Neat Chitosan Porous Materials: A Review of Preparation, Structure Characterization and Application
Abstract
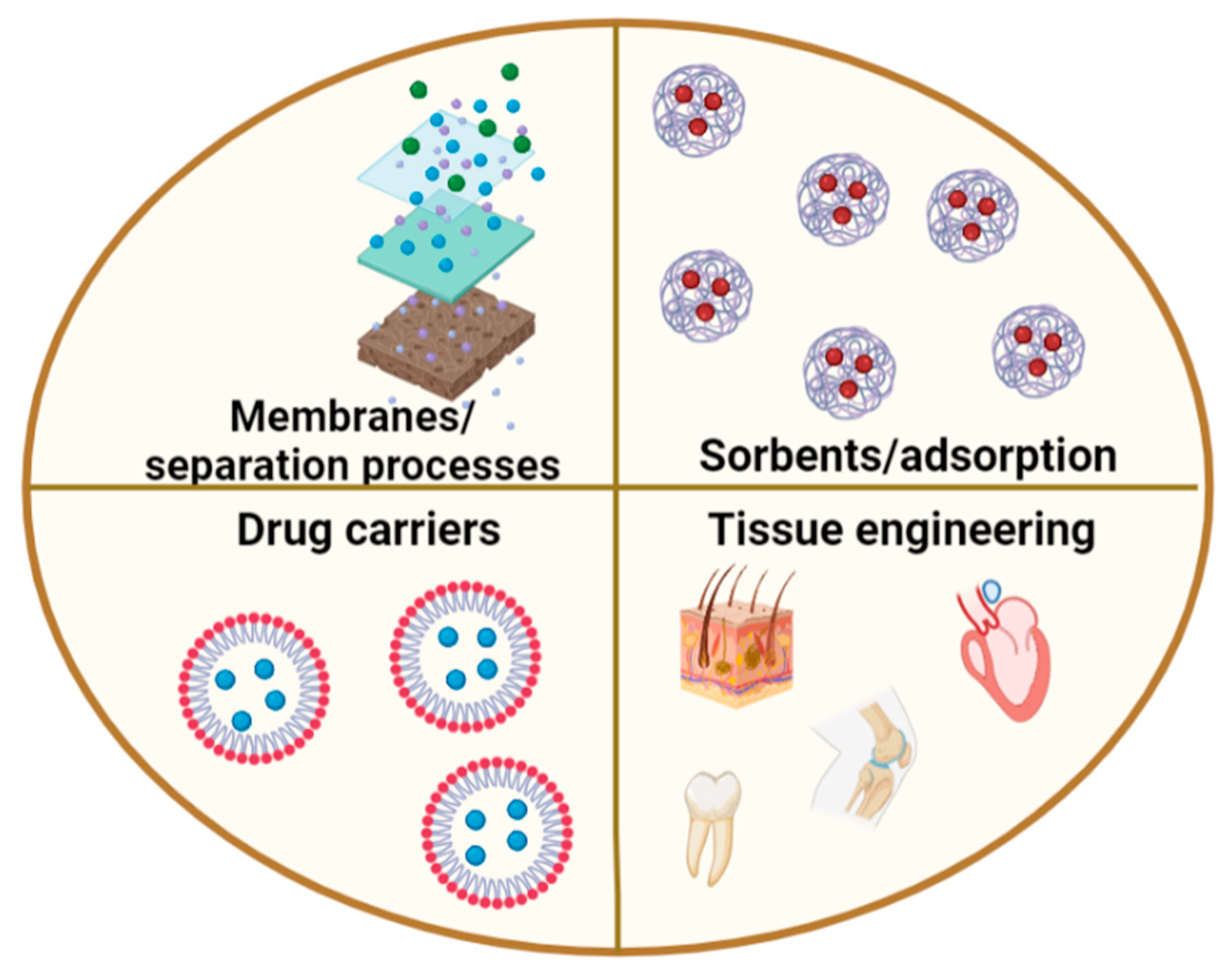
:1. Introduction
2. Freeze-Drying (Lyophilization)
2.1. Preparation of Chitosan-Derived Porous Materials by Lyophilization
2.2. Application of Porous Chitosan Materials Prepared by the Freeze-Drying Method
3. Cryogelation
3.1. Preparation of Chitosan-Derived Porous Materials via Cryogelation
3.2. Application of Porous Chitosan Materials Prepared by Cryogelation Method
4. Sol-Gel Method
4.1. Preparation of Chitosan-Derived Porous Materials Using the Sol-Gel Method
4.2. Application of Porous Chitosan Materials Prepared by Sol-Gel Method
5. Phase Inversion
5.1. Preparation of Chitosan-Derived Porous Materials Using Phase Inversion
5.2. Application of Porous Chitosan Materials Prepared by a Phase Separation Method
6. Extraction of a Porogen Agent
6.1. Preparation and Characterization of Chitosan-Derived Porous Materials Prepared by the Extraction of a Porogen Agent
6.2. Application of Porous Chitosan Materials Prepared by Extracting a Porogen Agent
7. Summary
Funding
Conflicts of Interest
References
- Kou, S.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Boudouaia, N.; Bengharez, Z.; Jellali, S. Preparation and characterization of chitosan extracted from shrimp shells waste and chitosan film: Application for Eriochrome black T removal from aqueous solutions. Appl. Water Sci. 2019, 9, 91. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Crognale, S.; Russo, C.; Petruccioli, M.; D’Annibale, A. Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints. Fermentation 2022, 8, 76. [Google Scholar] [CrossRef]
- Elsoud, M.M.A.; Kady, E.M.E. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019, 43, 1–12. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Rasti, H.; Parivar, K.; Baharara, J.; Iranshahi, M.; Namvar, F. Chitin from the Mollusc Chiton: Extraction, Characterization and Chitosan Preparation. Iran J. Pharm. Res. 2017, 16, 366–379. [Google Scholar]
- Kim, J.S.; Lee, S. Immobilization of Trypsin from Porcine Pancreas onto Chitosan Nonwoven by Covalent Bonding. Polymers 2019, 11, 1462. [Google Scholar] [CrossRef]
- Chang, X.X.; Mubarak, N.M.; Mazari, S.A.; Jatoi, A.S.; Ahmad, A.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Karri, R.R.; Siddiqui, M.T.H.; et al. A review on the properties and applications of chitosan, cellulose and deep eutectic solvent in green chemistry. J. Ind. Eng. Chem. 2021, 104, 362–380. [Google Scholar] [CrossRef]
- Aranaz; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Rahman, L.; Goswami, J. Recent development on physical and biological properties of chitosan-based composite films with natural extracts: A review. J. Bioact. Compat. Polym. 2021, 36, 225–236. [Google Scholar] [CrossRef]
