Secondary Metabolites with Biomedical Applications from Plants of the Sarraceniaceae Family
Abstract
:1. Introduction
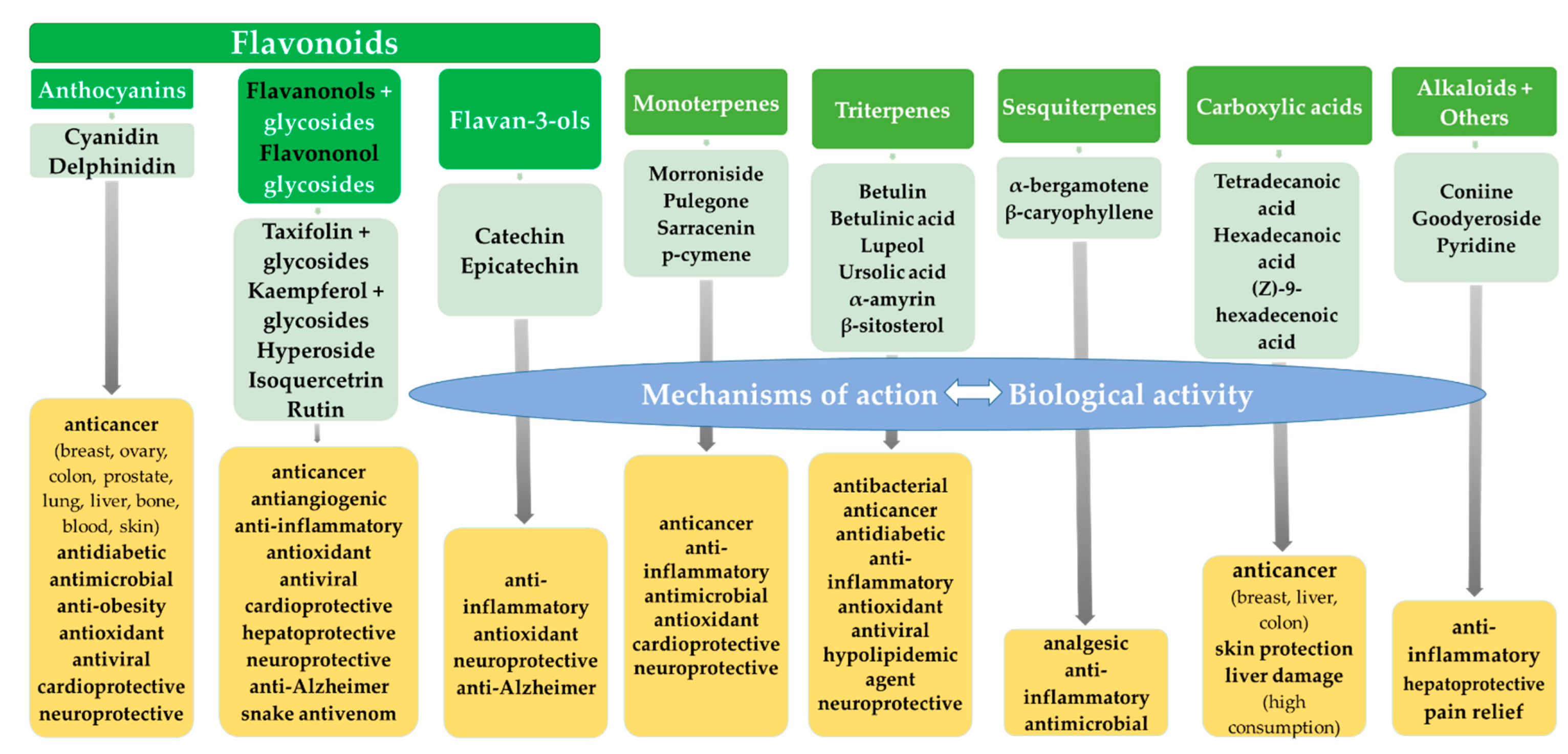
2. Flavonoids
2.1. Anthocyanidins
Biological Activity
2.2. Flavonols, Flavonol Glycosides and Flavononol Glycosides
2.2.1. Biological Activity of S. purpurea Extracts
2.2.2. Biological Activity of Taxifolin
2.2.3. Biological Activity of Kaempferol and Kaempferol Derivatives
2.2.4. Biological Activity of Quercetin-3-O-galactoside
2.2.5. Biological Activity of Quercetin-3-O-glucoside
2.2.6. Biological Activity of Quercetin-3-O-rutinoside
2.3. Biological Activity of Flavan-3-ols
3. Monoterpenes
Biological Activity
4. Triterpenes
Biological Activity
5. Sesquiterpenes
Biological Activity
6. Fatty Acids
Biological Activity
7. Alkaloids
8. Other Compounds
9. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Ellison, A.M.; Butler, E.D.; Hicks, E.J.; Naczi, R.F.C.; Calie, P.J.; Bell, C.D.; Davis, C.C. Phylogeny and Biogeography of the Carnivorous Plant Family Sarraceniaceae. PLoS ONE 2012, 7, e39291. [Google Scholar] [CrossRef]
- Naczi, R.F.C. Systematics and Evolution of Sarraceniaceae. In Carnivorous Plants. Physiology, Ecology and Evolution; Ellison, A.M., Adamec, L., Eds.; Oxford University Press: Oxford, UK, 2018; pp. 105–119. [Google Scholar]
- Debuhr, L.E. Distribution and Reproductive Biology of Darlingtonia Californica; Claremont Graduate School: Claremont, CA, USA, 1973. [Google Scholar]
- Mellichamp, T.L. Sarraceniaceae. Available online: http://floranorthamerica.org/Sarraceniaceae (accessed on 22 August 2022).
- McPherson, S.; Wistuba, A.; Fleischmann, A.; Nerz, J. Sarraceniaceae of South America; Redfern Natural History Productions: Poole, UK, 2011. [Google Scholar]
- Liu, S.; Smith, S.D. Phylogeny and Biogeography of South American Marsh Pitcher Plant Genus Heliamphora (Sarraceniaceae) Endemic to the Guiana Highlands. Mol. Phylogenet. Evol. 2021, 154, 106961. [Google Scholar] [CrossRef] [PubMed]
- DE, S. Carnivorous Plants of the United States and Canada; Timber Press: Portland, OR, USA, 2002. [Google Scholar]
- Rice, B.A. Reversing the Roles of Predator and Prey. In Cellular Origin, Life in Extreme Habitats and Astrobiology. All Flesh Is Grass: Plant-Animal Interrelationships; Seckbach, J., Dubinsk, Z., Eds.; Springer: Berlin, Germany, 2011; pp. 491–518. [Google Scholar]
- Hotti, H.; Gopalacharyulu, P.; Seppänen-Laakso, T.; Rischer, H. Metabolite Profiling of the Carnivorous Pitcher Plants Darlingtonia and Sarracenia. PLoS ONE 2017, 12, e0171078. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking Plant Secondary Metabolites and Plant Microbiomes: A Review. Front. Plant Sci. 2021, 12, 621276. [Google Scholar] [CrossRef]
- Teoh, E.S. Secondary Metabolites of Plants. In Medicinal Orchids of Asia; Springer International Publishing: Cham, Switzerland, 2016; pp. 59–73. ISBN 9783319242743. [Google Scholar]
- Moore, B.D.; Andrew, R.L.; Külheim, C.; Foley, W.J. Explaining Intraspecific Diversity in Plant Secondary Metabolites in an Ecological Context. New Phytol. 2014, 201, 733–750. [Google Scholar] [CrossRef]
- Hatcher, C.R.; Ryves, D.B.; Millett, J. The Function of Secondary Metabolites in Plant Carnivory. Ann. Bot. 2020, 125, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Dress, W.J.; Newell, S.J.; Nastase, A.J.; Ford, J.C. Analysis of Amino Acids in Nectar from Pitchers of Sarracenia Purpurea (Sarraceniaceae). Am. J. Bot. 1997, 84, 1701–1706. [Google Scholar] [CrossRef]
- Bennett, K.F.; Ellison, A.M. Nectar, Not Colour, May Lure Insects to Their Death. Biol. Lett. 2009, 5, 469–472. [Google Scholar] [CrossRef]
- Miles, D.H.; Kokpol, U.; Mody, V.; Hedin, P.A. Volatiles in Sarracenia Flava. Phytochemistry 1975, 14, 845–846. [Google Scholar] [CrossRef]
- Jaffé, K.; Blum, M.S.; Fales, H.M.; Mason, R.T.; Cabrera, A. On Insect Attractants from Pitcher Plants of the GenusHeliamphora (Sarraceniaceae). J. Chem. Ecol. 1995, 21, 379–384. [Google Scholar] [CrossRef]
- Jürgens, A.; El-Sayed, A.M.; Suckling, D.M. Do Carnivorous Plants Use Volatiles for Attracting Prey Insects? Funct. Ecol. 2009, 23, 875–887. [Google Scholar] [CrossRef]
- Kurup, R.; Johnson, A.J.; Sankar, S.; Hussain, A.A.; Kumar, C.S.; Sabulal, B. Fluorescent Prey Traps in Carnivorous Plants. Plant Biol. 2013, 15, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A. Carnivorous Plants and Their Biotic Interactions. J. Plant Interact. 2022, 17, 333–343. [Google Scholar] [CrossRef]
- Gaascht, F.; Dicato, M.; Diederich, M. Venus Flytrap (Dionaea Muscipula Solander Ex Ellis) Contains Powerful Compounds That Prevent and Cure Cancer. Front. Oncol. 2013, 3, 202. [Google Scholar] [CrossRef]
- Devi, S.P.; Kumaria, S.; Rao, S.R.; Tandon, P. Carnivorous Plants as a Source of Potent Bioactive Compound: Naphthoquinones. Trop. Plant Biol. 2016, 9, 267–279. [Google Scholar] [CrossRef]
- De Filippis, L.F. Plant-Environment Interaction: Responses and Approaches to Mitigate Stress. In Plant-Environment Interaction: Responses and Approaches to Mitigate Stress; Azooz, M.M., Ahmad, P., Eds.; John Wiley & Sons Ltd: Oxford, UK, 2016; pp. 263–299. [Google Scholar]
- Clarke, C.; Cross, A.; Rice, B. Conservation of Carnivorous Plants; Aaron, M., Ellison, L.A., Eds.; Oxford University Press: Oxford, UK, 2018; Volume 1. [Google Scholar]
- Leduc, C.; Coonishish, J.; Haddad, P.; Cuerrier, A. Plants Used by the Cree Nation of Eeyou Istchee (Quebec, Canada) for the Treatment of Diabetes: A Novel Approach in Quantitative Ethnobotany. J. Ethnopharmacol. 2006, 105, 55–63. [Google Scholar] [CrossRef]
- Muhammad, A.; Guerrero-Analco, J.A.; Martineau, L.C.; Musallam, L.; Madiraju, P.; Nachar, A.; Saleem, A.; Haddad, P.S.; Arnason, J.T. Antidiabetic Compounds from Sarracenia Purpurea Used Traditionally by the Eeyou Istchee Cree First Nation. J. Nat. Prod. 2012, 75, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Arndt, W.; Mitnik, C.; Denzler, K.L.; White, S.; Waters, R.; Jacobs, B.L.; Rochon, Y.; Olson, V.A.; Damon, I.K.; Langland, J.O. In Vitro Characterization of a Nineteenth-Century Therapy for Smallpox. PLoS ONE 2012, 7, e32610. [Google Scholar] [CrossRef]
- Kannan, L.; Kumar, A.; Kumar, A.; Jacobs, B.; Langland, J. Anti-Herpes Virus Activity of the Carnivorous Botanical, Sarracenia Purpurea. Sci. Rep. 2020, 10, 18953. [Google Scholar] [CrossRef]
- Melnic, E.; Gundareddy, V.; Fallick, F.; Hwang, R. Going Green Is Not Always Safe. Am. J. Gastroenterol. 2009, 104, S297. [Google Scholar] [CrossRef]
- Manchikanti, L.; Pampati, V.; Rivera, J.J.; McManus, C.D.; Damron, K.S.; Barnhill, R. Caudal Epidural Injections with Sarapin or Steroids in Chronic Low Back Pain. Pain Physician 2001, 4, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Campos, G.J.; Chacón, T.C.; Cova, F.J.; Flores, S.A.; Rojas, J.A.; Risso, A.J.; Zerpa González, H.A. Evaluation of the Local Analgesic Effects of a Commercial Aqueous Extract of Sarracenia Purpurea and Ammonium Sulfate in the Equine Abaxial Sesamoid Block Model. J. Equine Vet. Sci. 2013, 33, 1004–1007. [Google Scholar] [CrossRef]
- Harkins, J.D.; Mundy, G.D.; Stanley, S.D.; Sams, R.A.; Tobin, T. Lack of Local Anaesthetic Efficacy of Sarapin® in the Abaxial Sesamoid Block Model. J. Vet. Pharmacol. Ther. 1997, 20, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Manchikanti, K.N.; Pampati, V.; Damron, K.S.; McManus, C.D. A Double-Blind, Controlled Evaluation of the Value of Sarapin in Neural Blockade. Pain Physician 2004, 7, 59–62. [Google Scholar]
- Sheridan, P.M.; Griesbach, R.J. Anthocyanidins of Sarracenia L. Flowers and Leaves. HortScience 2001, 36, 384. [Google Scholar] [CrossRef]
- Shang, N.; Saleem, A.; Musallam, L.; Walshe-Roussel, B.; Badawi, A.; Cuerrier, A.; Arnason, J.T.; Haddad, P.S. Novel Approach to Identify Potential Bioactive Plant Metabolites: Pharmacological and Metabolomics Analyses of Ethanol and Hot Water Extracts of Several Canadian Medicinal Plants of the Cree of Eeyou Istchee. PLoS ONE 2015, 10, e0135721. [Google Scholar] [CrossRef]
- Morrison, S.A.; Li, H.; Webster, D.; Johnson, J.A.; Gray, C.A. Antimycobacterial Triterpenes from the Canadian Medicinal Plant Sarracenia Purpurea. J. Ethnopharmacol. 2016, 188, 200–203. [Google Scholar] [CrossRef]
- Tušek, M.; Curman, M.; Babić, M.; Tkalec, M. Photochemical Efficiency, Content of Photosynthetic Pigments and Phenolic Compounds in Different Pitcher Parts of Sarracenia Hybrids. Acta Bot. Croat. 2016, 75, 179–185. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.; Campra, P. Cytotoxicity Screening of Endemic Plants from Guayana Highlands. Trop. Biomed. 2009, 26, 149–154. [Google Scholar]
