Inflammatory Biomarkers in Exhaled Breath Condensate: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
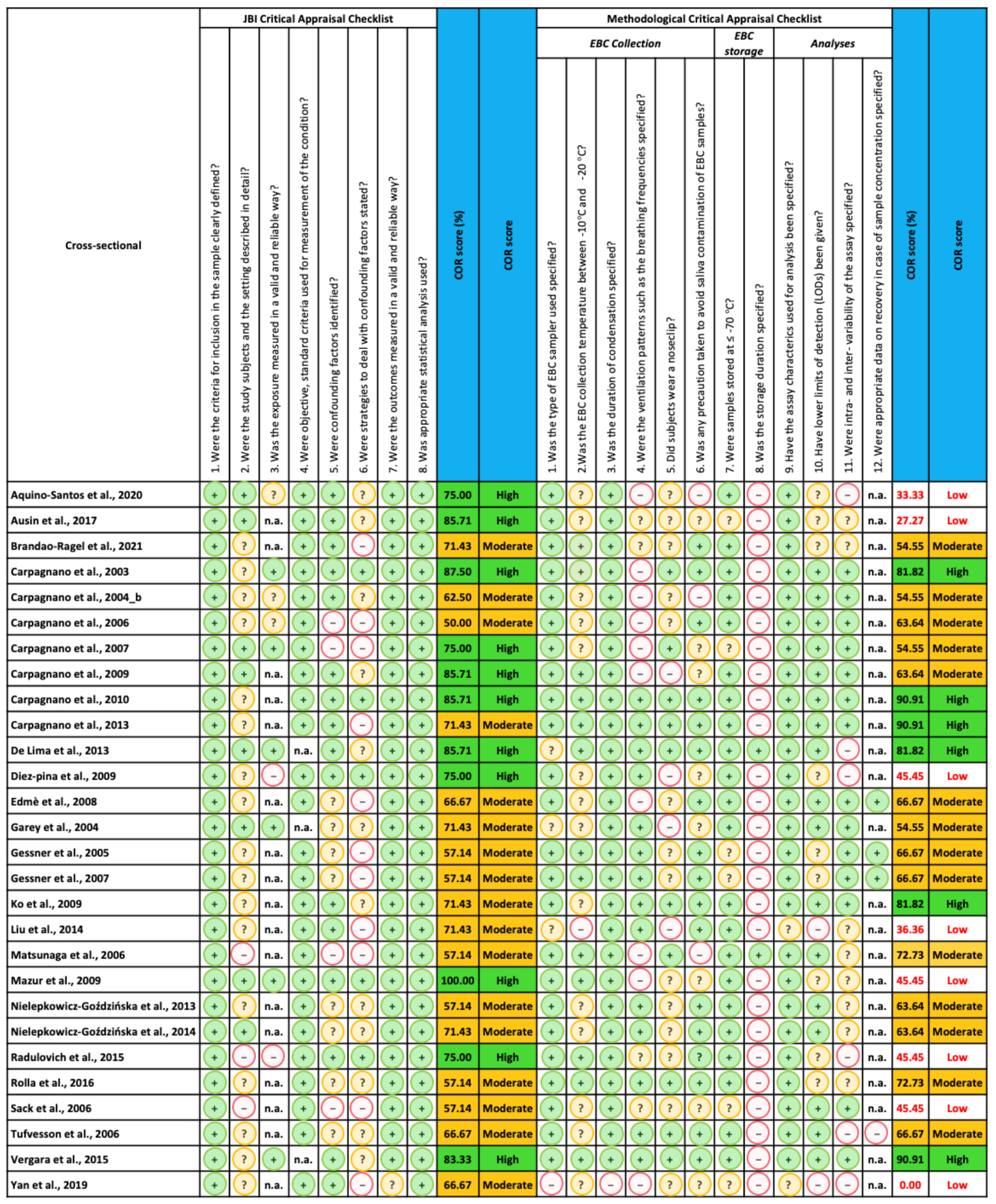
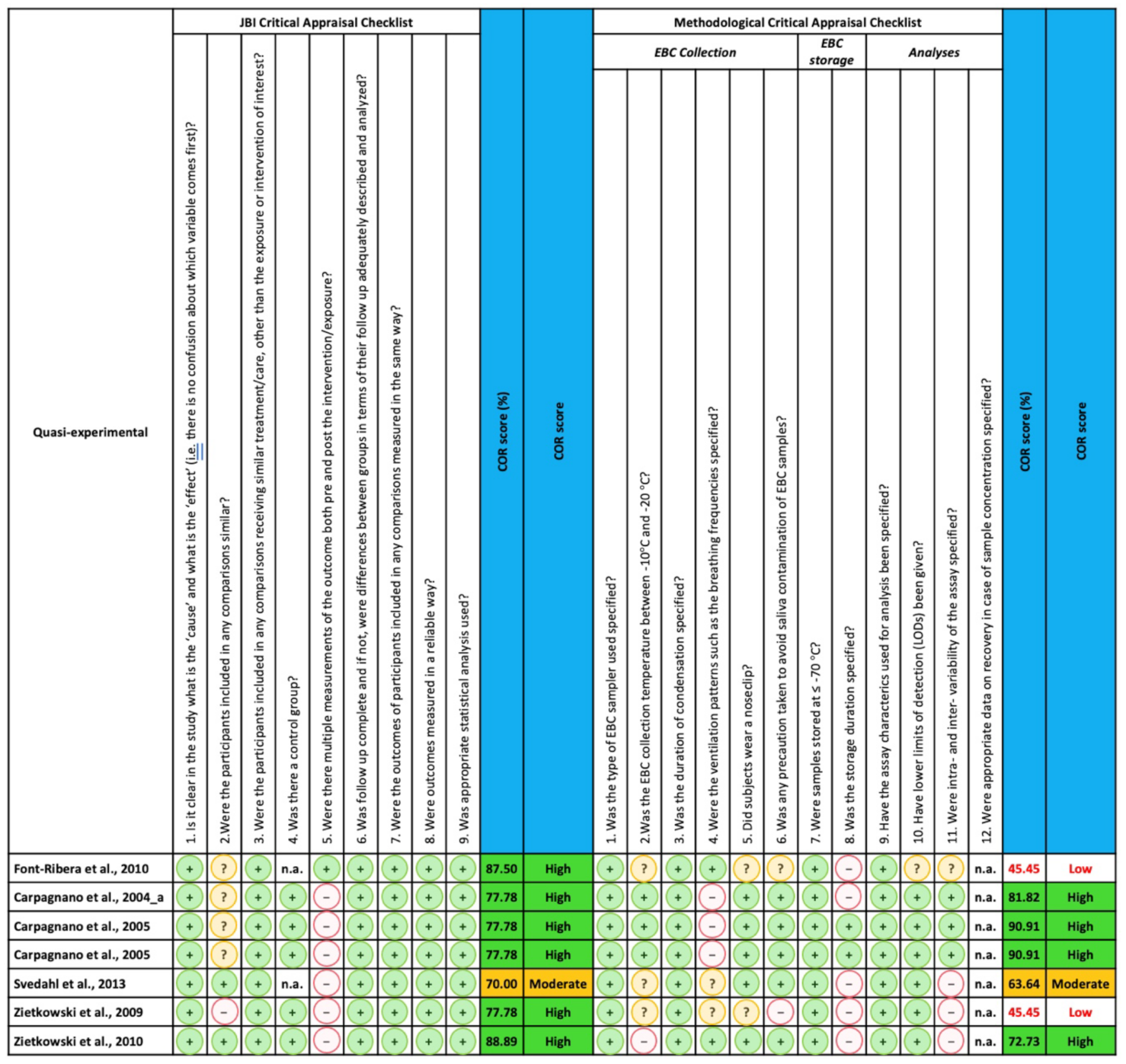
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Qualitative Synthesis
3.2. Study and Participant Characteristics
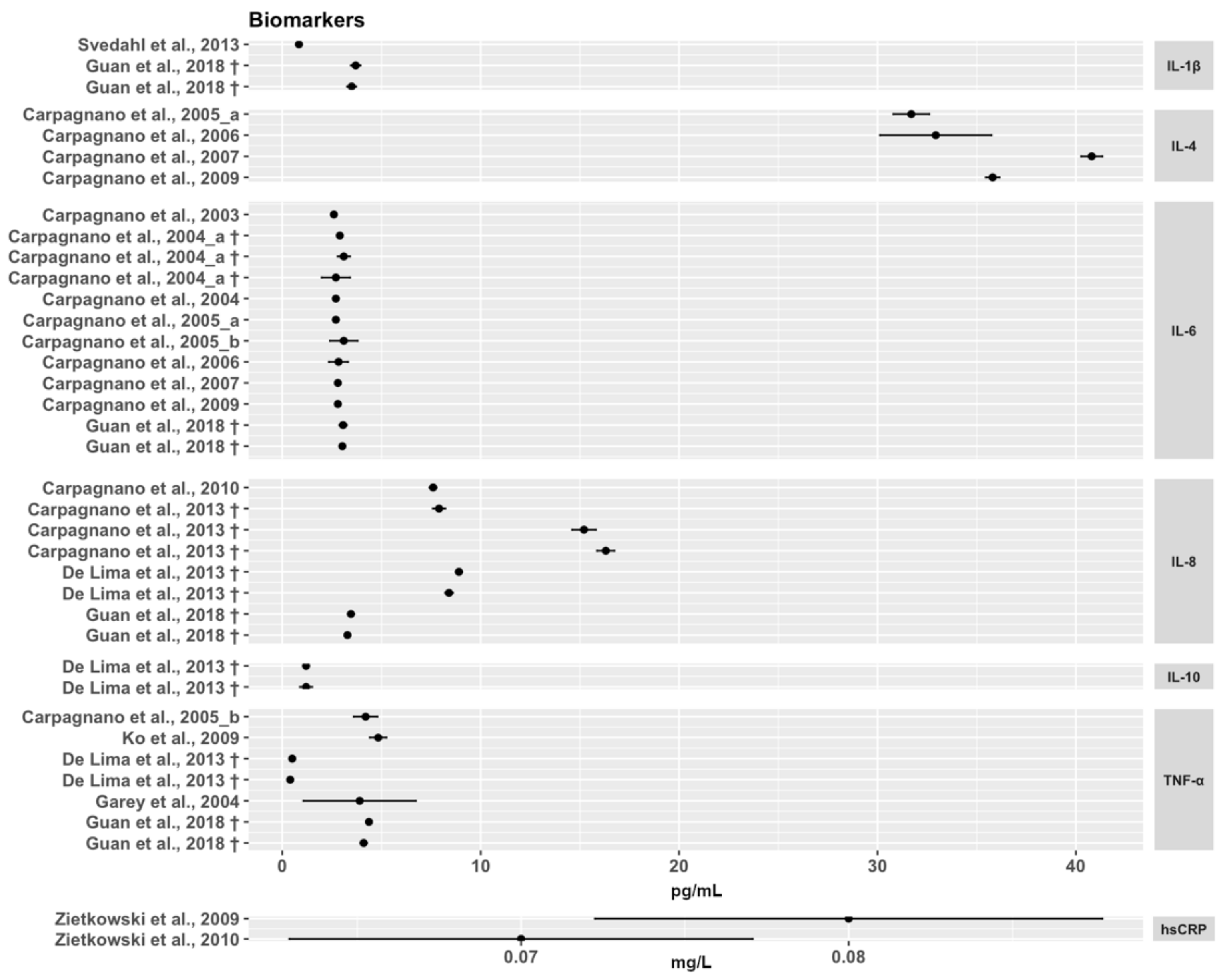
3.3. Inflammation Biomarkers in EBC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| PubMed | |
|---|---|
| 1 | “Tumor Necrosis Factor-alpha” [Mesh] |
| 2 | “tumor necrosis factor-alpha” [tiab] OR “tumor necrosis factor-a” [tiab] OR “TNF-alpha” [tiab] OR TNFalpha [tiab] OR TNF-a [tiab] OR TNFa [tiab] OR “tumor necrosis factor (TNF)-alpha” [tiab] |
| 3 | “C-Reactive Protein” [Mesh] |
| 4 | “C-Reactive Protein” [tiab] OR CRP [tiab] |
| 5 | “Cytokines” [MESH:noexp] |
| 6 | “Interleukins” [MESH:noexp] |
| 7 | cytokines [tiab] OR interleukins [tiab] |
| 8 | “Interleukin-1” [Mesh] |
| 9 | “interleukin-1beta” [tiab] OR “interleukin-1 beta” [tiab] OR “interleukin-1 b” [tiab] OR “interleukin-1b” [tiab] OR “IL-1beta” [tiab] OR “IL-1 beta” [tiab] OR “IL1beta” [tiab] OR “IL1 beta” [tiab] OR “IL-1b” [tiab] OR “IL-1 b” [tiab] OR “IL1b” [tiab] OR “IL1 b” [tiab] OR “interleukin (IL)-1beta” [tiab] OR “interleukin (IL)-1 beta” [tiab] |
| 10 | “Interleukin-4” [Mesh] |
| 11 | “interleukin-4” [tiab] OR “IL-4” [tiab] OR IL4 [tiab] OR “interleukin (IL)-4” [tiab] |
| 12 | “Interleukin-6” [Mesh] |
| 13 | “interleukin-6” [tiab] OR “IL-6” [tiab] OR IL6 [tiab] OR “interleukin (IL)-6” [tiab] |
| 14 | “Interleukin-8” [Mesh] |
| 15 | “interleukin-8” [tiab] OR “IL-8” [tiab] OR IL8 [tiab] OR “interleukin (IL)-8” [tiab] |
| 16 | “Interleukin-10” [Mesh] |
| 17 | “interleukin-10” [tiab] OR “IL-10” [tiab] OR IL10 [tiab] OR “interleukin (IL)-10” [tiab] |
| 18 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 |
| 19 | “exhaled breath condensate *” [tiab] OR EBC [tiab] OR EBCs [tiab] OR “exhaled breath” [tiab] OR “breath condensate *” [tiab] |
| 20 | “Breath Tests” [Mesh] |
| 21 | “Exhalation” [Mesh] |
| 22 | #19 OR #20 OR #21 |
| 23 | #18 AND #22 |
| 24 | “Animals” [Mesh] |
| 25 | “Humans” [Mesh] |
| 26 | #24 NOT #25 |
| 27 | #23 NOT #26 |
| 28 | “Adolescent” [Mesh] |
| 29 | “Child” [Mesh] |
| 30 | “Infant” [Mesh] |
| 31 | #28 OR #29 OR #30 |
| 32 | “Adult” [Mesh] |
| 33 | #31 NOT #32 |
| 34 | #27 NOT #33 |
| Embase | |
| 1 | ‘tumor necrosis factor’/exp |
| 2 | ‘tumor necrosis factor-alpha’:ti,ab,kw OR ‘tumor necrosis factor-a’:ti,ab,kw OR ‘TNF-alpha’:ti,ab,kw OR TNFalpha:ti,ab,kw OR TNF-a:ti,ab,kw OR TNFa:ti,ab,kw OR ‘tumor necrosis factor (TNF)-alpha’:ti,ab,kw |
| 3 | ‘C reactive protein’/exp |
| 4 | ‘C-Reactive Protein’:ti,ab,kw OR CRP:ti,ab,kw |
| 5 | ‘cytokine’/de |
| 6 | ‘interleukin derivative’/de |
| 7 | cytokines:ti,ab,kw OR interleukins:ti,ab,kw |
| 8 | ‘interleukin 1’/exp |
| 9 | ‘interleukin-1beta’:ti,ab,kw OR ‘interleukin-1 beta’:ti,ab,kw OR ‘interleukin-1 b’:ti,ab,kw OR ‘interleukin-1b’:ti,ab,kw OR ‘IL-1beta’:ti,ab,kw OR ‘IL-1 beta’:ti,ab,kw OR ‘IL1beta’:ti,ab,kw OR ‘IL1 beta’:ti,ab,kw OR ‘IL-1b’:ti,ab,kw OR ‘IL-1 b’:ti,ab,kw OR ‘IL1b’:ti,ab,kw OR ‘IL1 b’:ti,ab,kw OR ‘interleukin (IL)-1beta’:ti,ab,kw OR ‘interleukin (IL)-1 beta’:ti,ab,kw |
| 10 | ‘interleukin 4’/exp |
| 11 | ‘interleukin-4′:ti,ab,kw OR ‘IL-4′:ti,ab,kw OR ‘IL4′:ti,ab,kw OR ‘interleukin (IL)-4′:ti,ab,kw |
| 12 | ‘interleukin 6’/exp |
| 13 | ‘interleukin-6′:ti,ab,kw OR ‘IL-6′:ti,ab,kw OR ‘IL6′:ti,ab,kw OR ‘interleukin (IL)-6′:ti,ab,kw |
| 14 | ‘interleukin 8’/exp |
| 15 | ‘interleukin-8′:ti,ab,kw OR ‘IL-8′:ti,ab,kw OR ‘IL8′:ti,ab,kw OR ‘interleukin (IL)-8′:ti,ab,kw |
| 16 | ‘interleukin 10’/exp |
| 17 | ‘interleukin-10′:ti,ab,kw OR ‘IL-10′:ti,ab,kw OR ‘IL10′:ti,ab,kw OR ‘interleukin (IL)-10′:ti,ab,kw |
| 18 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 |
| 19 | ‘exhaled breath condensate’/exp |
| 20 | ‘exhaled breath condensate *’:ti,ab,kw OR EBC:ti,ab,kw OR EBCs:ti,ab,kw OR ‘exhaled breath’:ti,ab,kw OR ‘breath condensate *’:ti,ab,kw |
| 21 | ‘breath analysis’/exp |
| 22 | ‘exhalation’/exp |
| 23 | #19 OR #20 OR #21 OR #22 |
| 24 | #18 AND #23 |
| 25 | ‘animal’/de |
| 26 | ‘animal experiment’/exp |
| 27 | ‘nonhuman’/de |
| 28 | #25 OR #26 OR #27 |
| 29 | ‘human’/de |
| 30 | #28 NOT #29 |
| 31 | #23 NOT #30 |
| 32 | ‘adolescent’/exp |
| 33 | ‘child’/exp |
| 34 | #32 OR #33 |
| 35 | ‘adult’/exp |
| 36 | #34 NOT #35 |
| 37 | #31 NOT #36 |
| Cochrane CENTRAL | |
| #1 | MeSH descriptor: [Tumor Necrosis Factor-alpha] explode all trees |
| #2 | (“tumor necrosis factor-alpha” OR “tumor necrosis factor-a” OR “TNF-alpha” OR TNFalpha OR TNF-a OR TNFa):ti,ab,kw |
