How Can We Improve the Vaccination Response in Older People? Part II: Targeting Immunosenescence of Adaptive Immunity Cells
Abstract
1. Introduction
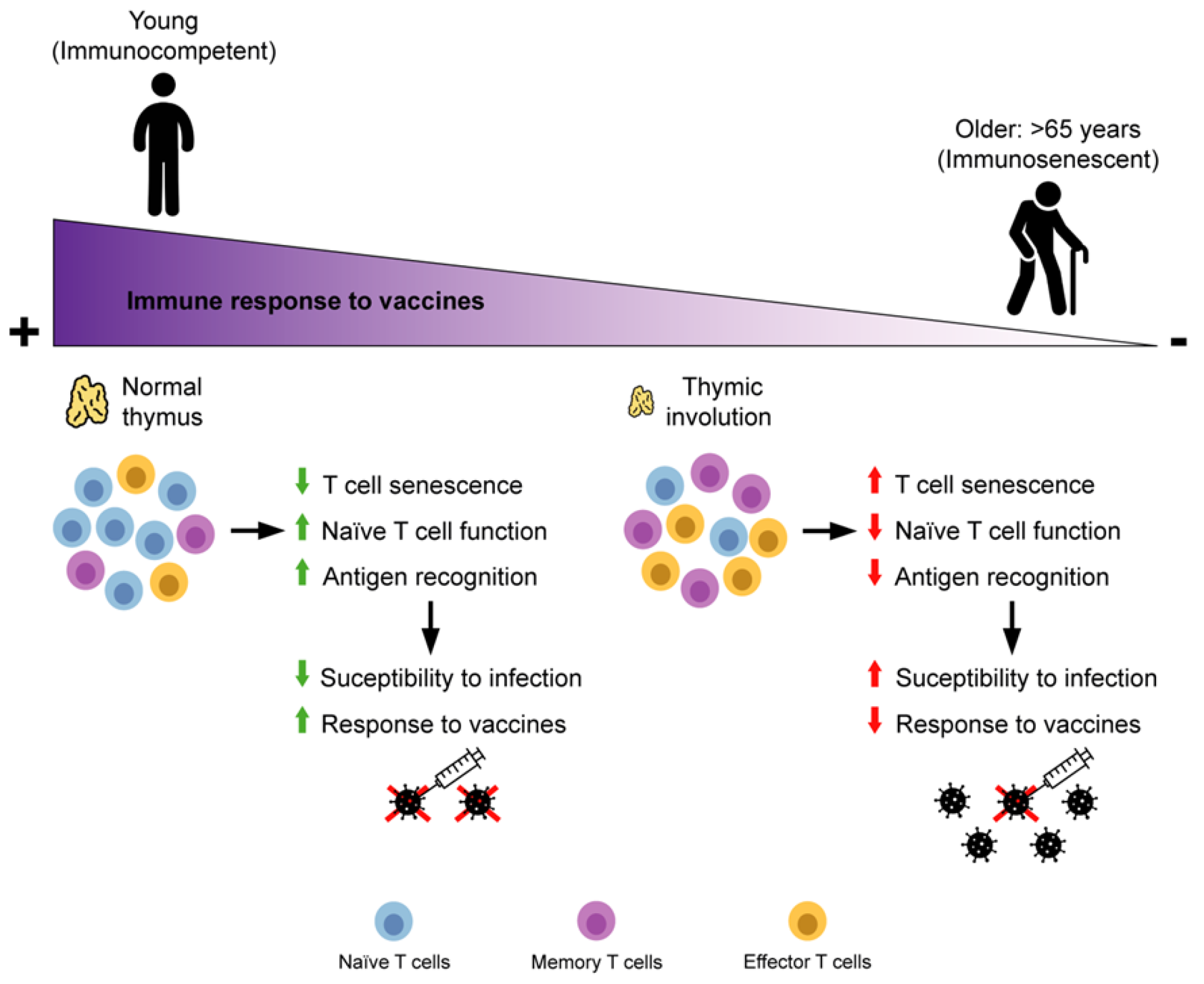
2. Adaptive Immunity Immunosenescence
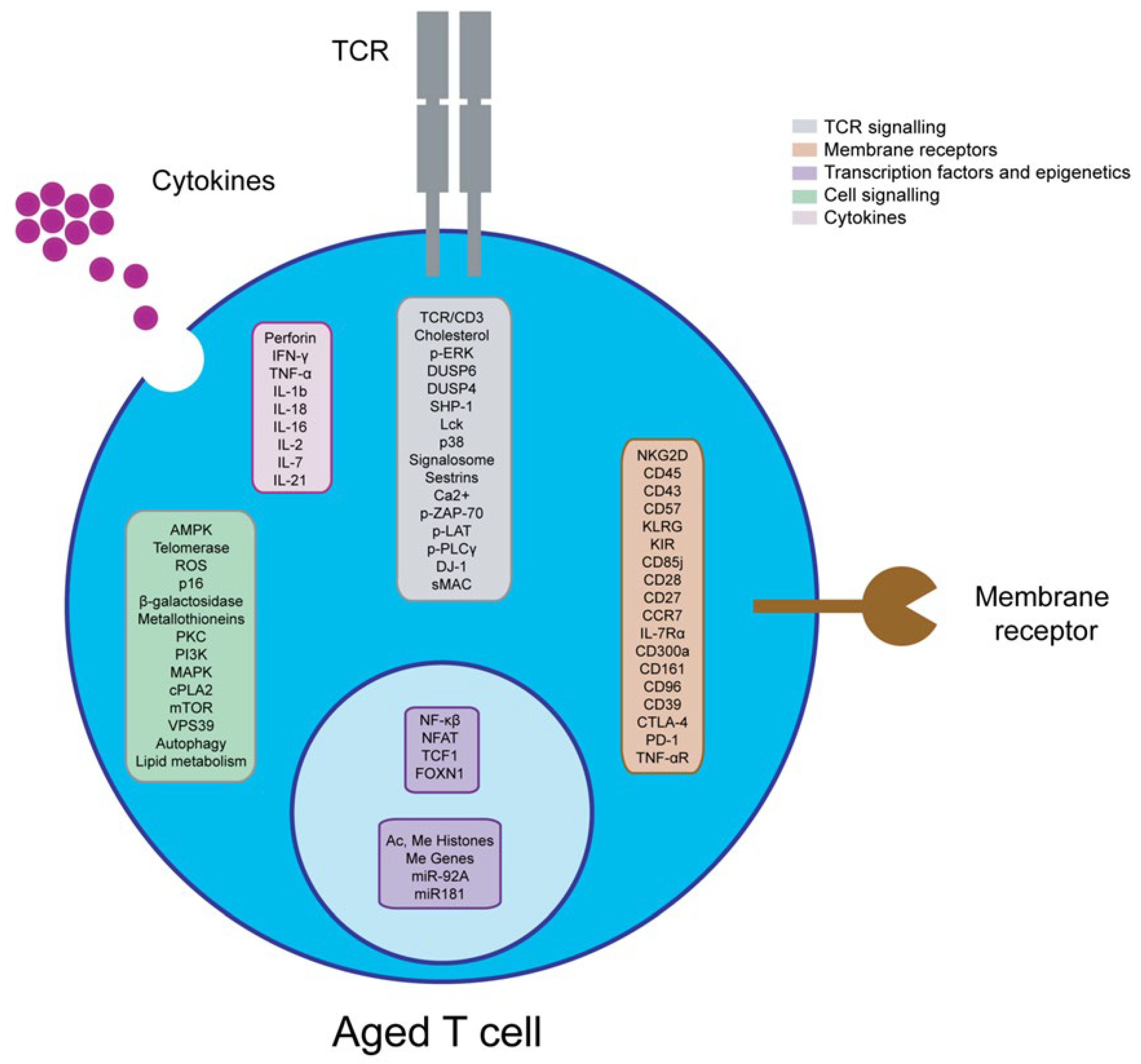
2.1. T Cells
2.2. Effect of Ageing and Viral Chronic Infections on T Cells
2.3. B Cells
2.4. Effect of Ageing and Viral Chronic Infections on B Cell
3. Immunosenescence of Adaptive Immunity and Vaccine Failure in Older People
4. Strategies to Reverse Immunosenescence of Adaptive Immunity in Older People
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rondy, M.; El Omeiri, N.; Thompson, M.G.; Levêque, A.; Moren, A.; Sullivan, S.G. Effectiveness of Influenza Vaccines in Preventing Severe Influenza Illness among Adults: A Systematic Review and Meta-Analysis of Test-Negative Design Case-Control Studies. J. Infect. 2017, 75, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Aiello, A.; Pawelec, G.; Ligotti, M.E. Vaccination in Old Age: Challenges and Promises. In Human Aging; Elsevier: Amsterdam, The Netherlands, 2021; pp. 129–153. [Google Scholar]
- O’Hagan, D.T.; Ott, G.S.; Van Nest, G.; Rappuoli, R.; Del Giudice, G. The History of MF59® Adjuvant: A Phoenix That Arose from the Ashes. Expert Rev. Vaccines 2013, 12, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Tsang, P.; Gorse, G.J.; Strout, C.B.; Sperling, M.; Greenberg, D.P.; Ozol-Godfrey, A.; DiazGranados, C.; Landolfi, V. Immunogenicity and Safety of Fluzone® Intradermal and High-Dose Influenza Vaccines in Older Adults ≥65 Years of Age: A Randomized, Controlled, Phase II Trial. Vaccine 2014, 32, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2020, 21, 83–100. [Google Scholar] [CrossRef]
- National Institute on Aging Vaccinations and Older Adults. Available online: https://www.nia.nih.gov/health/vaccinations-older-adults (accessed on 24 January 2022).
