Scholar] [CrossRef]
- Reicherzer, T.; Häffner, S.; Shahzad, T.; Gronbach, J.; Mysliwietz, J.; Hübener, C.; Hasbargen, U.; Gertheiss, J.; Schulze, A.; Bellusci, S.; et al. Activation of the NF-κB pathway alters the phenotype of MSCs in the tracheal aspirates of preterm infants with severe BPD. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L87–L101. [Google Scholar] [CrossRef]
- Kheirollahi, V.; Wasnick, R.M.; Biasin, V.; Vazquez-Armendariz, A.I.; Chu, X.; Moiseenko, A.; Weiss, A.; Wilhelm, J.; Zhang, J.-S.; Kwapiszewska, G.; et al. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat. Commun. 2019, 10, 2987. [Google Scholar] [CrossRef]
- Augustine, S.; Avey, M.T.; Harrison, B.; Locke, T.; Ghannad, M.; Moher, D.; Thébaud, B. Mesenchymal Stromal Cell Therapy in Bronchopulmonary Dysplasia: Systematic Review and Meta-Analysis of Preclinical Studies. Stem Cells Transl. Med. 2017, 6, 2079–2093. [Google Scholar] [CrossRef]
- Lesage, F.; Thébaud, B. Mesenchymal Stromal Cell-Derived Extracellular Vesicles for Neonatal Lung Disease: Tiny Particles, Major Promise, Rigorous Requirements for Clinical Translation. Cells 2022, 11, 1176. [Google Scholar] [CrossRef]
- Goetz, M.; Kremer, S.; Behnke, J.; Staude, B.; Shahzad, T.; Holzfurtner, L.; Chao, C.-M.; Morty, R.; Bellusci, S.; Ehrhardt, H. MSC Based Therapies to Prevent or Treat BPD—A Narrative Review on Advances and Ongoing Challenges. Int. J. Mol. Sci. 2021, 22, 1138. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Lee, M.H.; Sung, S.I.; Lee, B.S.; Kim, K.S.; Kim, A.-R.; Park, W.S. Stem Cells for Bronchopulmonary Dysplasia in Preterm Infants: A Randomized Controlled Phase II Trial. STEM CELLS Transl. Med. 2021, 10, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xia, Y.; Zhou, O.; Song, Y.; Zhang, X.; Tian, D.; Li, Q.; Shu, C.; Liu, E.; Yuan, X.; et al. Allogeneic human umbilical cord-derived mesenchymal stem cells for severe bronchopulmonary dysplasia in children: Study protocol for a randomized controlled trial (MSC-BPD trial). Trials 2020, 21, 125. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.P.; Bozyk, P.D.; Bentley, J.K.; Linn, M.J.; Goldsmith, A.M.; Schumacher, R.E.; Weiner, G.M.; Filbrun, A.G.; Hershenson, M.B. Isolation of Tracheal Aspirate Mesenchymal Stromal Cells Predicts Bronchopulmonary Dysplasia. Pediatrics 2010, 126, e1127–e1133. [Google Scholar] [CrossRef] [PubMed]
- Pierro, M.; Thébaud, B.; Soll, R. Mesenchymal stem cells for the prevention and treatment of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2017, 11, CD011932. [Google Scholar] [CrossRef] [PubMed]
- Sugar, S.S.; Heyob, K.M.; Cheng, X.; Lee, R.J.; Rogers, L.K. Perinatal inflammation alters histone 3 and histone 4 methylation patterns: Effects of MiR-29b supplementation. Redox Biol. 2021, 38, 101783. [Google Scholar] [CrossRef] [PubMed]