| #3 | MeSH descriptor: [C-Reactive Protein] explode all trees |
| #4 | (“C-Reactive Protein” OR CRP):ti,ab,kw |
| #5 | MeSH descriptor: [Cytokines] this term only |
| #6 | MeSH descriptor: [Interleukins] this term only |
| #7 | (cytokines OR interleukins):ti,ab,kw |
| #8 | MeSH descriptor: [Interleukin-1] explode all trees |
| #9 | (“interleukin-1beta” OR “interleukin-1 beta” OR “interleukin-1 b” OR “interleukin-1b” OR “IL-1beta” OR “IL-1 beta” OR “IL1beta” OR “IL1 beta” OR “IL-1b” OR “IL-1 b” OR “IL1b” OR “IL1 b”):ti,ab,kw |
| #10 | MeSH descriptor: [Interleukin-4] explode all trees |
| #11 | (“interleukin-4” OR “IL-4” OR IL4):ti,ab,kw |
| #12 | MeSH descriptor: [Interleukin-6] explode all trees |
| #13 | (“interleukin-6” OR “IL-6” OR IL6):ti,ab,kw |
| #14 | MeSH descriptor: [Interleukin-8] explode all trees |
| #15 | (“interleukin-8” OR “IL-8” OR IL8):ti,ab,kw |
| #16 | MeSH descriptor: [Interleukin-10] explode all trees |
| #17 | (“interleukin-10” OR “IL-10” OR IL10):ti,ab,kw |
| #18 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 |
| #19 | (“exhaled breath condensate *” OR EBC OR EBCs OR “exhaled breath” OR “breath condensate *”):ti,ab,kw |
| #20 | MeSH descriptor: [Breath Tests] explode all trees |
| #21 | MeSH descriptor: [Exhalation] explode all trees |
| #22 | #19 OR #20 OR #21 |
| #23 | #18 AND #22. |
Appendix B
| 1 | Was the type of EBC sampler used specified? |
| 2 | Was the EBC collection temperature between −10 °C and −20 °C? |
| 3 | Was the duration of condensation specified? |
| 4 | Were the ventilation patterns such as the breathing frequencies specified? |
| 5 | Did subjects wear a noseclip? |
| 6 | Was any precaution taken to avoid saliva contamination of EBC samples? |
| 7 | Were samples stored at ≤−70 °C? |
| 8 | Was the storage duration specified? |
| 9 | Have the assay characteristics used for analysis been specified? |
| 10 | Have lower limits of detection (LODs) been given? |
| 11 | Were intra- and inter-variability of the assay specified? |
| 12 | Were appropriate data on recovery in case of sample concentration specified? |
Appendix C
References
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Feghali, C.A.; Wright, T.M. Introduction 3. Discussion 3.1 Cytokines involved in acute inflammation 3.1.1 Interleukin-1 3.1.2 Tumor necrosis factor 3.1.3 Interleukin-6 3.1.4 Interleukin-11 3.1.5 Interleukin-8/chemokines 3.1.6 Eotaxin 3.1.7 Interleukin-16 3.1.8 Interleukin-17 3.1.9 C. Front. Biosci. 1997, 2, 12–26. [Google Scholar]
- Wieseler-Frank, J.; Maier, S.F.; Watkins, L.R. Central proinflammatory cytokines and pain enhancement. Neurosignals 2005, 14, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Zietkowski, Z.; Tomasiak-Lozowska, M.M.; Skiepko, R.; Mroczko, B.; Szmitkowski, M.; Bodzenta-Lukaszyk, A. High-sensitivity C-reactive protein in the exhaled breath condensate and serum in stable and unstable asthma. Respir. Med. 2009, 103, 379–385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Połomska, J.; Bar, K.; Sozańska, B. Exhaled breath condensate—a non-invasive approach for diagnostic methods in asthma. J. Clin. Med. 2021, 10, 2697. [Google Scholar] [CrossRef]
- Jackson, A.S.; Sandrini, A.; Campbell, C.; Chow, S.; Thomas, P.S.; Yates, D.H. Comparison of biomarkers in exhaled breath condensate and bronchoalveolar lavage. Am. J. Respir. Crit. Care Med. 2007, 175, 222–227. [Google Scholar] [CrossRef]
- Tenero, L.; Zaffanello, M.; Piazza, M.; Piacentini, G. Measuring airway inflammation in asthmatic children. Front. Pediatr. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Koczulla, R.; Dragonieri, S.; Schot, R.; Bals, R.; Gauw, S.A.; Vogelmeier, C.; Rabe, K.F.; Sterk, P.J.; Hiemstra, P.S. Comparison of exhaled breath condensate pH using two commercially available devices in healthy controls, asthma and COPD patients. Respir. Res. 2009, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Koutsokera, A.; Loukides, S.; Gourgoulianis, K.I.; Kostikas, K. Biomarkers in the exhaled breath condensate of healthy adults: Mapping the path towards reference values. Curr. Med. Chem. 2008, 15, 620–630. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Lamacchia, C.; Palmer, G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 2010, 6, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Yu, C.; Chen, G.; Lin, J.; Xie, Z.; Xia, T.; Luo, W.; Cai, X.; Liu, S. The inflammatory response induced by relmβ upregulates il-8 and il-1β expression in bronchial epithelial cells in copd. Int. J. COPD 2021, 16, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Perkins, C.; Wills-Karp, M.; Finkelman, F.D. IL-4 induces IL-13-independent allergic airway inflammation. J. Allergy Clin. Immunol. 2006, 118, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Cesta, M.C.; Zippoli, M.; Marsiglia, C.; Gavioli, E.M.; Mantelli, F.; Allegretti, M.; Balk, R.A. The Role of Interleukin-8 in Lung Inflammation and Injury: Implications for the Management of COVID-19 and Hyperinflammatory Acute Respiratory Distress Syndrome. Front. Pharmacol. 2022, 12, 1–7. [Google Scholar] [CrossRef]