- Wong, G.C.L.; Strickland, M.C.; Larbi, A. Changes in T Cell Homeostasis and Vaccine Responses in Old Age. Interdiscip. Top. Gerontol. Geriatr. 2020, 43, 36–55. [Google Scholar] [CrossRef]
- Aiello, A.; Ligotti, M.E.; Garnica, M.; Accardi, G.; Arasanz, H.; Bocanegra, A.; Blanco, E.; Calabro, A.; Chocarro, L.; Echaide, M.; et al. How can we improve vaccination response in old people? I part. Targeting Immunosenescence of Innate Immunity Cells. Int. J. Mol. Sci. 2022.
- Cossarizza, A.; Chang, H.D.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the Use of Flow Cytometry and Cell Sorting in Immunological Studies (Second Edition). Eur. J. Immunol. 2019, 49, 1457–1973. [Google Scholar] [CrossRef]
- Finak, G.; Langweiler, M.; Jaimes, M.; Malek, M.; Taghiyar, J.; Korin, Y.; Raddassi, K.; Devine, L.; Obermoser, G.; Pekalski, M.L.; et al. Standardizing Flow Cytometry Immunophenotyping Analysis from the Human ImmunoPhenotyping Consortium. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Golubovskaya, V.; Wu, L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers 2016, 8, 36. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Jameson, S.C.; Masopust, D. Understanding Subset Diversity in T Cell Memory. Immunity 2018, 48, 214–226. [Google Scholar] [CrossRef]
- Bell, L. CD4+ T Cells | British Society for Immunology. Available online: https://www.immunology.org/public-information/bitesized-immunology/cells/cd4-t-cells (accessed on 12 July 2022).
- Wissinger, E. CD8+ T Cells | British Society for Immunology. Available online: https://www.immunology.org/public-information/bitesized-immunology/cells/cd8-t-cells (accessed on 12 July 2022).
- Farber, D.L.; Yudanin, N.A.; Restifo, N.P. Human Memory T Cells: Generation, Compartmentalization and Homeostasis. Nat. Rev. Immunol. 2014, 14, 24–35. [Google Scholar] [CrossRef]
- Patterson, H.; Nibbs, R.; Mcinnes, I.; Siebert, S. Protein Kinase Inhibitors in the Treatment of Inflammatory and Autoimmune Diseases. Clin. Exp. Immunol. 2014, 176, 1–10. [Google Scholar] [CrossRef]
- Pearce, E.L.; Poffenberger, M.C.; Chang, C.H.; Jones, R.G. Fueling Immunity: Insights into Metabolism and Lymphocyte Function. Science 2013, 342, 1242454. [Google Scholar] [CrossRef]
- Kumar, B.V.; Connors, T.; Farber, D.L. Human T Cell Development, Localization, and Function throughout. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef]
- Pinti, M.; Appay, V.; Campisi, J.; Frasca, D.; Fülöp, T.; Sauce, D.; Larbi, A.; Weinberger, B.; Cossarizza, A. Aging of the Immune System: Focus on Inflammation and Vaccination. Eur. J. Immunol. 2016, 46, 2286–2301. [Google Scholar] [CrossRef]
- Bulati, M.; Caruso, C.; Colonna-Romano, G. From Lymphopoiesis to Plasma Cells Differentiation, the Age-Related Modifications of B Cell Compartment Are Influenced by “Inflamm-Ageing”. Ageing Res. Rev. 2017, 36, 125–136. [Google Scholar] [CrossRef]
- Akbar, A.N.; Henson, S.M. Are Senescence and Exhaustion Intertwined or Unrelated Processes That Compromise Immunity? Nat. Rev. Immunol. 2011, 11, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Rezzani, R.; Nardo, L.; Favero, G.; Peroni, M.; Rodella, L.F. Thymus and Aging: Morphological, Radiological, and Functional Overview. Age 2014, 36, 313. [Google Scholar] [CrossRef]
- Thomas, R.; Wang, W.; Su, D.M. Contributions of Age-Related Thymic Involution to Immunosenescence and Inflammaging. Immun. Ageing 2020, 17, 1–17. [Google Scholar] [CrossRef]
- Nikolich-Žugich, J. Ageing and Life-Long Maintenance of T-Cell Subsets in the Face of Latent Persistent Infections. Nat. Rev. Immunol. 2008, 8, 512. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.E.; Chiu, Y.L.; Frasca, D. How Does Cytomegalovirus Factor into Diseases of Aging and Vaccine Responses, and by What Mechanisms? GeroScience 2017, 39, 261. [Google Scholar] [CrossRef] [PubMed]
- Kadambari, S.; Klenerman, P.; Pollard, A.J. Why the Elderly Appear to Be More Severely Affected by COVID-19: The Potential Role of Immunosenescence and CMV. Rev. Med. Virol. 2020, 30, 2144. [Google Scholar] [CrossRef] [PubMed]
- Jergović, M.; Contreras, N.A.; Nikolich-Žugich, J. Impact of CMV upon Immune Aging: Facts and Fiction. Med. Microbiol. Immunol. 2019, 208, 263–269. [Google Scholar] [CrossRef]
- Caruso, C.; Ligotti, M.E.; Accardi, G.; Aiello, A.; Candore, G. An Immunologist’s Guide to Immunosenescence and Its Treatment. Expert Rev. Clin. Immunol. 2022, 18, 961–981. [Google Scholar] [CrossRef]
- Puleston, D.J.; Simon, A.K. Autophagy in the Immune System. Immunology 2014, 141, 1–8. [Google Scholar] [CrossRef]
- Macian, F. Autophagy in T Cell Function and Aging. Front. Cell Dev. Biol. 2019, 7, 00213. [Google Scholar] [CrossRef]
- Zhang, H.; Puleston, D.J.; Simon, A.K. Special Issue: Aging and Rejuvenation Autophagy and Immune Senescence. Trends Mol. Med. 2016, 22, 671–686. [Google Scholar] [CrossRef]
- Arata, Y.; Watanabe, A.; Motosugi, R.; Murakami, R.; Goto, T.; Hori, S.; Hirayama, S.; Hamazaki, J.; Murata, S. Defective Induction of the Proteasome Associated with T-Cell Receptor Signaling Underlies T-Cell Senescence. Genes to Cells 2019, 24, 801–813. [Google Scholar] [CrossRef]
- Witkowski, J.M.; Mikosik, A.; Bryl, E.; Fulop, T. Proteodynamics in Aging Human T Cells—The Need for Its Comprehensive Study to Understand the Fine Regulation of T Lymphocyte Functions. Exp. Gerontol. 2018, 107, 161–168. [Google Scholar] [CrossRef]
- Ciabattini, A.; Nardini, C.; Santoro, F.; Garagnani, P.; Franceschi, C.; Medaglini, D. Vaccination in the Elderly: The Challenge of Immune Changes with Aging. Semin. Immunol. 2018, 40, 83–94. [Google Scholar] [CrossRef]
- Franceschi, C.; Salvioli, S.; Garagnani, P.; de Eguileor, M.; Monti, D.; Capri, M. Immunobiography and the Heterogeneity of Immune Responses in the Elderly: A Focus on Inflammaging and Trained Immunity. Front. Immunol. 2017, 8, 1. [Google Scholar] [CrossRef]
- Taneja, V. Sex Hormones Determine Immune Response. Front. Immunol. 2018, 9, 01931. [Google Scholar] [CrossRef]
- Libert, C.; Dejager, L.; Pinheiro, I. The X Chromosome in Immune Functions: When a Chromosome Makes the Difference. Nat. Rev. Immunol. 2010, 10, 594–604. [Google Scholar] [CrossRef]
- Márquez, E.J.; Chung, C.; Marches, R.; Rossi, R.J.; Nehar-Belaid, D.; Eroglu, A.; Mellert, D.J.; Kuchel, G.A.; Banchereau, J.; Ucar, D. Sexual-Dimorphism in Human Immune System Aging. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Caruso, C.; Accardi, G.; Virruso, C.; Candore, G. Sex, Gender and Immunosenescence: A Key to Understand the Different Lifespan between Men and Women? Immun. Ageing 2013, 10, 1. [Google Scholar] [CrossRef]