- Kmiec, M.; Pighinelli, L.; Tedesco, M.F.; Silva, M.M.; Reis, V. Chitosan-properties and applications in dentistry. Adv. Tissue Eng. Regen. Med. 2017, 2, 205–211. [Google Scholar]
- Bravo-Anaya, L.M.; Fernández-Solís, K.G.; Rosselgong, J.; Nano-Rodríguez, J.L.E.; Carvajal, F.; Rinaudo, M. Chitosan-DNA polyelectrolyte complex: Influence of chitosan characteristics and mechanism of complex formation. Int. J. Biol. Macromol. 2019, 126, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Liew, M.W.J.; Venkatraman, S. Recent Advances in Chitosan-Based Carriers for Gene Delivery. Mar. Drugs 2019, 17, 381. [Google Scholar] [CrossRef]
- Hashem, F.M.; Nasr, M.; Khairy, A.; Alqurshi, A. In vitro cytotoxicity and transfection efficiency of pDNA encoded p53 gene-loaded chitosan-sodium deoxycholate nanoparticles. Int. J. Nanomed. 2019, 14, 4123–4131. [Google Scholar] [CrossRef]
- Ahmed, I.; Dildar, L.; Haque, A.; Patra, P.; Mukhopadhyay, M.; Hazra, S.; Kulkarni, M.; Thomas, S.; Plaisier, J.R.; Dutta, S.B.; et al. Chitosan-fatty acid interaction mediated growth of Langmuir monolayer and Langmuir-Blodgett films. J. Colloid Interface Sci. 2018, 514, 433–442. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Bao, X.; Xu, G.; Yao, P. Fatty acid and quaternary ammonium modified chitosan nanoparticles for insulin delivery. Colloids Surf. B Biointerfaces 2018, 170, 136–143. [Google Scholar] [CrossRef]
- Lamptey, R.N.L.; Gothwal, A.; Trivedi, R.; Arora, S.; Singh, J. Synthesis and Characterization of Fatty Acid Grafted Chitosan Polymeric Micelles for Improved Gene Delivery of VGF to the Brain through Intranasal Route. Biomedicines 2022, 10, 493. [Google Scholar] [CrossRef]
- Martins, G.O.; Petrônio, M.S.; Lima, A.M.F.; Junior, A.M.M.; Tiera, V.A.D.; Calmon, M.d.; Vilamaior, P.S.L.; Han, S.W.; Tiera, M.J. Amphipathic chitosans improve the physicochemical properties of siRNA-chitosan nanoparticles at physiological conditions. Carbohydr. Polym. 2019, 216, 332–342. [Google Scholar] [CrossRef]
- Gupta, A.; Pal, A.K.; Woo, E.M.; Katiyar, V. Effects of Amphiphilic Chitosan on Stereocomplexation and Properties of Poly(lactic acid) Nano-biocomposite. Sci. Rep. 2018, 8, 4351. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Naskar, S.; Kuotsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target 2019, 27, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-H.; Chao, H.-M.; Chern, E.; Hsu, S.-H. Chitosan 3D cell culture system promotes naïve-like features of human induced pluripotent stem cells: A novel tool to sustain pluripotency and facilitate differentiation. Biomaterials 2021, 268, 120575. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Lepeltier, E.; Loretz, B.; Desmaele, D.; Zapp, J.; Herrmann, J.; Couvreur, P.; Lehr, C.-M. Squalenoylation of Chitosan: A Platform for Drug Delivery? Biomacromolecules 2018, 16, 2930–2939. [Google Scholar] [CrossRef]