- Ogawa, Y.; Duru, E.A.; Ameredes, B.T. Role of IL-10 in the resolution of airway inflammation. Curr. Mol. Med. 2008, 8, 437–445. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Hoidal, J.R.; Mukherjee, T.K. Role of TNFα in pulmonary pathophysiology. Respir. Res. 2006, 7, 1–9. [Google Scholar] [CrossRef]
- Horváth, I.; Hunt, J.; Barnes, P.J.; Alving, K.; Antczak, A.; Baraldi, E.; Becher, G.; van Beurden, W.J.C.; Corradi, M.; Dekhuijzen, R.; et al. Exhaled breath condensate: Methodological recommendations and unresolved questions. Eur. Respir. J. 2005, 26, 523–548. [Google Scholar] [CrossRef]
- Horváth, I.; Barnes, P.J.; Loukides, S.; Sterk, P.J.; Högman, M.; Olin, A.C.; Amann, A.; Antus, B.; Baraldi, E.; Bikov, A.; et al. A European Respiratory Society technical standard: Exhaled biomarkers in lung disease. Eur. Respir. J. 2017, 49, 1600965. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, B.; Suvarna, A.; Phillips, R.; Juster, R.P.; McDermott, B.; Sarnyai, Z. Health risk behaviours and allostatic load: A systematic review. Neurosci. Biobehav. Rev. 2020, 108, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 1–13. [Google Scholar] [CrossRef]
- Aquino-Santos, H.C.; Tavares-Vasconcelos, J.S.; Brandão-Rangel, M.A.R.; Araújo-Rosa, A.C.; Morais-Felix, R.T.; Oliveira-Freitas, S.; Santa-Rosa, F.A.; Oliveira, L.V.F.; Bachi, A.L.L.; Alves, T.G.G.; et al. Chronic alteration of circadian rhythm is related to impaired lung function and immune response. Int. J. Clin. Pract. 2020, 74, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ausin, P.; Martinez-Llorens, J.; Sabate-Bresco, M.; Casadevall, C.; Barreiro, E.; Gea, J. Sex differences in function and structure of the quadriceps muscle in chronic obstructive pulmonary disease patients. Chron. Respir. Dis. 2017, 14, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Brandao-Rangel, M.A.R.; Moraes-Ferreira, R.; Oliveira-Junior, M.C.; Santos-Dias, A.; Bachi, A.L.L.; Gabriela-Pereira, G.; de Oliveira Freitas, S.; Araújo-Rosa, A.C.; Oliveira, L.V.F.; Frison, C.R.; et al. Pulmonary function changes in older adults with and without metabolic syndrome. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Kharitonov, S.A.; Foschino-Barbaro, M.P.; Resta, O.; Gramiccioni, E.; Barnes, P.J. Increase inflammatory markers in the exhaled breath condensate of cigarette smokers. Eur. Respir. J. 2003, 21, 589–593. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Kharitonov, S.A.; Foschino-Barbaro, M.P.; Resta, O.; Gramiccioni, E.; Barnes, P.J. Supplementary oxygen in healthy subjects and those with COPD increases oxidative stress and airway inflammation. Thorax 2004, 59, 1016–1019. [Google Scholar] [CrossRef][Green Version]
- Carpagnano, G.E.; Resta, O.; Foschino-Barbaro, M.P.; Spanevello, A.; Stefano, A.; Di Gioia, G.; Serviddio, G.; Gramiccioni, E. Exhaled Interleukine-6 and 8-isoprostane in chronic obstructive pulmonary disease: Effect of carbocysteine lysine salt monohydrate (SCMC-Lys). Eur. J. Pharmacol. 2004, 505, 169–175. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Foschino Barbaro, M.P.; Resta, O.; Gramiccioni, E.; Valerio, N.V.; Bracciale, P.; Valerio, G. Exhaled markers in the monitoring of airways inflammation and its response to steroid’s treatment in mild persistent asthma. Eur. J. Pharmacol. 2005, 519, 175–181. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Barbaro, M.P.F.; Cagnazzo, M.; Di Gioia, G.; Giliberti, T.; Di Matteo, C.; Resta, O. Use of exhaled breath condensate in the study of airway inflammation after hypertonic saline solution challenge. Chest 2005, 128, 3159–3166. [Google Scholar] [CrossRef] [PubMed]