- Reus, B.; Caserta, S.; Larsen, M.; Morrow, G.; Bano, A.; Hallensleben, M.; Rajkumar, C.; Pera, A.; Kern, F. In-Depth Profiling of T-Cell Responsiveness to Commonly Recognized CMV Antigens in Older People Reveals Important Sex Differences. Front. Immunol. 2021, 10, 3207. [Google Scholar] [CrossRef]
- Giefing-Kröll, C.; Berger, P.; Lepperdinger, G.; Grubeck-Loebenstein, B. How Sex and Age Affect Immune Responses, Susceptibility to Infections, and Response to Vaccination. Aging Cell 2015, 14, 309–321. [Google Scholar] [CrossRef]
- Leng, S.X.; Margolick, J.B. Aging, Sex, Inflammation, Frailty, and CMV and HIV Infections. Cell. Immunol. 2020, 348, 104024. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Lee, W.W.; Weyand, C.M. Aging and T-Cell Diversity. Exp. Gerontol. 2007, 42, 400–406. [Google Scholar] [CrossRef]
- Hussain, T.; Quinn, K.M. Similar but Different: Virtual Memory CD8 T Cells as a Memory-like Cell Population. Immunol. Cell Biol. 2019, 97, 675–684. [Google Scholar] [CrossRef]
- Czesnikiewicz-Guzik, M.; Lee, W.W.; Cui, D.; Hiruma, Y.; Lamar, D.L.; Yang, Z.Z.; Ouslander, J.G.; Weyand, C.M.; Goronzy, J.J. T Cell Subset-Specific Susceptibility to Aging. Clin. Immunol. 2008, 127, 107–118. [Google Scholar] [CrossRef]
- Schmitt, V.; Rink, L.; Uciechowski, P. The Th17/Treg Balance Is Disturbed during Aging. Exp. Gerontol. 2013, 48, 1379–1386. [Google Scholar] [CrossRef]
- Larbi, A.; Pawelec, G.; Wong, S.; Goldeck, D.; Tai, J.; Fulop, T. Impact of Age on T Cell Signaling: A General Defect or Specific Alterations? Ageing Res. Rev. 2011, 10, 370–378. [Google Scholar] [CrossRef]
- Tomoiu, A.; Larbi, A.; Fortin, C.; Dupuis, G.; Fulop, T. Do Membrane Rafts Contribute to Human Immunosenescence? Ann. N. Y. Acad. Sci. 2007, 1100, 98–110. [Google Scholar] [CrossRef]
- Li, G.; Yu, M.; Lee, W.W.; Tsang, M.; Krishnan, E.; Weyand, C.M.; Goronzy, J.J. Decline in MiR-181a Expression with Age Impairs T Cell Receptor Sensitivity by Increasing DUSP6 Activity. Nat. Med. 2012, 18, 1518–1524. [Google Scholar] [CrossRef]
- Yu, M.; Li, G.; Lee, W.-W.; Yuan, M.; Cui, D.; Weyand, C.M.; Goronzy, J.J. Signal Inhibition by the Dual-Specific Phosphatase 4 Impairs T Cell-Dependent B-Cell Responses with Age. Proc. Natl. Acad. Sci. USA 2012, 109, E879–E888. [Google Scholar] [CrossRef]
- Le Page, A.; Fortin, C.; Garneau, H.; Allard, N.; Tsvetkova, K.; Tan, C.; Larbi, A.; Dupuis, G.; Fülöp, T. Downregulation of Inhibitory SRC Homology 2 Domain-Containing Phosphatase-1 (SHP-1) Leads to Recovery of T Cell Responses in Elderly. Cell Commun. Signal. 2014, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Lanna, A.; Henson, S.M.; Escors, D.; Akbar, A.N. AMPK-TAB1 Activated P38 Drives Human T Cell Senescence. Nat Immunol 2014, 15, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Lanna, A.; Gomes, D.C.O.; Muller-Durovic, B.; McDonnell, T.; Escors, D.; Gilroy, D.W.; Lee, J.H.; Karin, M.; Akbar, A.N. A Sestrin-Dependent Erk/Jnk/P38 MAPK Activation Complex Inhibits Immunity during Ageing. Nat. Immunol. 2017, 18, 354. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.I.; De Maeyer, R.P.H.; Covre, L.P.; Nehar-Belaid, D.; Lanna, A.; Ward, S.; Marches, R.; Chambers, E.S.; Gomes, D.C.O.; Riddell, N.E.; et al. Sestrins Induce Natural Killer Function in Senescent-like CD8+ T Cells. Nat. Immunol. 2020, 21, 684–694. [Google Scholar] [CrossRef]
- Grossmann, A.; Maggio-Price, L.; Jinneman, J.C.; Rabinovitch, P.S. Influence of Aging on Intracellular Free Calcium and Proliferation of Mouse T-Cell Subsets from Various Lymphoid Organs. Cell. Immunol. 1991, 135, 118–131. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Li, G.; Yu, M.; Weyand, C.M. Signaling Pathways in Aged T Cells—A Reflection of T Cell Differentiation, Cell Senescence and Host Environment. Semin. Immunol. 2012, 24, 365. [Google Scholar] [CrossRef]
- Zeng, N.; Capelle, C.M.; Baron, A.; Kobayashi, T.; Cire, S.; Tslaf, V.; Leonard, C.; Coowar, D.; Koseki, H.; Westendorf, A.M.; et al. DJ-1 Depletion Prevents Immunoaging in T-Cell Compartments. EMBO Rep. 2022, 23, e53302. [Google Scholar] [CrossRef]
- Garcia, G.G.; Berger, S.B.; Sadighi Akha, A.A.; Miller, R.A. Age-Associated Changes in Glycosylation of CD43 and CD45 on Mouse CD4 T Cells. Eur. J. Immunol. 2005, 35, 622–631. [Google Scholar] [CrossRef]
- Bunet, R.; Nayrac, M.; Ramani, H.; Sylla, M.; Durand, M.; Chartrand-Lefebvre, C.; Routy, J.-P.; Landay, A.L.; Gauchat, J.-F.; Chomont, N.; et al. Loss of CD96 Expression as a Marker of HIV-Specific CD8+ T-Cell Differentiation and Dysfunction. Front. Immunol. 2021, 12, 2006. [Google Scholar] [CrossRef]
- Fang, F.; Yu, M.; Cavanagh, M.M.; Saunders, J.H.; Qi, Q.; Ye, Z.; Saux, S.L.; Sultan, W.; Turgano, E.; Dekker, C.L.; et al. Expression of CD39 on Activated T Cells Impairs Their Survival in Older Individuals. Cell Rep. 2016, 14, 1218. [Google Scholar] [CrossRef]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in Immunity and Inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef]
- Effros, R.B. Telomerase Induction in T Cells: A Cure for Aging and Disease? Exp. Gerontol. 2007, 42, 416. [Google Scholar] [CrossRef]
- Callender, L.A.; Carroll, E.C.; Beal, R.W.J.; Chambers, E.S.; Nourshargh, S.; Akbar, A.N.; Henson, S.M. Human CD8+ EMRA T Cells Display a Senescence-Associated Secretory Phenotype Regulated by P38 MAPK. Aging Cell 2018, 17, e12675. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Y.; Zheng, L.; Zheng, C.; Song, J.; Zhang, Q.; Kang, B.; Liu, Z.; Jin, L.; Xing, R.; et al. Global Characterization of T Cells in Non-Small-Cell Lung Cancer by Single-Cell Sequencing. Nat. Med. 2018, 24, 978–985. [Google Scholar] [CrossRef]
- Strioga, M.; Pasukoniene, V.; Characiejus, D. CD8+ CD28- and CD8+ CD57+ T Cells and Their Role in Health and Disease. Immunology 2011, 134, 17–32. [Google Scholar] [CrossRef]
- Akha, A.A.S.; Miller, R.A. Signal Transduction in the Aging Immune System. Curr. Opin. Immunol. 2005, 17, 486–491. [Google Scholar] [CrossRef]
- Preite, S.; Gomez-Rodriguez, J.; Cannons, J.L.; Schwartzberg, P.L. T and B-Cell Signaling in Activated PI3K Delta Syndrome: From Immunodeficiency to Autoimmunity. Immunol. Rev. 2019, 291, 154–173. [Google Scholar] [CrossRef]
- Shao, L.; Goronzy, J.J.; Weyand, C.M. DNA-Dependent Protein Kinase Catalytic Subunit Mediates T-Cell Loss in Rheumatoid Arthritis. EMBO Mol. Med. 2010, 2, 415–427. [Google Scholar] [CrossRef]