- Steinle, H.; Ionescu, T.-M.; Schenk, S.; Golombek, S.; Kunnakattu, S.-J.; Özbek, M.T.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Incorporation of Synthetic mRNA in Injectable Chitosan-Alginate Hybrid Hydrogels for Local and Sustained Expression of Exogenous Proteins in Cells. Int. J. Mol. Sci. 2018, 19, 1313. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N.; Mitropoulos, A.C. Chitosan adsorbents for dye removal: A review. Polym. Int. 2017, 66, 1800–1811. [Google Scholar] [CrossRef]
- Shin, J.-H.; Yang, J.E.; Park, J.E.; Jeong, S.-W.; Choi, S.-J.; Choi, Y.J.; Jeon, J. Rapid and Efficient Removal of Anionic Dye in Water Using a Chitosan-Coated Iron Oxide-Immobilized Polyvinylidene Fluoride Membrane. ACS Omega 2022, 7, 8759–8766. [Google Scholar] [CrossRef]
- Verma, S.; Dutta, R.K. Adsorptive Removal of Toxic Dyes Using Chitosan and Its Composites. In Green Materials for Wastewater Treatment. Environmental Chemistry for a Sustainable World; Naushad, M., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2020; Volume 38. [Google Scholar]
- Zhang, Y.; Zhao, M.; Cheng, Q.; Wang, C.; Li, H.; Han, X.; Fan, Z.; Su, G.; Pan, D.; Li, Z. Research progress of adsorption and removal of heavy metals by chitosan and its derivatives: A review. Chemosphere 2021, 279, 130927. [Google Scholar] [CrossRef] [PubMed]
- Borgohain, R.; Pattnaik, U.; Prasad, B.; Mandal, B. A review on chitosan-based membranes for sustainable CO2 separation applications: Mechanism, issues, and the way forward. Carbohydr. Polym. 2021, 267, 118178. [Google Scholar] [CrossRef] [PubMed]
- Rosli, N.A.H.; Loh, K.S.; Wong, W.Y.; Yunus, R.M.; Lee, T.K.; Ahmad, A.; Chong, S.T. Review of Chitosan-Based Polymers as Proton Exchange Membranes and Roles of Chitosan-Supported Ionic Liquids. Int. J. Mol. Sci. 2020, 21, 632. [Google Scholar] [CrossRef] [PubMed]
- Dudek, G.; Gnus, M.; Turczyn, R.; Strzelewicz, A.; Krasowska, M. Pervaporation with chitosan membranes containing iron oxide nanoparticles. Sep. Purif. Technol. 2014, 133, 8–15. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; González-Valdez, J.; Zamidi, A.M. High-performance pervaporation chitosan-based membranes: New insights and perspectives. Rev. Chem. Eng. 2021, 37, 959–974. [Google Scholar] [CrossRef]
- Long, Q.; Zhang, Z.; Qi, G.; Wang, Z.; Chen, Y.; Liu, Z.-Q. Fabrication of Chitosan Nanofiltration Membranes by the Film Casting Strategy for Effective Removal of Dyes/Salts in Textile Wastewater. ACS Sustain. Chem. Eng. 2020, 8, 2512–2522. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Applications of Chitosan as Food Packaging Materials. In Sustainable Agriculture Reviews; Crini, G., Lichtfouse, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 36. [Google Scholar]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef]
