Using the Maize Nested Association Mapping (NAM) Population to Partition Arbuscular Mycorrhizal Effects on Drought Stress Tolerance into Hormonal and Hydraulic Components
Abstract
1. Introduction
2. Results
2.1. Experiment 1
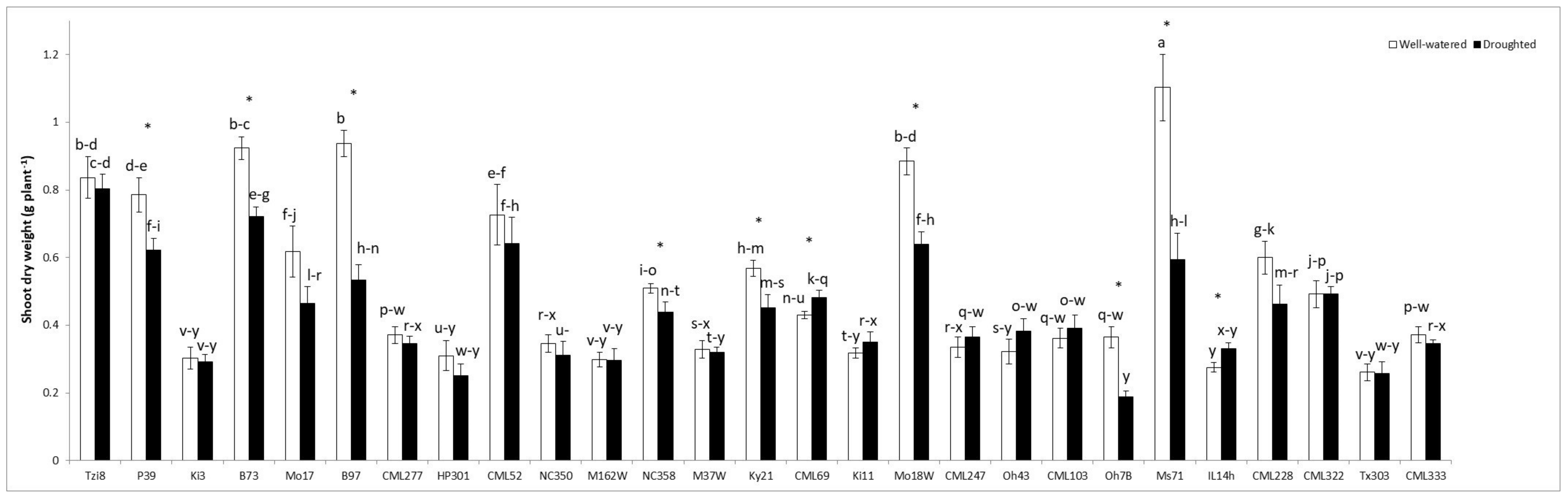
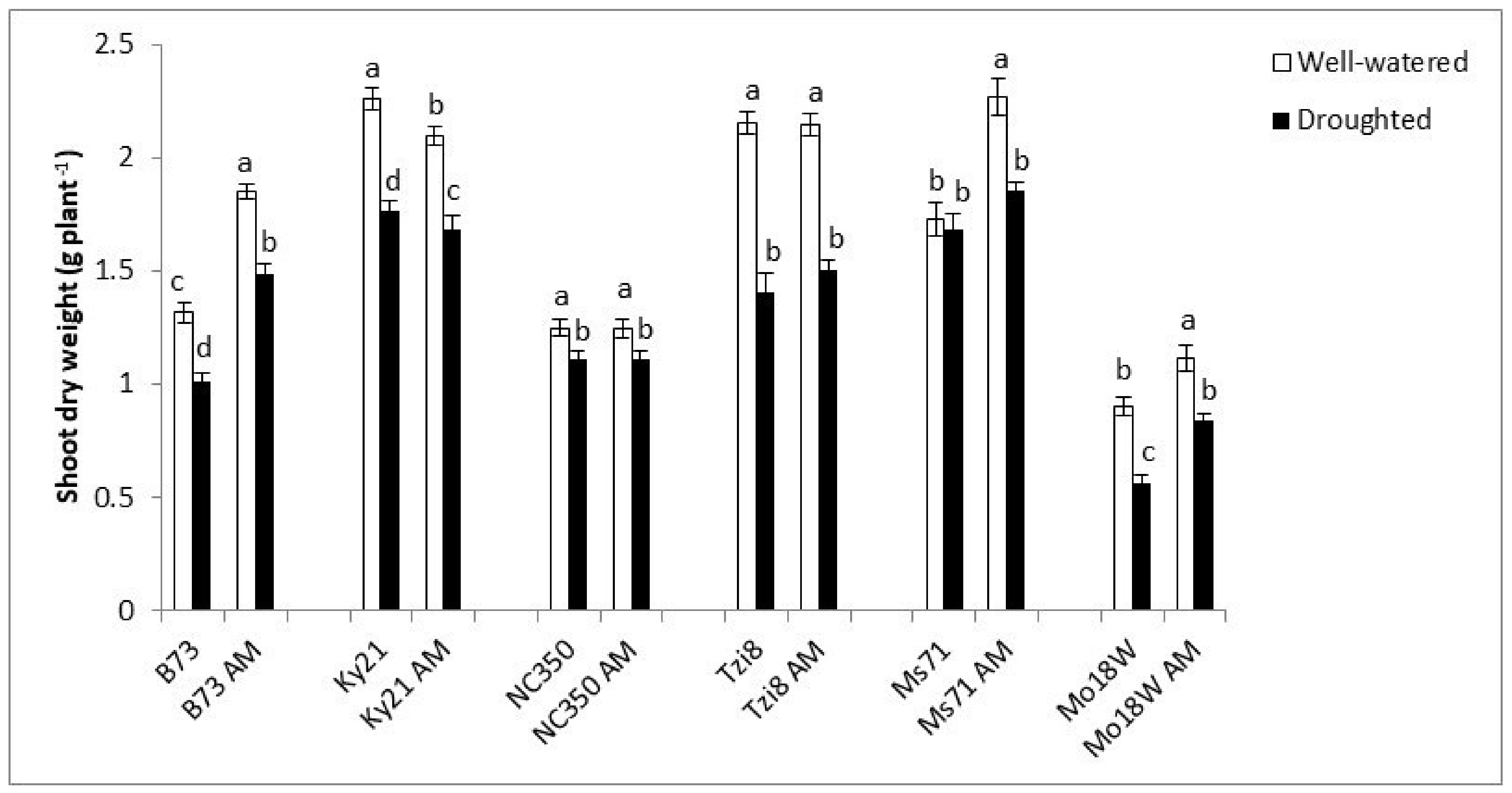
2.1.1. Plant Shoot Dry Weight (SDW)
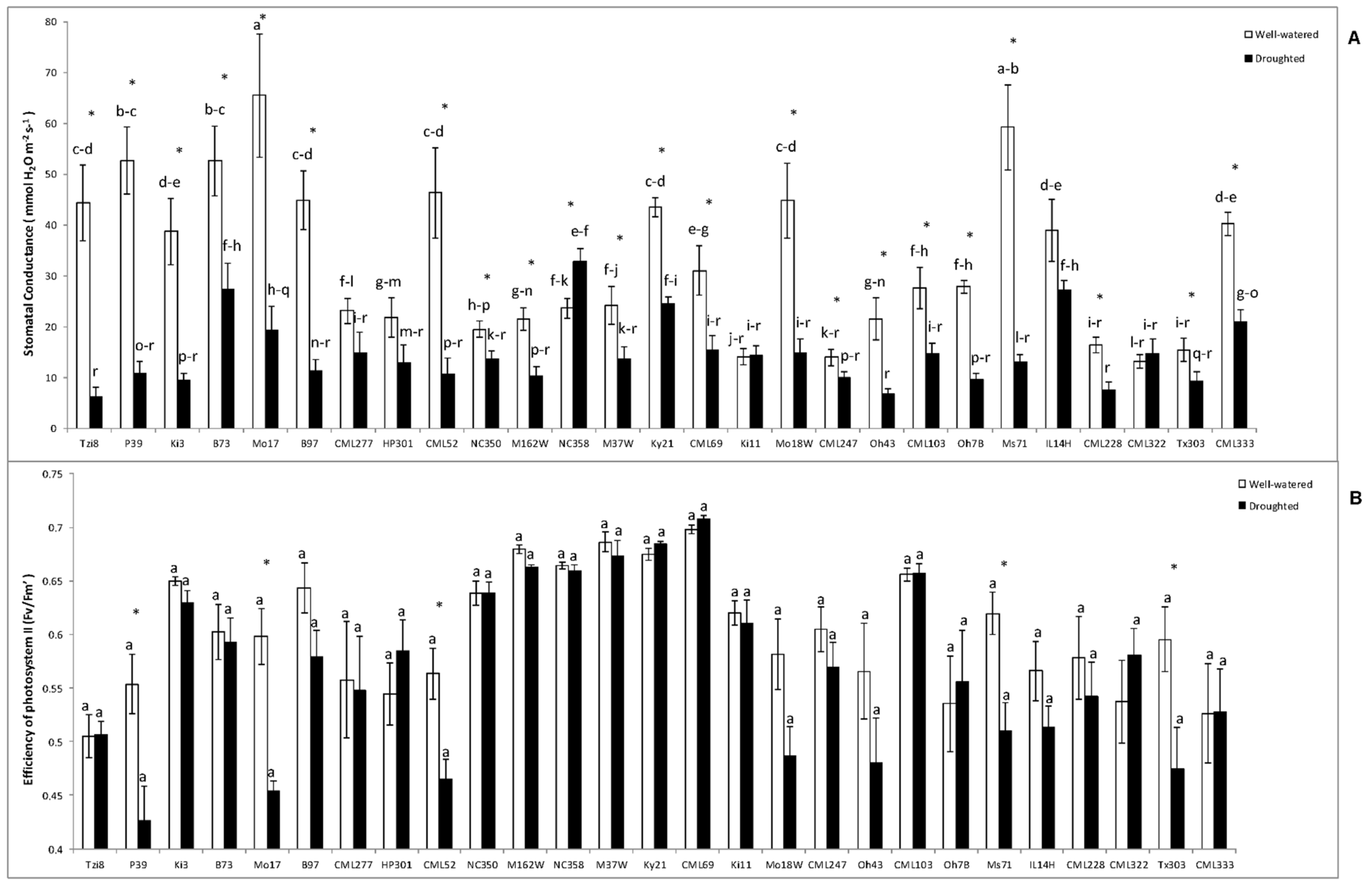
2.1.2. Stomatal Conductance (Gs) and Efficiency of Photosystem II
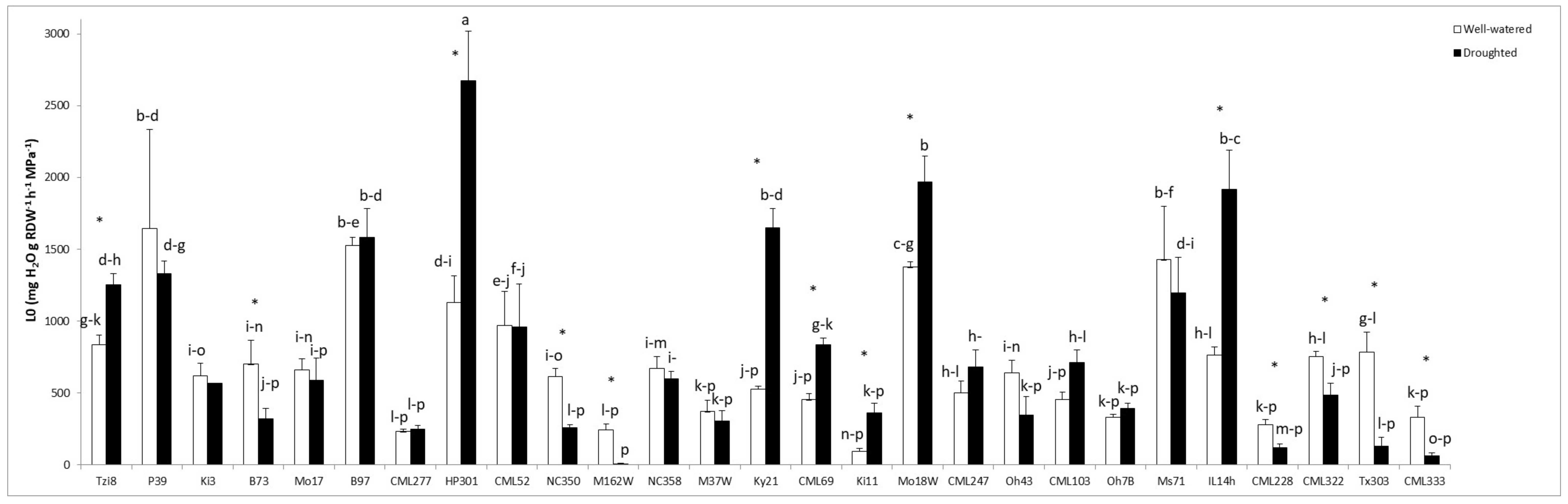
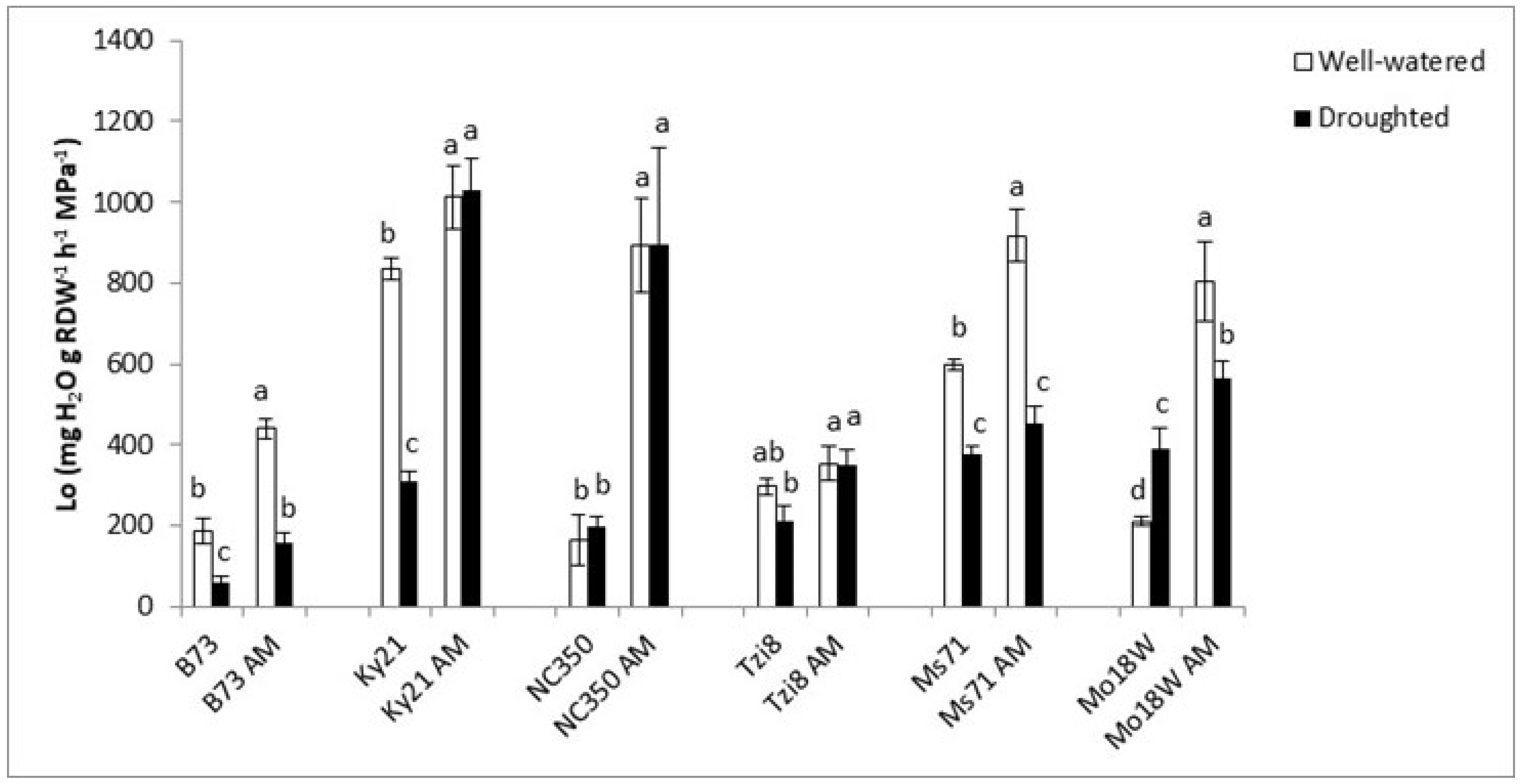
2.1.3. Osmotic Root Hydraulic Conductivity (Lo)
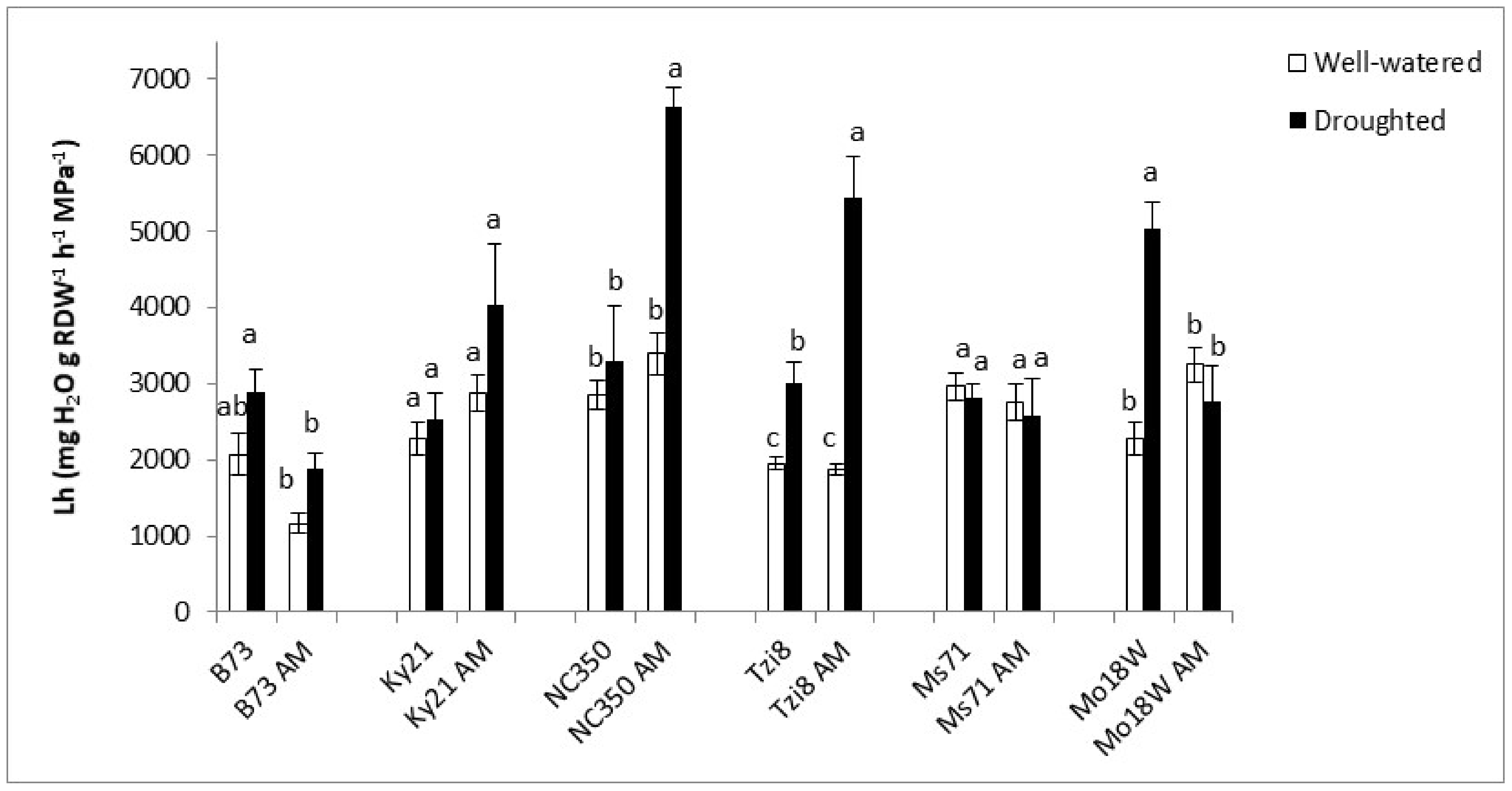
2.1.4. Hydrostatic Root Hydraulic Conductivity (Lh)
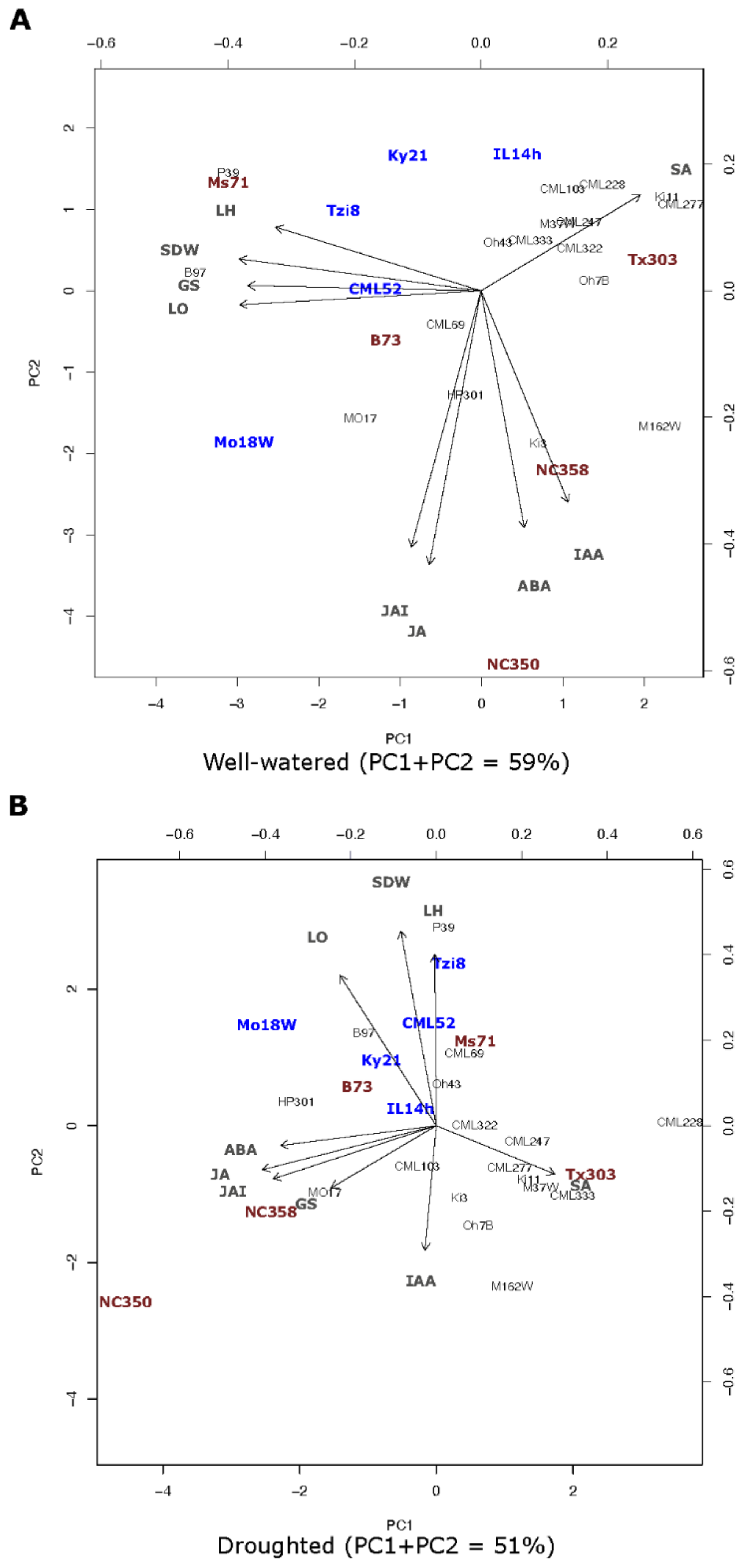
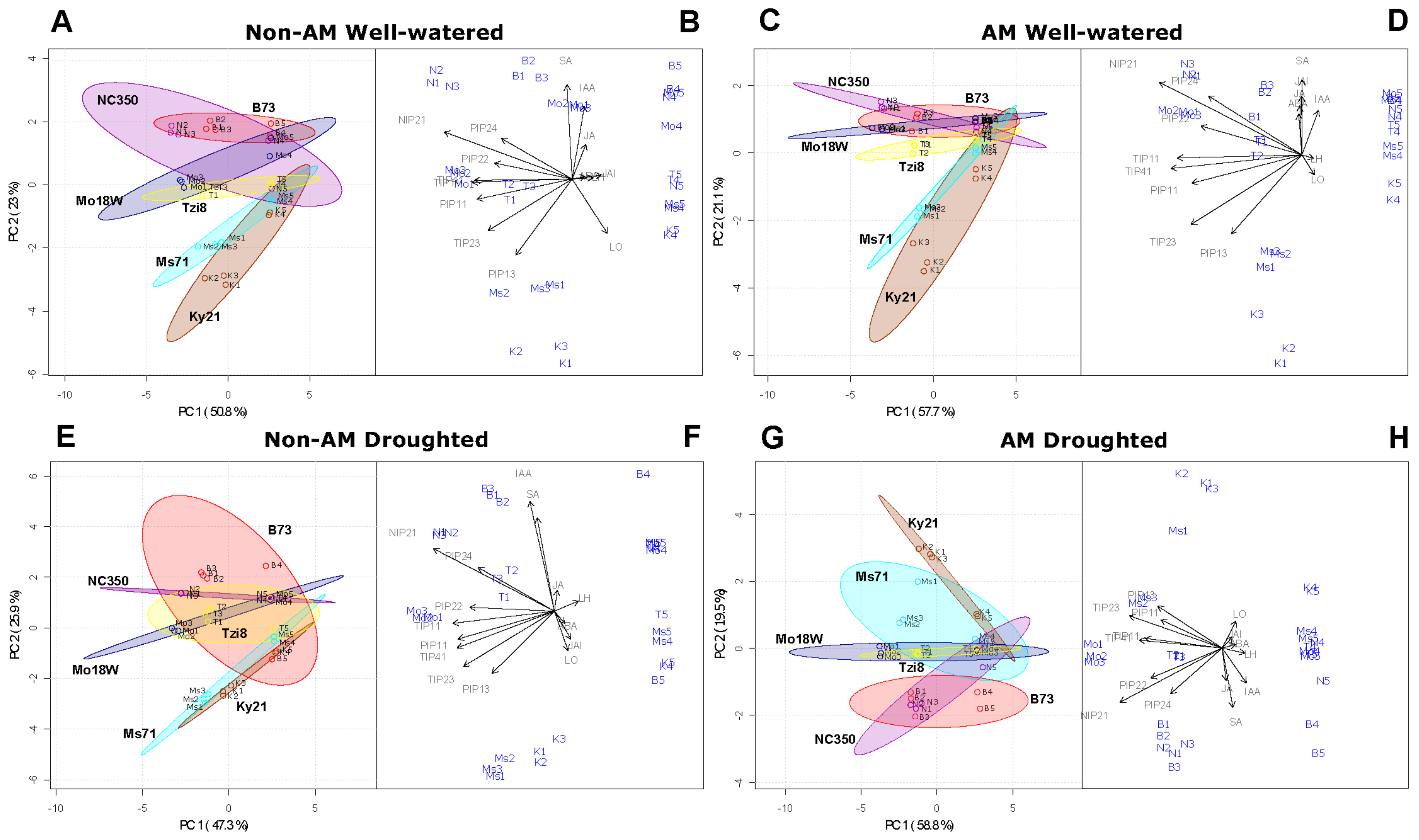
2.1.5. Principal Component Analysis
2.2. Experiment 2
2.2.1. AM Root Colonization and Shoot Dry Weight
2.2.2. Stomatal Conductance (Gs) and Efficiency of Photosystem II
2.2.3. Osmotic Root Hydraulic Conductivity (Lo)
2.2.4. Hydrostatic Root Hydraulic Conductivity (Lh)
2.2.5. Principal Component Analysis
3. Discussion
3.1. Importance of the AM Symbiosis and Aquaporins for Maize under Drought Stress
3.2. Maize Physiological and Hydraulic Responses to Drought and Mycorrhization
3.3. Maize Hormonal Responses to Drought and Mycorrhization
4. Materials and Methods
4.1. Design of the Experiments and Statistical Analysis
4.1.1. Experiment 1
4.1.2. Experiment 2
4.2. Soil and Biological Materials
4.3. Growth Conditions
4.4. Measurements
4.4.1. Biomass Production
4.4.2. Symbiotic Development
4.4.3. Stomatal Conductance
4.4.4. Photosynthetic Efficiency
4.4.5. Osmotic Root Hydraulic Conductivity (Lo)
4.4.6. Hydrostatic Root Hydraulic Conductivity (Lh)
4.4.7. Sap Hormonal Content
4.4.8. Quantitative Real-Time RT-PCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, S.; Zou, Y.-N.; Kuc, K.; Hashem, A.; Abd-Allah, E.-F.; Wu, Q.-S. Elucidating the mechanisms underlying enhanced drought tolerance in plants mediated by arbuscular mycorrhizal fungi. Front. Microbiol. 2021, 23, 809473. [Google Scholar] [CrossRef] [PubMed]
- Trenberth, K.E.; Dai, A.; Van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Estrada, B.; Aroca, R.; Barea, J.M.; Ruiz-Lozano, J.M. Native arbuscular mycorrhizal fungi isolated from a saline habitat improved maize antioxidant systems and plant tolerance to salinity. Plant Sci. 2013, 201–202, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Chen, C.; Wei, S.; Shang, X.; Sun, M.; Xia, R.; Liu, X.; Hao, D.; Chen, H.; Xie, Q. Identification of drought tolerant mechanisms in maize seedlings based on transcriptome analysis of recombination inbred lines. Front. Plant Sci. 2016, 7, 1080. