Pneumonia and Related Conditions in Critically Ill Patients—Insights from Basic and Experimental Studies
Abstract
:1. Introduction
2. Results
2.1. Theoretical Study: Pneumonia and Its Acute and Chronic Comorbidities in the COHD Database
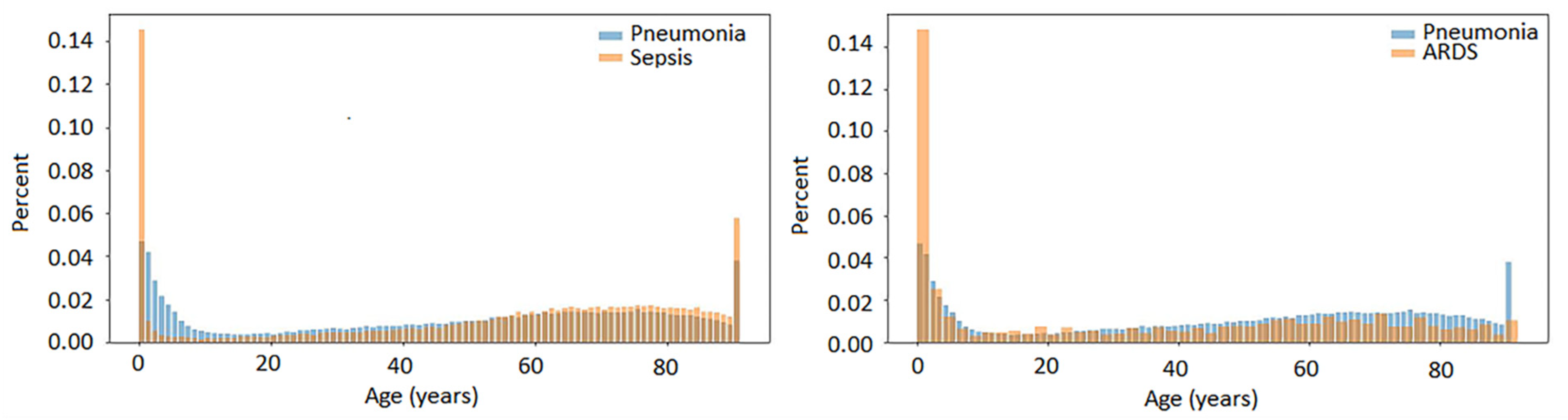
2.1.1. Overview of Pneumonia, Sepsis and ARDS and Co-Occurring Conditions
2.1.2. Temporal Associations of Pneumonia, Sepsis and ARDS
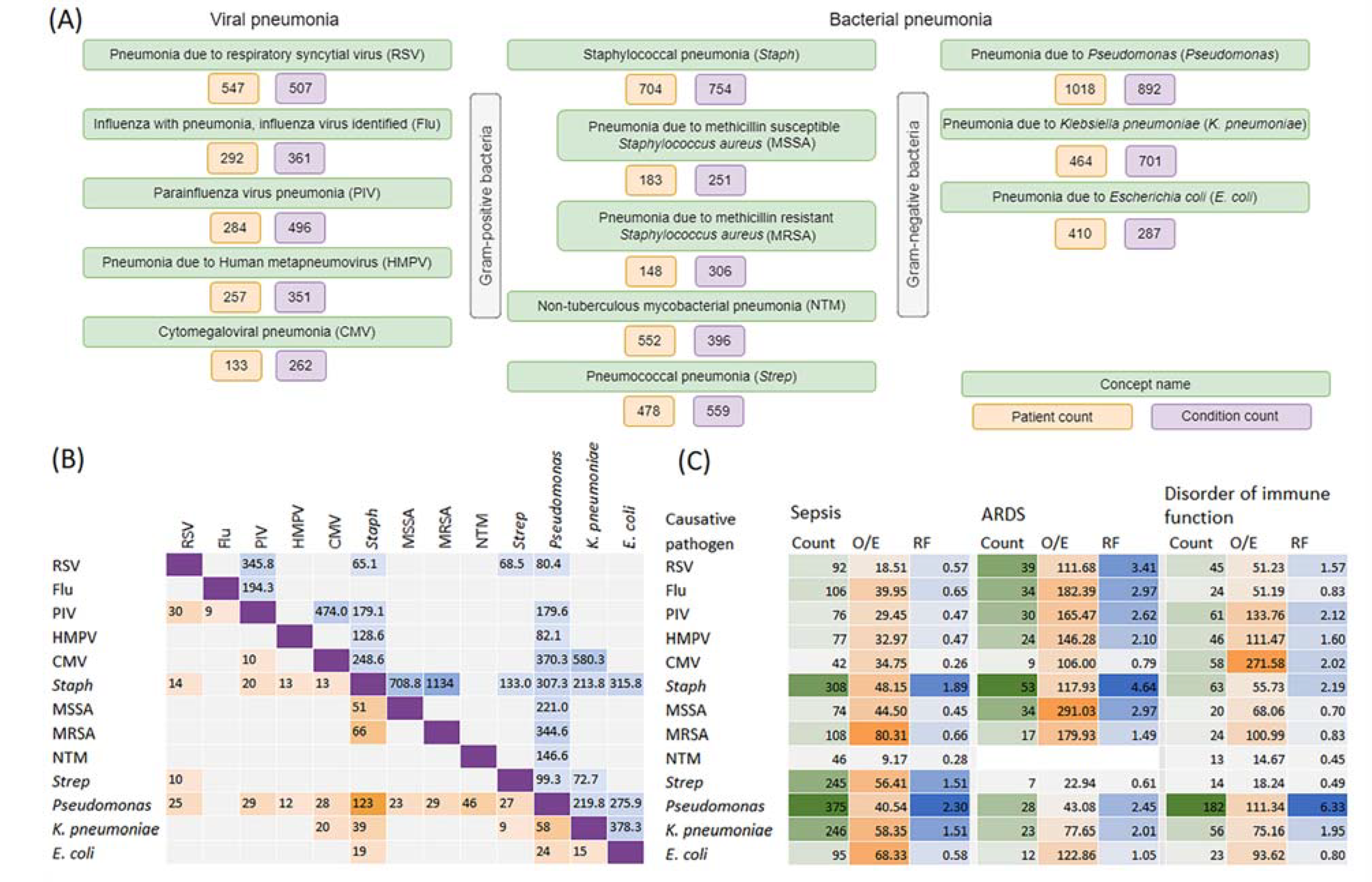
2.1.3. Pathogen-Specific Pneumonia and Its Co-Occurrence with Sepsis, ARDS and Disorders of Immune Function