- Janik, W.; Wojtala, A.; Pietruszka, A.; Dudek, G.; Sabura, E. Environmentally Friendly Melt-Processed Chitosan/Starch Composites Modified with PVA and Lignin. Polymers 2021, 13, 2685. [Google Scholar] [CrossRef]
- Janik, W.; Nowotarski, M.; Shyntum, D.Y.; Banaś, A.; Krukiewicz, K.; Kudła, S.; Dudek, G. Antibacterial and Biodegradable Polysaccharide-Based Films for Food Packaging Applications: Comparative Study. Materials 2022, 15, 3236. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Mahmodi, G.; Zarrintaj, P.; Taghizadeh, A.; Taghizadeh, M.; Manouchehri, S.; Dangwal, S.; Ronte, A.; Ganjali, M.R.; Ramsey, J.D.; Kim, S.-J.; et al. From microporous to mesoporous mineral frameworks: An alliance between zeolite and chitosan. Carbohydr. Res. 2020, 489, 107930. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Taghizadeh, A.; Yazdi, M.K.; Zarrintaj, P.; Stadler, F.J.; Ramsey, J.D.; Habibzadeh, S.; Rad, S.H.; Naderi, G.; Saeb, M.R.; et al. Chitosan-based inks for 3D printing and bioprinting. Green Chem. 2022, 24, 62–101. [Google Scholar] [CrossRef]
- Gaidhani, K.A.; Harwalker, M.; Bhambere, D.; Nirgude, P.S. Lyophilization/Freeze Drying—A Review. World J. Pharm. Res. 2015, 4, 516–543. [Google Scholar]
- Svagan, J.; Jensen, P.; Dvinskikh, S.V.; Furó, I.; Berglund, L.A. Towards tailored hierarchical in cellulose nanocomposite foams prepared by freezing/freeze drying. J. Mater. Chem. 2010, 20, 6646–6654. [Google Scholar] [CrossRef]
- Borisova, A.; de Bruyn, M.; Budarin, V.L.; Shuttleworth, P.S.; Dodson, J.R.; Segatto, M.L.; Clark, J.H. A sustainable freeze-drying route to porous polysaccharides with tailored hierarchical meso-and microporosity. Macromol. Rapid Commun. 2015, 36, 774–779. [Google Scholar] [CrossRef]
- Tang, X.C.; Pikal, M.J. Design of Freeze-Drying Processes for Pharmaceuticals: Practical Advice. Pharm. Res. 2004, 21, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Pisano, R.; Fissore, D.; Velardi, S.A.; Barresi, A.A. In-Line Optimization and Control of an Industrial Freeze-Drying Process for Pharmaceuticals. J. Pharm. Sci. 2010, 99, 4691–4709. [Google Scholar] [CrossRef]
- Sadikoglu, H.; Ozdemir, M.; Seker, M. Freeze-Drying of Pharmaceutical Products: Research and Development Needs. Dry. Technol. 2006, 24, 849–861. [Google Scholar] [CrossRef]
- Ratti, C. 3—Freeze drying for food powder production. In Handbook of Food Powders; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 57–84. [Google Scholar]
- Oikonomopoulou, V.P.; Krokida, M.K.; Karathanos, V.T. The influence of freeze drying conditions on microstructural changes of food products. Procedia Food Sci. 2011, 1, 647–654. [Google Scholar] [CrossRef]
- Jingjing, E.; Chen, J.; Chen, Z.; Ma, R.; Zhang, J.; Yao, C.; Wang, R.; Zhang, Q.; Yang, Y.; Li, J.; et al. Effects of different initial pH values on freeze-drying resistance of Lactiplantibacillus plantarum LIP-1 based on transcriptomics and proteomics. Food Res. Int. 2021, 149, 110694. [Google Scholar]