- Durrani-Kolarik, S.; Pool, C.A.; Gray, A.; Heyob, K.M.; Cismowski, M.J.; Pryhuber, G.; Lee, L.J.; Yang, Z.; Tipple, T.; Rogers, L.K. miR-29b supplementation decreases expression of matrix proteins and improves alveolarization in mice exposed to maternal inflammation and neonatal hyperoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L339–L349. [Google Scholar] [CrossRef]
- Doyle, L.W.; Cheong, J.L.; Hay, S.; Manley, B.J.; Halliday, H.L. Late (≥7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2021, 11, CD001145. [Google Scholar] [CrossRef]
- Razak, A.; Alshehri, N. Azithromycin for preventing bronchopulmonary dysplasia in preterm infants: A systematic review and meta-analysis. Pediatr. Pulmonol. 2021, 56, 957–966. [Google Scholar] [CrossRef]
- Pruski, P.; Correia, G.D.S.; Lewis, H.V.; Capuccini, K.; Inglese, P.; Chan, D.; Brown, R.G.; Kindinger, L.; Lee, Y.S.; Smith, A.; et al. Direct on-swab metabolic profiling of vaginal microbiome host interactions during pregnancy and preterm birth. Nat. Commun. 2021, 12, 5967. [Google Scholar] [CrossRef]
- Stelzer, I.A.; Ghaemi, M.S.; Han, X.; Ando, K.; Hédou, J.J.; Feyaerts, D.; Peterson, L.S.; Rumer, K.K.; Tsai, E.S.; Ganio, E.A.; et al. Integrated trajectories of the maternal metabolome, proteome, and immunome predict labor onset. Sci. Transl. Med. 2021, 13, eabd9898. [Google Scholar] [CrossRef]
- Ophelders, D.R.M.G.; Boots, A.W.; Hütten, M.C.; Al-Nasiry, S.; Jellema, R.K.; Spiller, O.B.; van Schooten, F.-J.; Smolinska, A.; Wolfs, T.G.A.M. Screening of Chorioamnionitis Using Volatile Organic Compound Detection in Exhaled Breath: A Pre-clinical Proof of Concept Study. Front. Pediatr. 2021, 9, 617906. [Google Scholar] [CrossRef] [PubMed]
- Förster, K.; Sass, S.; Ehrhardt, H.; Mous, D.S.; Rottier, R.J.; Oak, P.; Schulze, A.; Flemmer, A.W.; Gronbach, J.; Hübener, C.; et al. Early Identification of Bronchopulmonary Dysplasia Using Novel Biomarkers by Proteomic Screening. Am. J. Respir. Crit. Care Med. 2018, 197, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.V.; Kandasamy, J.; Dolma, K.; Ramani, M.; Kumar, R.; Wilson, L.; Aghai, Z.H.; Barnes, S.; Blalock, J.E.; Gaggar, A.; et al. Early airway microbial metagenomic and metabolomic signatures are associated with development of severe bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L810–L815. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hao, S.; Chen, X.; Cheng, M.; Xu, J.; Li, C.; Zheng, H.; Volpe, G.; Chen, A.; Liao, S.; et al. Spatial Transcriptome Uncovers the Mouse Lung Architectures and Functions. Front. Genet. 2022, 13, 858808. [Google Scholar] [CrossRef]
| Animals | Infection Route | Type and Dose of Microbial Insults | Time of Prenatal Infection | Fetal/Neonatal Outcome * | Molecular Changes of the Lung * | Structural and Functional Changes of the Lung * |
|---|---|---|---|---|---|---|
| Sprague- Dawley rats [44,78] | i.a. after laparotomy | LPS (055:B5) 1 µg/sac or IL1B 0.5 µg/sac | 16.5 dpc | Spontaneous delivery 3–4 d after i.a. LPS | At P1, P3 and P7: LPS ↑ a total of 28 pro- and anti-inflammatory cytokines and ΜΦ influx, ↑ Tie2 and Fgf, ↑ LOX activity | At P1, P3, and P7: Both LPS and IL1B ↓ terminal airspace and the no. of secondary septa, ↑ MLI |