- Muhammad, A.; Haddad, P.S.; Durst, T.; Arnason, J.T. Phytochemical Constituents of Sarracenia Purpurea L. (Pitcher Plant). Phytochemistry 2013, 94, 238–242. [Google Scholar] [CrossRef]
- Cieniak, C.; Walshe-Roussel, B.; Liu, R.; Muhammad, A.; Saleem, A.; Haddad, P.S.; Cuerrier, A.; Foster, B.C.; Arnason, J.T. Phytochemical Comparison of the Water and Ethanol Leaf Extracts of the Cree Medicinal Plant, Sarracenia Purpurea L. (Sarraceniaceae). J. Pharm. Pharm. Sci. 2015, 18, 484. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.S.; Asim, M.; Saleem, A.; Haddad, P.S.; Arnason, J.T.; Bennett, S. AL Characterizing the Cytoprotective Activity of Sarracenia Purpurea L., a Medicinal Plant That Inhibits Glucotoxicity in PC12 Cells. BMC Complement. Altern. Med. 2012, 12, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.-F.; Starks, C.M.; Williams, R.B.; Rice, S.M.; Norman, V.L.; Olson, K.M.; Hough, G.W.; Goering, M.G.; O’Neil-Johnson, M.; Eldridge, G.R. Secoiridoid Glycosides from the Pitcher Plant Sarracenia Alata. Helv. Chim. Acta 2009, 92, 273–280. [Google Scholar] [CrossRef]
- Miles, D.H.; Kokpol, U.; Bhattacharyya, J.; Atwood, J.L.; Stone, K.E.; Bryson, T.A.; Wilson, C. Structure of Sarracenin. An Unusual Enol Diacetal Monoterpene from the Insectivorous Plant Sarracenia Flava. J. Am. Chem. Soc. 1976, 98, 1569–1573. [Google Scholar] [CrossRef]
- Newman, T.; Ibrahim, S.; Wheeler, J.W.; McLaughlin, W.; Petersen, R.L.; Duffield, R.M. Identification of Sarracenin in Four Species of Sarracenia (Sarraceniaceae). Biochem. Syst. Ecol. 2000, 28, 193–195. [Google Scholar] [CrossRef]
- Łyczko, J.; Twardowski, J.P.; Skalny, B.; Galek, R.; Szumny, A.; Gruss, I.; Piesik, D.; Sendel, S. Sarracenia Alata (Alph. Wood) Alph.Wood Microcuttings as a Source of Volatiles Potentially Responsible for Insects’ Respond. Molecules 2021, 26, 2406. [Google Scholar] [CrossRef]
- Miles, D.H.; Kokpol, U.; Zalkow, L.H.; Steindel, S.J.; Nabors, J.B. Tumor Inhibitors I: Preliminary Investigation of Antitumor Activity of Sarracenia Flava. J. Pharm. Sci. 1974, 63, 613–615. [Google Scholar] [CrossRef]
- Miles, D.H.; Kokpol, U. Tumor Inhibitors II: Constituents and Antitumor Activity of Sarracenia Flava. J. Pharm. Sci. 1976, 65, 284–285. [Google Scholar] [CrossRef]
- Mody, N.V.; Henson, R.; Hedin, P.A.; Kokpol, U.; Miles, D.H. Isolation of the Insect Paralyzing Agent Coniine FromSarracenia Flava. Experientia 1976, 32, 829–830. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Das, S.K. Roles of Flavonoids in Plants. Int. J. Pharm. Sci. Tech. 2011, 6, 12–35. [Google Scholar]
- Dos Santos Nascimento, L.B.; Tattini, M. Beyond Photoprotection: The Multifarious Roles of Flavonoids in Plant Terrestrialization. Int. J. Mol. Sci. 2022, 23, 5284. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Halter, B.; Liu, D.; Gilbert, E.R.; Cline, M.A. Dietary Flavonoids as Modulators of Lipid Metabolism in Poultry. Front. Physiol. 2022, 13, 863860. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; de Oliveira, B.D.; da Silva, E.R.; Sacramento, N.T.B.; Bertoldi, M.C.; Pinto, U.M. Anti-Quorum Sensing Activity of Phenolic Extract from Eugenia Brasiliensis (Brazilian Cherry). Food Sci. Technol. 2016, 36, 337–343. [Google Scholar] [CrossRef]
- Quecan, B.X.V.; Santos, J.T.C.; Rivera, M.L.C.; Hassimotto, N.M.A.; Almeida, F.A.; Pinto, U.M. Effect of Quercetin Rich Onion Extracts on Bacterial Quorum Sensing. Front. Microbiol. 2019, 10, 867. [Google Scholar] [CrossRef]
- Newell, S.J.; Nastase, A.J. Efficiency of Insect Capture by Sarracenia Purpurea (Sarraceniaceae), the Northern Pitcher Plant. Am. J. Bot. 1998, 85, 88–91. [Google Scholar] [CrossRef]
- Ockermann, P.; Headley, L.; Lizio, R.; Hansmann, J. A Review of the Properties of Anthocyanins and Their Influence on Factors Affecting Cardiometabolic and Cognitive Health. Nutrients 2021, 13, 2831. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Ž.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The Therapeutic Potential of Anthocyanins: Current Approaches Based on Their Molecular Mechanism of Action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Abiotic Stress-induced Anthocyanins in Plants: Their Role in Tolerance to Abiotic Stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape Berry Secondary Metabolites and Their Modulation by Abiotic Factors in a Climate Change Scenario–A Review. Front. Plant Sci. 2021, 12, 643258. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and Biological Activities of Anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, H.; Fernandes, A.; Brás, N.F.; Mateus, N.; de Freitas, V.; Fernandes, I. Anthocyanins as Antidiabetic Agents—In Vitro and In Silico Approaches of Preventive and Therapeutic Effects. Molecules 2020, 25, 3813. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Dietary Anthocyanin-Rich Plants: Biochemical Basis and Recent Progress in Health Benefits Studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef]
- Schnell, D.E.; Sarracenia, L. Petal Extract Chromatography. Castanea 1978, 43, 107–115. [Google Scholar]
- Bendokas, V.; Stanys, V.; Mažeikienė, I.; Trumbeckaite, S.; Baniene, R.; Liobikas, J. Anthocyanins: From the Field to the Antioxidants in the Body. Antioxidants 2020, 9, 819. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hung, C.-H.; Hung, T.-W.; Lin, Y.-C.; Wang, C.-J.; Kao, S.-H. Dietary Delphinidin Inhibits Human Colorectal Cancer Metastasis Associating with Upregulation of MiR-204-3p and Suppression of the Integrin/FAK Axis. Sci. Rep. 2019, 9, 18954. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary Cyanidin 3-O-β-D-Glucoside-Rich Purple Corn Color Prevents Obesity and Ameliorates Hyperglycemia in Mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef]
- Afzal, M.; Redha, A.; AlHasan, R. Anthocyanins Potentially Contribute to Defense against Alzheimer’s Disease. Molecules 2019, 24, 4255. [Google Scholar] [CrossRef]
- Husain, A.; Chanana, H.; Khan, S.A.; Dhanalekshmi, U.M.; Ali, M.; Alghamdi, A.A.; Ahmad, A. Chemistry and Pharmacological Actions of Delphinidin, a Dietary Purple Pigment in Anthocyanidin and Anthocyanin Forms. Front. Nutr. 2022, 9, 746881. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Morroni, F.; Hrelia, S.; Angeloni, C.; Marchesi, A.; Cantelli-Forti, G.; Hrelia, P. Neuroprotective Effects of Anthocyanins and Their in Vivo Metabolites in SH-SY5Y Cells. Neurosci. Lett. 2007, 424, 36–40. [Google Scholar] [CrossRef]
- Kim, S.M.; Chung, M.J.; Ha, T.J.; Choi, H.N.; Jang, S.J.; Kim, S.O.; Chun, M.H.; Do, S.I.; Choo, Y.K.; Park, Y. Il Neuroprotective Effects of Black Soybean Anthocyanins via Inactivation of ASK1–JNK/P38 Pathways and Mobilization of Cellular Sialic Acids. Life Sci. 2012, 90, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Diaconeasa, Z.; Știrbu, I.; Xiao, J.; Leopold, N.; Ayvaz, Z.; Danciu, C.; Ayvaz, H.; Stǎnilǎ, A.; Nistor, M.; Socaciu, C. Anthocyanins, Vibrant Color Pigments, and Their Role in Skin Cancer Prevention. Biomedicines 2020, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.-C.; Kim, H.; Kim, Y.-J.; Park, S.-H.; Song, J.-H.; Lee, K.H.; Lee, I.H.; Lee, Y.-K.; So, K.A.; Choi, K.-C.; et al. Delphinidin Inhibits BDNF-Induced Migration and Invasion in SKOV3 Ovarian Cancer Cells. Bioorg. Med. Chem. Lett. 2017, 27, 5337–5343. [Google Scholar] [CrossRef] [PubMed]
- Maya-Cano, D.A.; Arango-Varela, S.; Santa-Gonzalez, G.A. Phenolic Compounds of Blueberries (Vaccinium Spp) as a Protective Strategy against Skin Cell Damage Induced by ROS: A Review of Antioxidant Potential and Antiproliferative Capacity. Heliyon 2021, 7, e06297. [Google Scholar] [CrossRef]
- Chen, Y.; Ge, Z.; Huang, S.; Zhou, L.; Zhai, C.; Chen, Y.; Hu, Q.; Cao, W.; Weng, Y.; Li, Y. Delphinidin Attenuates Pathological Cardiac Hypertrophy via the AMPK/NOX/MAPK Signaling Pathway. Aging 2020, 12, 5362–5383. [Google Scholar] [CrossRef]
- Les, F.; Cásedas, G.; Gómez, C.; Moliner, C.; Valero, M.S.; López, V. The Role of Anthocyanins as Antidiabetic Agents: From Molecular Mechanisms to in Vivo and Human Studies. J. Physiol. Biochem. 2021, 77, 109–131. [Google Scholar] [CrossRef]
- Moriwaki, S.; Suzuki, K.; Muramatsu, M.; Nomura, A.; Inoue, F.; Into, T.; Yoshiko, Y.; Niida, S. Delphinidin, One of the Major Anthocyanidins, Prevents Bone Loss through the Inhibition of Excessive Osteoclastogenesis in Osteoporosis Model Mice. PLoS ONE 2014, 9, e97177. [Google Scholar] [CrossRef]
- Murata, M.; Kosaka, R.; Kurihara, K.; Yamashita, S.; Tachibana, H. Delphinidin Prevents Disuse Muscle Atrophy and Reduces Stress-Related Gene Expression. Biosci. Biotechnol. Biochem. 2016, 80, 1636–1640. [Google Scholar] [CrossRef]
