Topical Administration of a Marine Oil Rich in Pro-Resolving Lipid Mediators Accelerates Wound Healing in Diabetic db/db Mice through Angiogenesis and Macrophage Polarization
Abstract
:1. Introduction
2. Results
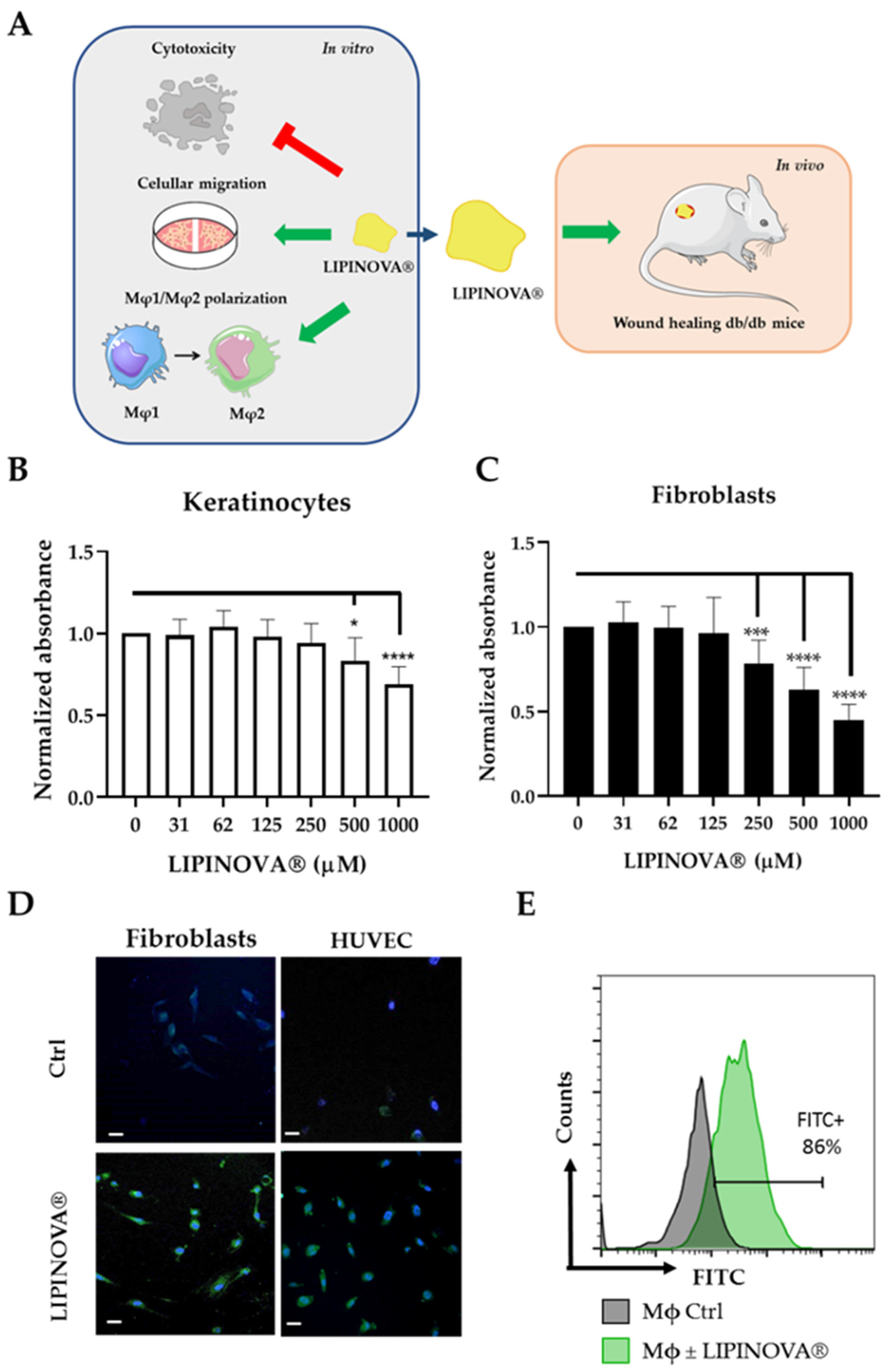
2.1. Study Design and Biocompatibility of LIPINOVA® In Vitro
2.2. LIPINOVA® Promotes Migration and Spreading of Fibroblast and Keratinocytes
2.3. Inflammatory Response to LIPINOVA® In Vitro
2.4. LIPINOVA® Improves Wound Healing Closure in Mice
2.5. LIPINOVA® Promotes Macrophage Polarization towards an Anti-Inflammatory Profile
3. Discussion
4. Materials and Methods
4.1. Ethical Statements
4.2. LIPINOVA® Manufacturing Process and SPM Analysis
4.3. Cell Culture of Adherent Primary Cultures
4.4. Isolation and Culture of Peripheral Blood Monocytes
4.5. Preparation of LIPINOVA® for In Vitro Assays
4.6. Cytotoxicity Assays
4.7. Flow Cytometry
4.8. Real Time Quantitative PCR
4.9. Measurement of Cytokines by Enzyme-Linked Immunosorbent Assay (ELISA)
4.10. In Vitro Wound Healing Assay
4.11. Animals
4.12. In Vivo Wound Healing
4.13. Histology and Morphometric Analysis
4.14. Analysis of Macrophage Mφ1/Mφ2
4.15. Analysis of Blood Vessel Density
4.16. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis†. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, U.A.; Dipietro, L.A. Diabetes and wound angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef]
- Nathan, D. Long-term complications of diabetes mellitus. N. Engl. J. Med. 1993, 328, 1676–1685. [Google Scholar] [CrossRef]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal Stem Cells Enhance Wound Healing Through Differentiation and Angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef]
- Stables, M.J.; Gilroy, D.W. Old and new generation lipid mediators in acute inflammation and resolution. Prog. Lipid Res. 2011, 50, 35–51. [Google Scholar]
- Wu, C.L.; Jain, D.; McNeill, J.N.; Little, D.; Anderson, J.A.; Huebner, J.L.; Kraus, V.B.; Rodriguiz, R.M.; Wetsel, W.C.; Guilak, F. Dietary fatty acid content regulates Wound repair and the pathogenesis of osteoarthritis following joint injury. Ann. Rheum. Dis. 2015, 74, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Komprda, T.; Sladek, Z.; Sevcikova, Z.; Svehlova, V.; Wijacki, J.; Guran, R.; Do, T.; Lackova, Z.; Polanska, H.; Vrlikova, L.; et al. Comparison of dietary oils with different polyunsaturated fatty acid n-3 and n-6 content in the rat model of cutaneous wound healing. Int. J. Mol. Sci. 2020, 21, 7911. [Google Scholar] [CrossRef] [PubMed]
- Oliver, L.; Dietrich, T.; Marañón, I.; Villarán, M.C.; Barrio, R.J. Producing omega-3 polyunsaturated fatty acids: A review of sustainable sources and future trends for the EPA and DHA market. Resources 2020, 9, 148. [Google Scholar] [CrossRef]