- Kim, C.; Jin, J.; Weyand, C.M.; Goronzy, J.J. The Transcription Factor TCF1 in T Cell Differentiation and Aging. Int. J. Mol. Sci. 2020, 21, 6497. [Google Scholar] [CrossRef]
- Nicoli, F.; Cabral-Piccin, M.P.; Papagno, L.; Gallerani, E.; Fusaro, M.; Folcher, V.; Dubois, M.; Clave, E.; Vallet, H.; Frere, J.J.; et al. Altered Basal Lipid Metabolism Underlies the Functional Impairment of Naive CD8 + T Cells in Elderly Humans. J. Immunol. 2022, 208, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hartman, C.; Li, L.; Albert, C.; Si, F.; Gao, A.; Huang, L.; Zhao, Y.; Lin, W.; Hsueh, E.; et al. Reprogramming Lipid Metabolism Prevents Effector T Cell Senescence and Enhances Tumor Immunotherapy. Sci. Transl. Med. 2021, 13, aaz6314. [Google Scholar] [CrossRef]
- Dozmorov, M.G.; Coit, P.; Maksimowicz-Mckinnon, K.; Sawalha, A.H. Age-Associated DNA Methylation Changes in Naive CD4+ T Cells Suggest an Evolving Autoimmune Epigenotype in Aging T Cells. Epigenomics 2017, 9, 429–445. [Google Scholar] [CrossRef]
- Suarez-Álvarez, B.; Rodríguez, R.M.; Schlangen, K.; Raneros, A.B.; Márquez-Kisinousky, L.; Fernández, A.F.; Díaz-Corte, C.; Aransay, A.M.; López-Larrea, C. Phenotypic Characteristics of Aged CD4+ CD28null T Lymphocytes Are Determined by Changes in the Whole-Genome DNA Methylation Pattern. Aging Cell 2017, 16, 293–303. [Google Scholar] [CrossRef]
- Hu, B.; Jadhav, R.R.; Gustafson, C.E.; Saux, S.L.; Ye, Z.; Li, X.; Tian, L.; Weyand, C.M.; Goronzy, J.J. Distinct Age-Related Epigenetic Signatures in CD4 and CD8 T Cells. Front. Immunol. 2020, 11, 585168. [Google Scholar] [CrossRef] [PubMed]
- Ohyashiki, M.; Ohyashiki, J.H.; Hirota, A.; Kobayashi, C.; Ohyashiki, K. Age-Related Decrease of MiRNA-92a Levels in Human CD8+T-Cells Correlates with a Reduction of Naïve T Lymphocytes. Immun. Ageing 2011, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Roghanian, A. B Cells | British Society for Immunology. Available online: https://www.immunology.org/public-information/bitesized-immunology/cells/b-cells (accessed on 12 July 2022).
- Bernasconi, N.L.; Traggiai, E.; Lanzavecchia, A. Maintenance of Serological Memory by Polyclonal Activation of Human Memory B Cells. Science 2002, 298, 2199–2202. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.K.; Duque, G. Age-Related Bone Loss: Old Bone, New Facts. Gerontology 2002, 48, 62–71. [Google Scholar] [CrossRef]
- Denkinger, M.D.; Leins, H.; Schirmbeck, R.; Florian, M.C.; Geiger, H. HSC Aging and Senescent Immune Remodeling. Trends Immunol. 2015, 36, 815–824. [Google Scholar] [CrossRef]
- Henry, C.J.; Casás-Selves, M.; Kim, J.; Zaberezhnyy, V.; Aghili, L.; Daniel, A.E.; Jimenez, L.; Azam, T.; McNamee, E.N.; Clambey, E.T.; et al. Aging-Associated Inflammation Promotes Selection for Adaptive Oncogenic Events in B Cell Progenitors. J. Clin. Investig. 2015, 125, 4666–4680. [Google Scholar] [CrossRef]
- Listì, F.; Candore, G.; Modica, M.A.; Russo, M.; Di Lorenzo, G.; Esposito-Pellitteri, M.; Colonna-Romano, G.; Aquino, A.; Bulati, M.; Lio, D.; et al. A Study of Serum Immunoglobulin Levels in Elderly Persons That Provides New Insights into B Cell Immunosenescence. Ann. N. Y. Acad. Sci. 2006, 1089, 487–495. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B. Inflammaging Decreases Adaptive and Innate Immune Responses in Mice and Humans. Biogerontology 2016, 17, 7–19. [Google Scholar] [CrossRef]
- Dunn-Walters, D.K. The Ageing Human B Cell Repertoire: A Failure of Selection? Clin. Exp. Immunol. 2016, 183, 50–56. [Google Scholar] [CrossRef]
- Frasca, D.; DIaz, A.; Romero, M.; Garcia, D.; Blomberg, B.B. B Cell Immunosenescence. Annu. Rev. Cell Dev. Biol. 2020, 36, 551. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B. Aging Induces B Cell Defects and Decreased Antibody Responses to Influenza Infection and Vaccination. Immun. Ageing 2020, 17, 1–10. [Google Scholar] [CrossRef]
- Shi, Y.; Yamazaki, T.; Okubo, Y.; Uehara, Y.; Sugane, K.; Agematsu, K. Regulation of Aged Humoral Immune Defense against Pneumococcal Bacteria by IgM Memory B Cell. J. Immunol. 2005, 175, 3262–3267. [Google Scholar] [CrossRef]
- Kaminski, D.A.; Wei, C.; Qian, Y.; Rosenberg, A.F.; Sanz, I. Advances in Human B Cell Phenotypic Profiling. Front. Immunol. 2012, 3, 302. [Google Scholar] [CrossRef]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef]
- Cevenini, E.; Caruso, C.; Candore, G.; Capri, M.; Nuzzo, D.; Duro, G.; Rizzo, C.; Colonna-Romano, G.; Lio, D.; Carlo, D.; et al. Age-Related Inflammation: The Contribution of Different Organs, Tissues and Systems. How to Face It for Therapeutic Approaches. Curr. Pharm. Des. 2010, 16, 609–618. [Google Scholar] [CrossRef]
- Derhovanessian, E.; Larbi, A.; Pawelec, G. Biomarkers of Human Immunosenescence: Impact of Cytomegalovirus Infection. Curr. Opin. Immunol. 2009, 21, 440–445. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weyand, C.M. Understanding Immunosenescence to Improve Responses to Vaccines. Nat. Immunol. 2013, 14, 428–436. [Google Scholar] [CrossRef]
- Haralambieva, I.H.; Painter, S.D.; Kennedy, R.B.; Ovsyannikova, I.G.; Lambert, N.D.; Goergen, K.M.; Oberg, A.L.; Poland, G.A. The Impact of Immunosenescence on Humoral Immune Response Variation after Influenza A/H1N1 Vaccination in Older Subjects. PLoS ONE 2015, 10, 0122282. [Google Scholar] [CrossRef]
- Nakaya, H.I.; Hagan, T.; Duraisingham, S.S.; Lee, E.K.; Kwissa, M.; Rouphael, N.; Frasca, D.; Gersten, M.; Mehta, A.K.; Gaujoux, R.; et al. Systems Analysis of Immunity to Influenza Vaccination across Multiple Years and in Diverse Populations Reveals Shared Molecular Signatures. Immunity 2015, 43, 1186–1198. [Google Scholar] [CrossRef]
- Chambers, E.S.; Akbar, A.N. Can Blocking Inflammation Enhance Immunity during Aging? J. Allergy Clin. Immunol. 2020, 145, 1323–1331. [Google Scholar] [CrossRef]
- Saurwein-Teissl, M.; Lung, T.L.; Marx, F.; Gschösser, C.; Asch, E.; Blasko, I.; Parson, W.; Böck, G.; Schönitzer, D.; Trannoy, E.; et al. Lack of Antibody Production Following Immunization in Old Age: Association with CD8(+)CD28(-) T Cell Clonal Expansions and an Imbalance in the Production of Th1 and Th2 Cytokines. J. Immunol. 2002, 168, 5893–5899. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Jojic, V.; Sharma, S.; Shen-Orr, S.S.; Angel, C.J.L.; Onengut-Gumuscu, S.; Kidd, B.A.; Maecker, H.T.; Concannon, P.; Dekker, C.L.; et al. Cytomegalovirus Infection Enhances the Immune Response to Influenza. Sci. Transl. Med. 2015, 7, 281ra43. [Google Scholar] [CrossRef] [PubMed]