2.2. Experimental Study: Host Immune Response Measured Using TRECs/KRECs in Patients with Community-Acquired and Nosocomial Pneumonia
2.2.1. Demographic and Clinical Data
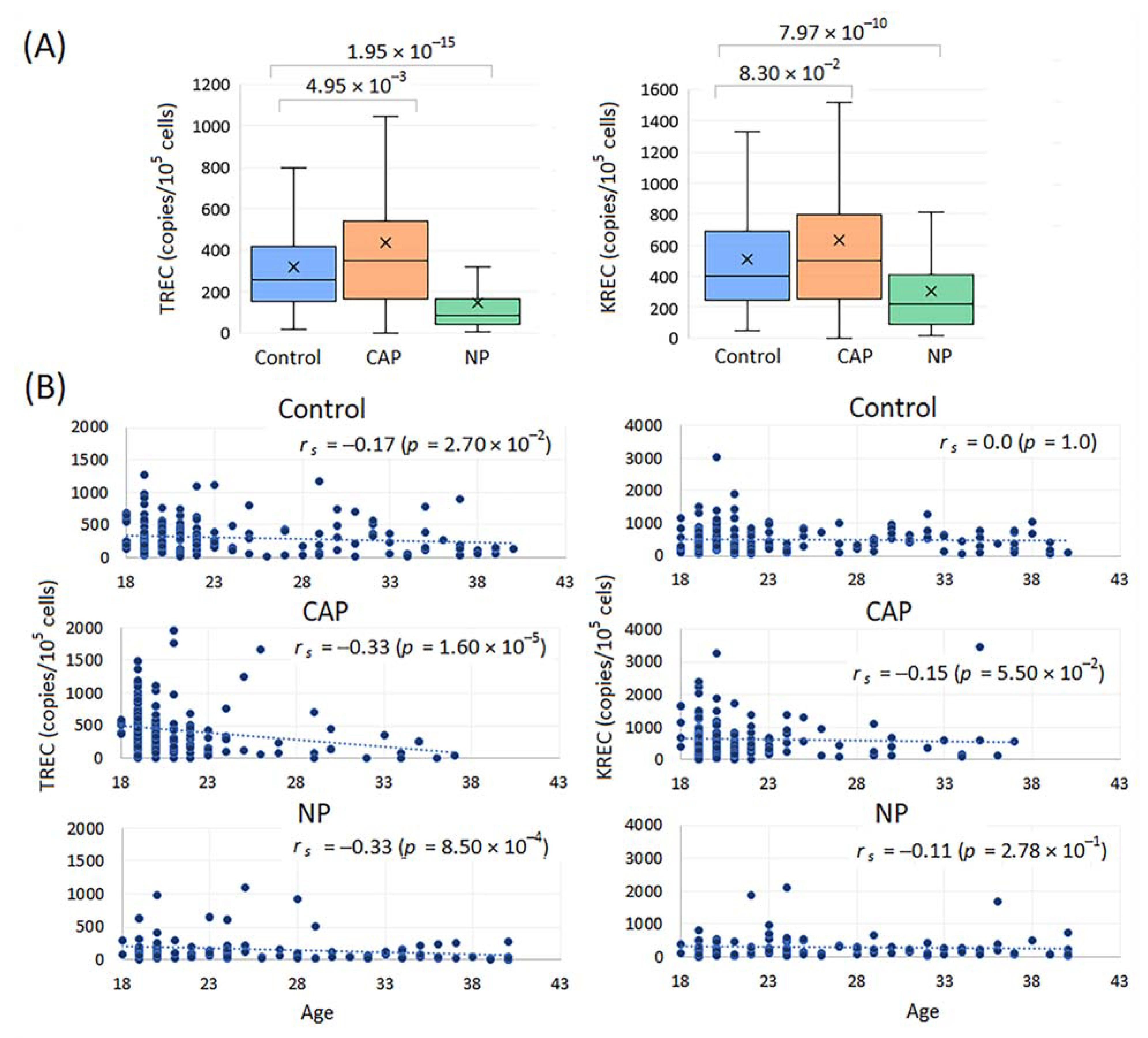
2.2.2. TREC and KREC Levels in Healthy Controls, CAP and NP Patients
2.2.3. TREC and KREC Levels in Pneumonia with Varying Severity and Outcome
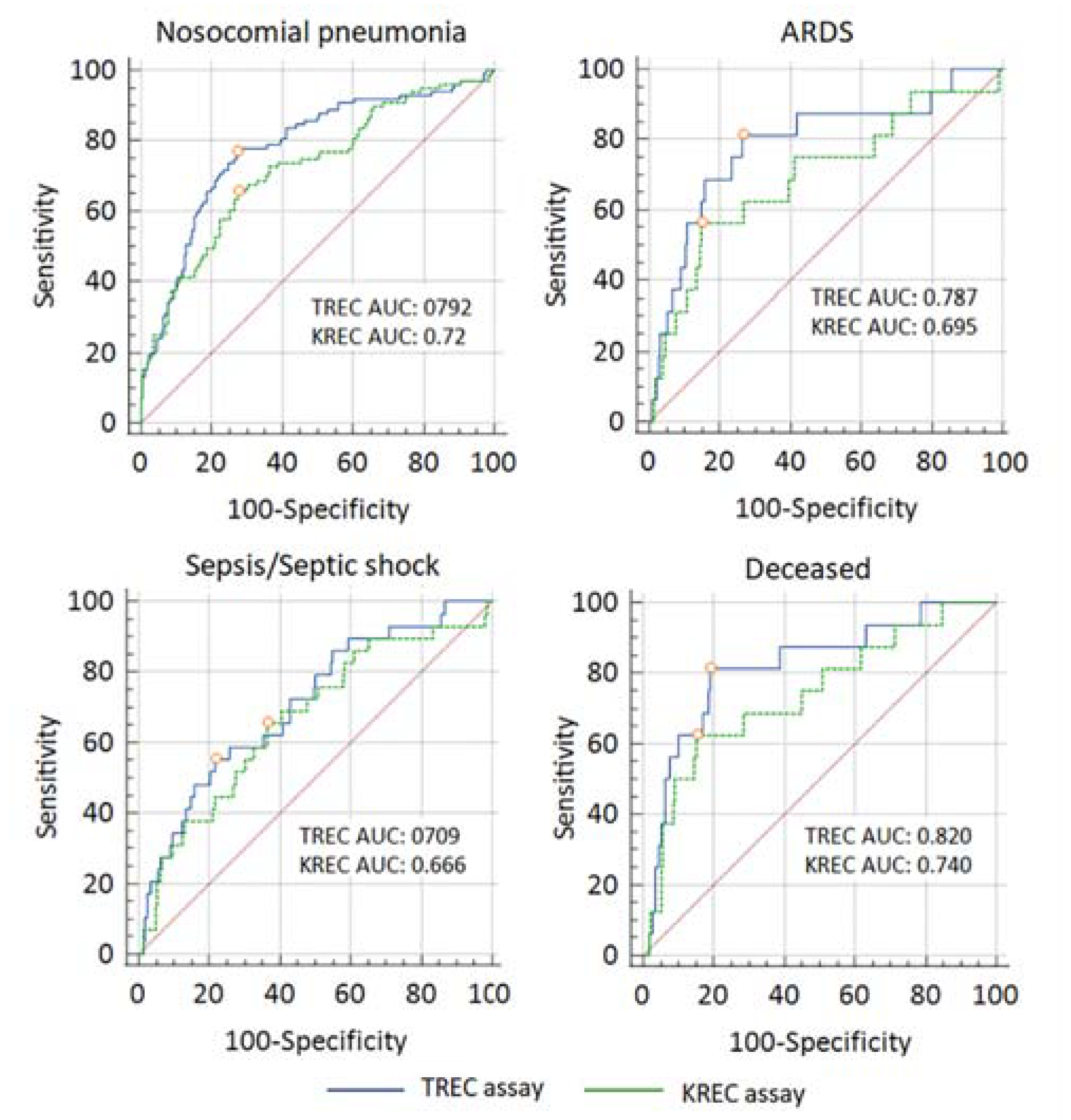
2.2.4. Diagnostic Accuracy of TREC and KREC Assays
3. Discussion
4. Materials and Methods
4.1. Theoretical Study with Columbia Open Health Data (COHD)
4.2. Experimental Study
4.2.1. Patients and Controls
4.2.2. Generation of Control Constructs for TRECs and KRECs
4.2.3. Real-Time Quantitative PCR (RQ-PCR) for TRECs and KRECs
4.2.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menéndez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Primers 2021, 7, 25. [Google Scholar] [PubMed]
- Heo, J.Y.; Song, J.Y. Disease Burden and Etiologic Distribution of Community-Acquired Pneumonia in Adults: Evolving Epidemiology in the Era of Pneumococcal Conjugate Vaccines. Infect. Chemother. 2018, 50, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Executive Summary: Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Segala, F.V.; Bavaro, D.F.; Di Gennaro, F.; Salvati, F.; Marotta, C.; Saracino, A.; Murri, R.; Fantoni, M. Impact of SARS-CoV-2 Epidemic on Antimicrobial Resistance: A Literature Review. Viruses 2021, 13, 2110. [Google Scholar] [CrossRef]
- Regunath, H.; Oba, Y. Community-Acquired Pneumonia. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430749/ (accessed on 28 July 2022).