- Ward, O.P.; Singh, A. Omega-3/6 fatty acids: Alternative sources of production. Process Biochem. 2005, 40, 3627–3652. [Google Scholar] [CrossRef]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: Bridging the gap between supply and demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef]
- Huang, T.; Wang, T.; Heianza, Y.; Zheng, Y.; Sun, D.; Kang, J.H.; Pasquale, L.R.; Rimm, E.B.; Manson, J.A.E.; Hu, F.B.; et al. Habitual consumption of long-chain n-3 PUFAs and fish attenuates genetically associated long-term weight gain. Am. J. Clin. Nutr. 2019, 109, 665–673. [Google Scholar] [CrossRef]
- Parolini, C. Biotechnology Approaches for the Treatment of Dyslipidemia. Cardiovasc. Drugs Ther. 2021, 35, 167–183. [Google Scholar] [CrossRef]
- Enguita, M.; Razquin, N.; Pamplona, R.; Quiroga, J.; Prieto, J.; Fortes, P. The cirrhotic liver is depleted of docosahexaenoic acid (DHA), a key modulator of NF-κB and TGFβ pathways in hepatic stellate cells. Cell Death Dis. 2019, 10, 14. [Google Scholar] [CrossRef]
- Conte, M.S.; Desai, T.A.; Wu, B.; Schaller, M.; Werlin, E. Pro-resolving lipid mediators in vascular disease. J. Clin. Investig. 2018, 128, 3727–3735. [Google Scholar] [CrossRef]
- Parolini, C. Marine n-3 polyunsaturated fatty acids: Efficacy on inflammatory-based disorders. Life Sci. 2020, 263, 118591. [Google Scholar] [CrossRef]
- Otranto, M.; Do Nascimento, A.P.; Monte-Alto-Costa, A. Effects of supplementation with different edible oils on cutaneous wound healing. Wound Repair Regen. 2010, 18, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.D.S.; Bandeira, L.G.; Monte-Alto-Costa, A.; Romana-Souza, B. Supplementation with olive oil, but not fish oil, improves cutaneous wound healing in stressed mice. Wound Repair Regen. 2014, 22, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Wang, G.; Li, L.; Lin, G.; Deng, Z. Omega-3 polyunsaturated fatty acids reduce vascular endothelial growth factor production and suppress endothelial wound repair. J. Cardiovasc. Transl. Res. 2013, 6, 287–293. [Google Scholar] [CrossRef]
- McDaniel, J.C.; Belury, M.; Ahijevych, K.; Blakely, W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008, 16, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, M.J.; Hellmann, J.; Kosuri, M.; Bhatnagar, A.; Spite, M. Proresolution Therapy for the Treatment of Delayed Healing of Diabetic Wounds. Diabetes 2013, 62, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.W.; Supp, D.M. Role of Arginine and Omega-3 Fatty Acids in Wound Healing and Infection. Adv. Wound Care 2014, 3, 682–690. [Google Scholar] [CrossRef]
- Stańdo, M.; Piatek, P.; Namiecinska, M.; Lewkowicz, P.; Lewkowicz, N. Omega-3 polyunsaturated fatty acids epa and dha as an adjunct to non-surgical treatment of periodontitis: A randomized clinical trial. Nutrients 2020, 12, 2614. [Google Scholar] [CrossRef]