- Strindhall, J.; Ernerudh, J.; Mörner, A.; Waalen, K.; Löfgren, S.; Matussek, A.; Bengner, M. Humoral Response to Influenza Vaccination in Relation to Pre-Vaccination Antibody Titres, Vaccination History, Cytomegalovirus Serostatus and CD4/CD8 Ratio. Infect. Dis. 2016, 48, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.C. T-Cell-Inducing Vaccines—What’s the Future. Immunology 2012, 135, 19. [Google Scholar] [CrossRef]
- Behar, S.M.; Woodworth, J.S.M.; Wu, Y. The next Generation: Tuberculosis Vaccines That Elicit Protective CD8+ T Cells. Expert Rev. Vaccines 2007, 6, 441. [Google Scholar] [CrossRef]
- Kennedy, J.S.; Frey, S.E.; Yan, L.; Rothman, A.L.; Cruz, J.; Newman, F.K.; Orphin, L.; Belshe, R.B.; Ennis, F.A. Induction of Human T Cell-Mediated Immune Responses after Primary and Secondary Smallpox Vaccination. J. Infect. Dis. 2004, 190, 1286–1294. [Google Scholar] [CrossRef]
- Colditz, G.A.; Brewer, T.F.; Berkey, C.S.; Wilson, M.E.; Burdick, E.; Fineberg, H.V.; Mosteller, F. Efficacy of BCG Vaccine in the Prevention of Tuberculosis: Meta-Analysis of the Published Literature. JAMA 1994, 271, 698–702. [Google Scholar] [CrossRef]
- Lang, P.O.; Govind, S.; Michel, J.P.; Aspinall, R.; Mitchell, W.A. Immunosenescence: Implications for Vaccination Programmes in Adults. Maturitas 2011, 68, 322–330. [Google Scholar] [CrossRef]
- McConkey, S.J.; Reece, W.H.H.; Moorthy, V.S.; Webster, D.; Dunachie, S.; Butcher, G.; Vuola, J.M.; Blanchard, T.J.; Gothard, P.; Watkins, K.; et al. Enhanced T-Cell Immunogenicity of Plasmid DNA Vaccines Boosted by Recombinant Modified Vaccinia Virus Ankara in Humans. Nat. Med. 2003, 9, 729–735. [Google Scholar] [CrossRef]
- Karwacz, K.; Mukherjee, S.; Apolonia, L.; Blundell, M.P.; Bouma, G.; Escors, D.; Collins, M.K.; Thrasher, A.J. Nonintegrating Lentivector Vaccines Stimulate Prolonged T-Cell and Antibody Responses and Are Effective in Tumor Therapy. J. Virol. 2009, 83, 3094–3103. [Google Scholar] [CrossRef]
- MacDonald, D.C.; Singh, H.; Whelan, M.A.; Escors, D.; Arce, F.; Bottoms, S.E.; Barclay, W.S.; Maini, M.; Collins, M.K.; Rosenberg, W.C. Harnessing Alveolar Macrophages for Sustained Mucosal T-Cell Recall Confers Long-Term Protection to Mice against Lethal Influenza Challenge without Clinical Disease. Mucosal Immunol. 2014, 7, 89–100. [Google Scholar] [CrossRef][Green Version]
- Haq, K.; McElhaney, J. Immunosenescence: Influenza Vaccination and the Elderly. Curr. Opin. Immunol. 2014, 29, 38–42. [Google Scholar] [CrossRef]
- McElhaney, J.E.; Kuchel, G.A.; Zhou, X.; Swain, S.L.; Haynes, L. T-Cell Immunity to Influenza in Older Adults: A Pathophysiological Framework for Development of More Effective Vaccines. Front. Immunol. 2016, 7, 41. [Google Scholar] [CrossRef]
- Park, H.L.; Shim, S.H.; Lee, E.Y.; Cho, W.; Park, S.; Jeon, H.J.; Ahn, S.Y.; Kim, H.; Nam, J.H. Obesity-Induced Chronic Inflammation Is Associated with the Reduced Efficacy of Influenza Vaccine. Hum. Vaccin. Immunother. 2014, 10, 1181. [Google Scholar] [CrossRef]
- Bartleson, J.M.; Radenkovic, D.; Covarrubias, A.J.; Furman, D.; Winer, D.A.; Verdin, E. SARS-CoV-2, COVID-19 and the Aging Immune System. Nat. Aging 2021, 1, 769–782. [Google Scholar] [CrossRef]
- Haynes, L.; Swain, S.L. Aged-Related Shifts in T Cell Homeostasis Lead to Intrinsic T Cell Defects. Semin. Immunol. 2012, 24, 350. [Google Scholar] [CrossRef]
- Lefebvre, J.S.; Masters, A.R.; Hopkins, J.W.; Haynes, L. Age-Related Impairment of Humoral Response to Influenza Is Associated with Changes in Antigen Specific T Follicular Helper Cell Responses. Sci. Rep. 2016, 6, 25051. [Google Scholar] [CrossRef]
- Yager, E.J.; Ahmed, M.; Lanzer, K.; Randall, T.D.; Woodland, D.L.; Blackman, M.A. Age-Associated Decline in T Cell Repertoire Diversity Leads to Holes in the Repertoire and Impaired Immunity to Influenza Virus. J. Exp. Med. 2008, 205, 711–723. [Google Scholar] [CrossRef]
- Van Werkhoven, C.H.; Huijts, S.M.; Bolkenbaas, M.; Grobbee, D.E.; Bonten, M.J.M. The Impact of Age on the Efficacy of 13-Valent Pneumococcal Conjugate Vaccine in Elderly. Clin. Infect. Dis. 2015, 61, 1835–1838. [Google Scholar] [CrossRef]
- Lu, Y.J.; Gross, J.; Bogaert, D.; Finn, A.; Bagrade, L.; Zhang, Q.; Kolls, J.K.; Srivastava, A.; Lundgren, A.; Forte, S.; et al. Interleukin-17A Mediates Acquired Immunity to Pneumococcal Colonization. PLoS Pathog. 2008, 4, e1000159. [Google Scholar] [CrossRef]
- Weinberg, A.; Lazar, A.A.; Zerbe, G.O.; Hayward, A.R.; Chan, I.S.F.; Vessey, R.; Silber, J.L.; MacGregor, R.R.; Chan, K.; Gershon, A.A.; et al. Influence of Age and Nature of Primary Infection on Varicella-Zoster Virus—Specific Cell-Mediated Immune Responses. J. Infect. Dis. 2010, 201, 1024. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.-J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barberà, J.; et al. Efficacy of an Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Chlibek, R.; Smetana, J.; Pauksens, K.; Rombo, L.; Van den Hoek, J.A.R.; Richardus, J.H.; Plassmann, G.; Schwarz, T.F.; Ledent, E.; Heineman, T.C. Safety and Immunogenicity of Three Different Formulations of an Adjuvanted Varicella-Zoster Virus Subunit Candidate Vaccine in Older Adults: A Phase II, Randomized, Controlled Study. Vaccine 2014, 32, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Cavanagh, M.M.; Saux, S.L.; NamKoong, H.; Kim, C.; Turgano, E.; Liu, Y.; Wang, C.; Mackey, S.; Swan, G.E.; et al. Diversification of the Antigen-Specific T Cell Receptor Repertoire after Varicella Zoster Vaccination. Sci. Transl. Med. 2016, 8, 332ra46. [Google Scholar] [CrossRef]
- Weinberger, B. Vaccines for the Elderly: Current Use and Future Challenges. Immun. Ageing 2018, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence and Human Vaccine Immune Responses. Immun. Ageing 2019, 16, 1–16. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Ferracci, F.; Blomberg, B.B. MicroRNAs MiR-155 and MiR-16 Decrease AID and E47 in B Cells from Elderly Individuals. J. Immunol. 2015, 195, 2134–2140. [Google Scholar] [CrossRef]
- Ponnappan, S.; Ponnappan, U. Aging and Immune Function: Molecular Mechanisms to Interventions. Antioxid Redox Signal 2011, 14, 1551–1585. [Google Scholar] [CrossRef]
- CDC COVID-19 Provisional Counts-Weekly Updates by Select Demographic and Geographic Characteristics. Available online: https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm (accessed on 14 February 2022).