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Li Bassi, G.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar]
- Ferguson, N.D.; Fan, E.; Camporota, L.; Antonelli, M.; Anzueto, A.; Beale, R.; Brochard, L.; Brower, R.; Esteban, A.; Gattinoni, L.; et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med. 2012, 38, 1573–1582. [Google Scholar] [CrossRef]
- Hendrickson, K.W.; Peltan, I.D.; Brown, S.M. The Epidemiology of Acute Respiratory Distress Syndrome Before and After Coronavirus Disease 2019. Crit. Care Clin. 2021, 37, 703–716. [Google Scholar]
- Máca, J.; Jor, O.; Holub, M.; Sklienka, P.; Burša, F.; Burda, M.; Janout, V.; Ševčík, P. Past and Present ARDS Mortality Rates: A Systematic Review. Respir. Care 2017, 62, 113–122. [Google Scholar]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Executive Summary: Surviving Sepsis Campaign: International Guidelines for the Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, 1974–1982. [Google Scholar]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019—Results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Respiratory distress syndrome. Nat. Rev. Dis. Primers 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Dadonaite, B.; Roser, M. Pneumonia. Available online: https://ourworldindata.org/pneumonia (accessed on 28 July 2022).
- Ewig, S.; Birkner, N.; Strauss, R.; Schaefer, E.; Pauletzki, J.; Bischoff, H.; Schraeder, P.; Welte, T.; Hoeffken, G. New perspectives on community-acquired pneumonia in 388,406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax 2009, 64, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- van Gageldonk-Lafeber, A.B.; Bogaerts, M.A.; Verheij, R.A.; van der Sande, M.A. Time trends in primary-care morbidity, hospitalization and mortality due to pneumonia. Epidemiol. Infect. 2009, 137, 1472–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reechaipichitkul, W.; Thavornpitak, Y.; Sutra, S. Burden of adult pneumonia in Thailand: A nationwide hospital admission data 2010. J. Med. Assoc. Thai. 2014, 97, 283–292. [Google Scholar]
- Ramirez, J.A.; Wiemken, T.L.; Peyrani, P.; Arnold, F.W.; Kelley, R.; Mattingly, W.A.; Nakamatsu, R.; Pena, S.; Guinn, B.E.; Furmanek, S.P.; et al. Adults Hospitalized With Pneumonia in the United States: Incidence, Epidemiology, and Mortality. Clin. Infect. Dis. 2017, 65, 1806–1812. [Google Scholar] [CrossRef]
- Palmer, S.; Albergante, L.; Blackburn, C.C.; Newman, T.J. Thymic involution and rising disease incidence with age. Proc. Natl. Acad. Sci. USA 2018, 115, 1883–1888. [Google Scholar] [CrossRef]
- Palmer, S.; Cunniffe, N.; Donnelly, R. COVID-19 hospitalization rates rise exponentially with age, inversely proportional to thymic T-cell production. J. R. Soc. Interface 2021, 18, 20200982. [Google Scholar] [CrossRef]
- Vivier, E.; Malissen, B. Innate and adaptive immunity: Specificities and signaling hierarchies revisited. Nat. Immunol. 2005, 6, 17–21. [Google Scholar] [CrossRef]
- Jung, D.; Alt, F.W. Unraveling V(D)J recombination; insights into gene regulation. Cell 2004, 116, 299–311. [Google Scholar] [CrossRef]