- Samarpita, S.; Rasool, M. Cyanidin Attenuates IL-17A Cytokine Signaling Mediated Monocyte Migration and Differentiation into Mature Osteoclasts in Rheumatoid Arthritis. Cytokine 2021, 142, 155502. [Google Scholar] [CrossRef] [PubMed]
- Calland, N.; Sahuc, M.-E.; Belouzard, S.; Pène, V.; Bonnafous, P.; Mesalam, A.A.; Deloison, G.; Descamps, V.; Sahpaz, S.; Wychowski, C.; et al. Polyphenols Inhibit Hepatitis C Virus Entry by a New Mechanism of Action. J. Virol. 2015, 89, 10053–10063. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Calvo, Á.; Jiménez de Oya, N.; Martín-Acebes, M.A.; Garcia-Moruno, E.; Saiz, J.-C. Antiviral Properties of the Natural Polyphenols Delphinidin and Epigallocatechin Gallate against the Flaviviruses West Nile Virus, Zika Virus, and Dengue Virus. Front. Microbiol. 2017, 8, 1314. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, T.; Calderaro, A.; Barreca, D.; Tellone, E.; Trombetta, D.; Ficarra, S.; Smeriglio, A.; Mandalari, G.; Gattuso, G. Biotechnological Applications and Health-Promoting Properties of Flavonols: An Updated View. Int. J. Mol. Sci. 2022, 23, 1710. [Google Scholar] [CrossRef] [PubMed]
- Roewer, N.; Broscheit, J. Use of Delphinidin against Staphylococcus Aureus. U.S. Patent Application No. 14/389,492, 2013. [Google Scholar]
- Hollman, P.C.; Arts, I.C. Flavonols, Flavones and Flavanols-Nature, Occurrence and Dietary Burden. J. Sci. Food Agric. 2000, 80, 1081–1093. [Google Scholar] [CrossRef]
- Rashid, M.I.; Fareed, M.I.; Rashid, H.; Aziz, H.; Ehsan, N.; Khalid, S.; Ghaffar, I.; Ali, R.; Gul, A.; Hakeem, K.R. Flavonoids and Their Biological Secrets. In Plant and Human Health; Springer International Publishing: Cham, Switzerland, 2019; Volume 2, pp. 579–605. [Google Scholar]
- Romeo, J.T.; Bacon, J.D.; Mabry, T.J. Ecological Considerations of Amino Acids and Flavonoids in Sarracenia Species. Biochem. Syst. Ecol. 1977, 5, 117–120. [Google Scholar] [CrossRef]
- Hall, B.; Rapinski, M.; Spoor, D.; Eid, H.; Saleem, A.; Arnason, J.T.; Foster, B.; Cuerrier, A.; Haddad, P.S.; Harris, C.S. A Multivariate Approach to Ethnopharmacology: Antidiabetic Plants of Eeyou Istchee. Front. Pharmacol. 2022, 12, 511078. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Martineau, L.C.; Saleem, A.; Muhammad, A.; Vallerand, D.; Benhaddou-Andaloussi, A.; Nistor, L.; Afshar, A.; Arnason, J.T.; Haddad, P.S. Stimulation of AMP-Activated Protein Kinase and Enhancement of Basal Glucose Uptake in Muscle Cells by Quercetin and Quercetin Glycosides, Active Principles of the Antidiabetic Medicinal Plant Vaccinium Vitis-Idaea. Mol. Nutr. Food Res. 2010, 54, 991–1003. [Google Scholar] [CrossRef]
- Eid, H.M.; Haddad, P.S. Mechanisms of Action of Indigenous Antidiabetic Plants from the Boreal Forest of Northeastern Canada. Adv. Endocrinol. 2014, 2014, 272968. [Google Scholar] [CrossRef]
- Li, S.; Pasquin, S.; Eid, H.M.; Gauchat, J.-F.; Saleem, A.; Haddad, P.S. Anti-Apoptotic Potential of Several Antidiabetic Medicinal Plants of the Eastern James Bay Cree Pharmacopeia in Cultured Kidney Cells. BMC Complement. Altern. Med. 2018, 18, 37. [Google Scholar] [CrossRef]
- Thuan, N.H.; Shrestha, A.; Trung, N.T.; Tatipamula, V.B.; Van Cuong, D.; Canh, N.X.; Van Giang, N.; Kim, T.; Sohng, J.K.; Dhakal, D. Advances in Biochemistry and the Biotechnological Production of Taxifolin and Its Derivatives. Biotechnol. Appl. Biochem. 2022, 69, 848–861. [Google Scholar] [CrossRef] [PubMed]
- Butterweck, V.; Jürgenliemk, G.; Nahrstedt, A.; Winterhoff, H. Flavonoids from Hypericum Perforatum Show Antidepressant Activity in the Forced Swimming Test. Planta Med. 2000, 66, 3–6. [Google Scholar] [CrossRef]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological Basis and New Insights of Taxifolin: A Comprehensive Review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef] [PubMed]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, İ.; Alwasel, S.H. Antioxidant Activity of Taxifolin: An Activity–Structure Relationship. J. Enzyme Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Kolhir, V.K.; Bykov, V.A.; Baginskaja, A.I.; Sokolov, S.Y.; Glazova, N.G.; Leskova, T.E.; Sakovich, G.S.; Tjukavkina, N.A.; Kolesnik, Y.A.; Rulenko, I.A. Antioxidant Activity of a Dihydroquercetin Isolated from Larix Gmelinii (Rupr.) Rupr. Wood. Phyther. Res. 1996, 10, 478–482. [Google Scholar] [CrossRef]
- Dok-Go, H.; Lee, K.H.; Kim, H.J.; Lee, E.H.; Lee, J.; Song, Y.S.; Lee, Y.-H.; Jin, C.; Lee, Y.S.; Cho, J. Neuroprotective Effects of Antioxidative Flavonoids, Quercetin, (+)-Dihydroquercetin and Quercetin 3-Methyl Ether, Isolated from Opuntia Ficus-Indica Var. Saboten. Brain Res. 2003, 965, 130–136. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Wang, W.-Y.; Chang, C.-C.; Liou, K.-T.; Sung, Y.-J.; Liao, J.-F.; Chen, C.-F.; Chang, S.; Hou, Y.-C.; Chou, Y.-C.; et al. Taxifolin Ameliorates Cerebral Ischemia-Reperfusion Injury in Rats through Its Anti-Oxidative Effect and Modulation of NF-Kappa B Activation. J. Biomed. Sci. 2006, 13, 127–141. [Google Scholar] [CrossRef]
- Zhang, H.-Q.; Wang, Y.-J.; Yang, G.-T.; Gao, Q.-L.; Tang, M.-X. Taxifolin Inhibits Receptor Activator of NF-ΚB Ligand-Induced Osteoclastogenesis of Human Bone Marrow-Derived Macrophages in Vitro and Prevents Lipopolysaccharide-Induced Bone Loss in Vivo. Pharmacology 2019, 103, 101–109. [Google Scholar] [CrossRef]
- Pan, S.; Zhao, X.; Ji, N.; Shao, C.; Fu, B.; Zhang, Z.; Wang, R.; Qiu, Y.; Jin, M.; Kong, D. Inhibitory Effect of Taxifolin on Mast Cell Activation and Mast Cell-Mediated Allergic Inflammatory Response. Int. Immunopharmacol. 2019, 71, 205–214. [Google Scholar] [CrossRef]
- Yang, C.-L.; Lin, Y.-S.; Liu, K.-F.; Peng, W.-H.; Hsu, C.-M. Hepatoprotective Mechanisms of Taxifolin on Carbon Tetrachloride-Induced Acute Liver Injury in Mice. Nutrients 2019, 11, 2655. [Google Scholar] [CrossRef]
- Itaya, S.; Igarashi, K. Effects of Taxifolin on the Serum Cholesterol Level in Rats. Biosci. Biotechnol. Biochem. 1992, 56, 1492–1494. [Google Scholar] [CrossRef]
- Igarashi, K.; Uchida, Y.; Murakami, N.; Mizutani, K.; Masuda, H. Effect of Astilbin in Tea Processed from Leaves of Engelhardtia Chrysolepis on the Serum and Liver Lipid Concentrations and on the Erythrocyte and Liver Antioxidative Enzyme Activities of Rats. Biosci. Biotechnol. Biochem. 1996, 60, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Sunil, C.; Xu, B. An Insight into the Health-Promoting Effects of Taxifolin (Dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [CrossRef] [PubMed]
- Wasimul, H.; Shakti, P.P.; Barij, N.S. Evaluation of Taxifolin and Phloretin as Antiangiogenic Flavonoids: An in Vivo, in Vitro Experimental Analysis. Int. J. Pharm. Pharm. Sci. 2015, 7, 72–79. [Google Scholar]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Punia, S.; Mukherjee, T.K. Kaempferol—A Dietary Anticancer Molecule with Multiple Mechanisms of Action: Recent Trends and Advancements. J. Funct. Foods 2017, 30, 203–219. [Google Scholar] [CrossRef]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent Progress Regarding Kaempferol for the Treatment of Various Diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, G.-H. Evaluation of Antioxidant and Inhibitory Activities for Different Subclasses Flavonoids on Enzymes for Rheumatoid Arthritis. J. Food Sci. 2010, 75, H212–H217. [Google Scholar] [CrossRef]
- Shukla, R.; Pandey, V.; Vadnere, G.P.; Lodhi, S. Role of Flavonoids in Management of Inflammatory Disorders. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Elsevier: Amsterdam, The Netherlands, 2019; pp. 293–322. [Google Scholar]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Kong, L.; Luo, C.; Li, X.; Zhou, Y.; He, H. The Anti-Inflammatory Effect of Kaempferol on Early Atherosclerosis in High Cholesterol Fed Rabbits. Lipids Health Dis. 2013, 12, 115. [Google Scholar] [CrossRef]
- Navarro, S.L.; Schwarz, Y.; Song, X.; Wang, C.-Y.; Chen, C.; Trudo, S.P.; Kristal, A.R.; Kratz, M.; Eaton, D.L.; Lampe, J.W. Cruciferous Vegetables Have Variable Effects on Biomarkers of Systemic Inflammation in a Randomized Controlled Trial in Healthy Young Adults. J. Nutr. 2014, 144, 1850–1857. [Google Scholar] [CrossRef]
- Hosseinpour-Niazi, S.; Mirmiran, P.; Fallah-Ghohroudi, A.; Azizi, F. Non-Soya Legume-Based Therapeutic Lifestyle Change Diet Reduces Inflammatory Status in Diabetic Patients: A Randomised Cross-over Clinical Trial. Br. J. Nutr. 2015, 114, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Geybels, M.S.; Verhage, B.A.J.; Arts, I.C.W.; van Schooten, F.J.; Goldbohm, R.A.; van den Brandt, P.A. Dietary Flavonoid Intake, Black Tea Consumption, and Risk of Overall and Advanced Stage Prostate Cancer. Am. J. Epidemiol. 2013, 177, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Vellosa, J.C.R.; Regasini, L.O.; Khalil, N.M.; Bolzani, V.d.S.; Khalil, O.A.K.; Manente, F.A.; Pasquini Netto, H.; Oliveira, O.M.M.d.F. Antioxidant and Cytotoxic Studies for Kaempferol, Quercetin and Isoquercitrin. Eclét. Quím. 2011, 36, 07–20. [Google Scholar] [CrossRef]