- Lins, M.P.; Smaniotto, S. Potential Impact of SARS-CoV-2 Infection on the Thymus. Can. J. Microbiol. 2021, 67, 23–28. [Google Scholar] [CrossRef]
- Moss, P. The T Cell Immune Response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of Antibody Response to BNT162b2 Vaccine after Six Months: A Longitudinal Prospective Study. Lancet Reg. Health–Eur. 2021, 10, 100208. [Google Scholar] [CrossRef]
- Soiza, R.L.; Scicluna, C.; Thomson, E.C. Efficacy and Safety of COVID-19 Vaccines in Older People. Age Ageing 2021, 50, 279–283. [Google Scholar] [CrossRef]
- Urra, J.M.; Cabrera, C.M.; Porras, L.; Ródenas, I. Selective CD8 Cell Reduction by SARS-CoV-2 Is Associated with a Worse Prognosis and Systemic Inflammation in COVID-19 Patients. Clin. Immunol. 2020, 217, 108486. [Google Scholar] [CrossRef]
- Westmeier, J.; Paniskaki, K.; Karaköse, Z.; Werner, T.; Sutter, K.; Dolff, S.; Overbeck, M.; Limmer, A.; Liu, J.; Zheng, X.; et al. Impaired Cytotoxic CD8+ T Cell Response in Elderly COVID-19 Patients. MBio 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Jo, N.; Zhang, R.; Ueno, H.; Yamamoto, T.; Weiskopf, D.; Nagao, M.; Yamanaka, S.; Hamazaki, Y. Aging and CMV Infection Affect Pre-Existing SARS-CoV-2-Reactive CD8 + T Cells in Unexposed Individuals. Front. aging 2021, 2. [Google Scholar] [CrossRef]
- Löhr, P.; Schiele, S.; Arndt, T.T.; Grützner, S.; Claus, R.; Römmele, C.; Müller, G.; Schmid, C.; Dennehy, K.M.; Rank, A. Impact of Age and Gender on Lymphocyte Subset Counts in Patients with COVID-19. Cytom. Part A 2021. [Google Scholar] [CrossRef]
- Andryukov, B.G.; Besednova, N.N. Older Adults: Panoramic View on the COVID-19 Vaccination. AIMS Public Health 2021, 8, 388. [Google Scholar] [CrossRef]
- Jahrsdörfer, B.; Fabricius, D.; Scholz, J.; Ludwig, C.; Grempels, A.; Lotfi, R.; Körper, S.; Adler, G.; Schrezenmeier, H. BNT162b2 Vaccination Elicits Strong Serological Immune Responses Against SARS-CoV-2 Including Variants of Concern in Elderly Convalescents. Front. Immunol. 2021, 12, 743422. [Google Scholar] [CrossRef]
- Demaret, J.; Corroyer-Simovic, B.; Alidjinou, E.K.; Goffard, A.; Trauet, J.; Miczek, S.; Vuotto, F.; Dendooven, A.; Huvent-Grelle, D.; Podvin, J.; et al. Impaired Functional T-Cell Response to SARS-CoV-2 After Two Doses of BNT162b2 MRNA Vaccine in Older People. Front. Immunol. 2021, 12, 778679. [Google Scholar] [CrossRef]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; Baker, S.; Dougan, G.; et al. Age-Related Immune Response Heterogeneity to SARS-CoV-2 Vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 MRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and Immunogenicity of ChAdOx1 NCoV-19 Vaccine Administered in a Prime-Boost Regimen in Young and Old Adults (COV002): A Single-Blind, Randomised, Controlled, Phase 2/3 Trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Bignon, A.; Régent, A.; Klipfel, L.; Desnoyer, A.; De La Grange, P.; Martinez, V.; Lortholary, O.; Dalloul, A.; Mouthon, L.; Balabanian, K. DUSP4-Mediated Accelerated T-Cell Senescence in Idiopathic CD4 Lymphopenia. Blood 2015, 125, 2507–2518. [Google Scholar] [CrossRef] [PubMed]
- Henson, S.M.; Lanna, A.; Riddell, N.E.; Franzese, O.; Macaulay, R.; Griffiths, S.J.; Puleston, D.J.; Watson, A.S.; Simon, A.K.; Tooze, S.A.; et al. P38 Signaling Inhibits MTORC1-Independent Autophagy in Senescent Human CD8+ T Cells. J. Clin. Investig. 2014, 124, 4004. [Google Scholar] [CrossRef] [PubMed]
- Henson, S.M.; Macaulay, R.; Riddell, N.E.; Nunn, C.J.; Akbar, A.N. Blockade of PD-1 or P38 MAP Kinase Signaling Enhances Senescent Human CD8+ T-Cell Proliferation by Distinct Pathways. Eur. J. Immunol. 2015, 45, 1441–1451. [Google Scholar] [CrossRef]
- Vukmanovic-Stejic, M.; Chambers, E.S.; Suárez-Fariñas, M.; Sandhu, D.; Fuentes-Duculan, J.; Patel, N.; Agius, E.; Lacy, K.E.; Turner, C.T.; Larbi, A.; et al. Enhancement of Cutaneous Immunity during Aging by Blocking P38 Mitogen-Activated Protein (MAP) Kinase-Induced Inflammation. J. Allergy Clin. Immunol. 2018, 142, 844–856. [Google Scholar] [CrossRef]
- Parish, S.T.; Wu, J.E.; Effros, R.B. Modulation of T Lymphocyte Replicative Senescence via TNF-α Inhibition: Role of Caspase-3. J. Immunol. 2009, 182, 4237–4243. [Google Scholar] [CrossRef]
- Mannick, J.B.; Del Giudice, G.; Lattanzi, M.; Valiante, N.M.; Praestgaard, J.; Huang, B.; Lonetto, M.A.; Maecker, H.T.; Kovarik, J.; Carson, S.; et al. MTOR Inhibition Improves Immune Function in the Elderly. Sci. Transl. Med. 2014, 6, 268ra179. [Google Scholar] [CrossRef]
- Jin, J.; Kim, C.; Xia, Q.; Gould, T.M.; Cao, W.; Zhang, H.; Li, X.; Weiskopf, D.; Grifoni, A.; Sette, A.; et al. Activation of MTORC1 at Late Endosomes Misdirects T Cell Fate Decision in Older Individuals. Sci. Immunol. 2021, 6, abg0791. [Google Scholar] [CrossRef]
- Netti, G.S.; Infante, B.; Troise, D.; Mercuri, S.; Panico, M.; Spadaccino, F.; Catalano, V.; Gigante, M.; Simone, S.; Pontrelli, P.; et al. MTOR Inhibitors Improve Both Humoral and Cellular Response to SARS-CoV-2 Messenger RNA BNT16b2 Vaccine in Kidney Transplant Recipients. Am. J. Transplant 2022, 22, 1475–1482. [Google Scholar] [CrossRef]
- Zhang, H.; Alsaleh, G.; Feltham, J.; Sun, Y.; Napolitano, G.; Riffelmacher, T.; Charles, P.; Frau, L.; Hublitz, P.; Yu, Z.; et al. Polyamines Control EIF5A Hypusination, TFEB Translation, and Autophagy to Reverse B Cell Senescence. Mol. Cell 2019, 76, 110–125.e9. [Google Scholar] [CrossRef]
- Xiong, Y.; Xiong, Y.; Zhang, H.; Zhao, Y.; Han, K.; Zhang, J.; Zhao, D.; Yu, Z.; Geng, Z.; Wang, L.; et al. HPMSCs-Derived Exosomal MiRNA-21 Protects Against Aging-Related Oxidative Damage of CD4 + T Cells by Targeting the PTEN/PI3K-Nrf2 Axis. Front. Immunol. 2021, 12, 780897. [Google Scholar] [CrossRef]