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-Lozano, J.M. Enhanced drought stress tolerance by the arbuscular mycorrhizal symbiosis in a drought-sensitive maize cultivar is related to a broader and differential regulation of host plant aquaporins than in a drought-tolerant cultivar. Front. Plant Sci. 2017, 8, 1056. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Flint-Garcia, S.A.; Thuillet, A.-C.; Yu, J.; Pressoir, G.; Romero, S.M.; Mitchell, S.E.; Doebley, J.; Kresovich, S.; Goodman, M.M.; Buckler, E.S. Maize association population: A high-resolution platform for quantitative trait locus dissection. Plant J. 2005, 44, 1054–1064. [Google Scholar] [CrossRef]
- McMullen, M.D.; Kresovich, S.; Villeda, H.S.; Bradbury, P.; Li, H.; Sun, Q.; Flint-Garcia, S.; Thornsberry, J.; Acharya, C.; Bottoms, C.; et al. Genetic properties of the maize nested association mapping population. Science 2009, 325, 737–740. [Google Scholar] [CrossRef]
- Varma, A. Mycorrhiza: State of the Art, Genetics and Molecular Biology, Eco-Funcion, Biotecnology, Eco-Physiology, Structure and Systematics, 3rd ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Vimal, S.R.; Singh, J.S.; Arora, N.K.; Singh, S. Soil-plant-microbe interactions in stressed agriculture management: A review. Pedosphere 2017, 27, 177–192. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R. Plant aquaporins and mycorrhizae: Their regulation and involvement in plant physiology and performance. In Plant Aquaporins; From Transport to Signaling; Signaling and Communication in Plants Series; Chaumont, F., Tyerman, S.D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 333–353. [Google Scholar]
- Aroca, R.; Porcel, R.; Ruiz-lozano, J.M. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses. New Phytol. 2007, 173, 808–816. [Google Scholar] [CrossRef]
- Santander, C.; Aroca, R.; Ruiz-Lozano, J.M.; Olave, J.; Cartes, P.; Borie, F.; Cornejo, P. Arbuscular mycorrhiza effects on plant performance under osmotic stress. Mycorrhiza 2017, 27, 639–657. [Google Scholar] [CrossRef]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.J.; Mingsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef]
- Chaumont, F.; Barrieu, F.; Wojcik, E.; Chrispeels, M.J.; Jung, R. Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol. 2001, 125, 1206–1215. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.-T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef]
- Singh, R.K.; Deshmukh, R.; Muthamilarasan, R.; Rani, K.; Prasad, M. Versatile roles of aquaporins in physiological processes and stress tolerance in plants. Plant Physiol. Biochem. 2020, 149, 178–189. [Google Scholar] [CrossRef]
- Hachez, C.; Veselov, D.; Ye, Q.; Reinhardt, H.; Knipfer, T.; Fricke, W.; Chaumont, F. Short-term control of maize cell and root water permeability through plasma membrane aquaporin isoforms. Plant Cell Environ. 2012, 35, 185–198. [Google Scholar] [CrossRef]