- Carpagnano, G.E.; Resta, O.; Ventura, M.T.; Amoruso, A.C.; Di Gioia, G.; Giliberti, T.; Refolo, L.; Foschino-Barbaro, M.P. Airway inflammation in subjects with gastro-oesophageal reflux and gastro-oesophageal reflux-related asthma. J. Intern. Med. 2006, 259, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Carpagnano, G.E.; Resta, O.; Gelardi, M.; Spanevello, A.; Di Gioia, G.; Giliberti, T.; Depalo, A.; Barbaro, M.P.F. Exhaled inflammatory markers in aspirin-induced asthma syndrome. Am. J. Rhinol. 2007, 21, 542–547. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Carratú, P.; Gelardi, M.; Spanevello, A.; Di Gioia, G.; Condreva, T.; Resta, O.; Barbaro, M.P.F. Increased IL-6 and IL-4 in exhaled breath condensate of patients with nasal polyposis. Monaldi Arch. Chest Dis.-Pulm. Ser. 2009, 71, 3–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carpagnano, G.E.; Spanevello, A.; Sabato, R.; Depalo, A.; Palladino, G.P.; Bergantino, L.; Foschino Barbaro, M.P. Systemic and airway inflammation in sleep apnea and obesity: The role of ICAM-1 and IL-8. Transl. Res. 2010, 155, 35–43. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Turchiarelli, V.; Spanevello, A.; Palladino, G.P.; Barbaro, M.P.F. Aging and airway inflammation. Aging Clin. Exp. Res. 2013, 25, 239–245. [Google Scholar] [CrossRef]
- De Lima, T.M.; Kazama, C.M.; Koczulla, A.R.; Hiemstra, P.S.; Macchione, M.; Godoy Fernandes, A.L.; de Santos, U.P.; Bueno-Garcia, M.L.; Zanetta, D.M.; Saldiva de André, C.D.; et al. PH in exhaled breath condensate and nasal lavage as a biomarker of air pollution-related inflammation in street traffic-controllers and office-workers. Clinics 2013, 68, 1488–1494. [Google Scholar] [CrossRef]
- Diez-Pina, J.M.; Fernandez-Aceñero, M.J.; Llorente-Alonso, M.J.; Diaz-Lobato, S.; Mayoralas, S.; Florez, A. Tumor necrosis factor alpha as a marker of systemic and local inflammation in “healthy” smokers. Int. J. Gen. Med. 2009, 2, 9–14. [Google Scholar] [CrossRef]
- Edmé, J.L.; Tellart, A.S.; Launay, D.; Neviere, R.; Grutzmacher, C.; Boulenguez, C.; Labalette, M.; Hachulla, E.; Hatron, P.Y.; Dessaint, J.P.; et al. Cytokine concentrations in exhaled breath condensates in systemic sclerosis. Inflamm. Res. 2008, 57, 151–156. [Google Scholar] [CrossRef]
- Font-Ribera, L.; Kogevinas, M.; Zock, J.P.; Gómez, F.P.; Barreiro, E.; Nieuwenhuijsen, M.J.; Fernandez, P.; Lourencetti, C.; Pérez-Olabarría, M.; Bustamante, M.; et al. Short-term changes in respiratory biomarkers after swimming in a chlorinated pool. Environ. Health Perspect. 2010, 118, 1538–1544. [Google Scholar] [CrossRef]
- Garey, K.W.; Neuhauser, M.M.; Robbins, R.A.; Danziger, L.H.; Rubinstein, I. Markers of Inflammation in Exhaled Breath Condensate of Young Healthy Smokers. Chest 2004, 125, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Gessner, C.; Scheibe, R.; Wötzel, M.; Hammerschmidt, S.; Kuhn, H.; Engelmann, L.; Hoheisel, G.; Gillissen, A.; Sack, U.; Wirtz, H. Exhaled breath condensate cytokine patterns in chronic obstructive pulmonary disease. Respir. Med. 2005, 99, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Gessner, C.; Hammerschmidt, S.; Kuhn, H.; Hoheisel, G.; Gillissen, A.; Sack, U.; Wirtz, H. Breath condensate nitrite correlates with hyperinflation in chronic obstructive pulmonary disease. Respir. Med. 2007, 101, 2271–2278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guan, T.; Hu, S.; Han, Y.; Wang, R.; Zhu, Q.; Hu, Y.; Fan, H.; Zhu, T. The effects of facemasks on airway inflammation and endothelial dysfunction in healthy young adults: A double-blind, randomized, controlled crossover study. Part. Fibre Toxicol. 2018, 15, 1–12. [Google Scholar] [CrossRef]
- Ko, F.W.S.; Leung, T.F.; Wong, G.W.K.; Ngai, J.; To, K.W.; Ng, S.; Hui, D.S.C. Measurement of tumor necrosis factor-α, leukotriene B4, and interleukin 8 in the exhaled breath condensate in patients with acute exacerbations of chronic obstructive pulmonary disease. Int. J. COPD 2009, 4, 79–86. [Google Scholar] [CrossRef]
- Liu, H.C.; Lu, M.C.; Lin, Y.C.; Wu, T.C.; Hsu, J.Y.; Jan, M.S.; Chen, C.M. Differences in IL-8 in serum and exhaled breath condensate from patients with exacerbated COPD or asthma attacks. J. Formos. Med. Assoc. 2014, 113, 908–914. [Google Scholar] [CrossRef]
- Matsunaga, K.; Yanagisawa, S.; Ichikawa, T.; Ueshima, K.; Akamatsu, K.; Hirano, T.; Nakanishi, M.; Yamagata, T.; Minakata, Y.; Ichinose, M. Airway cytokine expression measured by means of protein array in exhaled breath condensate: Correlation with physiologic properties in asthmatic patients. J. Allergy Clin. Immunol. 2006, 118, 84–90. [Google Scholar] [CrossRef]
- Mazur, W.; Stark, H.; Sovijärvi, A.; Myllärniemi, M.; Kinnula, V.L. Comparison of 8-isoprostane and interleukin-8 in induced sputum and exhaled breath condensate from asymptomatic and symptomatic smokers. Respiration 2009, 78, 209–216. [Google Scholar] [CrossRef]
- Nielepkowicz-Goździńska, A.; Fendler, W.; Robak, E.; Kulczycka-Siennicka, L.; Gorski, P.; Pietras, T.; Brzeziańska, E.; Antczak, A. Exhaled cytokines in systemic lupus erythematosus with lung involvement. Pol. Arch. Med. Wewn. 2013, 123, 141–148. [Google Scholar] [CrossRef]