- Sharma, V.; Joseph, C.; Ghosh, S.; Agarwal, A.; Mishra, M.K.; Sen, E. Kaempferol Induces Apoptosis in Glioblastoma Cells through Oxidative Stress. Mol. Cancer Ther. 2007, 6, 2544–2553. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Lu, H.-F.; Chou, Y.-C.; Shih, Y.-L.; Bau, D.-T.; Chen, J.-C.; Hsu, S.-C.; Chung, J.-G. Kaempferol Induces DNA Damage and Inhibits DNA Repair Associated Protein Expressions in Human Promyelocytic Leukemia HL-60 Cells. Am. J. Chin. Med. 2015, 43, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xue, L. Kaempferol Suppresses Proliferation and Induces Cell Cycle Arrest, Apoptosis, and DNA Damage in Breast Cancer Cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2019, 27, 629–634. [Google Scholar] [CrossRef]
- Sharma, N.; Biswas, S.; Al-Dayan, N.; Alhegaili, A.S.; Sarwat, M. Antioxidant Role of Kaempferol in Prevention of Hepatocellular Carcinoma. Antioxidants 2021, 10, 1419. [Google Scholar] [CrossRef]
- Yang, G.; Xing, J.; Aikemu, B.; Sun, J.; Zheng, M. Kaempferol Exhibits a Synergistic Effect with Doxorubicin to Inhibit Proliferation, Migration, and Invasion of Liver Cancer. Oncol. Rep. 2021, 45, 32. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular Endothelial Growth Factor as a Target for Anticancer Therapy. Oncologist 2004, 9, 2–10. [Google Scholar] [CrossRef]
- Luo, H.; Rankin, G.O.; Juliano, N.; Jiang, B.-H.; Chen, Y.C. Kaempferol Inhibits VEGF Expression and in Vitro Angiogenesis through a Novel ERK-NFκB-CMyc-P21 Pathway. Food Chem. 2012, 130, 321–328. [Google Scholar] [CrossRef]
- Ramos, S. Effects of Dietary Flavonoids on Apoptotic Pathways Related to Cancer Chemoprevention. J. Nutr. Biochem. 2007, 18, 427–442. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, J.E.T.; Rodrigues, A.L.M.; de Lisboa, D.S.; Liberato, H.R.; Falcão, M.J.C.; da Silva, C.R.; Nobre Júnior, H.V.; Braz Filho, R.; de Paula Junior, V.F.; Alves, D.R.; et al. Chemical Composition and Antifungal In Vitro and In Silico, Antioxidant, and Anticholinesterase Activities of Extracts and Constituents of Ouratea Fieldingiana (DC.) Baill. Evid. Based Complement. Altern. Med. 2018, 2018, 1748487. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, L.; Huleihel, M.; Zaccai, M.; Ben-Shabat, S. Potent Antiviral Flavone Glycosides from Ficus Benjamina Leaves. Fitoterapia 2012, 83, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.-K.; Kwak, S.; Kwon, O.-J.; Bae, J.-S. Hyperoside Inhibits High-Glucose-Induced Vascular Inflammation In Vitro and In Vivo. Inflammation 2014, 37, 1389–1400. [Google Scholar] [CrossRef]
- Jin, X.; Yan, E.; Wang, H.; Sui, H.; Liu, Z.; Gao, W.; Jin, Y. Hyperoside Exerts Anti-Inflammatory and Anti-Arthritic Effects in LPS-Stimulated Human Fibroblast-like Synoviocytes in Vitro and in Mice with Collagen-Induced Arthritis. Acta Pharmacol. Sin. 2016, 37, 674–686. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yue, Z.; Guo, M.; Fang, L.; Bai, L.; Li, X.; Tao, Y.; Wang, S.; Liu, Q.; Zhi, D.; et al. Dietary Flavonoid Hyperoside Induces Apoptosis of Activated Human LX-2 Hepatic Stellate Cell by Suppressing Canonical NF- κ B Signaling. Biomed Res. Int. 2016, 2016, 1068528. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, Y.; Yang, T.; Huang, X.; Zhou, J.; Yang, C.; Zhou, J.; Liu, Y.; Shi, S. Inhibitory Effects of Quercetin and Its Major Metabolite Quercetin-3-O-β-D-glucoside on Human UDP-glucuronosyltransferase 1A Isoforms by Liquid Chromatography-tandem Mass Spectrometry. Exp. Ther. Med. 2021, 22, 842. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and Anti-Inflammatory Activities of Quercetin and Its Derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Krasteva, I.; Nikolova, I.; Danchev, N.; Nikolov, S. Phytochemical Analysis of Ethyl Acetate Extract from Astragalus Corniculatus Bieb. and Brain Antihypoxic Activity. Acta Pharm. 2004, 54, 151–156. [Google Scholar]
- Lee, S.; Lee, J.; Lee, H.; Sung, J. Relative Protective Activities of Quercetin, Quercetin-3-glucoside, and Rutin in Alcohol-induced Liver Injury. J. Food Biochem. 2019, 43, e13002. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Kim, S.J.; Kim, J.H. Quercetin-3-O-Glucoside Suppresses Pancreatic Cancer Cell Migration Induced by Tumor-Deteriorated Growth Factors In Vitro. Oncol. Rep. 2016, 35, 2473–2479. [Google Scholar] [CrossRef]
- Nile, A.; Nile, S.H.; Shin, J.; Park, G.; Oh, J.-W. Quercetin-3-Glucoside Extracted from Apple Pomace Induces Cell Cycle Arrest and Apoptosis by Increasing Intracellular ROS Levels. Int. J. Mol. Sci. 2021, 22, 10749. [Google Scholar] [CrossRef] [PubMed]
- You, H.J.; Ahn, H.J.; Ji, G.E. Transformation of Rutin to Antiproliferative Quercetin-3-Glucoside by Aspergillus Niger. J. Agric. Food Chem. 2010, 58, 10886–10892. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.E.; Kuster, R.M.; Yamamoto, K.A.; Salles, T.S.; Campos, R.; de Meneses, M.D.; Soares, M.R.; Ferreira, D. Quercetin and Quercetin 3-O-Glycosides from Bauhinia Longifolia (Bong.) Steud. Show Anti-Mayaro Virus Activity. Parasit. Vectors 2014, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Zhou, X.; Zhao, C.; Chen, H.; Zhao, Y.; Gong, X. Anti-Inflammatory, Antiviral and Quantitative Study of Quercetin-3-O-β-D-Glucuronide in Polygonum perfoliatum L. Fitoterapia 2011, 82, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Kroeker, A.; He, S.; Kozak, R.; Audet, J.; Mbikay, M.; Chrétien, M. Prophylactic Efficacy of Quercetin 3-β- O-d -Glucoside against Ebola Virus Infection. Antimicrob. Agents Chemother. 2016, 60, 5182–5188. [Google Scholar] [CrossRef] [Green Version]
- Wong, G.; He, S.; Siragam, V.; Bi, Y.; Mbikay, M.; Chretien, M.; Qiu, X. Antiviral Activity of Quercetin-3-β-O-D-Glucoside against Zika Virus Infection. Virol. Sin. 2017, 32, 545–547. [Google Scholar] [CrossRef]
- Sholkamy, E.N.; Muthukrishnan, P.; Abdel-Raouf, N.; Nandhini, X.; Ibraheem, I.B.M.; Mostafa, A.A. Antimicrobial and Antinematicidal Metabolites from Streptomyces Cuspidosporus Strain SA4 against Selected Pathogenic Bacteria, Fungi and Nematode. Saudi J. Biol. Sci. 2020, 27, 3208–3220. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Tyszczuk, K. Sensitive Voltammetric Determination of Rutin at an in Situ Plated Lead Film Electrode. J. Pharm. Biomed. Anal. 2009, 49, 558–561. [Google Scholar] [CrossRef]
- Meng, X.-L.; Yu, M.-M.; Liu, Y.-C.; Gao, Y.-L.; Chen, X.-S.; Shou, S.-T.; Chai, Y.-F. Rutin Inhibits Cardiac Apoptosis and Prevents Sepsis-Induced Cardiomyopathy. Front. Physiol. 2022, 13, 834077. [Google Scholar] [CrossRef] [PubMed]
- Kreft, I.; Fabjan, N.; Yasumoto, K. Rutin Content in Buckwheat (Fagopyrum Esculentum Moench) Food Materials and Products. Food Chem. 2006, 98, 508–512. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. The Beneficial Role of Rutin, A Naturally Occurring Flavonoid in Health Promotion and Disease Prevention: A Systematic Review and Update. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Watson, R.R., Preedy, V., Eds.; Elsevier: London, UK, 2019; pp. 457–479. [Google Scholar]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef] [PubMed]
- Peres, D.A.; de Oliveira, C.A.; da Costa, M.S.; Tokunaga, V.K.; Mota, J.P.; Rosado, C.; Consiglieri, V.O.; Kaneko, T.M.; Velasco, M.V.R.; Baby, A.R. Rutin Increases Critical Wavelength of Systems Containing a Single UV Filter and with Good Skin Compatibility. Ski. Res. Technol. 2016, 22, 325–333. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, S.-N.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J.; Lee, H.K.; Kim, J.; Kwon, S.B.; Kim, M.J.; et al. Biological Effects of Rutin on Skin Aging. Int. J. Mol. Med. 2016, 38, 357–363. [Google Scholar] [CrossRef]
- Pinzaru, I.; Chioibas, R.; Marcovici, I.; Coricovac, D.; Susan, R.; Predut, D.; Georgescu, D.; Dehelean, C. Rutin Exerts Cytotoxic and Senescence-Inducing Properties in Human Melanoma Cells. Toxics 2021, 9, 226. [Google Scholar] [CrossRef]
- Karakurt, S. Modulatory Effects of Rutin on the Expression of Cytochrome P450s and Antioxidant Enzymes in Human Hepatoma Cells. Acta Pharm. 2016, 66, 491–502. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Sitarek, P.; Skała, E.; Toma, M.; Wielanek, M.; Pytel, D.; Wieczfińska, J.; Szemraj, J.; Śliwiński, T. Induction of Apoptosis by in Vitro and in Vivo Plant Extracts Derived from Menyanthes Trifoliata L. in Human Cancer Cells. Cytotechnology 2019, 71, 165–180. [Google Scholar] [CrossRef]