- Varela-López, A.; Giampieri, F.; Bullón, P.; Battino, M.; Quiles, J.L. Role of lipids in the onset, progression and treatment of periodontal disease. A systematic review of studies in humans. Int. J. Mol. Sci. 2016, 17, 1202. [Google Scholar] [CrossRef]
- Titos, E.; Rius, B.; González-Périz, A.; López-Vicario, C.; Morán-Salvador, E.; Martínez-Clemente, M.; Arroyo, V.; Clària, J. Resolvin D1 and Its Precursor Docosahexaenoic Acid Promote Resolution of Adipose Tissue Inflammation by Eliciting Macrophage Polarization toward an M2-Like Phenotype. J. Immunol. 2011, 187, 5408–5418. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Serhan, C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2015, 1851, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: Concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie 2020, 178, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.S.; Zahner, G.J.; Gasper, W.J.; Harris, W.S.; Conte, M.S.; Hills, N.K.; Grenon, S.M. Relationship between the omega-3 index and specialized pro-resolving lipid mediators in patients with peripheral arterial disease taking fish oil supplements. J. Clin. Lipidol. 2017, 11, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Spite, M.; Clària, J.; Serhan, C.N. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef]
- Serhan, C.N.; Dalli, J.; Karamnov, S.; Choi, A.; Park, C.; Xu, Z.; Ji, R.; Zhu, M.; Petasis, N.A. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012, 26, 1755–1765. [Google Scholar] [CrossRef]
- Akagi, D.; Chen, M.; Toy, R.; Chatterjee, A.; Conte, M.S. Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB J. 2015, 29, 2504–2513. [Google Scholar] [CrossRef]
- Tejedor, S.; Dolz-Pérez, I.; Decker, C.G.; Hernándiz, A.; Diez, J.L.; Álvarez, R.; Castellano, D.; García, N.A.; Ontoria-Oviedo, I.; Nebot, V.J.; et al. Polymer Conjugation of Docosahexaenoic Acid Potentiates Cardioprotective Therapy in Preclinical Models of Myocardial Ischemia/Reperfusion Injury. Adv. Healthc. Mater. 2021, 10, e2002121. [Google Scholar] [CrossRef]
- Souza, P.R.; Marques, R.M.; Gomez, E.A.; Colas, R.A.; De Matteis, R.; Zak, A.; Patel, M.; Collier, D.J.; Dalli, J. Enriched Marine Oil Supplements Increase Peripheral Blood Specialized Pro-Resolving Mediators Concentrations and Reprogram Host Immune Responses: A Randomized Double-Blind Placebo-Controlled Study. Circ. Res. 2020, 126, 75–90. [Google Scholar] [CrossRef]
- Schaller, M.S.; Chen, M.; Colas, R.A.; Sorrentino, T.A.; Lazar, A.A.; Grenon, S.M.; Dalli, J.; Conte, M.S. Treatment with a marine oil supplement alters lipid mediators and leukocyte phenotype in healthy patients and those with peripheral artery disease. J. Am. Heart Assoc. 2020, 9, e016113. [Google Scholar] [CrossRef]