- Nielepkowicz-Goździńska, A.; Fendler, W.; Robak, E.; Kulczycka-Siennicka, L.; Górski, P.; Pietras, T.; Brzeziańska, E.; Antczak, A. Exhaled IL-8 in systemic lupus erythematosus with and without pulmonary fibrosis. Arch. Immunol. Ther. Exp. (Warsz) 2014, 62, 231–238. [Google Scholar] [CrossRef]
- Radulovic, M.; Bauman, W.A.; Wecht, J.M.; LaFountaine, M.; Kahn, N.; Hobson, J.; Singh, K.; Renzi, C.; Yen, C.; Schilero, G.J. Biomarkers of inflammation in persons with chronic tetraplegia. J. Breath Res. 2015, 9, 36001. [Google Scholar] [CrossRef] [PubMed]
- Rolla, G.; Fusaro, E.; Nicola, S.; Bucca, C.; Peroni, C.; Parisi, S.; Cassinis, M.C.; Ferraris, A.; Angelino, F.; Heffler, E.; et al. Th-17 cytokines and interstitial lung involvement in systemic sclerosis. J. Breath Res. 2016, 10, 46013. [Google Scholar] [CrossRef] [PubMed]
- Sack, U.; Scheibe, R.; Wötzel, M.; Hammerschmidt, S.; Kuhn, H.; Emmrich, F.; Hoheisel, G.; Wirtz, H.; Gessner, C. Multiplex analysis of cytokines in exhaled breath condensate. Cytom. Part A 2006, 69, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Svedahl, S.R.; Svendsen, K.; Tufvesson, E.; Romundstad, P.R.; Sjaastad, A.K.; Qvenild, T.; Hilt, B. Inflammatory markers in blood and exhaled air after short-term exposure to cooking fumes. Ann. Occup. Hyg. 2013, 57, 230–239. [Google Scholar] [CrossRef]
- Tufvesson, E.; Bjermer, L. Methodological improvements for measuring eicosanoids and cytokines in exhaled breath condensate. Respir. Med. 2006, 100, 34–38. [Google Scholar] [CrossRef]
- Vergara, D.; Ávila, D.; Escobar, E.; Carrasco-Pozo, C.; Sánchez, A.; Gotteland, M. The intake of maqui (Aristotelia chilensis) berry extract normalizes H2O2 and IL-6 concentrations in exhaled breath condensate from healthy smokers—An explorative study. Nutr. J. 2015, 14, 1–5. [Google Scholar] [CrossRef]
- Yan, F.; Pidayi, M.; Xia, Y.; Hu, X.; Yang, Z. The prognosis value of C-reactive protein and endothelin-1 in chronic obstructive pulmonary disease patients with pulmonary artery pressure. Pak. J. Pharm. Sci. 2019, 32, 1697–1701. [Google Scholar]
- Zietkowski, Z.; Skiepko, R.; Tomasiak-Lozowska, M.M.; Mroczko, B.; Szmitkowski, M.; Bodzenta-Lukaszyk, A. Changes in high-sensitivity C-reactive protein in serum and exhaled breath condensate after intensive exercise in patients with allergic asthma. Int. Arch. Allergy Immunol. 2010, 153, 75–85. [Google Scholar] [CrossRef]
- Zammit, C.; Liddicoat, H.; Moonsie, I.; Makker, H. Obesity and respiratory diseases. Am. J. Clin. Hypn. 2011, 53, 335–343. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, D.; Wu, X.; Zhang, X.; Zhou, Q.; Luo, Y.; Yang, X.; Chock, C.J.; Liu, M.; Yang, X.O. Leptin Promotes Allergic Airway Inflammation through Targeting the Unfolded Protein Response Pathway. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Habib, A.R.; Kalish, L.; Alvarado, R.; Campbell, R.; Grayson, J.; Sacks, R.; Harvey, R.J. The association between body size and chronic upper airway disorders. Aust. J. Otolaryngol. 2021, 4, 1–11. [Google Scholar] [CrossRef]
- Vezir, E.; Civelek, E.; Dibek Misirlioglu, E.; Toyran, M.; Capanoglu, M.; Karakus, E.; Kahraman, T.; Ozguner, M.; Demirel, F.; Gursel, I.; et al. Effects of Obesity on Airway and Systemic Inflammation in Asthmatic Children. Int. Arch. Allergy Immunol. 2021, 182, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Busse, P.J.; Mathur, S.K. Age-related changes in immune function: Effect on airway inflammation. J. Allergy Clin. Immunol. 2010, 126, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.; Yates, D.H.; Thomas, P.S. Reproducibility of exhaled breath condensate markers. Eur. Respir. J. 2008, 32, 1124–1126. [Google Scholar] [CrossRef]
- Ahmadzai, H.; Huang, S.; Hettiarachchi, R.; Lin, J.L.; Thomas, P.S.; Zhang, Q. Exhaled breath condensate: A comprehensive update. Clin. Chem. Lab. Med. 2013, 51, 1343–1361. [Google Scholar] [CrossRef]
- Montuschi, P. Review: Analysis of exhaled breath condensate in respiratory medicine: Methodological aspects and potential clinical applications. Ther. Adv. Respir. Dis. 2007, 1, 5–23. [Google Scholar] [CrossRef]
- De Jager, W.; Bourcier, K.; Rijkers, G.T.; Prakken, B.J.; Seyfert-Margolis, V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009, 10, 52. [Google Scholar] [CrossRef]
- Grob, N.M.; Aytekin, M.; Dweik, R.A. Biomarkers in exhaled breath condensate: A review of collection, processing and analysis. J. Breath Res. 2008, 2, 37004. [Google Scholar] [CrossRef]