- Kurowska, M.M. Aquaporins in cereals—Important players in maintaining cell homeostasis under abiotic stress. Genes 2021, 12, 477. [Google Scholar] [CrossRef]
- Fox, A.R.; Maistriaux, L.C.; Chaumont, F. Toward understanding of the high number of plant aquaporin isoforms and multiple regulation mechanisms. Plant Sci. 2017, 264, 179–187. [Google Scholar] [CrossRef]
- Zwiazek, J.J.; Xu, H.; Tan, X.; Navarro-Ródenas, A.; Morte, A. Significance of oxygen transport through aquaporins. Sci. Rep. 2017, 7, 40411. [Google Scholar] [CrossRef]
- Maurel, C.; Plassard, C. Aquaporins: For more than water at the plant–fungus interface. New Phytol. 2011, 190, 815–817. [Google Scholar] [CrossRef]
- Bárzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.; Ruiz-Lozano, J.M. New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize. Mol. Plant-Microbe Interact. 2014, 27, 349–363. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Aroca, R.; Zamarreño, A.M.; García-Mina, J.M.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis and salicylic acid regulate aquaporins and root hydraulic properties in maize plants subjected to drought. Agric. Water Manag. 2018, 202, 271–284. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Aroca, R.; Delgado-Huertas, A.; Ruiz-Lozano, J.M. Elucidating the possible involvement of maize aquaporins and arbuscular mycorrhizal symbiosis in the plant ammonium and urea transport under drought stress conditions. Plants 2020, 9, 148. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Aroca, R.; Ruiz-Lozano, J.M. Elucidating the possible involvement of maize aquaporins in the plant boron transport and homeostasis mediated by Rhizophagus irregularis under drought stress conditions. Int. J. Mol. Sci. 2020, 21, 1748. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Müller, M. Hormonal cross-talk in plant development and stress responses. Front. Plant Sci. 2013, 4, 529. [Google Scholar] [CrossRef]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef]
- Foo, E.; Ross, J.J.; Jones, W.T.; Reid, J.B. Plant hormones in arbuscular mycorrhizal symbioses: An emerging role for gibberellins. Ann. Bot. 2013, 111, 769–779. [Google Scholar] [CrossRef]
- Pozo, M.J.; López-Ráez, J.A.; Azcón, C.; García-Garrido, J.M. Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol. 2015, 205, 1431–1436. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, F.; Sun, X.; Shang, D.; He, F.; Li, X.; Zhang, J.; Jiang, X. Effects of S-abscisic acid (S-ABA) on seed germination, seedling growth, and ASR1 gene expression under drought stress in maize. J. Plant Growth Regul. 2019, 38, 1300–1313. [Google Scholar] [CrossRef]