- Callan, N.; Hanes, D.; Bradley, R. Early evidence of efficacy for orally administered SPM-enriched marine lipid fraction on quality of life and pain in a sample of adults with chronic pain. J. Transl. Med. 2020, 18, 401. [Google Scholar] [CrossRef] [PubMed]
- Kendall, A.C.; Kiezel-Tsugunova, M.; Brownbridge, L.C.; Harwood, J.L.; Nicolaou, A. Lipid functions in skin: Differential effects of n-3 polyunsaturated fatty acids on cutaneous ceramides, in a human skin organ culture model. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Trousdale, R.K.; Jacobs, S.; Simhaee, D.A.; Wu, J.K.; Lustbader, J.W. Wound Closure and Metabolic Parameter Variability in a db/db Mouse Model for Diabetic Ulcers. J. Surg. Res. 2009, 151, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Van De Vyver, M.; Boodhoo, K.; Frazier, T.; Hamel, K.; Kopcewicz, M.; Levi, B.; Maartens, M.; Machcinska, S.; Nunez, J.; Pagani, C.; et al. Histology Scoring System for Murine Cutaneous Wounds. Stem Cells Dev. 2021, 30, 23. [Google Scholar] [CrossRef] [PubMed]
- Hankenson, K.D.; Watkins, B.A.; Schoenlein, I.A.; Allen, K.G.D.; Turek, J.J. Omega-3 fatty acids enhance ligament fibroblast collagen formation in association with changes in interleukin-6 production. Proc. Soc. Exp. Biol. Med. 2000, 223, 88–95. [Google Scholar] [CrossRef]
- Severing, A.L.; Rembe, J.D.; Füllerer, M.; Stürmer, E.K. Impact of the chronic wound microenvironment and marine omega-3 fatty acids on skin cell regeneration processes. Exp. Dermatol. 2022, 31, 725–735. [Google Scholar] [CrossRef]
- Gómez-Ferrer, M.; Amaro-Prellezo, E.; Dorronsoro, A.; Sánchez-Sánchez, R.; Vicente, Á.; Cosín-Roger, J.; Barrachina, M.D.; Baquero, M.C.; Valencia, J.; Sepúlveda, P. Hif-overexpression and pro-inflammatory priming in human mesenchymal stromal cells improves the healing properties of extracellular vesicles in experimental crohn’s disease. Int. J. Mol. Sci. 2021, 22, 11269. [Google Scholar] [CrossRef]
- Averina, E.S.; Kutyrev, I.A. Perspectives on the use of marine and freshwater hydrobiont oils for development of drug delivery systems. Biotechnol. Adv. 2011, 29, 548–557. [Google Scholar] [CrossRef]
- Hellmann, J.; Sansbury, B.E.; Wong, B.; Li, X.; Singh, M.; Nuutila, K.; Chiang, N.; Eriksson, E.; Serhan, C.N.; Spite, M. Biosynthesis of D-Series Resolvins in Skin Provides Insights into their Role in Tissue Repair. J. Investig. Dermatol. 2018, 138, 2051–2060. [Google Scholar] [CrossRef]
- Liu, M.; Saeki, K.; Matsunobu, T.; Okuno, T.; Koga, T.; Sugimoto, Y.; Yokoyama, C.; Nakamizo, S.; Kabashima, K.; Narumiya, S.; et al. 12-hydroxyheptadecatrienoic acid promotes epidermal wound healing by accelerating keratinocyte migration via the BLT2 receptor. J. Exp. Med. 2014, 211, 1063–1078. [Google Scholar] [CrossRef]