- Serana, F.; Chiarini, M.; Zanotti, C.; Sottini, A.; Bertoli, D.; Bosio, A.; Caimi, L.; Imberti, L. Use of V(D)J recombination excision circles to identify T- and B-cell defects and to monitor the treatment in primary and acquired immunodeficiencies. J. Transl. Med. 2013, 11, 119. [Google Scholar] [CrossRef]
- Kwok, J.S.Y.; Cheung, S.K.F.; Ho, J.C.Y.; Tang, I.W.H.; Chu, P.W.K.; Leung, E.Y.S.; Lee, P.P.W.; Cheuk, D.K.L.; Lee, V.; Ip, P.; et al. Establishing Simultaneous T Cell Receptor Excision Circles (TREC) and K-Deleting Recombination Excision Circles (KREC) Quantification Assays and Laboratory Reference Intervals in Healthy Individuals of Different Age Groups in Hong Kong. Front. Immunol. 2020, 11, 1411. [Google Scholar] [CrossRef] [PubMed]
- Currier, R.; Puck, J.M. SCID newborn screening: What we’ve learned. J. Allergy Clin. Immunol. 2021, 147, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Somech, R. T-cell receptor excision circles in primary immunodeficiencies and other T-cell immune disorders. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 517–524. [Google Scholar] [CrossRef]
- Khadzhieva, M.B.; Kalinina, E.V.; Larin, S.S.; Sviridova, D.A.; Gracheva, A.S.; Chursinova, J.V.; Stepanov, V.A.; Redkin, I.V.; Avdeikina, L.S.; Rumyantsev, A.G.; et al. TREC/KREC Levels in Young COVID-19 Patients. Diagnostics 2021, 11, 1486. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, A.A.; Tikhonova, E.; Kudryavtsev, I.; Kudlay, D.; Korsunsky, I.; Beleniuk, V.; Borisov, A. TREC/KREC Levels and T and B Lymphocyte Subpopulations in COVID-19 Patients at Different Stages of the Disease. Viruses 2022, 14, 646. [Google Scholar] [CrossRef]
- Ta, C.N.; Dumontier, M.; Hripcsak, G.; Tatonetti, N.P.; Weng, C. Columbia Open Health Data, clinical concept prevalence and co-occurrence from electronic health records. Sci. Data 2018, 5, 180273. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Musher, D.M.; Shachkina, S.; Chirinos, J.A. Acute pneumonia and the cardiovascular system. Lancet 2013, 381, 496–505. [Google Scholar] [CrossRef]
- Taub, D.D.; Longo, D.L. Insights into thymic aging and regeneration. Immunol. Rev. 2005, 205, 72–93. [Google Scholar] [CrossRef]
- Falci, C.; Gianesin, K.; Sergi, G.; Giunco, S.; De Ronch, I.; Valpione, S.; Soldà, C.; Fiduccia, P.; Lonardi, S.; Zanchetta, M.; et al. Immune senescence and cancer in elderly patients: Results from an exploratory study. Exp. Gerontol. 2013, 48, 1436–1442. [Google Scholar] [CrossRef]
- Salnikova, L.E.; Kolobkov, D.S.; Sviridova, D.A.; Abilev, S.K. An overview of germline variations in genes of primary immunodeficiences through integr ative analysis of ClinVar, HGMD® and dbSNP databases. Hum. Genet. 2021, 140, 1379–1393. [Google Scholar] [CrossRef] [PubMed]
- Goggins, J.A.; Kurtz, J.R.; McLachlan, J.B. Control of Persistent Salmonella Infection Relies on Constant Thymic Output Despite Increased Peripheral Antigen-Specific T Cell Immunity. Pathogens 2020, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, P.; Roux, H.; Couëdel-Courteille, A.; Dutrieux, J.; Naudin, C.; Charmeteau de Muylder, B.; Cheynier, R.; Squara, P.; Marullo, S. Protective reactive thymus hyperplasia in COVID-19 acute respiratory distress syndrome. Crit. Care 2021, 25, 4. [Google Scholar] [CrossRef] [PubMed]