- Turan, I.; Demir, S.; Kilinc, K.; Burnaz, N.A.; Yaman, S.O.; Akbulut, K.; Mentese, A.; Aliyazicioglu, Y.; Deger, O. Antiproliferative and Apoptotic Effect of Morus Nigra Extract on Human Prostate Cancer Cells. Saudi Pharm. J. 2017, 25, 241–248. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Gu, Y.; Lv, Z.; Chen, Y.; Gao, H. Expression of NF-KappaB and P38 under Intervention of Rutin in Lung Cancer Therapy. Biomed. Res. 2017, 28, 2344–2347. [Google Scholar]
- Corsale, I.; Carrieri, P.; Martellucci, J.; Piccolomini, A.; Verre, L.; Rigutini, M.; Panicucci, S. Flavonoid Mixture (Diosmin, Troxerutin, Rutin, Hesperidin, Quercetin) in the Treatment of I–III Degree Hemorroidal Disease: A Double-Blind Multicenter Prospective Comparative Study. Int. J. Colorectal Dis. 2018, 33, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Nasri Nasrabadi, P.; Zareian, S.; Nayeri, Z.; Salmanipour, R.; Parsafar, S.; Gharib, E.; Asadzadeh Aghdaei, H.; Zali, M.R. A Detailed Image of Rutin Underlying Intracellular Signaling Pathways in Human SW480 Colorectal Cancer Cells Based on MiRNAs-lncRNAs-mRNAs-TFs Interactions. J. Cell. Physiol. 2019, 234, 15570–15580. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, Y.; Zhao, C.; Yang, Y.; Xiong, C.; Zhang, D.; Feng, S.; Wu, J.; Wang, X. Rutin Supplementation Reduces Oxidative Stress, Inflammation and Apoptosis of Mammary Gland in Sheep During the Transition Period. Front. Vet. Sci. 2022, 9, 907299. [Google Scholar] [CrossRef] [PubMed]
- Sachetto, A.T.A.; Rosa, J.G.; Santoro, M.L. Rutin (Quercetin-3-Rutinoside) Modulates the Hemostatic Disturbances and Redox Imbalance Induced by Bothrops Jararaca Snake Venom in Mice. PLoS Negl. Trop. Dis. 2018, 12, e0006774. [Google Scholar] [CrossRef]
- Sachetto, A.T.A.; Miyamoto, J.G.; Tashima, A.K.; de Souza, A.O.; Santoro, M.L. The Bioflavonoids Rutin and Rutin Succinate Neutralize the Toxins of B. Jararaca Venom and Inhibit Its Lethality. Front. Pharmacol. 2022, 13, 828269. [Google Scholar] [CrossRef]
- Di Pierro, F.; Derosa, G.; Maffioli, P.; Bertuccioli, A.; Togni, S.; Riva, A.; Allegrini, P.; Khan, A.; Khan, S.; Khan, B.A.; et al. Possible Therapeutic Effects of Adjuvant Quercetin Supplementation Against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study. Int. J. Gen. Med. 2021, 14, 2359–2366. [Google Scholar] [CrossRef]
- Di Pierro, F.; Iqtadar, S.; Khan, A.; Ullah Mumtaz, S.; Masud Chaudhry, M.; Bertuccioli, A.; Derosa, G.; Maffioli, P.; Togni, S.; Riva, A.; et al. Potential Clinical Benefits of Quercetin in the Early Stage of COVID-19: Results of a Second, Pilot, Randomized, Controlled and Open-Label Clinical Trial. Int. J. Gen. Med. 2021, 14, 2807–2816. [Google Scholar] [CrossRef]
- Kumari, A.; Rajput, V.S.; Nagpal, P.; Kukrety, H.; Grover, S.; Grover, A. Dual Inhibition of SARS-CoV-2 Spike and Main Protease through a Repurposed Drug, Rutin. J. Biomol. Struct. Dyn. 2022, 40, 4987–4999. [Google Scholar] [CrossRef]
- Youn, H.S.; Lee, J.Y.; Saitoh, S.I.; Miyake, K.; Kang, K.W.; Choi, Y.J.; Hwang, D.H. Suppression of MyD88- and TRIF-Dependent Signaling Pathways of Toll-like Receptor by (−)-Epigallocatechin-3-Gallate, a Polyphenol Component of Green Tea. Biochem. Pharmacol. 2006, 72, 850–859. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lu, T.-H.; Su, C.-C.; Lay, I.-S.; Lin, H.-Y.; Fang, K.-M.; Ho, T.-J.; Chen, K.-L.; Su, Y.-C.; Chiang, W.-C.; et al. Lotus Leaf (Nelumbo Nucifera) and Its Active Constituents Prevent Inflammatory Responses in Macrophages via JNK/NF-ΚB Signaling Pathway. Am. J. Chin. Med. 2014, 42, 869–889. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-Y.; Sang, L.-X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Zhou, Y.; Luo, X.; Ruan, Y.; Zhou, L.; Wang, Q.; Yan, Y.J.; Liu, Q.; Chen, J. Against NF-ΚB/Thymic Stromal Lymphopoietin Signaling Pathway, Catechin Alleviates the Inflammation in Allergic Rhinitis. Int. Immunopharmacol. 2018, 61, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-Inflammatory Action of Green Tea. Antiinflamm. Antiallergy Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Savickas, A.; Vetchý, D.; Masteikova, R.; Kasauskas, A.; Bernatoniene, J. Direct Effects of (−)-Epicatechin and Procyanidin B2 on the Respiration of Rat Heart Mitochondria. Biomed Res. Int. 2015, 2015, 232836. [Google Scholar] [CrossRef]
- Xie, H.; Li, X.; Ren, Z.; Qiu, W.; Chen, J.; Jiang, Q.; Chen, B.; Chen, D. Antioxidant and Cytoprotective Effects of Tibetan Tea and Its Phenolic Components. Molecules 2018, 23, 179. [Google Scholar] [CrossRef] [PubMed]
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Antioxidant Role of Catechin in Health and Disease. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 267–271. [Google Scholar]
- Ono, K.; Yoshiike, Y.; Takashima, A.; Hasegawa, K.; Naiki, H.; Yamada, M. Potent Anti-Amyloidogenic and Fibril-Destabilizing Effects of Polyphenols in Vitro: Implications for the Prevention and Therapeutics of Alzheimer’s Disease. J. Neurochem. 2003, 87, 172–181. [Google Scholar] [CrossRef]
- Omar, S.H. Biophenols. In Discovery and Development of Neuroprotective Agents from Natural Products; Elsevier: Amsterdam, The Netherlands, 2018; pp. 103–148. [Google Scholar]
- Han, S.; Kollmer, M.; Markx, D.; Claus, S.; Walther, P.; Fändrich, M. Amyloid Plaque Structure and Cell Surface Interactions of β-Amyloid Fibrils Revealed by Electron Tomography. Sci. Rep. 2017, 7, 43577. [Google Scholar] [CrossRef] [Green Version]
- Ide, K.; Matsuoka, N.; Yamada, H.; Furushima, D.; Kawakami, K. Effects of Tea Catechins on Alzheimer’s Disease: Recent Updates and Perspectives. Molecules 2018, 23, 2357. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research Advances in Their Phytochemistry, Biological Activities, and Pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef] [PubMed]
- Tor-Anyiin, T.A.; Igoli, J.O.; Anyam, J.V.; Anyam, J.N. Isolation and Antimicrobial Activity of Sarracenin from Root Bark of Strychnos Spinosa. J. Chem. Soc. Niger. 2015, 40, 71–75. [Google Scholar]
- Dong, Y.; Feng, Z.-L.; Chen, H.-B.; Wang, F.-S.; Lu, J.-H. Corni Fructus: A Review of Chemical Constituents and Pharmacological Activities. Chin. Med. 2018, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B. Pharmacology and Applications of Naturally Occurring Iridoids, 1st ed.; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Liu, T.; Sun, F.; Cui, J.; Zheng, S.; Li, Z.; Guo, D.; Tian, X.; Zhu, Z.; Zheng, W.; Wang, Y.; et al. Morroniside Enhances Angiogenesis and Improves Cardiac Function Following Acute Myocardial Infarction in Rats. Eur. J. Pharmacol. 2020, 872, 172954. [Google Scholar] [CrossRef] [PubMed]
- Pi, W.-X.; Feng, X.-P.; Ye, L.-H.; Cai, B.-C. Combination of Morroniside and Diosgenin Prevents High Glucose-Induced Cardiomyocytes Apoptosis. Molecules 2017, 22, 163. [Google Scholar] [CrossRef]
- Hu, N.; Ren, S.; Li, W.; Zhang, T.; Zhao, C. Morroniside Promotes Bone Marrow Mesenchymal Stem Cell Proliferation in Rats. Mol. Med. Rep. 2013, 7, 1565–1570. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Lin, J.; Lin, M. Morroniside Promotes the Osteogenesis by Activating PI3K/Akt/MTOR Signaling. Biosci. Biotechnol. Biochem. 2021, 85, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zeng, G.; Liu, Z.; Zhang, B.; Cui, X.; Zhao, H.; Zheng, X.; Song, G.; Kang, J.; Xia, C. Protein Kinase B and Extracellular Signal-regulated Kinase Contribute to the Chondroprotective Effect of Morroniside on Osteoarthritis Chondrocytes. J. Cell. Mol. Med. 2015, 19, 1877–1886. [Google Scholar] [CrossRef]
- Park, E.; Lee, C.G.; Han, S.J.; Yun, S.H.; Hwang, S.; Jeon, H.; Kim, J.; Choi, C.W.; Yang, S.; Jeong, S.-Y. Antiosteoarthritic Effect of Morroniside in Chondrocyte Inflammation and Destabilization of Medial Meniscus-Induced Mouse Model. Int. J. Mol. Sci. 2021, 22, 2987. [Google Scholar] [CrossRef]
- Yang, C.; Kuai, X.; Gao, W.; Yu, J.; Wang, Q.; Li, L.; Zhang, L. Morroniside-Induced PP2A Activation Antagonizes Tau Hyperphosphorylation in a Cellular Model of Neurodegeneration. J. Alzheimer’s Dis. 2016, 51, 33–44. [Google Scholar] [CrossRef]
- Chen, K.; Lu, Y.; Liu, C.; Zhang, L.; Fang, Z.; Yu, G. Morroniside Prevents H2O2 or Aβ1–42-Induced Apoptosis via Attenuating JNK and P38 MAPK Phosphorylation. Eur. J. Pharmacol. 2018, 834, 295–304. [Google Scholar] [CrossRef]