- Claussen, I.A.; Ustad, T.S.; Strommen, I.; Walde, P.M. Atmospheric Freeze Drying—A Review. Dry. Technol. 2007, 25, 947–957. [Google Scholar] [CrossRef]
- Oyinloye, T.M.; Yoon, W.B. Effect of Freeze Drying on Quality and Grinding Process of Food Produce: A Review. Processes 2020, 8, 354. [Google Scholar] [CrossRef]
- Khan, M.I.H.; Wellard, R.M.; Nagy, S.A.; Joardder, M.U.H.; Karim, M.A. Experimental investigation of bound and free water transport process during drying of hygroscopic food material. Int. J. Therm. Sci. 2017, 117, 266–273. [Google Scholar] [CrossRef]
- Garg, T.; Chanana, A.; Joshi, R. Preparation of Chitosan Scaffolds for Tissue Engineering using Freeze Drying Technology. IOSR J. Pharm. 2012, 2, 72–73. [Google Scholar] [CrossRef]
- Song, W.; Xu, J.; Gao, L.; Zhang, Q.; Tong, J.; Ren, L. Preparation of Freeze-Dried Porous Chitosan Microspheres for the Removal of Hexavalent Chromium. Appl. Sci. 2021, 11, 4217. [Google Scholar] [CrossRef]
- Tomaz, A.F.; de Carvalho, S.M.S.; Barbosa, M.C.; Silva, S.M.L.; Gutierrez, M.A.S.; de Lima, A.G.B.; Fook, M.V.L. Ionically Crosslinked Chitosan Membranes Used as Drug Carriers for Cancer Therapy Application. Materials 2018, 11, 2051. [Google Scholar] [CrossRef]
- Zeng, M.; Yuan, X.; Yang, Z.; Qi, C. Novel macroporous palladium cation crosslinked chitosan membranes for heterogeneous catalysis application. Int. J. Biol. Macromol. 2014, 68, 189–197. [Google Scholar] [CrossRef]
- Madihally, S.V.; Matthew, H.W.T. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Riniki, K.; Dutta, P.K. Chitosan based scaffolds by lyophilization and sc. CO2 assisted methods for tissue engineering applications. J. Macromol. Sci. 2010, 47, 429–434. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, H.; Wang, J.; Qu, W. Production of chitosan scaffolds by lyophilization or electrospinning; which is better for peripheral nerve regeneration? Neural Regen. Res. 2021, 16, 1093–1098. [Google Scholar]
- Ji, C.; Shi, J. Thermal-crosslinked porous chitosan scaffolds for soft tissue engineering applications. Mater. Sci. Eng. C 2013, 33, 3780–3785. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, I.; Sergi, R.; D’Abusco, A.S.; Mariano, A.; Martinelli, A.; Piozzi, A.; Francolini, I. Chitosan scaffolds with enhanced mechanical strength and elastic response by combination of freeze gelation, photo-crosslinking and freeze-drying. Carbohydr. Polym. 2021, 267, 118156. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, H. One-step synthesis of protein-encapsulated microspheres in a porous scaffolds by freeze-drying double emulsions and tuneble protein release. Chem. Commun. 2013, 49, 8833–8835. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, X.Y.; Shi, S.; Kong, X.Y.; Guo, G.; Huang, M.J.; Luo, F.; Wei, Y.Q.; Zhao, X.; Qian, Z.Y. Preparation and characterization of a novel chitosan scaffold. Carbohydr. Polym. 2010, 80, 860–865. [Google Scholar] [CrossRef]