- Gul, K.A.; Sonerud, T.; Fjærli, H.O.; Nakstad, B.; Abrahamsen, T.G.; Inchley, C.S. Thymus activity measured by T-cell receptor excision circles in patients with different severities of respiratory syncytial virus infection. BMC Infect. Dis. 2017, 17, 18. [Google Scholar] [CrossRef]
- Girardot, T.; Rimmelé, T.; Venet, F.; Monneret, G. Apoptosis-induced lymphopenia in sepsis and other severe injuries. Apoptosis 2017, 22, 295–305. [Google Scholar] [CrossRef]
- Mohammadinejad, P.; Ataeinia, B.; Kaynejad, K.; Zeinoddini, A.; Sadeghi, B.; Hosseini, M.; Rezaei, N.; Aghamohammadi, A. Antibiotic resistance in patients with primary immunodeficiency disorders versus immunocompetent patients. Expert Rev. Clin. Immunol. 2015, 11, 1163–1172. [Google Scholar] [CrossRef]
- Smelaya, T.V.; Belopolskaya, O.B.; Smirnova, S.V.; Kuzovlev, A.N.; Moroz, V.V.; Golubev, A.M.; Pabalan, N.A.; Salnikova, L.E. Genetic dissection of host immune response in pneumonia development and progression. Sci. Rep. 2016, 6, 35021. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Fine, M.J.; Auble, T.E.; Yealy, D.M.; Hanusa, B.H.; Weissfeld, L.A.; Singer, D.E.; Coley, C.M.; Marrie, T.J.; Kapoor, W.N. A prediction rule to identify low-risk patients with community-acquired pneumonia. N. Engl. J. Med. 1997, 336, 243–250. [Google Scholar] [CrossRef]
- Chuchalin, A.G.; Sinopal’nikov, A.I.; Kozlov, R.S.; Avdeev, S.N.; Tyurin, I.E.; Rudnov, V.A.; Rachina, S.A.; Fesenko, O.V. Russian Respiratory Society Interregional association on clinical microbiology and antimicrobial chemotherapy. Clinical guidelines on diagnosis, treatment and prevention of severe community-acquired pneumonia in adults. Pulmonologiya 2014, 4, 13–48. (In Russian) [Google Scholar] [CrossRef]
- Gelfand, B.R. Nosocomial Pneumonia in Adults; Russian National Guidelines; MIA: Moscow, Russia, 2016. (In Russian) [Google Scholar]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]


| Feature | CAP (%) | NP (%) | p |
|---|---|---|---|
| Total number | 164 | 99 | |
| Age | 21.29 ± 3.89 | 26.68 ± 6.68 | 4.52 × 10−14 |
| Sex ratio (M) | 159 (96.9) | 93 (93.9) | 0.34 |
| Current smoking status | |||
| Yes | 126 | 44 | 0.09 |
| No | 25 | 17 | - |
| No data | 13 | 38 | - |
| Pre-existing conditions | |||
| Cardiovascular diseases | 0 (0.0) | 2 (2.0) | 0.14 |
| Gastric/duodenal ulcer | 1 (0.6) | 2 (2.0) | 0.56 |
| Neurological conditions | 2 (1.2) | 1 (1.0) | 1.0 |
| Obesity | 1 (0.6) | 3 (3.0) | 0.15 |
| Musculoskeletal disorders | 1 (0.6) | 2 (2.0) | 0.56 |
| Benign neoplasms | 0 (0.0) | 1 (1.0) | 0.38 |
| Genitourinary system diseases | 1 (0.6) | 2 (2.0) | 0.56 |
| Initial diagnosis | |||
| Trauma | 0 (0.0) | 90 (90.9) | - |
| Acute poisoning | 0 (0.0) | 2 (2.0) | - |
| Surgery | 0 (0.0) | 7 (7.1) | - |
| CAP | 164 (100) | 0 (0.0) | - |
| Disease history | |||
| Day of CAP on admission | 8.01 ± 7.48 | - | |
| Day of NP development | - | 4.93 ± 2.48 | |
| General information on infectious pathogens in BAL fluid | |||
| Microbiological data | 114 (69.5) | 46 (45.5) | 2.62 × 10−4 |
| Gram-positive bacilli (GPB) | 72 (63.2) | 3 (6.5) | 5.51 × 10−9 |
| Gram-negative bacilli (GNB) | 1 (0.9) | 35 (76.1) | 1.83 × 10−24 |
| Mixed GPB + GNB | 41 (36.0) | 8 (17.4) | 0.023 |