- Son, H.J.; Lee, J.; Lee, S.Y.; Kim, E.K.; Park, M.J.; Kim, K.W.; Park, S.H.; Cho, M. La Metformin Attenuates Experimental Autoimmune Arthritis through Reciprocal Regulation of Th17/Treg Balance and Osteoclastogenesis. Mediators Inflamm. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Radtke, A.J.; Anderson, C.F.; Riteau, N.; Rausch, K.; Scaria, P.; Kelnhofer, E.R.; Howard, R.F.; Sher, A.; Germain, R.N.; Duffy, P. Adjuvant and Carrier Protein-Dependent T-Cell Priming Promotes a Robust Antibody Response against the Plasmodium Falciparum Pfs25 Vaccine Candidate. Sci. Rep. 2017, 7, 40312. [Google Scholar] [CrossRef]
- Gustafson, C.E.; Weyand, C.M.; Goronzy, J.J. T Follicular Helper Cell Development and Functionality in Immune Aging. Clin. Sci. 2018, 132, 1925. [Google Scholar] [CrossRef]
- Gärtner, B.C.; Weinke, T.; Wahle, K.; Kwetkat, A.; Beier, D.; Schmidt, K.J.; Schwarz, T.F. Importance and Value of Adjuvanted Influenza Vaccine in the Care of Older Adults from a European Perspective—A Systematic Review of Recently Published Literature on Real-World Data. Vaccine 2022, 40, 2999–3008. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging Concepts in the Science of Vaccine Adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Weinberger, B. Adjuvant Strategies to Improve Vaccination of the Elderly Population. Curr. Opin. Pharmacol. 2018, 41, 34–41. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of Adjuvanticity: A Review on Adjuvants in Licensed Vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Hennessy, E.J.; Parker, A.E. Targeting Toll-like Receptors: Emerging Therapeutics? Nat. Rev. Drug Discov. 2010, 9, 293–307. [Google Scholar] [CrossRef]
- Nanishi, E.; Angelidou, A.; Rotman, C.; Dowling, D.J.; Levy, O.; Ozonoff, A. Precision Vaccine Adjuvants for Older Adults: A Scoping Review. Clin. Infect. Dis. 2022, 75, S72–S80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.J.X.; Li, C.; To, K.K.W.; Zhu, H.S.; Lee, A.C.Y.; Li, C.G.; Chan, J.F.W.; Hung, I.F.N.; Yuen, K.Y. Toll-like Receptor 7 Agonist Imiquimod in Combination with Influenza Vaccine Expedites and Augments Humoral Immune Responses against Influenza A(H1N1)Pdm09 Virus Infection in BALB/c Mice. Clin. Vaccine Immunol. 2014, 21, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.M.; Booy, R. Effects of Exercise on Vaccine-Induced Immune Responses. Hum. Vaccin. Immunother. 2013, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Minuzzi, L.G. Effects of Lifelong Training on Senescence and Mobilization of T Lymphocytes in Response to Acute Exercise. Exerc. Immunol. Rev. 2018, 24, 72–84. [Google Scholar] [PubMed]
- Haase, H.; Rink, L. The Immune System and the Impact of Zinc during Aging. Immun. Ageing 2009, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Hernanz, A.; Guayerbas, N.; Victor, V.M.; Arnalich, F. Vitamin E Ingestion Improves Several Immune Functions in Elderly Men and Women. Free Radic. Res. 2008, 42, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Huijskens, M.J.A.J.; Walczak, M.; Sarkar, S.; Atrafi, F.; Senden-Gijsbers, B.L.M.G.; Tilanus, M.G.J.; Bos, G.M.J.; Wieten, L.; Germeraad, W.T.V. Ascorbic Acid Promotes Proliferation of Natural Killer Cell Populations in Culture Systems Applicable for Natural Killer Cell Therapy. Cytotherapy 2015, 17, 613–620. [Google Scholar] [CrossRef]
- Farges, M.C.; Minet-Quinard, R.; Walrand, S.; Thivat, E.; Ribalta, J.; Winklhofer-Roob, B.; Rock, E.; Vasson, M.P. Immune Status Is More Affected by Age than by Carotenoid Depletion-Repletion in Healthy Human Subjects. Br. J. Nutr. 2012, 108, 2054–2065. [Google Scholar] [CrossRef]
- Yuan, J.; Lu, L.; Zhang, Z.; Zhang, S. Dietary Intake of Resveratrol Enhances the Adaptive Immunity of Aged Rats. Rejuvenation Res. 2012, 15, 507–515. [Google Scholar] [CrossRef]
- Stahl, E.C.; Brown, B.N. Cell Therapy Strategies to Combat Immunosenescence. Organogenesis 2015, 11, 159. [Google Scholar] [CrossRef]
- Duffy, M.; Ritter, T.; Ceredig, R.; Griffin, M. Mesenchymal Stem Cell Effects on T-Cell Effector Pathways. Stem Cell Res. Ther. 2011, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, L.; Ruan, L.; Oh, J.; Dong, X.; Zhuge, Q.; Su, D.M. Extracellular Vesicles Extracted from Young Donor Serum Attenuate Inflammaging via Partially Rejuvenating Aged T-Cell Immunotolerance. FASEB J. 2018, 32, 5899–5912. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, Y.; Hu, Z.; Wu, M.; Wang, C.; Feng, Z.; Mao, C.; Tan, Y.; Liu, Y.; Chen, L.; et al. Thymosin Alpha 1 Reduces the Mortality of Severe Coronavirus Disease 2019 by Restoration of Lymphocytopenia and Reversion of Exhausted T Cells. Clin. Infect. Dis. 2020, 71, 2150–2157. [Google Scholar] [CrossRef]
- Bajaj, V.; Gadi, N.; Spihlman, A.P.; Wu, S.C.; Choi, C.H.; Moulton, V.R. Aging, Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections? Front. Physiol. 2021, 11, 1–23. [Google Scholar] [CrossRef]
- Lorenzo, E.C.; Torrance, B.L.; Keilich, S.R.; Al-Naggar, I.; Harrison, A.; Xu, M.; Bartley, J.M.; Haynes, L. Senescence-Induced Changes in CD4 T Cell Differentiation Can Be Alleviated by Treatment with Senolytics. Aging Cell 2022, 21, 13525. [Google Scholar] [CrossRef]
- Avivi, I.; Zisman-Rozen, S.; Naor, S.; Dai, I.; Benhamou, D.; Shahaf, G.; Tabibian-Keissar, H.; Rosenthal, N.; Rakovsky, A.; Hanna, A.; et al. Depletion of B Cells Rejuvenates the Peripheral B-Cell Compartment but Is Insufficient to Restore Immune Competence in Aging. Aging Cell 2019, 18, 12959. [Google Scholar] [CrossRef]
| Cell Phenotype | Changes | Causes and/or Effects |
|---|---|---|
| T cell CD3+ | =/↓ | Reduction of haematopoietic stem cell progenitors; Defects in thymic stromal niches. Reduced T-cell responses; |
| CD4+ Naïve CD4+/CD45RA+/CCR7+/CD27+/CD28+ | ↓ | Thymic involution; Phenotypic conversion of naïve T cells into memory phenotype. Reduced responses to new antigens and neoantigens; Increased susceptibility to infections. |
| CD8+ Naïve CD8+/CD45RA+/CCR7+/CD27+/CD28+ | ↓↓ | |