| Sprague- Dawley rats [57] | i.a. after laparotomy | LPS (055:B5) 1 µg/sac | 16.5 dpc | Spontaneous delivery Mortality at birth: 52% Mortality at P7 and P14: 75% Weight at P7 and P14: normal | At P1:Fgfr4, Vegfr2 and Hmox1 ↓, IL6 ↑ At P7 and P14: oxidative markers nitrotyrosine and 8-OHdG ↑ | At P7 and P14: MLI ↑ |
| Sprague- Dawley rats [58,59] | i.a. after laparotomy | LPS (055:B5) 10 µg/sac | E20 # | C-section at E22 Mortality at birth: 11% Mortality at P2–P14: 57% Weight at P2–P14: ↓ | At P1: SOD activity and MnSOD ↑ VEGF and VEGFR2 ↓, eNOS unaffected | At P7 and P14: alveolar count and vascular density ↓, MLI and vascular wall thickness ↑ |
| Sprague- Dawley rat [68,69,77] | i.a. after laparotomy | LPS (055:B5) 10 µg/sac | E20 # | C-section at E22 Mortality at birth: 30% Mortality at P2 and P14: 42% Weight at P14: ↓ | At P1: sFLT1 ↑, VEGF ↓, HIF1A and HIF2A ↓ | At P14: alveolar count and vascular density ↓, MLI, vessel wall thickness and RVH ↑, Rrs ↑, Crs ↓ |
| WKAH/htm rats [73] | i.a. after laparotomy | LPS (055:B5) 1 µg/sac | E21 # | C-section at E22 Mortality at birth and P7–P70: 11% and 30–40% Weight at P14–P60: ↓ | At P1, 3, 7, 14, 21, 45, and 60: alveolar surface density ↓ and radius ↑, alveolar numerical density ↓, no abnormal collagen distribution | |
| Sprague- Dawley rats [74] | i.a. after laparotomy | LPS (055:B5) 1 µg/sac | E20 # | Spontaneous delivery 2 d after i.a. LPS. Mortality at birth: 25% | At P8: IL1B and ΜΦ count ↑ At P8 and P14: TNF and IL6 unaffected | At P8 and P14: alveolar counts ↓, Rrs ↑, Crs unaffected |
| Sprague- Dawley rats [76] | i.a. after laparotomy | LPS (0111:B4) 10 µg/sac | E20 # | Spontaneous term delivery Mortality at birth: 28.9% Weight at P1–P7: unaffected | At P3 and P7: GM-CSF ↓, CXCL10 and ARG1 ↑, TNF, IL1B, IL10, VEGF unaffected, arginine and ADMA ↓ | At P3 and P7: alveolar size and MLI ↑, secondary crests ↓ |
| C57BL/6 mice [60] | i.a. under ultrasound, i.p. | LPS (0111:B4) 100 ng/sac, LPS (055:B5) 10 µg/mouse | 16.5 dpc | PTD before 18 dpc: 87.5% Mortality at birth: >85% PTD before 18 dpc: 80% Mortality at birth: >85% | At 17.5 dpc:Il1b, Il6, Ccl2, 3 and 5, Cxcl1 ↑. Cytokine response: i.a. LPS > i.p. LPS | |
| C3H/HeN mice [61,62] | i.p. | LPS (0111:B4) 80 µg/kg | 16 dpc | 20% of dams didn’t deliver Weight at 2 w and 4 w: normal Weight at 8 w: ↓ | At 18 dpc: Tnf, Cxcl1 and miR29b ↓, Tgfb1, Il1b and Col1a1 ↑ At P7: Il1b, Tgfb1 and Col3a1 ↑ At P14: mediators above unaffected At 4 w: ΜΦ ↑ | At P14: septal thickness and Rrs ↑, air space and Crs ↓ At 4 w: air space and septal thickness ↑ collagen, lung Rrs and Crs unaffected At 8 w: no. alveoli ↓, septal thickness ↑, fibrosis and alveolar simplification in microCT, Rrs and Crs unaffected |