| Biomarkers | Role | Description |
|---|---|---|
| CRP | Pro-inflammatory | Detection of bacteria and damaged human cells and complement activation. Circulating concentration rises in response to infection and is associated with risk of coronary heart disease [6]. |
| IL-1β | Pro-inflammatory | Response to exogenous and endogenous noxious stimuli and induction of IL-6 and IL-8 secretion by bronchial epithelial cells [14,15]. |
| IL-4 | Anti-inflammatory | Response to allergic airway inflammation [16]. |
| IL-6 | Pro-inflammatory | Response to several stimuli, including exercise, allergens, and respiratory viruses [5]. |
| IL-8 | Pro-inflammatory | Neutrophil recruitment with an important role in pathological and physiological conditions [15,17]. |
| IL-10 | Anti-inflammatory | Immune-suppressive cytokine, which reduces the recruitment of effector T cells and counteracts the effects of TNF-α and IL-1β Response to allergic challenge [18]. |
| TNF-α | Pro-inflammatory | Pleiotropic immune activator, involved in many airway disorders [19]. |
| Biomarker | n° of Studies | n° of Studies (%) with Data > LOD | n° of Studies (%) with Data < LOD | n° of Studies (%) without LOD Declared |
|---|---|---|---|---|
| CRP | 3 | 2 (66.7%) | - | 1 (33.3%) |
| IL-1β | 12 | 2 (16.7%) | 5 (41.7%) | 5 (41.7%) |
| IL-4 | 11 | 6 (54.5%) | 2 (18.2%) | 3 (27.3%) |
| IL-6 | 19 | 11 (57.9%) | 2 (10.5%) | 6 (31.6%) |
| IL-8 | 16 | 5 (31.3%) | 4 (25.0%) | 7 (43.8%) |
| IL-10 | 12 | 2 (16.7%) | 2 (16.7%) | 8 (66.7%) |
| TNF-α | 18 | 6 (33.3%) | 3 (16.7%) | 9 (50.0%) |
| Authors, Year | Country | n° Subjects (M;F) | Age | Collection Device | Collection Temperature | Storage Temperature | Analytical Method | Data | LOD | SCORE Quality Assessment JBI | SCORE Authors’ Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CRP | |||||||||||
| Zietkowski et al., 2009 [7] | Poland | 15 (6;9) | 33.13 (6.71) † | EcoScreen; Eric Jaeger GmbH, Hoechberg, Germany | 0 °C | −80 °C | highly sensitive CRP assay (Konelab, Waltham, MA, USA) | 0.08 ± 0.03 mg/L | 0.05 mg/L | 77.78 High | 45.45 Low |
| Zietkowski et al., 2010 [58] | Poland | 8 (4;4) | 29.9 (7.1) † | EcoScreen; Eric Jaeger GmbH, Hoechberg, Germany | 0 °C | −80 °C | highly sensitive CRP assay (Konelab, Waltham, MA, USA) | 0.07 ± 0.03 mg/L | 0.02 mg/L | 88.89 High | 72.73 High |
| IL-1β | |||||||||||
| Guan et al., 2018 [44] | China | 15 (7;8) | 20 ± 1 | ECOScreen (Jager, Germany) | NA | −80 °C | BD Cytometric Bead Array, BD-Biosciences, San Jose, CA, USA | 3.71 (2.31) pg/mL | 2.4 pg/mL | 84.62 High | 54.55 Medium |
| Guan et al., 2018 [44] | China | 15 (7;8) | 20 ± 1 | ECOScreen (Jager, Germany) | NA | −80 °C | BD Cytometric Bead Array, BD-Biosciences, San Jose, CA, USA | 3.34 (2.26) pg/mL | 2.4 pg/mL | 84.62 High | 54.55 Medium |
| Svedahl et al., 2013 [54] | Norway | 24 (14;10) | 23.8 ± 2.5 | ECoScreen; Jager, Wurzburg, Germany | NA | −70 °C | Quantikine HS from R&D Systems (Minneapolis, MN, USA) | 0.84; CI= 0.64–1.10 pg/mL | 0.05 pg/mL | 77.78 High | 63.64 Medium |
| IL-4 | |||||||||||
| Carpagnano et al., 2005_a [30] | Italy | 15 (5;10) | 35 ± 6 | EcoScreen (Jaeger, Wurzburg, Germany) | −20 °C | −70 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 31.7 ± 3.5 pg/mL | 20 pg/mL | 77.78 High | 90.91 High |
| Carpagnano et al., 2006 [32] | Italy | 17 (8;9) | 37 ± 9 | EcoScreen (Jaeger, Wurzburg, Germany) | On ice | −70 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 31.6 (27.5–39.7)pg/mL | 20 pg/mL | 50.00 Medium | 63.64 Medium |
| Carpagnano et al., 2007 [33] | Italy | 10 (5;5) | 44 ± 8 | EcoScreen (Jaeger, Wurzburg, Germany) | NA | −70 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 40.8 ± 1.7 pg/mL | 15 pg/mL | 75.00 High | 54.55 Medium |
| Carpagnano et al., 2009 [34] | Italy | 10 (-;-) | 43 ± 9 | EcoScreen (Jaeger, Wurzburg, Germany) | −20 °C | −80 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 35.8 ± 1.1 pg/mL | 20 pg/mL | 85.71 High | 63.64 Medium |
| Edmè et al., 2008 * [39] | France | 19 (-;-) | 38.3 ± 13.6 | EcoScreen (Jaeger, Wurzburg, Germany) | NA | −80 °C | Cytometric Bead Arrays (CBA) Becton Dickinson, San Jose, CA | 32.1 (23 76) † pg/mL | 5 pg/mL | 66.67 Medium | 66.67 Medium |
| Matsunaga et al., 2006 [47] | Japan | 10 (3;7) | 34.4 ± 6.6 | EcoScreen, (Jaeger, Germany) | −20 °C | −70 °C | Human Inflammation Antibody III (ray Biontec Inc, Norcross, GA, USA) | 5.2 ± 1.7 pg/mL | 1pg/mL | 57.14 Medium | 72.73 Medium |
| IL-6 | |||||||||||
| Carpagnano et al., 2003 [27] | Italy | 14 (8;6) | 45 ± 6 | EcoScreen (Jaeger, Wurzburg, Germany) | −20 °C | −70 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 2.6 ± 0.2 pg/mL | 1.5 pg/mL | 87.50 High | 81.82 High |