- Augimeri, G.; Plastina, P.; Gionfriddo, G.; Rovito, D.; Giordano, C.; Fazio, A.; Barone, I.; Catalano, S.; Andò, S.; Bonofiglio, D.; et al. N-eicosapentaenoyl dopamine, a conjugate of dopamine and eicosapentaenoic acid (EPA), exerts anti-inflammatory properties in mouse and human macrophages. Nutrients 2019, 11, 2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendall, A.C.; Pilkington, S.M.; Murphy, S.A.; Carratore, F.D.; Sunarwidhi, A.L.; Kiezel-Tsugunova, M.; Urquhart, P.; Watson, R.E.B.; Breitling, R.; Rhodes, L.E.; et al. Dynamics of the human skin mediator lipidome in response to dietary ω-3 fatty acid supplementation. FASEB J. 2019, 33, 13014–13027. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Chen, C.C.; Li, T.K.; Wang, P.H.; Liu, L.R.; Chang, F.Y.; Wang, Y.C.; Yu, Y.H.; Lin, S.P.; Mersmann, H.J.; et al. Docosahexaenoic acid suppresses the expression of FoxO and its target genes. J. Nutr. Biochem. 2012, 23, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.A.; Bhonde, R.R. Omega-3 polyunsaturated fatty acids promote angiogenesis in placenta derived mesenchymal stromal cells. Pharmacol. Res. 2018, 132, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Strassburg, K.; Huijbrechts, A.M.L.; Kortekaas, K.A.; Lindeman, J.H.; Pedersen, T.L.; Dane, A.; Berger, R.; Brenkman, A.; Hankemeier, T.; Van Duynhoven, J.; et al. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis: Application in cardiac surgery. Anal. Bioanal. Chem. 2012, 404, 1413–1426. [Google Scholar] [CrossRef]
- Castellano, D.; Sanchis, A.; Blanes, M.; Pérez del Caz, M.D.; Ruiz-Saurí, A.; Piquer-Gil, M.; Pelacho, B.; Marco, B.; Garcia, N.; Ontoria-Oviedo, I.; et al. Electrospun poly(hydroxybutyrate) scaffolds promote engraftment of human skin equivalents via macrophage M2 polarization and angiogenesis. J. Tissue Eng. Regen. Med. 2018, 12, e983–e994. [Google Scholar] [CrossRef]
- Ontoria-Oviedo, I.; Dorronsoro, A.; Sánchez, R.; Ciria, M.; Gómez-Ferrer, M.; Buigues, M.; Grueso, E.; Tejedor, S.; García-García, F.; González-King, H.; et al. Extracellular Vesicles Secreted by Hypoxic AC10 Cardiomyocytes Modulate Fibroblast Cell Motility. Front. Cardiovasc. Med. 2018, 5, 152. [Google Scholar] [CrossRef] [Green Version]




| Fatty Acids | % Area | |
|---|---|---|
| Myristic | C14:0 | 0.44 |
| Myristoleic | C14:1 | n.d. |
| Pentadecanoic | C15:0 | n.d. |
| Palmitic | C16:0 | 1.31 |
| Palmitoleic | C16:1 n7 | 0.61 |
| Hexadecaenoic | C16:4 n1 | 0.23 |
| Margaric | C17:0 | 0.06 |
| Margaroleic | C17:1 | 0.12 |
| Stearic | C18:0 | 1.00 |
| Oleic | C18:1 n9 | 2.05 |
| Vaccenic | C18:1 n7 | 0.65 |
| Linoleic | C18:2 n6 | 0.25 |
| Gamma-linolenic | C18:3 n6 | 0.08 |
| Linolenic | C18:3 n3 | 0.15 |
| Stearidonic | C18:4 n3 | 0.67 |
| Arachidic | C20:0 | 0.73 |
| Eicosenoic | C20:1 n9 | 2.32 |
| Gondonic | C20:1 n7 | 0.38 |
| Arachidonic | C20:4 n6 | 0.96 |