| LysEGFP-reporter C57BL/6N mice [63] | i.p. | LPS (0111:B4) 0.25 mg/kg or 1 mg/kg | 13 or 17 dpc | 2× i.p. 1 mg/kg: PTD at 14 and 18 dpc: 30 and 86%, mortality at 14 and 18 dpc: >90% and 60–70%. 2× i.p. 0.25 mg/kg: no PTD | At 16, 17 and 18 dpc: neutrophil infiltration ↑ after 2× i.p. LPS 0.25 mg/kg | |
| C57BL/6 mice [64] | i.p. | LPS (055:B5) 200 µg/kg | 16 dpc | Viable litters Weight at P1, 5, 14: normal Weight at P21: ↓ | At P1:Tlr4, Tnf, Il1b, Il6, Tgfb1 ↑ At P5: Il1b ↑, other mediators unaffected At P14: CTGF ↓, ACTA2 ↑ At P21: Col3a1, Tgfb1, Ctgf ↑ | At P21: ASM area ↑, alveolar count unaffected, Rrs ↑, inspiratory capacity and Crs ↓ |
| C57BL/6 mice [65] | i.p. | LPS (source unknown) 100 µg/kg | E18 # | Spontaneous delivery 3 d after i.p. LPS | At P21: iNOS and Tgfb1 ↑, Tnf, Il1b and Il6 unaffected | At P21: vessel reactivity ↓, airway reactivity unaffected |
| C57BL/6J mice [66] | i.p. | LPS (0111:B4) 150 µg/kg | E14 # | Viable litters | At P3: TNF, IL1A, IL1RA, IL6, CXCL2, CXCR2, CCL4, sICAM1, C5a, TREM1 ↑ | At P28: alveolar number, size and surface unaffected |
| BALB/cJ mice [40,67] | i.a. after laparotomy | LPS (055:B5) 100 pg/sac | 15 dpc | No PTD Mortality at birth <5% | At 17 dpc: TIE2 ↑ At 18 dpc: SFTPA and TLR4 ↑, Tnf ↑, Il1b ↑ | |
| C57BL/6 and FVB mice [70] | i.p | LPS (0111:B4) 25 µg per mouse | 16 or 17 dpc | PTD within 17 h of i.p. LPS Mortality at birth: 61% | 8h after i.p.: Tlr2, Tlr4, Sftpd ↓ | |
| ErbB4 transgenic mice [71,72] | i.a. under guidance of patent blue dye | LPS (0127:B8) 4 µg/sac | 17 dpc | C-Section 24 h after i.a. LPS | At P1: TNF and ΜΦ influx ↑ | At P1: mesenchyme volume and septal thickness ↑, alveolar septa ↓, elastic fibers ↓ |
| CD-1 mice [75] | i.u. after laparotomy | LPS (055:B5) 25 µg per mouse | E17 # | PTD before E19: 55% Mortality at P1: 60% | 6 h after LPS: Hippo signaling (Yap1,Taz) ↓ At E18: Il1b, CXCR2 and HIF1B↑, SFTPB and vimentin (endothelial marker) ↓ | At P1: alveolarnumber ↓, septal thickness ↑ |
| CD-1 mice [79] | i.a. after laparotomy | UP 5000 cfu/sac | E13.5 # | No PTD. Pups’ survival unaffected | At 17.5 dpc and P3.5: IL1A, IL1B, IL6, CCL2, CXCL2 and TGFB1 ↑ At P3.5: myeloperoxidase ↑ | At P14.5: MLI unaffected |
| BALB/c mice [80] | i.vag. | GBS 3 × 104 cells | 17 and 18 dpc | No PTD Mortality at birth, P1, P4: 5%, 21% and 40% Weight at birth and P10: ↓ | At P1: atelectasis, narrow airway lumen, edema and hemorrhage |
| Type and Doses of Microbial Insults | Time of Prenatal Infection | Groups | Molecular Change of the Lung * | Structural and Functional Change of the Lung * |
|---|---|---|---|---|
| Single infectious stimulants | ||||
| LPS (055:B5) 10 mg/sac, or IL1A or IL1B (15 µg or 150 µg/sac) [98] | E118 | Control 7 d LPS 7 d IL1A (two doses) 7 d IL1B (two doses) | At E125: 150 µg IL1A, 150 µg IL1B and LPS ↑ leukocyte influx, ↑ Sat PC and SPs mRNA, inflammatory effect: LPS = IL1A > IL1B | At E125: lung compliance and volume ↑, lung function and Sat PC was positively correlated with leukocytes |