- Wang, W.; Sun, F.; An, Y.; Ai, H.; Zhang, L.; Huang, W.; Li, L. Morroniside Protects Human Neuroblastoma SH-SY5Y Cells against Hydrogen Peroxide-Induced Cytotoxicity. Eur. J. Pharmacol. 2009, 613, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-X.; Wang, R.; Xi, J.; Shen, L.; Zhu, A.-Y.; Qi, Q.; Wang, Q.-Y.; Zhang, L.-J.; Wang, F.-C.; Lü, H.-Z.; et al. Morroniside Protects SK-N-SH Human Neuroblastoma Cells against H2O2-Induced Damage. Int. J. Mol. Med. 2017, 39, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.-X.; Shi, Y.-J.; Chen, J.; Song, X.; Shen, L.; Qi, Q.; Ding, S.-Q.; Wang, Q.-Y.; Wang, R.; Lü, H.-Z.; et al. The Neuroprotective Role of Morroniside against Spinal Cord Injury in Female Rats. Neurochem. Int. 2021, 148, 105105. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, H.; Jing, J.; Yu, L.; Wu, X.; Lu, Z. Morroniside Regulates Hair Growth and Cycle Transition via Activation of the Wnt/β-Catenin Signaling Pathway. Sci. Rep. 2018, 8, 13785. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gao, Q.; Shang, Z.; Liu, J.; Zhou, S.; Dang, J.; Liu, L.; Lange, I.; Srividya, N.; Lange, B.M.; et al. Functional Characterization and Structural Insights Into Stereoselectivity of Pulegone Reductase in Menthol Biosynthesis. Front. Plant Sci. 2021, 12, 780970. [Google Scholar] [CrossRef]
- Božović, M.; Ragno, R. Calamintha Nepeta (L.) Savi and Its Main Essential Oil Constituent Pulegone: Biological Activities and Chemistry. Molecules 2017, 22, 290. [Google Scholar] [CrossRef]
- Franzios, G.; Mirotsou, M.; Hatziapostolou, E.; Kral, J.; Scouras, Z.G.; Mavragani-Tsipidou, P. Insecticidal and Genotoxic Activities of Mint Essential Oils. J. Agric. Food Chem. 1997, 45, 2690–2694. [Google Scholar] [CrossRef]
- Gunderson, C.A.; Samuelian, J.H.; Evans, C.K.; Brattsten, L.B. Effects of the Mint Monoterpene Pulegone on Spodoptera Eridania (Lepidoptera: Noctuidae). Environ. Entomol. 1985, 14, 859–861. [Google Scholar] [CrossRef]
- Scortichini, M.; Rossi, M.P. Preliminary in Vitro Evaluation of the Antimicrobial Activity of Terpenes and Terpenoids towards Erwinia Amylovora (Burrill) Winslow et Al. J. Appl. Bacteriol. 1991, 71, 109–112. [Google Scholar] [CrossRef]
- Dhingra, A.K.; Chopra, B.; Bhardwaj, S.; Dhar, K.L. Synthesis and Characterization of Novel Pulegone Derivatives as Substitutes of 4-(1,1 Dimethylethyl) Cyclohexan-1-Ol Acetate. J. Pharm. Res. 2011, 4, 19–21. [Google Scholar]
- González-Chávez, M.M.; Cárdenas-Ortega, N.C.; Méndez-Ramos, C.A.; Pérez-Gutiérrez, S. Fungicidal Properties of the Essential Oil of Hesperozygis Marifolia on Aspergillus Flavus Link. Molecules 2011, 16, 2501–2506. [Google Scholar] [CrossRef] [PubMed]
- de Urbina, A.V.O.; Martin, M.L.; Montero, M.J.; Carron, R.; Sevilla, M.A.; San Roman, L. Antihistaminic Activity of Pulegone on the Guinea-Pig Ileum. J. Pharm. Pharmacol. 2011, 42, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Park, H.-J.; Abdul, Q.A.; Jung, H.A.; Choi, J.S. Pulegone Exhibits Anti-Inflammatory Activities through the Regulation of NF-ΚB and Nrf-2 Signaling Pathways in LPS-Stimulated RAW 264.7 Cells. Nat. Prod. Sci. 2018, 24, 28. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.; Barbieri, R.; Silva, A.; Nabavi, S.; Tsetegho Sokeng, A.; Izadi, M.; Jafari, N.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health Beneficial and Pharmacological Properties of P-Cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef]
- Nazaruk, J.; Borzym-Kluczyk, M. The Role of Triterpenes in the Management of Diabetes Mellitus and Its Complications. Phytochem. Rev. 2015, 14, 675–690. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and Betulinic Acid: Triterpenoids Derivatives with a Powerful Biological Potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Pilarska, K.M.; Panić, M.; Redovniković, I.R.; Wróbel-Kwiatkowska, M. Characterization of Carnivorous Plants Sarracenia Purpurea L. Transformed with Agrobacterium Rhizogenes. Mapp. Intimacies 2021. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Chiang, W.-Y.; Chen, P.-J.; Lin, E.-S.; Huang, C.-Y. Anticancer and Antioxidant Activities of the Root Extract of the Carnivorous Pitcher Plant Sarracenia Purpurea. Plants 2022, 11, 1668. [Google Scholar] [CrossRef]
- Liby, K.; Honda, T.; Williams, C.R.; Risingsong, R.; Royce, D.B.; Suh, N.; Dinkova-Kostova, A.T.; Stephenson, K.K.; Talalay, P.; Sundararajan, C.M.; et al. Novel Semisynthetic Analogues of Betulinic Acid with Diverse Cytoprotective, Antiproliferative, and Proapoptotic Activities. Mol. Cancer Ther. 2007, 6, 2113–2119. [Google Scholar] [CrossRef]
- Shankar, E.; Zhang, A.; Franco, D.; Gupta, S. Betulinic Acid-Mediated Apoptosis in Human Prostate Cancer Cells Involves P53 and Nuclear Factor-Kappa B (NF-ΚB) Pathways. Molecules 2017, 22, 264. [Google Scholar] [CrossRef] [PubMed]
- Bębenek, E.; Chrobak, E.; Piechowska, A.; Głuszek, S.B. Betulin: A Natural Product with Promising Anticancer Activity against Colorectal Cancer Cells. Med. Stud. Med. 2020, 36, 298–302. [Google Scholar] [CrossRef]
- Dash, S.K.; Chattopadhyay, S.; Dash, S.S.; Tripathy, S.; Das, B.; Mahapatra, S.K.; Bag, B.G.; Karmakar, P.; Roy, S. Self Assembled Nano Fibers of Betulinic Acid: A Selective Inducer for ROS/TNF-Alpha Pathway Mediated Leukemic Cell Death. Bioorg. Chem. 2015, 63, 85–100. [Google Scholar] [CrossRef]
- Zhan, X.; Li, J.; Zhang, S.; Xing, P.; Xia, M. Betulinic Acid Exerts Potent Antitumor Effects on Paclitaxel-resistant Human Lung Carcinoma Cells (H460) via G2/M Phase Cell Cycle Arrest and Induction of Mitochondrial Apoptosis. Oncol. Lett. 2018, 16, 3628–3634. [Google Scholar] [CrossRef]
- Saeed, M.E.M.; Mahmoud, N.; Sugimoto, Y.; Efferth, T.; Abdel-Aziz, H. Betulinic Acid Exerts Cytotoxic Activity Against Multidrug-Resistant Tumor Cells via Targeting Autocrine Motility Factor Receptor (AMFR). Front. Pharmacol. 2018, 9, 481. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Pang, Q.; Wang, Y.; Yan, X. Betulinic Acid Induces Apoptosis by Regulating PI3K/Akt Signaling and Mitochondrial Pathways in Human Cervical Cancer Cells. Int. J. Mol. Med. 2017, 40, 1669–1678. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Li, Q.-J.; Feng, Y.-L.; Pan, F. Betulinic Acid Promotes TRAIL Function on Liver Cancer Progression Inhibition through P53/Caspase-3 Signaling Activation. Biomed. Pharmacother. 2017, 88, 349–358. [Google Scholar] [CrossRef]
- Yang, C.; Li, Y.; Fu, L.; Jiang, T.; Meng, F. Betulinic Acid Induces Apoptosis and Inhibits Metastasis of Human Renal Carcinoma Cells in Vitro and in Vivo. J. Cell. Biochem. 2018, 119, 8611–8622. [Google Scholar] [CrossRef]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.-R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and Its Derivatives as Novel Compounds with Different Pharmacological Effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef]
- Viji, V.; Helen, A.; Luxmi, V.R. Betulinic Acid Inhibits Endotoxin-Stimulated Phosphorylation Cascade and pro-Inflammatory Prostaglandin E2 Production in Human Peripheral Blood Mononuclear Cells. Br. J. Pharmacol. 2011, 162, 1291–1303. [Google Scholar] [CrossRef]
- Nader, M.A.; Baraka, H.N. Effect of Betulinic Acid on Neutrophil Recruitment and Inflammatory Mediator Expression in Lipopolysaccharide-Induced Lung Inflammation in Rats. Eur. J. Pharm. Sci. 2012, 46, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Z.; Xiong, F.; Chen, C.; Chao, X.; Huang, J.; Huang, H. Betulinic Acid Ameliorates Experimental Diabetic-Induced Renal Inflammation and Fibrosis via Inhibiting the Activation of NF-ΚB Signaling Pathway. Mol. Cell. Endocrinol. 2016, 434, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Kaundal, M.; Deshmukh, R.; Akhtar, M. Protective Effect of Betulinic Acid against Intracerebroventricular Streptozotocin Induced Cognitive Impairment and Neuronal Damage in Rats: Possible Neurotransmitters and Neuroinflammatory Mechanism. Pharmacol. Rep. 2018, 70, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Navabi, S.P.; Sarkaki, A.; Mansouri, E.; Badavi, M.; Ghadiri, A.; Farbood, Y. The Effects of Betulinic Acid on Neurobehavioral Activity, Electrophysiology and Histological Changes in an Animal Model of the Alzheimer’s Disease. Behav. Brain Res. 2018, 337, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Oloyede, H.O.B.; Ajiboye, H.O.; Salawu, M.O.; Ajiboye, T.O. Influence of Oxidative Stress on the Antibacterial Activity of Betulin, Betulinic Acid and Ursolic Acid. Microb. Pathog. 2017, 111, 338–344. [Google Scholar] [CrossRef]
- Chintharlapalli, S.; Papineni, S.; Ramaiah, S.K.; Safe, S. Betulinic Acid Inhibits Prostate Cancer Growth through Inhibition of Specificity Protein Transcription Factors. Cancer Res. 2007, 67, 2816–2823. [Google Scholar] [CrossRef]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective Cytotoxicity of Betulinic Acid on Tumor Cell Lines, but Not on Normal Cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Małaczewska, J.; Kaczorek-Łukowska, E.; Kazuń, B. High Cytotoxicity of Betulin towards Fish and Murine Fibroblasts: Is Betulin Safe for Nonneoplastic Cells? BMC Vet. Res. 2021, 17, 198. [Google Scholar] [CrossRef]