- Riemann, M.; Dhakarey, R.; Hazman, M.; Miro, B.; Kohli, A.; Nick, P. Exploring jasmonates in the hormonal network of drought and salinity responses. Front. Plant Sci. 2015, 6, 1077. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Romera, B.; Ruiz-Lozano, J.M.; Zamarreño, A.M.; García-Mina, J.M.; Aroca, R. Arbuscular mycorrhizal symbiosis and methyl jasmonate avoid the inhibition of root hydraulic conductivity caused by drought. Mycorrhiza 2016, 26, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Faried, H.N.; Ayyub, C.M.; Amjad, M.; Ahmed, R.; Wattoo, F.M.; Butt, M.; Bashir, M.; Shaheen, M.R.; Waqas, M.A. Salicylic acid confers salt tolerance in potato plants by improving water relations, gaseous exchange, antioxidant activities and osmoregulation. J. Sci. Food Agric. 2017, 97, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, M.T.; Coenen, C. Genetic evidence for auxin involvement in arbuscular mycorrhiza initiation. New Phytol. 2011, 189, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, M.; Gutjahr, C.; Couzigou, J.M.; Zouine, M.; Lauressergues, D.; Timmers, A.; Audran, C.; Bouzayen, M.; Bécard, G.; Combier, J.P. Auxin perception is required for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Physiol. 2014, 166, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Zhang, F.; Zhang, D.J.; Srivastava, A.; Wu, Q.S.; Zou, Y.N. Mycorrhiza stimulates root-hair growth and IAA synthesis and transport in trifoliate orange under drought stress. Sci. Rep. 2018, 8, 1978. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, G.; Erice, G.; Aroca, R.; Zamarreño, A.M.; García-Mina, J.M.; Ruiz-Lozano, J.M. Radial water transport in arbuscular mycorrhizal maize plants under drought stress conditions is affected by indole-acetic acid (IAA) application. J. Plant Physiol. 2020, 246–247, 153115. [Google Scholar] [CrossRef]
- Lobell, D.B.; Burke, M.B.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing climate change adaptation needs for food security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef]
- Boomsma, C.R.; Vyn, T.J. Maize drought tolerance: Potential improvements through arbuscular mycorrhizal symbiosis? Field Crop. Res. 2008, 108, 14–31. [Google Scholar] [CrossRef]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-Lozano, J.M. Contribution of the arbuscular mycorrhizal symbiosis to the regulation of radial root water transport in maize plants under water deficit. Environ. Exp. Bot. 2019, 167, 103821. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Ding, L.; Chaumont, F.; Aroca, R.; Ruiz-Lozano, J.M. The arbuscular mycorrhizal symbiosis alters aquaporins activity and root cell water permeability in maize plants subjected to water deficit. Plant Cell Environ. 2019, 42, 2274–2290. [Google Scholar] [CrossRef]