| LPS (055:B5) 10 mg/sac [100] | E92 | Control 2 d LPS | At E94: TNF, IL1B, IL8 and CCL2 ↑, no effect on anti-oxidant enzyme mRNA, SPs mRNA, VEGF and CTGF | At E94: lung air space unaffected |
| LPS (055:B5) 5 mg/sac [90] | E122 | Control 7 d LPS | At E129: IL8 and neutrophils and myeloid cell influx ↑, CD3+ T cells and FoxP3 + Treg cells ↑ | At E129: lung gas volume ↑, desaturated phospholipids ↑, morphometry unaffected |
| LPS (055:B5) 10 mg/sac [88,89] | E118 and/or E123 | Control 2 d LPS 7 d LPS 2 d + 7 d LPS | At E124 ± 1: 2 d LPS ↑TNF, IL1B, IL6, IL8, and A20 with lower values at 7 d LPS, 2 d + 7 d LPS hat no effect indicating LPS tolerance. LPS ↓ CAV1, ↑ TGFB1/SMAD2/3, and A-SMase/ceramide,↑HMOX1 | |
| LPS (055:B5) 10 mg/sac [99] | E107 and/or E114 | Control 7 d LPS 14d LPS | At E120: 7 d and 14 d LPS ↑ SHH and its mediators GLI1 and GLI2, 14d LPS ↑ BMP4, FGF10 and ELN, 7 d LPS ↓ BMP4 and ELN, both 7 d and 14 d LPS ↓ elastin foli, 14 d LPS ↑ collagen deposition | |
| LPS (055:B5) 0.1,1,4 or 10 mg/sac [91,96] | 5 h, 1, 3, 7 d before E125 | Control 5 h, 1, 3, 7 d LPS | At E125: dose-dependent effect to ↑ inflammatory cell influx, TNF, IL1B and IL8, SPs mRNA and H2O2, which peaked at 24–72 h after LPS | At E125: 4 and 10 mg/sac LPS ↑ lung gas volume and compliance, other doses didn’t |
| LPS (055:B5) 20 mg/sac [97] | E110, E118, E121, E123 or E124 | Control 1, 2, 4, 7, 15 d LPS | At E125: SFTPD↑ 1 d after i.a. LPS and remained at peak up to 7 d, other SPs peaked 2 d after i.a. LPS and persisted in lower level up to 15 d | At E125: Sat PC and lung gas volume ↑ 4 d after i.a. LPS and further ↑ up to 15 d |
| LPS (055:B5) 1 mg/d pumped i.a., or 10 mg/sac 1xper week [94] | E80 to E10, E100 to E128 | Control Continuous LPS, Multiple LPS | At E100: Continuous 20 d LPS ↑ neutrophil influx, ↓ eNOS and VEGFR2. At E130: multiple 20 d LPS ↑ neutrophil influx, ↑ Sat PC, eNOS, VEGFR2, E138 and E145: mild inflammation | At E100: ↑ Sat PC, ↓ saccule numbers, At E 130, 138, and 145: no lung abnormality |
| LPS (055:B5) 0.6 mg/d pumped i.a., or multiple 1 mg/sac injection [95] | E80 to E100, or E60, E80, E100 | Control Continuous LPS, 25 d, 45 d, 65 d LPS 25 d + 65 d LPS | At E125: all LPS groups ↑ inflammatory cell influx, Sat PC and SPs. 25d + 65 d LPS showed no significant impact vs. either alone | At E125: continuous LPS ↓ alveolar number, surface and wall thickness, and lung compliance |
| Multiple infectious stimuli | ||||
| UP 2 × 105 CCUs/sac, or LPS (055:B5) 10 mg/sac [86,87] | E80 or 83, E118 or E123 | Control 42 d/45 d UP 2 d LPS 7 d LPS UP + 2 d LPS UP + 7 d LPS | At E125: 2 d LPS or 2 d LPS + UP ↑ IL6, IL8, CCL2 and inflammatory cells, UP or 7 d LPS had relatively milder inflammatory effect. Both UP and LPS ↓ P63+ basal cells and TTF1+ club cells, ↓ SOX9 cells, Ki67+ cells, AT1 and AT2 cells, ↑ SPs, ↓ TGFB1, VEGFR2, ANG1. UP + LPS had synergistic effect than either insult. | At E125: Both UP and LPS ↓ vessel density, but ↑ lung gas volume, only UP + 7 d LPS ↑ MLI |