| Carpagnano et al., 2004_a [28] | Italy | 18(5;13) | 46 ± 6 | EcoScreen (Jaeger, Wurzburg, Germany) | On ice | −70 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 2.9 ± 0.6 pg/mL | 1.5 pg/mL | 77.78 High | 81.82 High |
| Carpagnano et al., 2004_a [28] | Italy | 5 (2;3) | 47 ± 3 | EcoScreen (Jaeger, Wurzburg, Germany) | On ice | −70 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 3.1 ± 0.6 pg/mL | 1.5 pg/mL | 77.78 High | 81.82 High |
| Carpagnano et al., 2004_b [29] | Italy | 15 (8;7) | 48 ± 7 | EcoScreen (Jaeger, Wurzburg, Germany) | −20 °C | −70 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 2.7 ± 0.6 pg/mL | 1.5 pg/mL | 62.50 Medium | 54.55 Medium |
| Carpagnano et al., 2005_a [30] | Italy | 15 (5;10) | 35 ± 6 | EcoScreen (Jaeger, Wurzburg, Germany) | −20 °C | −70 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 2.7 ± 0.6 pg/mL | 1.5 pg/mL | 77.78 High | 90.91 High |
| Carpagnano et al., 2005_b [31] | Italy | 7 (5;2) | 42 ± 5 | EcoScreen (Jaeger, Wurzburg, Germany) | −20 °C | −70 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 3.1 ± 0.7 pg/mL | 1.5 pg/mL | 77.78 High | 90.91 High |
| Carpagnano et al., 2006 [32] | Italy | 17 (8;9) | 37 ± 9 | EcoScreen (Jaeger, Wurzburg, Germany) | On ice | −70 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 2.6 (1.9-4.0) pg/mL | 1.5 pg/mL | 50.00 Medium | 63.64 Medium |
| Carpagnano et al., 2007 [33] | Italy | 10 (5;5) | 44 ± 8 | EcoScreen (Jaeger, Wurzburg, Germany) | NA | −70 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 2.8 ± 0.1 pg/mL | 1.5 pg/mL | 75.00 High | 54.55 Medium |
| Carpagnano et al., 2009 [34] | Italy | 10 (-;-) | 43 ± 9 | EcoScreen (Jaeger, Wurzburg, Germany) | −20 °C | −80 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 2.8 ± 0.1 pg/mL | 1.5 pg/mL | 85.71 High | 63.64 Medium |
| Edmè et al., 2008 * [39] | France | 19 (-;-) | 38.3 ± 13.6 | EcoScreen (Jaeger, Wurzburg, Germany) | NA | −80 °C | Cytometric Bead Arrays (CBA) Becton pg/mL Dickinson, San Jose, CA, USA | 111.7 (70-362) † pg/mL | 5 pg/mL | 66.67 Medium | 66.67 Medium |
| Guan et al., 2018 [44] | China | 15 (7;8) | 20 ± 1 | ECOScreen (Jager, Germany) | NA | −80 °C | BD Cytometric Bead Array, BD-Biosciences, San Jose, CA, USA | 3.09 (3.08) pg/mL | 2.4 pg/mL | 84.62 High | 54.55 Medium |
| Guan et al., 2018 [44] | China | 15 (7;8) | 20 ± 1 | ECOScreen (Jager, Germany) | NA | −80 °C | BD Cytometric Bead Array, BD-Biosciences, San Jose, CA, USA | 3.08 (2.03) pg/mL | 2.4 pg/mL | 84.62 High | 54.55 Medium |
| Matsunaga et al., 2006 [47] | Japan | 10 (3;7) | 34.4 ± 6.6 | EcoScreen, (Jaeger, Germany) | −20 °C | −70 °C | Human Inflammation Antibody III (ray Biontec Inc, Norcross, GA, USA) | 5.2 ± 1.2 pg/mL | 1 pg/mL | 57.14 Medium | 72.73 Medium |
| IL-8 | |||||||||||
| Carpagnano et al., 2010 [35] | Italy | 8 (5;3) | 42 ± 4 | EcoScreen (Jaeger, Wurzburg, Germany) | −20 °C | −70 °C | EIA kit (Human Interleukin-8, Bender med-Systems, Vienna, Austria) | 7.6 ± 0.5 pg/mL | 1.3 pg/mL | 85.71 High | 90.91 High |
| Carpagnano et al., 2013 [36] | Italy | 10 (5;5) | 26 ± 4.9 | EcoScreen (Jaeger, Wurzburg, Germany) | −20 °C | −70 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 7.9 ± 1.0 pg/mL | 1.5 pg/mL | 71.43 Medium | 90.91 High |
| Carpagnano et al., 2013 [36] | Italy | 10 (4;6) | 52 ± 5.9 | EcoScreen (Jaeger, Wurzburg, Germany) | −20 °C | −70 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 15.2 ± 1.9 pg/mL | 1.5 pg/mL | 71.43 Medium | 90.91 High |
| Carpagnano et al., 2013 [36] | Italy | 10 (5;5) | 67 ± 4.6 | EcoScreen (Jaeger, Wurzburg, Germany) | −20 °C | −70 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 16.3 ± 1.4 pg/mL | 1.5 pg/mL | 71.43 Medium | 90.91 High |
| De lima et al., 2013 [37] | Brazil | 73 (73;0) | 42 ± 7 | EcoScreen (Jaeger, Wurzburg, Germany) | NA | −80 °C | High sensitivity enzyme-immunoassays (Quantikine HS, R&D Systems Inc. Minneapolis, MN, USA) | 8.9 ± 1.8 pg/mL | 3.50 pg/mL | 85.71 High | 81.82 High |
| De lima et al., 2013 [37] | Brazil | 14 (14;0) | 30 ± 5 | EcoScreen (Jaeger, Wurzburg, Germany) | NA | −80 °C | High sensitivity enzyme-immunoassays (Quantikine HS, R&D Systems Inc. Minneapolis, MN, USA) | 8.4 ± 0.9 pg/mL | 3.50 pg/mL | 85.71 High | 81.82 High |
| Guan et al., 2018 [44] | China | 15 (7;8) | 20 ± 1 | ECOScreen (Jager, Germany) | NA | −80 °C | BD Cytometric Bead Array, BD-Biosciences, San Jose, CA, USA | 3.58 (1.95) pg/mL | 2.4 pg/mL | 84.62 High | 54.55 Medium |