- Fernández, M.A.; de las Heras, B.; Garcia, M.D.; Sáenz, M.T.; Villar, A. New Insights into the Mechanism of Action of the Anti-Inflammatory Triterpene Lupeol. J. Pharm. Pharmacol. 2010, 53, 1533–1539. [Google Scholar] [CrossRef]
- Ahmad, R.; Khan, A.; Lee, H.J.; Ur Rehman, I.; Khan, I.; Alam, S.I.; Kim, M.O. Lupeol, a Plant-Derived Triterpenoid, Protects Mice Brains against Aβ-Induced Oxidative Stress and Neurodegeneration. Biomedicines 2020, 8, 380. [Google Scholar] [CrossRef]
- Saleem, M.; Kweon, M.H.; Yun, J.M.; Adhami, V.M.; Khan, N.; Syed, D.N.; Mukhtar, H. A Novel Dietary Triterpene Lupeol Induces Fas-Mediated Apoptotic Death of Androgen-Sensitive Prostate Cancer Cells and Inhibits Tumor Growth in a Xenograft Model. Cancer Res. 2005, 65, 11203–11213. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Murtaza, I.; Tarapore, R.S.; Suh, Y.; Adhami, V.M.; Johnson, J.J.; Siddiqui, I.A.; Khan, N.; Asim, M.; Hafeez, B.B.; et al. Lupeol Inhibits Proliferation of Human Prostate Cancer Cells by Targeting -Catenin Signaling. Carcinogenesis 2009, 30, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, Y.J.; Chung, S.O.; Park, S.U. Recent Studies on Ursolic Acid and Its Biological and Pharmacological Activity. EXCLI J. 2016, 15, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Schito, A.M.; Caviglia, D.; Piatti, G.; Zorzoli, A.; Marimpietri, D.; Zuccari, G.; Schito, G.C.; Alfei, S. Efficacy of Ursolic Acid-Enriched Water-Soluble and Not Cytotoxic Nanoparticles against Enterococci. Pharmaceutics 2021, 13, 1976. [Google Scholar] [CrossRef]
- Weng, H.; Tan, Z.-J.; Hu, Y.-P.; Shu, Y.-J.; Bao, R.-F.; Jiang, L.; Wu, X.-S.; Li, M.-L.; Ding, Q.; Wang, X.; et al. Ursolic Acid Induces Cell Cycle Arrest and Apoptosis of Gallbladder Carcinoma Cells. Cancer Cell Int. 2014, 14, 96. [Google Scholar] [CrossRef]
- Castro, A.J.G.; Frederico, M.J.S.; Cazarolli, L.H.; Mendes, C.P.; Bretanha, L.C.; Schmidt, É.C.; Bouzon, Z.L.; de Medeiros Pinto, V.A.; da Fonte Ramos, C.; Pizzolatti, M.G.; et al. The Mechanism of Action of Ursolic Acid as Insulin Secretagogue and Insulinomimetic Is Mediated by Cross-Talk between Calcium and Kinases to Regulate Glucose Balance. Biochim. Biophys. Acta-Gen. Subj. 2015, 1850, 51–61. [Google Scholar] [CrossRef]
- Kim, S.-G.; Kim, M.-J.; Jin, D.-C.; Park, S.-N.; Cho, E.-G.; Freire, M.O.; Jang, S.-J.; Park, Y.-J.; Kook, J.-K. Antimicrobial Effect of Ursolic Acid and Oleanolic Acid against Methicillin-Resistant Staphylococcus Aureus. Korean J. Microbiol. 2012, 48, 212–215. [Google Scholar] [CrossRef]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial Activity of Oleanolic and Ursolic Acids: An Update. Evid. Based Complement. Altern. Med. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Jiménez-Arellanes, A.; Luna-Herrera, J.; Cornejo-Garrido, J.; López-García, S.; Castro-Mussot, M.E.; Meckes-Fischer, M.; Mata-Espinosa, D.; Marquina, B.; Torres, J.; Hernández-Pando, R. Ursolic and Oleanolic Acids as Antimicrobial and Immunomodulatory Compounds for Tuberculosis Treatment. BMC Complement. Altern. Med. 2013, 13, 258. [Google Scholar] [CrossRef] [Green Version]
- Shakhtshneider, T.P.; Kuznetsova, S.A.; Zamay, A.S.; Zamay, T.N.; Spivak, E.A.; Mikhailenko, M.A.; Malyar, Y.N.; Kuznetsov, B.N.; Chesnokov, N.V.; Boldyrev, V.V. New Composites of Betulin Esters with Arabinogalactan as Highly Potent Anti-Cancer Agents. Nat. Prod. Res. 2016, 30, 1382–1387. [Google Scholar] [CrossRef]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The Pentacyclic Triterpenoids in Herbal Medicines and Their Pharmacological Activities in Diabetes and Diabetic Complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar] [CrossRef] [PubMed]
- Dinku, W.; Isaksson, J.; Rylandsholm, F.G.; Bouř, P.; Brichtová, E.; Choi, S.U.; Lee, S.-H.; Jung, Y.-S.; No, Z.S.; Svendsen, J.S.M.; et al. Anti-Proliferative Activity of a Novel Tricyclic Triterpenoid Acid from Commiphora Africana Resin against Four Human Cancer Cell Lines. Appl. Biol. Chem. 2020, 63, 16. [Google Scholar] [CrossRef]
- Biskup, E.; Gołębiowski, M.; Gniadecki, R.; Stepnowski, P.; Łojkowska, E. Triterpenoid A-amyrin Stimulates Proliferation of Human Keratinocytes but Does Not Protect Them against UVB Damage. Acta Biochim. Pol. 2012, 59, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.O.; Oliveira, Y.I.S.; Adjafre, B.L.; de Moraes, M.E.A.; Aragão, G.F. Pharmacological Effects of the Isomeric Mixture of Alpha and Beta Amyrin from Protium Heptaphyllum: A Literature Review. Fundam. Clin. Pharmacol. 2019, 33, 4–12. [Google Scholar] [CrossRef]
- Babu, S.; Jayaraman, S. An Update on β-Sitosterol: A Potential Herbal Nutraceutical for Diabetic Management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef]
- Vivancos, M.; Moreno, J.J. β-Sitosterol Modulates Antioxidant Enzyme Response in RAW 264.7 Macrophages. Free Radic. Biol. Med. 2005, 39, 91–97. [Google Scholar] [CrossRef]
- Ponnulakshmi, R.; Shyamaladevi, B.; Vijayalakshmi, P.; Selvaraj, J. In Silico and in Vivo Analysis to Identify the Antidiabetic Activity of Beta Sitosterol in Adipose Tissue of High Fat Diet and Sucrose Induced Type-2 Diabetic Experimental Rats. Toxicol. Mech. Methods 2019, 29, 276–290. [Google Scholar] [CrossRef]
- Loizou, S.; Lekakis, I.; Chrousos, G.P.; Moutsatsou, P. β-Sitosterol Exhibits Anti-Inflammatory Activity in Human Aortic Endothelial Cells. Mol. Nutr. Food Res. 2010, 54, 551–558. [Google Scholar] [CrossRef]
- Takemoto, Y.; Kishi, C.; Sugiura, Y.; Yoshioka, Y.; Matsumura, S.; Moriyama, T.; Zaima, N. Distribution of Inhaled Volatile β-Caryophyllene and Dynamic Changes of Liver Metabolites in Mice. Sci. Rep. 2021, 11, 1728. [Google Scholar] [CrossRef]
- Hendriks, H.; Malingré, T.M.; Batterman, S.; Bos, R. Mono- and Sesqui-Terpene Hydrocarbons of the Essential Oil of Cannabis Sativa. Phytochemistry 1975, 14, 814–815. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β -Caryophyllene and β -Caryophyllene Oxide-Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Xing, C.; Qin, C.; Li, X.; Zhang, F.; Linhardt, R.J.; Sun, P.; Zhang, A. Chemical Composition and Biological Activities of Essential Oil Isolated by HS-SPME and UAHD from Fruits of Bergamot. LWT 2019, 104, 38–44. [Google Scholar] [CrossRef]
- Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor Activity of Palmitic Acid Found as a Selective Cytotoxic Substance in a Marine Red Alga. Anticancer Res. 2002, 22, 2587–2590. [Google Scholar] [PubMed]
- Karna, S.; Lim, W.B.; Kim, J.S.; Kim, S.W.; Zheng, H.; Bae, K.H.; Cho, M.S.; Oh, H.K.; Kim, O.S.; Choi, H.R.; et al. C16 Saturated Fatty Acid Induced Autophagy in A549 Cells through Topoisomerase I Inhibition. Food Nutr. Sci. 2012, 3, 1220–1227. [Google Scholar] [CrossRef]
- Tan, J.; Tian, Y.; Cai, R.; Luo, R.; Guo, J. Chemical Composition and Antiproliferative Effects of a Methanol Extract of Aspongopus Chinensis Dallas. Evid. Based Complement. Altern. Med. 2019, 2019, 2607086. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, Y.; Han, D.; Lu, J.; Yin, S.; Hu, H.; Zhao, C. Combination of Palmitic Acid and Methylseleninic Acid Induces Mitochondria-Dependent Apoptosis via Attenuation of the IRE1α Arm and Enhancement of CHOP in Hepatoma. ACS Omega 2021, 6, 15708–15715. [Google Scholar] [CrossRef]
- Mericli, F.; Becer, E.; Kabadayı, H.; Hanoglu, A.; Yigit Hanoglu, D.; Ozkum Yavuz, D.; Ozek, T.; Vatansever, S. Fatty Acid Composition and Anticancer Activity in Colon Carcinoma Cell Lines of Prunus Dulcis Seed Oil. Pharm. Biol. 2017, 55, 1239–1248. [Google Scholar] [CrossRef]
- Al-Hwaiti, M.S.; Alsbou, E.M.; Abu Sheikha, G.; Bakchiche, B.; Pham, T.H.; Thomas, R.H.; Bardaweel, S.K. Evaluation of the Anticancer Activity and Fatty Acids Composition of “Handal” (Citrullus Colocynthis L.) Seed Oil, a Desert Plant from South Jordan. Food Sci. Nutr. 2021, 9, 282–289. [Google Scholar] [CrossRef]
- Zeng, L.; Wu, G.-Z.; Goh, K.J.; Lee, Y.M.; Ng, C.C.; You, A.B.; Wang, J.; Jia, D.; Hao, A.; Yu, Q.; et al. Saturated Fatty Acids Modulate Cell Response to DNA Damage: Implication for Their Role in Tumorigenesis. PLoS ONE 2008, 3, e2329. [Google Scholar] [CrossRef]