- Polcyn, W.; Paluch-Lubawa, E.; Lehmann, T.; Mikula, R. Arbuscular mycorrhiza in highly fertilized maize cultures alleviates short-term drought effects but does not improve fodder yield and quality. Front. Plant Sci. 2019, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcón, R.; Aroca, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: New challenges in physiological and molecular studies. J. Exp. Bot. 2012, 63, 4033–4044. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcón, R.; Bárzana, G.; Aroca, R. Contribution of arbuscular mycorrhizal symbiosis to plant drought tolerance: State of the art. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2012; pp. 335–362. [Google Scholar]
- Yooyongwech, S.; Samphumphuang, T.; Tisarum, R.; Theerawitaya, C.; Cha-um, S. Arbuscular mycorrhizal fungi (AMF) improved water deficit tolerance in two different sweet potato genotypes involves osmotic adjustments via soluble sugar and free proline. Sci. Hortic. 2016, 198, 107–117. [Google Scholar] [CrossRef]
- Aroca, R.; Alguacil, M.M.; Vernieri, P.; Ruiz-Lozano, J.M. Plant responses to drought stress and exogenous ABA application are modulated differently by mycorrhization in tomato and an ABA-deficient mutant (Sitiens). Microb. Ecol. 2008, 56, 704–719. [Google Scholar] [CrossRef]
- Aroca, R.; Vernieri, P.; Ruiz-Lozano, J.M. Mycorrhizal and nonmycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J. Exp. Bot. 2008, 59, 2029–2041. [Google Scholar] [CrossRef][Green Version]
- Bárzana, G.; Aroca, R.; Paz, J.A.; Chaumont, F.; Martinez-Ballesta, M.C.; Carvajal, M.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well-watered and drought stress conditions. Ann. Bot. 2012, 109, 1009–1017. [Google Scholar] [CrossRef]
- Bárzana, G.; Aroca, R.; Ruiz-Lozano, J.M. Localized and non-localized effects of arbuscular mycorrhizal symbiosis on accumulation of osmolytes and aquaporins and on antioxidant systems in maize plants subjected to total or partial root drying. Plant Cell Environ. 2015, 38, 1613–1627. [Google Scholar] [CrossRef]
- Uehlein, N.; Fileschi, K.; Eckert, M.; Bienert, G.P.; Bertl, A.; Kaldenhoff, R. Arbuscular mycorrhizal symbiosis and plant aquaporin expression. Phytochemistry 2007, 68, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Santoni, V.; Maurel, C. Plant aquaporins: Roles in plant physiology. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar] [CrossRef] [PubMed]
- Afzal, Z.; Howton, T.; Sun, Y.; Mukhtar, M. The roles of aquaporins in plant stress responses. J. Dev. Biol. 2016, 4, 9. [Google Scholar] [CrossRef]