- Seol, Y.-J.; Lee, J.-Y.; Park, Y.-J.; Lee, Y.-M.; Ku, Y.; Rhyu, I.-C.; Lee, S.-J.; Han, S.-B.; Chung, C.-P. Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnol. Lett. 2004, 26, 1037–1041. [Google Scholar] [CrossRef]
- Kim, S.E.; Park, J.H.; Cho, Y.W.; Chung, H.; Jeong, S.Y.; Lee, E.B.; Kwon, I.C. Porous chitosan scaffold containing microspheres loaded with transforming growth factor-β1: Implications for cartilage tissue engineering. J. Control. Release 2003, 91, 365–374. [Google Scholar] [CrossRef]
- Heimbuck, M.; Priddy-Arrington, T.R.; Sawyer, B.J.; Caldorera-Moore, M.E. Effects of post-processing methods on chitosan-genipin hydrogel properties. Mater. Sci. Eng. C 2019, 98, 612–618. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Fabrication of porous chitosan particles using a novel two-step porogen leaching and lyophilization method with the label-free multivariate spectral assessment of live adhered cells. Colloids Surf. B 2021, 208, 112094. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, H. Green synthesis of chitosan-based nanofibers and their applications. Green Chem. 2010, 12, 1207–1214. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, Y.; Liu, B. Preparation of chitosan microcarriers by high voltage electrostatic field and freeze drying. J. Biosci. Bioeng. 2019, 128, 504–509. [Google Scholar] [CrossRef]
- Ren, L.; Xu, J.; Zhang, Y.; Zhou, J.; Chen, D.; Chang, Z. Preparation and characterization of porous chitosan microspheres and adsorption performance for hexavalent chromium. Int. J. Biol. Macromol. 2019, 135, 898–906. [Google Scholar] [CrossRef]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Huang, J.; Xue, C.; Wang, Y.; Wang, S.; Bao, S.; Chen, R.; Li, Y.; Gu, Y. Skin derived precursor Schwann cell-generated acellular matrix modified chitosan/silk scaffolds for bridging rat sciatic nerve gap. Neurosci. Res. 2018, 135, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ji, Y.; Zhao, Y.; Liu, Y.; Ding, F.; Gu, X.; Yang, Y. The influence of substrate stiffness on the behavior and functions of Schwann cells in culture. Biomaterials 2012, 33, 6672–6681. [Google Scholar] [CrossRef] [PubMed]
- Benhabbour, S.R.; Sheardown, H.; Adronov, A. Cell adhesion and proliferation on hydrophilic dendritically modified surfaces. Biomaterials 2008, 29, 4177–4186. [Google Scholar] [CrossRef]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef]
- Chen, Y.; Qi, Y.; Yan, X.; Ma, H.; Chen, J.; Liu, B.; Xue, Q. Green fabrication of porous chitosan/graphene oxide xerogels for drug delivery. J. Appl. Surf. Sci. 2014, 131, 40006. [Google Scholar]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 50. † Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 10, 77. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Okay, O. Basic Principles of Cryotropic Gelation. Adv. Polym. Sci. 2014, 263, 103. [Google Scholar]