| UP 2 × 105 CCUs/sac, and/or LPS (055:B5) 10 mg/sac [92] | E70, E92 or E87 | Control 24 d UP 2 d LPS 7 d LPS 24 d UP + 2 d LPS 24 d UP + 7 d LPS | At E94: LPS and 24 d UP + LPS ↑ CD3+ T cell and neutrophils, UP + LPS ↓ VEGF, VEGFR2, ANG1 and TIE2, 24 d UP + LPS had synergistic effect vs. either insult, 7 d LPS vs. 2 d LPS ↓ VEGFR2, ANG1 and TIE2 | At 94: UP + 2 d LPS ↑ fibrosis vs. 2 d LPS alone. 7 d LPS vs. 2 d LPS ↑ wall-to-lumen ratio of pulmonary arterioles |
| UP 2 × 107 CCUs/sac, and/or LPS (055:B5)10 mg/sac [93] | E54 or E 117, E122 | Control 7 d UP 70 d UP 2 d LPS 7 d UP + 2 d LPS 70 d UP + 2 d LPS | At E124 ± 1: UP alone induced mild inflammatory responses, LPS alone ↑ IL1B, IL6, IL8, and IL10, CCL2, IL1RA, ↑ CD3+ T cells, neutrophils and myeloid cells, ↑ TGFB1 Effect: 7 d UP + LPS = LPS alone, 70 d UP + LPS antagonized LPS alone | At E124 ± 1: LPS alone ↓ lung gas volume, other groups didn’t |
| Animal Model of IAI (N of Each Arm) | Interventional and Control Arms of IAI | Effect on Fetal and Neonatal Lung (Treatment vs. Control Arm) |
|---|---|---|
| Mice | ||
| i.a. LPS at 17 dpc [71] N = 4–11 | i.a. adult BMSCs or HPSCs control (each 2 × 106 cells) at the same time of i.a. LPS | At 18 dpc: BMSCs ↑ lung morphological maturation, but ↓ SFTPC. The effect was more pronounced in the presence of ErbB4 receptor |
| i.p. LPS at E14 and 65% O2 P1–P28 N = 3–20 [106,107] | Postnatal daily s.c. IL1RA 10 mg/kg or saline control 1× per day for 28 d | At P28: IL1RA ↓ alveolar size, ↑ alveolar number and surface area, ↓ collagen thickness and ACTA2, ↓ vascular resistance, but no effect on airway hyperreactivity. At P60: improved pulmonary vascular structure |
| i.a. LPS at 16.5 dpc [84] N = 8–10 | Dam i.p. NLRP3 inflammasome inhibitor MCC950 50 mg/kg or PBS control 1–2 h before LPS | Preterm delivery before 18.5 dpc and mortality at birth ↓ by 30% |
| i.u. LPS at E17 [75] N = 11 | Dam i.p. melatonin 10 mg/kg or saline control at E17 before LPS # | Preterm delivery before E19 and mortality up to P1 ↓ by about 60%. At P1: ↓ IL1B, CXCL2 and septal thickness ↑ SFTPB and endothelial cells |
| i.a. LPS at 16 dpc [135,136] and 85% O2 P1–P14 N = 4–8 | Postnatal treatment of intranasal miR29b (1 × 109) in viral/lipid vector or vector control on P3 | At P28: only miR29b in viral vectors ↓ septal thickness and showed trend to ↑ alveolarization, ↓ defects in matrix structure |
| Rats | ||
| i.a. LPS at E20 [17] N = 12–20 | i.a. MSCs-derived extracellular vesicles (0.25 × 106/sac) or none at E20 at the same time of LPS | At P14: treatment restored alveolar and vascular growth, lung function, and RVH to normal level as seen in LPS non-exposed pups |
| i.a. LPS at E20 [68] N = 10 | Prenatally: anti–sFLT1 mAb i.a. 1.5 µg/sac or saline after LPS Postnatally: anti–sFLT1 mAb i.p. (1 or 10 mg/kg), control IgG or saline 2× per week for 2 weeks | Prenatal but not postnatal treatment ↑ neonatal survival from birth up to 2 weeks by about 50% At P14: pre- and postnatal treatment ↑ alveolar count, vessel density, lung function, and ↓ RVH |