- Steudle, E.; Peterson, C.A. How does water get through roots? J. Exp. Bot. 1998, 49, 775–788. [Google Scholar] [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Regulation of root water uptake under abiotic stress conditions. J. Exp. Bot. 2012, 63, 43–57. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef]
- Ranathunge, K.; Kotula, L.; Steudle, E.; Lafitte, R. Water permeability and reflection coefficient of the outer part of young rice roots are differently affected by closure of water channels (aquaporins) or blockage of apoplastic pores. J. Exp. Bot. 2004, 55, 433–447. [Google Scholar] [CrossRef]
- Vadez, V.; Kholova, J.; Zaman-Allah, M.; Belko, N. Water: The most important “molecular” component of water stress tolerance research. Funct. Plant Biol. 2013, 40, 1310–1322. [Google Scholar] [CrossRef]
- Tyerman, S. Plant aquaporins: Their molecular biology, biophysics and significance for plant water relations. J. Exp. Bot. 1999, 50, 1055–1071. [Google Scholar] [CrossRef]
- Javot, H.; Maurel, C. The role of aquaporins in root water uptake. Ann. Bot. 2002, 90, 301–313. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Azcón, R.; Ruiz-Lozano, J.M. PIP aquaporin gene expression in arbuscular mycorrhizal Glycine max and Lactuca sativa plants in relation to drought stress tolerance. Plant Mol. Biol. 2006, 60, 389–404. [Google Scholar] [CrossRef]
- Blee, K.A.; Anderson, A.J. Regulation of arbuscule formation by carbon in the plant. Plant J. 1998, 16, 523–530. [Google Scholar] [CrossRef]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007, 175, 81–93. [Google Scholar] [CrossRef]
- Yepes-Molina, L.; Bárzana, G.; Carvajal, M. Controversial regulation of gene expression and protein transduction of aquaporins under drought and salinity stress. Plants 2020, 9, 1662. [Google Scholar] [CrossRef]
- Fetter, K.; Van Wilder, V.; Moshelion, M.; Chaumont, F. Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 2004, 16, 215–228. [Google Scholar] [CrossRef]
- Zelazny, E.; Borst, J.W.; Muylaert, M.; Batoko, H.; Hemminga, M.A.; Chaumont, F. FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proc. Natl. Acad. Sci. USA 2007, 104, 12359–12364. [Google Scholar] [CrossRef]
- Kreszies, T.; Schreiber, L.; Ranathunge, K. Suberized transport barriers in Arabidopsis, barley and rice roots: From the model plant to crop species. J. Plant Physiol. 2018, 227, 75–83. [Google Scholar] [CrossRef]
- Jang, J.Y.; Lee, S.H.; Rhee, J.Y.; Chung, G.C.; Ahn, S.J.; Kang, H. Transgenic Arabidopsis and tobacco plants overexpressing an aquaporin respond differently to various abiotic stresses. Plant Mol. Biol. 2007, 64, 621–632. [Google Scholar] [CrossRef]
- Jang, J.Y.; Rhee, J.Y.; Kim, D.G.G.; Chung, G.C.; Lee, J.H.; Kang, H. Ectopic Expression of a foreign aquaporin disrupts the natural expression patterns of endogenous aquaporin genes and alters plant responses to different stress conditions. Plant Cell Physiol. 2007, 48, 1331–1339. [Google Scholar] [CrossRef]