| Single infection | 48 (42.1) | 6 (13.0) | 3.84 × 10−4 |
| Main pathogens in BAL fluid | |||
| Streptococcus pneumoniae | 32 (28.1) | 2 (4.3) | 5.09 × 10−4 |
| Streptococcus pyogenes | 30 (26.3) | 2 (4.3) | 9.64 × 10−4 |
| Streptococcus spp. | 17 (14.9) | 1 (2.2) | 0.025 |
| Staphylococcus aureus ➢ MRSA | 27 (23.7) ➢ 0 (0.0) | 6 (13.0) ➢ 5 (10.9) | 0.19 ➢ 1.70 × 10−3 |
| Haemophilus influenzae | 24 (21.1) | 5 (10.9) | 0.17 |
| Enterobacter spp. | 6 (4.2) | 3 (6.5) | 0.72 |
| Pseudomonas aeruginosa | 1 (0.9) | 15 (32.6) | 1.38 × 10−8 |
| Klebsiella pneumoniae | 7 (6.1) | 11 (23.9) | 3.90 × 10−3 |
| Escherichia coli | 9 (7.9) | 8 (17.4) | 0.09 |
| Acinetobacter baumannii | 6 (4.2) | 11 (23.9) | 1.18 × 10−3 |
| Other | 5 (4.4) | 7 (15.2) | 0.04 |
| Clinical data | |||
| APACHE-II | 9.37 ± 5.13 | 16.70 ± 5.55 | 2.85 × 10−19 |
| PSI score 1 | 34 (20.7) | 0 (0.0) | 3.85 × 10−8 |
| PSI score 2 | 42 (25.6) | 6 (6.1) | 5.42 × 10−5 |
| PSI score 3 | 60 (36.6) | 47 (47.5) | 0.09 |
| PSI score 4 | 24 (14.6) | 32 (32.3) | 1.0 × 10−3 |
| PSI score 5 | 4 (2.4) | 14 (14.1) | 5.48 × 10−4 |
| Bilateral | 47 (28.7) | 73 (73.7) | 8.44 × 10−9 |
| Mechanical ventilation | 10 (6.1) | 35 (35.4) | 3.47 × 10−9 |
| Duration of mechanical ventilation, days | 1.3 ± 0.64 | 10.03 ± 4.82 | 6.0 × 10−6 |
| ICU admission | 31 (18.9) | 85 (85.9) | 3.11 × 10−9 |
| ICU length of stay, days | 4.10 ± 2.75 | 18.28 ± 14.35 | 2.40 × 10−9 |
| Hospital length of stay, days | 14.01 ± 6.62 | 48.30 ± 19.59 | 3.0 × 10−39 |
| ARDS | 2 (1.2) | 14 (14.1) | 3.45 × 10−5 |
| Sepsis/septic shock | 0 (0.0) | 29 (29.3) | 2.56 × 10−14 |
| Deceased | 2 (1.2) | 14 (14.1) | 3.50 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashatnikova, D.A.; Khadzhieva, M.B.; Kolobkov, D.S.; Belopolskaya, O.B.; Smelaya, T.V.; Gracheva, A.S.; Kalinina, E.V.; Larin, S.S.; Kuzovlev, A.N.; Salnikova, L.E. Pneumonia and Related Conditions in Critically Ill Patients—Insights from Basic and Experimental Studies. Int. J. Mol. Sci. 2022, 23, 9896. https://doi.org/10.3390/ijms23179896
Kashatnikova DA, Khadzhieva MB, Kolobkov DS, Belopolskaya OB, Smelaya TV, Gracheva AS, Kalinina EV, Larin SS, Kuzovlev AN, Salnikova LE. Pneumonia and Related Conditions in Critically Ill Patients—Insights from Basic and Experimental Studies. International Journal of Molecular Sciences. 2022; 23(17):9896. https://doi.org/10.3390/ijms23179896
Chicago/Turabian StyleKashatnikova, Darya A., Maryam B. Khadzhieva, Dmitry S. Kolobkov, Olesya B. Belopolskaya, Tamara V. Smelaya, Alesya S. Gracheva, Ekaterina V. Kalinina, Sergey S. Larin, Artem N. Kuzovlev, and Lyubov E. Salnikova. 2022. "Pneumonia and Related Conditions in Critically Ill Patients—Insights from Basic and Experimental Studies" International Journal of Molecular Sciences 23, no. 17: 9896. https://doi.org/10.3390/ijms23179896
APA StyleKashatnikova, D. A., Khadzhieva, M. B., Kolobkov, D. S., Belopolskaya, O. B., Smelaya, T. V., Gracheva, A. S., Kalinina, E. V., Larin, S. S., Kuzovlev, A. N., & Salnikova, L. E. (2022). Pneumonia and Related Conditions in Critically Ill Patients—Insights from Basic and Experimental Studies. International Journal of Molecular Sciences, 23(17), 9896. https://doi.org/10.3390/ijms23179896