| Eicosatetraenoic | C20:4 n3 | 1.13 |
| Eicosapentaenoic | C20:5 n3 | 20.08 |
| Behenic | C22:0 | 0.90 |
| Erucic | C22:1 n11 | 2.84 |
| Adrenic | C22:4 n6 | 0.52 |
| Docosapentaenoic | C22:5 n6 | 0.98 |
| Docosapentaenoic | C22:5 n3 | 7.57 |
| Lignoceric | C24:0 | 0.46 |
| Docosahexaenoic | C22:6 n3 | 45.47 |
| Nervonic | C24:1 n9 | 0.23 |
| Total ω3 | 75.07 | |
| Total ω6 | 0.98 | |
| Total ω9 | 4.60 | |
| SFA | 4.90 | |
| MUFA | 9.20 | |
| PUFA | 78.09 | |
| Factors Evaluated (at 15 Days) | Ctrl | LIPINOVA® |
|---|---|---|
| Epithelial thickness (µm) | 72.86 ± 27.80 | 62.19 ± 19.48 |
| Granulation tissue (µm) | 439.44 ± 133.95 | 310.14 ± 125.58 |
| Scar elevation index | 1.35 ± 0.12 | 1.29 ± 0.20 |
| Re-epithelization (0–2) | 0.89 ± 1.05 | 1.78 ± 0.71 |
| Keratinization (0–2) | 1.11 ± 0.93 | 1.78 ± 0.71 |
| Collagen deposition (0–2) | 0.70 ± 0.28 | 0.66 ± 0.19 |
| Remodeling (0–2) | 0.78 ± 0.44 | 0.89 ± 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ontoria-Oviedo, I.; Amaro-Prellezo, E.; Castellano, D.; Venegas-Venegas, E.; González-Santos, F.; Ruiz-Saurí, A.; Pelacho, B.; Prósper, F.; Pérez del Caz, M.D.; Sepúlveda, P. Topical Administration of a Marine Oil Rich in Pro-Resolving Lipid Mediators Accelerates Wound Healing in Diabetic db/db Mice through Angiogenesis and Macrophage Polarization. Int. J. Mol. Sci. 2022, 23, 9918. https://doi.org/10.3390/ijms23179918
Ontoria-Oviedo I, Amaro-Prellezo E, Castellano D, Venegas-Venegas E, González-Santos F, Ruiz-Saurí A, Pelacho B, Prósper F, Pérez del Caz MD, Sepúlveda P. Topical Administration of a Marine Oil Rich in Pro-Resolving Lipid Mediators Accelerates Wound Healing in Diabetic db/db Mice through Angiogenesis and Macrophage Polarization. International Journal of Molecular Sciences. 2022; 23(17):9918. https://doi.org/10.3390/ijms23179918
Chicago/Turabian StyleOntoria-Oviedo, Imelda, Elena Amaro-Prellezo, Delia Castellano, Elena Venegas-Venegas, Fernando González-Santos, Amparo Ruiz-Saurí, Beatriz Pelacho, Felipe Prósper, María Dolores Pérez del Caz, and Pilar Sepúlveda. 2022. "Topical Administration of a Marine Oil Rich in Pro-Resolving Lipid Mediators Accelerates Wound Healing in Diabetic db/db Mice through Angiogenesis and Macrophage Polarization" International Journal of Molecular Sciences 23, no. 17: 9918. https://doi.org/10.3390/ijms23179918
APA StyleOntoria-Oviedo, I., Amaro-Prellezo, E., Castellano, D., Venegas-Venegas, E., González-Santos, F., Ruiz-Saurí, A., Pelacho, B., Prósper, F., Pérez del Caz, M. D., & Sepúlveda, P. (2022). Topical Administration of a Marine Oil Rich in Pro-Resolving Lipid Mediators Accelerates Wound Healing in Diabetic db/db Mice through Angiogenesis and Macrophage Polarization. International Journal of Molecular Sciences, 23(17), 9918. https://doi.org/10.3390/ijms23179918