| Guan et al., 2018 [44] | China | 15 (7;8) | 20 ± 1 | ECOScreen (Jager, Germany) | NA | −80 °C | BD Cytometric Bead Array, BD-Biosciences, San Jose, CA, USA | 3.15 (1.95) pg/mL | 2.4 pg/mL | 84.62 High | 54.55 Medium |
| Matsunaga et al., 2006 [47] | Japan | 10 (3;7) | 34.4 ± 6.6 | EcoScreen, (Jaeger, Germany) | −20 °C | −70 °C | Human Inflammation Antibody III (ray Biontec Inc, Norcross, GA, USA) | 5.4 ± 1.8 pg/mL | 1 pg/mL | 57.14 Medium | 72.73 Medium |
| IL-10 | |||||||||||
| De lima et al., 2013 [37] | Brazil | 14 (14;0) | 30 ± 5 | EcoScreen (Jaeger, Wurzburg, Germany) | NA | −80 °C | High sensitivity enzyme-immunoassays (Quantikine HS, R&D Systems Inc. Minneapolis, MN, USA) | 1.0 (1.4) pg/mL | 0.50 pg/mL | 85.71 High | 81.82 High |
| De lima et al., 2013 [37] | Brazil | 73 (73;0) | 42 ± 7 | EcoScreen (Jaeger, Wurzburg, Germany) | NA | −80 °C | High sensitivity enzyme-immunoassays (Quantikine HS, R&D Systems Inc. Minneapolis, MN, USA) | 1.2 (1.6) pg/mL | 0.5 pg/mL | 85.71 High | 81.82 High |
| Edmè et al., 2008 * [39] | France | 19 (-;-) | 38.3 ± 13.6 | EcoScreen (Jaeger, Wurzburg, Germany) | NA | −80 °C | Cytometric Bead Arrays (CBA) Becton Dickinson, San Jose, CA, USA | 24.3 (13-492) † pg/mL | 5 pg/mL | 66.67 Medium | 66.67 Medium |
| TNF-α | |||||||||||
| Carpagnano et al., 2005_b [31] | Italy | 7 (5;2) | 42 ± 5 | EcoScreen (Jaeger, Wurzburg, Germany) | −20 °C | −70 °C | EIA (Cayman Chemical, Ann Arbor, MI, USA) | 4.2 ± 0.6 pg/mL | 1.5 pg/mL | 77.78 High | 90.91 High |
| De lima et al., 2013 [37] | Brazil | 14 (14;0) | 30 ± 5 | EcoScreen (Jaeger, Wurzburg, Germany) | NA | −80 °C | High sensitivity enzyme-immunoassays (Quantikine HS, R&D Systems Inc. Minneapolis, MN, USA) | 0.4 (0.2) pg/mL | 0.20 pg/mL | 85.71 High | 81.82 High |
| De lima et al., 2013 [37] | Brazil | 73 (73;0) | 42 ± 7 | EcoScreen (Jaeger, Wurzburg, Germany) | NA | −80 °C | High sensitivity enzyme-immunoassays (Quantikine HS, R&D Systems Inc. Minneapolis, MN, USA) | 0.5 (0.4) pg/mL | 0.106 pg/mL | 85.71 High | 81.82 High |
| Edmè et al., 2008 * [39] | France | 19 (-;-) | 38.3 ± 13.6 | EcoScreen (Jaeger, Wurzburg, Germany) | NA | −80 °C | Cytometric Bead Arrays (CBA) Becton Dickinson, San Jose, CA, USA | 44.6 (32-91) † pg/mL | 5 pg/mL | 66.67 Medium | 66.67 Medium |
| Garey et al., 2004 [41] | USA | 9 (5;4) | 22.0 ± 1.9 | Breath condensate was collected using a novel method where the subject inspires repeatedly to TLC and exhales into 1.5 m Teflon perfluoroalkoxy (PFA) tubing with 0.5 cm internal diameter | Immersed in ice | −70 °C | ELISA (R&D System Minneapolis, MN) | 3.9 ± 8.5 pg/mL | 2 pg/mL | 71.43 Medium | 54.55 Medium |
| Guan et al., 2018 [44] | China | 15 (7;8) | 20 ± 1 | ECOScreen (Jager, Germany) | NA | −80 °C | BD Cytometric Bead Array, BD-Biosciences, San Jose, CA, USA | 4.36 (1.79) pg/mL | 2.4 pg/mL | 84.62 High | 54.55 Medium |
| Guan et al., 2018 [44] | China | 15 (7;8) | 20 ± 1 | ECOScreen (Jager, Germany) | NA | −80 °C | BD Cytometric Bead Array, BD-Biosciences, San Jose, CA, USA | 4.14 (2.56) pg/mL | 2.4 pg/mL | 84.62 High | 54.55 Medium |
| Ko et al., 2009 [45] | China | 14 (9;5) | 75.2 ± 4.1 | EcoScreen (VIASYS Healthcare, Conshohochen, PA, USA) | NA | −70 °C | BioSource International, Camarillo, CA, USA | 4.84 (3.86-5.81) pg/mL | 0.09 pg/mL | 71.43 Medium | 81.82 High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghelli, F.; Panizzolo, M.; Garzaro, G.; Squillacioti, G.; Bellisario, V.; Colombi, N.; Bergamaschi, E.; Guseva Canu, I.; Bono, R. Inflammatory Biomarkers in Exhaled Breath Condensate: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 9820. https://doi.org/10.3390/ijms23179820
Ghelli F, Panizzolo M, Garzaro G, Squillacioti G, Bellisario V, Colombi N, Bergamaschi E, Guseva Canu I, Bono R. Inflammatory Biomarkers in Exhaled Breath Condensate: A Systematic Review. International Journal of Molecular Sciences. 2022; 23(17):9820. https://doi.org/10.3390/ijms23179820
Chicago/Turabian StyleGhelli, Federica, Marco Panizzolo, Giacomo Garzaro, Giulia Squillacioti, Valeria Bellisario, Nicoletta Colombi, Enrico Bergamaschi, Irina Guseva Canu, and Roberto Bono. 2022. "Inflammatory Biomarkers in Exhaled Breath Condensate: A Systematic Review" International Journal of Molecular Sciences 23, no. 17: 9820. https://doi.org/10.3390/ijms23179820
APA StyleGhelli, F., Panizzolo, M., Garzaro, G., Squillacioti, G., Bellisario, V., Colombi, N., Bergamaschi, E., Guseva Canu, I., & Bono, R. (2022). Inflammatory Biomarkers in Exhaled Breath Condensate: A Systematic Review. International Journal of Molecular Sciences, 23(17), 9820. https://doi.org/10.3390/ijms23179820