| CD4+ TCM CD4+/CD45RA−/CCR7+/CD27+/CD28+ | =/↑ | Effects of immunobiography. Reduced responses to cognate antigens; Increased susceptibility to infections, autoimmune disorders, chronic diseases, cardiovascular disease and cancer. |
| CD8+ TCM CD8+/CD45RA−/CCR7+/CD27+/CD28+ | =/↑ | |
| CD4+ TEM CD4+/CD45RA−/CCR7−/CD27−/CD28− | =/↑ | |
| CD8+ TEM CD8+/CD45RA−/CCR7−/CD27−/CD28− | ↑↑ | |
| CD4+ TEMRA CD4+/CD45RA+/CCR7−/CD27−/CD28− | =/↑ | Reactivation of persistent virus infections. Reduced responses to cognate antigens; Increased susceptibility to infections, autoimmune disorders, chronic diseases, cardiovascular disease, and cancer. |
| CD8+ TEMRA CD8+/CD45RA+/CCR7−/CD27−/CD28− | ↑↑ | |
| B cells CD19+ | ↓ | Reduction in haematopoietic stem cell progenitors; Reduced B-cell responses. |
| Naïve CD19+/IgDHigh/IgMHigh/CD27− or CD19+/IgG−/IgA−/CD27− | ↓ | Phenotypic conversion of naïve B cells into memory phenotype; Increased susceptibility to infectious diseases; Reduced ability to respond to new pathogens and reduced protection of vaccination. |
| Memory unswitched CD19+/IgDLow/IgMHigh/CD27+ | = | Maintained immune response against well-known antigens. |
| Memory switched CD19+/IgD−(Switched Igs, IgG+/IgA+/IgE+)/CD27+ | =/↓ | |
| IgM-only memory CD19+/IgD−/IgM+/CD27+ | =/↓ | |
| Double negative CD19+/IgD−/(Switched Igs, IgG+/IgA+/IgE+)/CD27− | ↑ | Negatively associated with the serum response to the influenza vaccine; Secretion of pro-inflammatory cytokines |
| Strategy | Effect | |
|---|---|---|
| Senescent T cell | DUSP6 inhibition | Recovery of T cell signalling |
| DUSP4 inhibition | Recovery of T cell signalling | |
| SHP-1 inhibition | Increased secretion of IL-2 and proliferation of CD4+ T cells | |
| MAPK p38 inhibition | Reversion of CD8+ T cell senescence | |
| MAPK p38 and PD-1 inhibition | Proliferation of TEMRA CD8+ T cells | |
| AMPK-TAB1-MAPK p38 complex inhibition | Proliferation of highly-differentiated T cells | |
| Sestrins–MAPK complex inhibition | Recovery of T cell activity Increase in influenza vaccine efficacy in mice | |
| Sestrins inhibition | Recovery of TCR signalling | |
| DJ-1 inhibition | Restoration of TCR | |
| PD-1 inhibition | Increase of cytokine production | |
| TNF-alpha inhibition | Postponement of CD28 downregulation | |
| mTOR inhibition | Improvement in immune response after influenza and SARS-CoV 2 vaccination | |
| mTOR and PI3K inhibition | Control of infection | |
| VPS39 inhibition | Higher levels of memory T cells | |
| Autophagy inhibition | Expansion of antigen specific CD8+ t cells | |
| cPLA2 inhibition | Prevention of T cell decline | |
| PTEN/PI3K-NRF2 axis activation | Loss of senescence markers expression | |
| AMPK activation | Decrease of Th17 differentiation and increase in Tregs | |
| Senolytic drugs | Depletion of senescent cells | |
| Thymosin | Increase in CD4+ and CD8+ T cells in older COVID-19 patients | |
| Adjuvants | Lipophilic adjuvants and TLR4 agonist | Improvement of T follicular responses to malaria vaccines in mice |
| AS01 adjuvant | Increase in CD4+ T cells for herpes zoster virus vaccination | |
| MF59 | Persistence of B cell and CD4+ T cell responses | |
| AS03 | Persistence of B cell and CD4+ T cell responses | |
| Flagellin | Increase in IFN-γ producing memory CD4+ T cells | |
| GLA-SE | Th1-biased T cell responses and enhances cytokine and granzyme B secretion | |
| Imiquimod | Increase in IFN-y expression and IgG isotype switching | |
| Lifestyle | Exercise | Decrease in the number of senescent lymphocytesIncreased levels of IL-7 and IL-15 Apoptosis of exhausted T cells |
| Zinc | Increase in CD4 and CD8 numbers | |
| Vitamin E | IL-2 production Naïve T cell activation and proliferation | |
| Vitamin C | Reduction of inflammaging T helper maturation | |
| Carotenoid | Mature T cell phenotype | |
| Polyphenols | Increase in IL-2 and IFN-gamma | |
| Polyunsaturated fatty acids | Proliferation of T lymphocytes | |
| Adoptive T cell therapy | Stem cell memory cells | |
| Virus-specific T cells | ||
| Mesenchymal stem cells | Reduction in the expression of senescent markers in CD4+ T cells Moderation of inflammaging in mice | |
| B cell | Induction of autophagy | Improvement in B cell response |
| Depletion | Rejuvenation of B cell population | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garnica, M.; Aiello, A.; Ligotti, M.E.; Accardi, G.; Arasanz, H.; Bocanegra, A.; Blanco, E.; Calabrò, A.; Chocarro, L.; Echaide, M.; et al. How Can We Improve the Vaccination Response in Older People? Part II: Targeting Immunosenescence of Adaptive Immunity Cells. Int. J. Mol. Sci. 2022, 23, 9797. https://doi.org/10.3390/ijms23179797
Garnica M, Aiello A, Ligotti ME, Accardi G, Arasanz H, Bocanegra A, Blanco E, Calabrò A, Chocarro L, Echaide M, et al. How Can We Improve the Vaccination Response in Older People? Part II: Targeting Immunosenescence of Adaptive Immunity Cells. International Journal of Molecular Sciences. 2022; 23(17):9797. https://doi.org/10.3390/ijms23179797
Chicago/Turabian StyleGarnica, Maider, Anna Aiello, Mattia Emanuela Ligotti, Giulia Accardi, Hugo Arasanz, Ana Bocanegra, Ester Blanco, Anna Calabrò, Luisa Chocarro, Miriam Echaide, and et al. 2022. "How Can We Improve the Vaccination Response in Older People? Part II: Targeting Immunosenescence of Adaptive Immunity Cells" International Journal of Molecular Sciences 23, no. 17: 9797. https://doi.org/10.3390/ijms23179797
APA StyleGarnica, M., Aiello, A., Ligotti, M. E., Accardi, G., Arasanz, H., Bocanegra, A., Blanco, E., Calabrò, A., Chocarro, L., Echaide, M., Kochan, G., Fernandez-Rubio, L., Ramos, P., Pojero, F., Zareian, N., Piñeiro-Hermida, S., Farzaneh, F., Candore, G., Caruso, C., & Escors, D. (2022). How Can We Improve the Vaccination Response in Older People? Part II: Targeting Immunosenescence of Adaptive Immunity Cells. International Journal of Molecular Sciences, 23(17), 9797. https://doi.org/10.3390/ijms23179797