- Maurel, C.; Javot, H.; Lauvergeat, V.; Gerbeau, P.; Tournaire, C.; Santoni, V.; Heyes, J. Molecular physiology of aquaporins in plants. Int. Rev. Cytol. 2002, 215, 105–148. [Google Scholar] [PubMed]
- Heinen, R.B.; Bienert, G.P.; Cohen, D.; Chevalier, A.S.; ·Uehlein, N.; Hachez, C.; Kaldenhoff, R.; Le Thiec, D.; Chaumont, F. Expression and characterization of plasma membrane aquaporins in stomatal complexes of Zea mays. Plant Mol. Biol. 2014, 86, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, T.; Shinozaki, K. Perception and transduction of abscisic acid signals: Keys to the function of the versatile plant hormone ABA. Trends Plant Sci. 2007, 12, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H. Mechanism of ABA signal transduction: Agricultural highlights for improving drought tolerance. J. Plant Biol. 2014, 57, 1–8. [Google Scholar] [CrossRef]
- Horváth, E.; Csiszár, J.; Gallé, Á.; Poór, P.; Szepesi, Á.; Tari, I. Hardening with salicylic acid induces concentration-dependent changes in abscisic acid biosynthesis of tomato under salt stress. J. Plant Physiol. 2015, 183, 54–63. [Google Scholar] [CrossRef]
- Rowe, J.H.; Topping, J.F.; Liu, J.; Lindsey, K. Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin. New Phytol. 2016, 211, 225–239. [Google Scholar] [CrossRef]
- Sánchez-Romera, B.; Ruiz-Lozano, J.M.; Li, G.; Luu, D.T.; Martínez-Ballesta, M.C.; Carvajal, M.; Zamarreño, A.M.; García-Mina, J.M.; Maurel, C.; Aroca, R. Enhancement of root hydraulic conductivity by methyl jasmonate and the role of calcium and abscisic acid in this process. Plant Cell Environ. 2014, 37, 995–1008. [Google Scholar] [CrossRef]
- Mahdieh, M.; Mostajeran, A. Abscisic acid regulates root hydraulic conductance via aquaporin expression modulation in Nicotiana tabacum. J. Plant Physiol. 2009, 166, 1993–2003. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Alguacil, M.M.; Bárzana, G.; Vernieri, P.; Aroca, R. Exogenous ABA accentuates the differences in root hydraulic properties between mycorrhizal and non mycorrhizal maize plants through regulation of PIP aquaporins. Plant Mol. Biol. 2009, 70, 565–579. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Armada, E.; Zamarreño, A.M.; García-Mina, J.M.; Aroca, R. Local root ABA/cytokinin status and aquaporins regulate poplar responses to mild drought stress independently of the ectomycorrhizal fungus Laccaria bicolor. J. Exp. Bot. 2019, 70, 6437–6446. [Google Scholar] [CrossRef]