| i.a. LPS at E20 N = 8–20 [77] | Postnatal i.p. rhIGF1/BP3 (0.02, 0.2, 2, or 20 mg/kg) or saline control for 2 weeks | At P14: eNOS and IGF1 ↑, RAC and vessel density was restored to normal values as in saline control, RVH, Rrs and Crs was improved in a dose-related manner |
| i.a. LPS at E20 [69] N = 15 | Prenatally: DMOG 10 mg/sac or GSK360A 1 mg/sac or saline control after LPS * Postnatally: DMOG 5 mg/kg or GSK360A 5 mg/kg i.p. or saline control every 2 days for 2 weeks | At P14: Pre- and postnatal treatment ↑ alveolar count, vessel density, lung function, and ↓ RVH. Prenatal treatment ↑ HIF1A and HIF2A, VEGF, and eNOS, and improved placental structure |
| i.a. LPS or IL1B at 16.5 dpc, N = 7–8 [44] | i.a. anti-IL1B 0.5 µg/sac, or CCR5 antagonist DAPTA 1 µg/sac, or RIP3 inhibitor GSK872 2 µg/sac, or BTA 0.4 µg (nature product), or saline control at 16.5 dpc | At P1, P3, and P7: all drugs ↓ inflammation, ↑ terminal airspace and no. of secondary septa, ↓ MLI |
| i.a. or i.p. LPS on the dam at 16.5 dpc [60] N = 8–10 | i.p. Exendin-4 30 µg/kg or saline control 6 h after LPS | Exendin-4 ↓ preterm deliveries and ↑ neonatal survival from birth to P15 At P15: Exendin-4 ↓inflammation in pups with i.a. but not i.p. LPS exposure |
| Sheep | ||
| i.a. LPS at E122 [90] N = 5–7 | Fetus received 250,000 IU/kg/d of IL2 or heparinized saline at E118 via umbilical catheter for 4 d before LPS | At E129: systemic IL2 did not inhibit inflammatory cell and IL8 responses in fetal lungs, but ↑ lung function |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Rivetti, S.; Lingampally, A.; Tacke, S.; Kojonazarov, B.; Bellusci, S.; Ehrhardt, H. Insights into the Black Box of Intra-Amniotic Infection and Its Impact on the Premature Lung: From Clinical and Preclinical Perspectives. Int. J. Mol. Sci. 2022, 23, 9792. https://doi.org/10.3390/ijms23179792
Dong Y, Rivetti S, Lingampally A, Tacke S, Kojonazarov B, Bellusci S, Ehrhardt H. Insights into the Black Box of Intra-Amniotic Infection and Its Impact on the Premature Lung: From Clinical and Preclinical Perspectives. International Journal of Molecular Sciences. 2022; 23(17):9792. https://doi.org/10.3390/ijms23179792
Chicago/Turabian StyleDong, Ying, Stefano Rivetti, Arun Lingampally, Sabine Tacke, Baktybek Kojonazarov, Saverio Bellusci, and Harald Ehrhardt. 2022. "Insights into the Black Box of Intra-Amniotic Infection and Its Impact on the Premature Lung: From Clinical and Preclinical Perspectives" International Journal of Molecular Sciences 23, no. 17: 9792. https://doi.org/10.3390/ijms23179792
APA StyleDong, Y., Rivetti, S., Lingampally, A., Tacke, S., Kojonazarov, B., Bellusci, S., & Ehrhardt, H. (2022). Insights into the Black Box of Intra-Amniotic Infection and Its Impact on the Premature Lung: From Clinical and Preclinical Perspectives. International Journal of Molecular Sciences, 23(17), 9792. https://doi.org/10.3390/ijms23179792