- Fatima, S.; Hu, X.; Huang, C.; Zhang, W.; Cai, J.; Huang, M.; Gong, R.-H.; Chen, M.; Ho, A.H.M.; Su, T.; et al. High-Fat Diet Feeding and Palmitic Acid Increase CRC Growth in Β2AR-Dependent Manner. Cell Death Dis. 2019, 10, 711. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Domínguez, D.; Elosúa-Bayes, M.; Beckedorff, F.; Laudanna, C.; Bigas, C.; Douillet, D.; Greco, C.; Symeonidi, A.; Hernández, I.; et al. Dietary Palmitic Acid Promotes a Prometastatic Memory via Schwann Cells. Nature 2021, 599, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Torres, S.; Baulies, A.; Alarcón-Vila, C.; Elena, M.; Fabriàs, G.; Casas, J.; Caballeria, J.; Fernandez-Checa, J.C.; García-Ruiz, C. Myristic Acid Potentiates Palmitic Acid-Induced Lipotoxicity and Steatohepatitis Associated with Lipodystrophy by Sustaning de Novo Ceramide Synthesis. Oncotarget 2015, 6, 41479–41496. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhou, M.; Song, L. A Review of Fatty Acids Influencing Skin Condition. J. Cosmet. Dermatol. 2020, 19, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- Frommer, K.W.; Hasseli, R.; Schäffler, A.; Lange, U.; Rehart, S.; Steinmeyer, J.; Rickert, M.; Sarter, K.; Zaiss, M.M.; Culmsee, C.; et al. Free Fatty Acids in Bone Pathophysiology of Rheumatic Diseases. Front. Immunol. 2019, 10, 2757. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Jiang, X.; Chen, K.; Liang, Q.; Zhang, S.; Zheng, J.; Ma, X.; Jiang, H.; Wu, H.; Tong, Q. The Role of Palmitoleic Acid in Regulating Hepatic Gluconeogenesis through SIRT3 in Obese Mice. Nutrients 2022, 14, 1482. [Google Scholar] [CrossRef]
- Astudillo, A.M.; Meana, C.; Bermúdez, M.A.; Pérez-Encabo, A.; Balboa, M.A.; Balsinde, J. Release of Anti-Inflammatory Palmitoleic Acid and Its Positional Isomers by Mouse Peritoneal Macrophages. Biomedicines 2020, 8, 480. [Google Scholar] [CrossRef]
- Bermúdez, M.A.; Pereira, L.; Fraile, C.; Valerio, L.; Balboa, M.A.; Balsinde, J. Roles of Palmitoleic Acid and Its Positional Isomers, Hypogeic and Sapienic Acids, in Inflammation, Metabolic Diseases and Cancer. Cells 2022, 11, 2146. [Google Scholar] [CrossRef]
- Watanabe, T.; Yano, S.; Kawai, T.; Jinbo, Y.; Nonomura, Y. Selective Antibacterial Activity of Palmitoleic Acid in Emulsions and Other Formulations. J. Surfactants Deterg. 2021, 24, 973–979. [Google Scholar] [CrossRef]
- Reynolds, T. Hemlock Alkaloids from Socrates to Poison Aloes. Phytochemistry 2005, 66, 1399–1406. [Google Scholar] [CrossRef]
- Erkent, U.; Iskit, A.B.; Onur, R.; Ilhan, M. The Effect of Coniine on Presynaptic Nicotinic Receptors. Z. Naturforsch. C 2016, 71, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hotti, H.; Rischer, H. The Killer of Socrates: Coniine and Related Alkaloids in the Plant Kingdom. Molecules 2017, 22, 1962. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-M.; Irino, N.; Furusho, N.; Hayashi, J.; Shoyama, Y. Pharmacologically Active Compounds in the Anoectochilus and Goodyera Species. J. Nat. Med. 2008, 62, 132–148. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Sun, Y.; Xu, L.; Sun, Y.; Li, T.; Peng, P.; Lou, H. Synthesis of Nature Product Kinsenoside Analogues with Anti-Inflammatory Activity. Bioorg. Med. Chem. 2021, 29, 115854. [Google Scholar] [CrossRef]
- De, S.; Kumar, S.K.A.; Shah, S.K.; Kazi, S.; Sarkar, N.; Banerjee, S.; Dey, S. Pyridine: The Scaffolds with Significant Clinical Diversity. RSC Adv. 2022, 12, 15385–15406. [Google Scholar] [CrossRef]
- Zaman, W.; Ye, J.; Saqib, S.; Liu, Y.; Shan, Z.; Hao, D.; Chen, Z.; Xiao, P. Predicting Potential Medicinal Plants with Phylogenetic Topology: Inspiration from the Research of Traditional Chinese Medicine. J. Ethnopharmacol. 2021, 281, 114515. [Google Scholar] [CrossRef]
- Zaman, W.; Ye, J.; Ahmad, M.; Saqib, S.; Shinwari, Z.K.; Chen, Z. Phylogenetic Exploration of Traditional Chinese Medicinal Plants: A Case Study on Lamiaceae (Angiosperms). Pak. J. Bot. 2022, 54, 1033–1040. [Google Scholar] [CrossRef]
- Uhnak, K.S. Micropropagation of Carnivorous Plants; University of Rhode Island: Kingston, Jamaica, 2003. [Google Scholar]
- Northcutt, C.; Davies, D.; Gagliardo, R.; Bucalo, K.; Determann, R.O.; Cruse-Sanders, J.M.; Pullman, G.S. Germination in Vitro, Micropropagation, and Cryogenic Storage for Three Rare Pitcher Plants: Sarracenia Oreophila (Kearney) Wherry (Federally Endangered), S. Leucophylla Raf., and S. Purpurea Spp. Venosa (Raf.) Wherry. HortScience 2012, 47, 74–80. [Google Scholar] [CrossRef]
- Miclea, I.; Bernat, R. In Vitro Multiplication of the Pitcher Plant Sarracenia Purpurea. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2018, 75, 134–136. [Google Scholar] [CrossRef]
- Makowski, W.; Królicka, A.; Nowicka, A.; Zwyrtková, J.; Tokarz, B.; Pecinka, A.; Banasiuk, R.; Tokarz, K.M. Transformed Tissue of Dionaea Muscipula J. Ellis as a Source of Biologically Active Phenolic Compounds with Bactericidal Properties. Appl. Microbiol. Biotechnol. 2021, 105, 1215–1226. [Google Scholar] [CrossRef]
- Krolicka, A.; Szpitter, A.; Stawujak, K.; Baranski, R.; Gwizdek-Wisniewska, A.; Skrzypczak, A.; Kaminski, M.; Lojkowska, E. Teratomas of Drosera Capensis var. Alba as a Source of Naphthoquinone: Ramentaceone. Plant Cell Tiss. Organ Cult. 2010, 103, 285–292. [Google Scholar] [CrossRef]
- Miguel, S.; Nisse, E.; Biteau, F.; Rottloff, S.; Mignard, B.; Gontier, E.; Hehn, A.; Bourgaud, F. Assessing Carnivorous Plants for the Production of Recombinant Proteins. Front. Plant Sci. 2019, 10, 793. [Google Scholar] [CrossRef]
- Hirsikorpi, M.; Kämäräinen, T.; Teeri, T.; Hohtola, A. Agrobacterium-Mediated Transformation of Round Leaved Sundew (Drosera Rotundifolia L.). Plant Sci. 2002, 16, 537–542. [Google Scholar] [CrossRef]
- Blehová, A.; Švubová, R.; Lukačová, Z.; Moravčíková, J.; Matušíková, I. Transformation of Sundew: Pitfalls and Promises. Plant Cell Tiss. Organ Cult. 2015, 120, 681–687. [Google Scholar] [CrossRef]
- Miguel, S.; Michel, C.; Biteau, F.; Hehn, A.; Bourgaud, F. In Vitro Plant Regeneration and Agrobacterium-Mediated Genetic Transformation of a Carnivorous Plant, Nepenthes Mirabilis. Sci. Rep. 2020, 10, 17482. [Google Scholar] [CrossRef]
- Rosa, A.B.; Malek, L.; Qin, W. The Development of the Pitcher Plant Sarracenia Purpurea into a Potentially Valuable Recombi-Nant Protein Production System. Biotechnol. Mol. Biol. Rev. 2009, 3, 105–110. [Google Scholar]
- Eilenberg, H.; Pnini-Cohen, S.; Rahamim, Y.; Sionov, E.; Segal, E.; Carmeli, S.; Zilberstein, A. Induced Production of Antifungal Naphthoquinones in the Pitchers of the Carnivorous Plant Nepenthes Khasiana. J. Exp. Bot. 2010, 61, 911–922. [Google Scholar] [CrossRef] [Green Version]

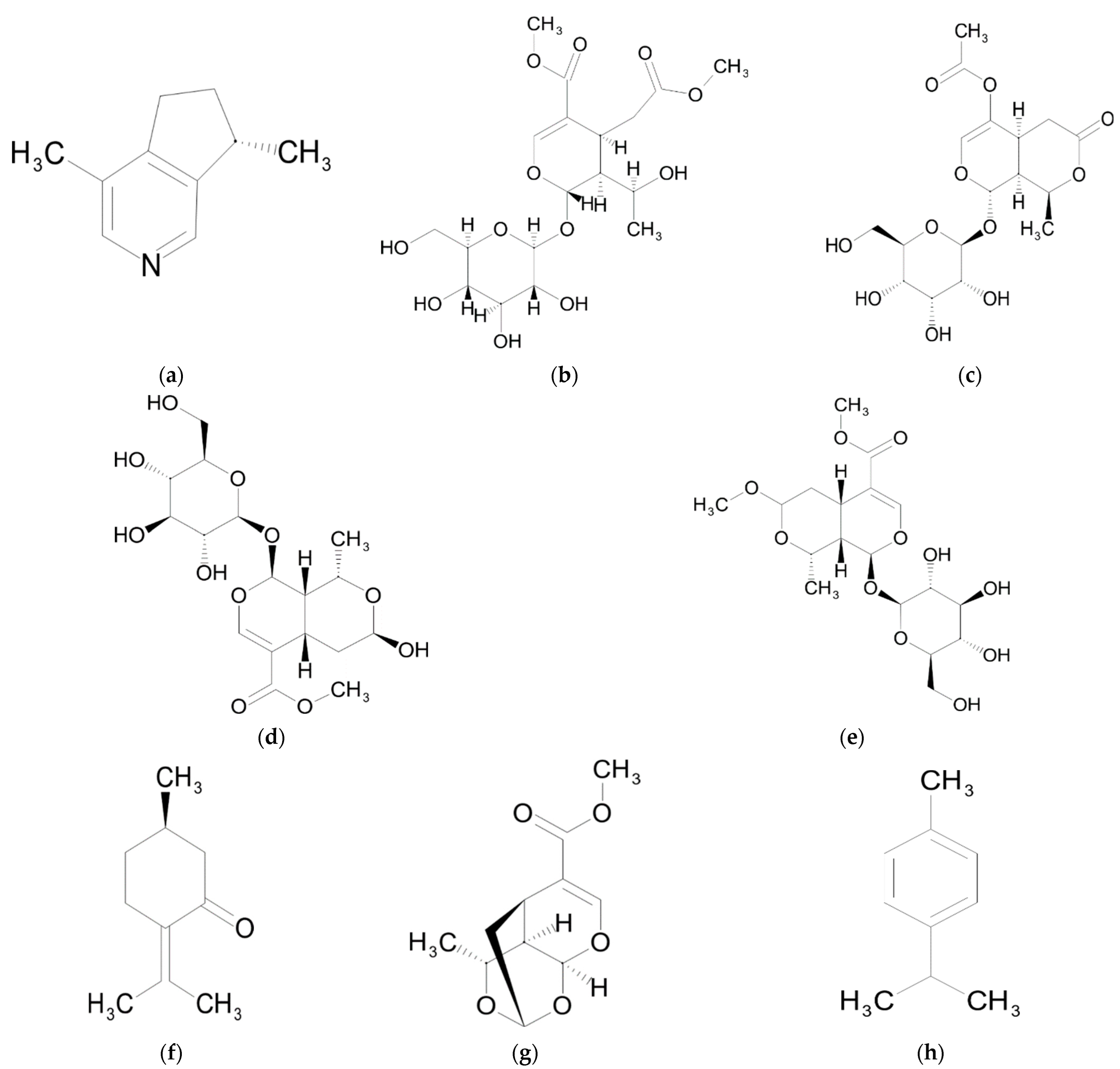
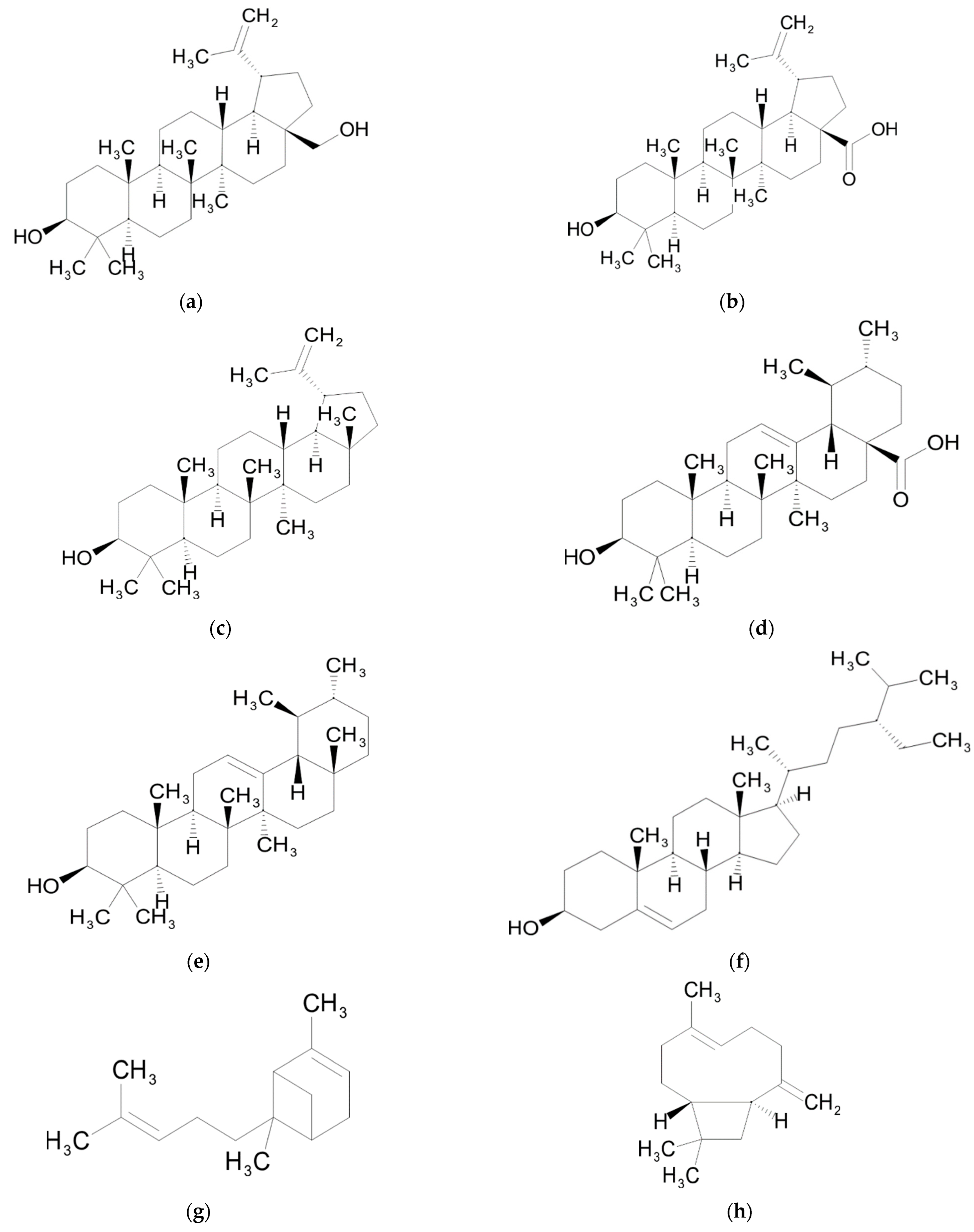
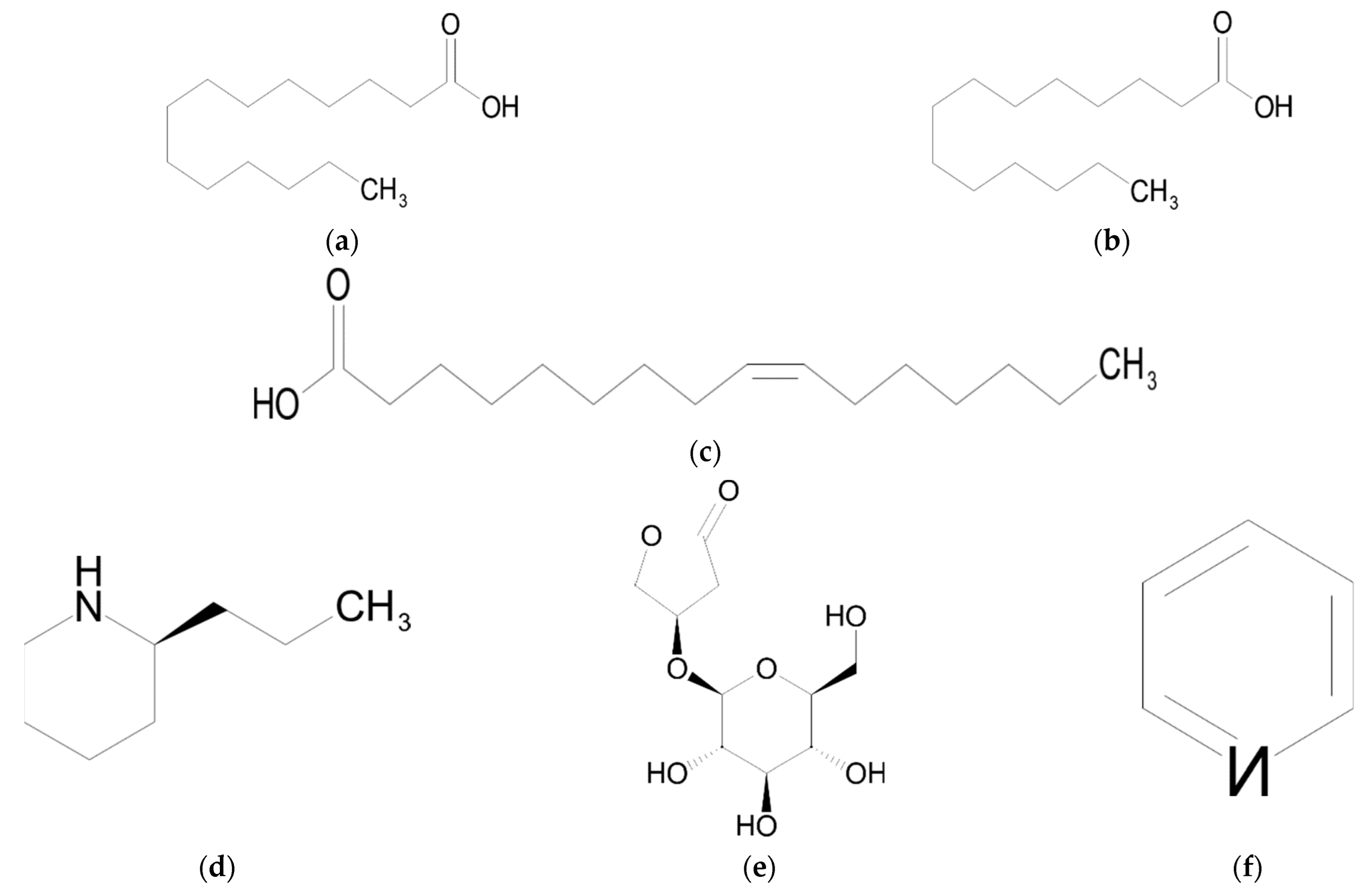
| Chemical Class | Compound | Reference |
|---|---|---|
| Flavonoids | Cyanidin | [34] |
| Delphinidin | [34] | |
| Taxifolin (dihydroquercetin) | [39] | |
| Taxifolin-3-O-glucoside | [26] | |
| Taxifolin-7-O-galactoside | [39,40] | |
| Gossypetin-3-O-galactoside | [39] | |
| Isorhamnetin-3-O-glucoside | [26] | |
| Kaempferol-3-O-(6″-caffeoylglucoside) | [26] | |
| Kaempferol-3-O-rutinoside | [26,40] | |
| Quercetin 3-O-α-L-arabinopyranoside | [35] | |
| Quercetin-3-O-arabinoside | [39] | |
| Quercetin-3-O-galactoside (hyperoside) | [26,39,40,41] | |
| Quercetin-3-O-glucoside (isoquercetrin) | [40] | |
| Quercetin-3-O-rutinoside (rutin) | [26] | |
| Tamarixetin-3-O-galactoside | [26,39,40] | |
| (+)-Catechin | [40] | |
| (−)-Epicatechin | [40,41] | |
| Monoterpenes | 7α-O-methylmorroniside | [26,42] |
| 7β-O-methylmorroniside | [26,42] | |
| Actinidine | [9] | |
| Alatenoside | [42] | |
| Alpigenoside | [9,42] | |
| Kingiside | [42] | |
| Morroniside | [26,41,42] | |
| Pulegone | [9] | |
| Sarracenin | [9,43,44] | |
| p-cymene | [45] | |
| Triterpenes | Betulin | [46] |
| Betulinic acid | [39,40,46] | |
| Lupeol | [47] | |
| Ursolic acid | [39,40] | |
| α-amyrin | [47] | |
| β-sitosterol | [47] | |
| Sequiterpenes | α-bergamotene | [45] |
| β-caryophyllene | [45] | |
| Carboxylic acids (fatty acids) | Tetradecanoic acid (myristic acid) | [9] |
| Hexadecanoic acid (palmitic acid) | [9] | |
| (Z)-9-hexadecenoic acid (palmitoleic acid) | [9] | |
| Alkaloids | Coniine | [9,48] |
| Lagumicine | [9] | |
| Other compounds | (Z)-13-docosenamide (erucamide) | [9] |
| 6′-O-caffeoylgoodyeroside | [26] | |
| Goodyeroside | [26,41] | |
| Nonanal | [9] | |
| Pyridine | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miclea, I. Secondary Metabolites with Biomedical Applications from Plants of the Sarraceniaceae Family. Int. J. Mol. Sci. 2022, 23, 9877. https://doi.org/10.3390/ijms23179877
Miclea I. Secondary Metabolites with Biomedical Applications from Plants of the Sarraceniaceae Family. International Journal of Molecular Sciences. 2022; 23(17):9877. https://doi.org/10.3390/ijms23179877
Chicago/Turabian StyleMiclea, Ileana. 2022. "Secondary Metabolites with Biomedical Applications from Plants of the Sarraceniaceae Family" International Journal of Molecular Sciences 23, no. 17: 9877. https://doi.org/10.3390/ijms23179877
APA StyleMiclea, I. (2022). Secondary Metabolites with Biomedical Applications from Plants of the Sarraceniaceae Family. International Journal of Molecular Sciences, 23(17), 9877. https://doi.org/10.3390/ijms23179877