- Boursiac, Y.; Boudet, J.; Postaire, O.; Luu, D.-T.; Tournaire-Roux, C.; Maurel, C. Stimulus-induced downregulation of root water transport involves reactive oxygen species-activated cell signaling and plasma membrane intrinsic protein internalization. Plant J. 2008, 56, 207–218. [Google Scholar] [CrossRef]
- Péret, B.; Li, G.; Zhao, J.; Band, L.R.; Voß, U.; Postaire, O.; Luu, D.-T.; Da Ines, O.; Casimiro, I.; Lucas, M.; et al. Auxin regulates aquaporin function to facilitate lateral root emergence. Nat. Cell Biol. 2012, 14, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Du, Y.; Tejos, R.; Beck, M.; Himschoot, E.; Li, H.; Robatzek, S.; Vanneste, S.; Friml, J. Salicylic acid interferes with clathrin-mediated endocytic protein trafficking. Proc. Natl. Acad. Sci. USA 2013, 110, 7946–7951. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stat. Circ. 1950, 347, 1–32. [Google Scholar]
- Porcel, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J. Exp. Bot. 2004, 55, 1743–1750. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—Calculation of qP and Fv’/Fm’ without measuring Fo’. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Albacete, A.; Ghanem, M.E.; Martinez-Andujar, C.; Acosta, M.; Sanchez-Bravo, J.; Martinez, V.; Lutts, S.; Dodd, I.C.; Perez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Lozano, J.M.; Quiroga, G.; Erice, G.; Pérez-Tienda, J.; Zamarreño, Á.M.; García-Mina, J.M.; Aroca, R. Using the Maize Nested Association Mapping (NAM) Population to Partition Arbuscular Mycorrhizal Effects on Drought Stress Tolerance into Hormonal and Hydraulic Components. Int. J. Mol. Sci. 2022, 23, 9822. https://doi.org/10.3390/ijms23179822
Ruiz-Lozano JM, Quiroga G, Erice G, Pérez-Tienda J, Zamarreño ÁM, García-Mina JM, Aroca R. Using the Maize Nested Association Mapping (NAM) Population to Partition Arbuscular Mycorrhizal Effects on Drought Stress Tolerance into Hormonal and Hydraulic Components. International Journal of Molecular Sciences. 2022; 23(17):9822. https://doi.org/10.3390/ijms23179822
Chicago/Turabian StyleRuiz-Lozano, Juan Manuel, Gabriela Quiroga, Gorka Erice, Jacob Pérez-Tienda, Ángel María Zamarreño, José María García-Mina, and Ricardo Aroca. 2022. "Using the Maize Nested Association Mapping (NAM) Population to Partition Arbuscular Mycorrhizal Effects on Drought Stress Tolerance into Hormonal and Hydraulic Components" International Journal of Molecular Sciences 23, no. 17: 9822. https://doi.org/10.3390/ijms23179822
APA StyleRuiz-Lozano, J. M., Quiroga, G., Erice, G., Pérez-Tienda, J., Zamarreño, Á. M., García-Mina, J. M., & Aroca, R. (2022). Using the Maize Nested Association Mapping (NAM) Population to Partition Arbuscular Mycorrhizal Effects on Drought Stress Tolerance into Hormonal and Hydraulic Components. International Journal of Molecular Sciences, 23(17), 9822. https://doi.org/10.3390/ijms23179822