- Santos-López, G.; Argüelles-Monal, W.; Carvajal-Millan, E.; López-Franco, Y.L.; Recillas-Mota, M.T.; Lizardi-Mendoza, J. Aerogels from Chitosan Solutions in Ionic Liquids. Polymers 2017, 9, 722. [Google Scholar] [CrossRef]
- Nielsen, N.E. Cross-linking-effect on physical properties of polymers. Polym. Rev. 1969, 3, 69–103. [Google Scholar] [CrossRef]
- Rabek, J.F. Contemporary Knowledge about Polymers; Scientific Publishing House PWN: Warszawa, Poland, 2009; pp. 128–138. [Google Scholar]
- Ho, M.-H.; Kuo, P.-Y.; Hsieh, H.-J.; Hsien, T.-Y.; Hou, L.-T.; Lai, J.-Y.; Wang, D.-M. Preparation of porous scaffolds by using freeze-extraction and freeze-gelation methods. Biomaterials 2004, 25, 129–138. [Google Scholar] [CrossRef]
- Chen, P.H.; Hwang, Y.-H.; Kuo, T.-Y.; Liu, F.-H.; Lai, J.-Y.; Hsieh, H.-J. Improvement in the Properties of Chitosan Membranes Using Natural Organic Acid Solutions as Solvents for Chitosan Dissolution. J. Med. Bioeng. 2007, 27, 23–28. [Google Scholar]
- Pinto, R.V.; Gomes, P.S.; Fernandes, M.H.; Costa, M.E.V.; Almeida, M.M. Glutaraldehyde-crosslinking chitosan scaffolds reinforced with calcium phosphate spray-dried granules for bone tissue applications. Mater. Sci. Eng. C 2020, 109, 110557. [Google Scholar] [CrossRef]
- Pierre, A.C. Introduction to Sol-Gel Processing; Springer Nature: London, UK, 2020. [Google Scholar]
- Parashar, M.; Shukla, V.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Cividanes, L.S.; Campos, T.M.B.; Rodrigues, L.A.; Brunelli, D.D.; Thim, G.P. Review of mullite synthesis routes by sol–gel method. J. Sol-Gel Sci. Technol. 2010, 55, 111–125. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Montembault, A.; Viton, C.; Domard, A. Rheometric Study of the Gelation of Chitosan in Aqueous Solution without Cross-Linking Agent. Biomacromolecules 2005, 6, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Dobashi, T.; Tomita, N.; Maki, Y.; Chang, C.P.; Yamamoto, T. An analysis of anisotropic gel forming process of chitosan. Carbohydr. Polym. 2011, 84, 709–712. [Google Scholar] [CrossRef]
- Cui, Z.; Xiang, Y.; Si, J.; Yang, M.; Zhang, Q.; Zhang, T. Ionic interactions between sulfuric acid and chitosan membranes. Carbohydr. Polym. 2008, 73, 111–116. [Google Scholar] [CrossRef]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Sauraj; Kumar, B.; Negi, Y.S. Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Hamdine, M.; Heuzey, M.-C.; Bégin, A. Viscoelastic properties of phosphoric and oxalic acid-based chitosan hydrogels. Rheol. Acta 2006, 45, 659. [Google Scholar] [CrossRef]
- Beppu, M.M.; Vieira, R.S.; Aimoli, C.G.; Santana, C.C. Crosslinking of chitosan membranes using glutaraldehyde: Effect on ion permeability and water absorption. J. Membr. Sci. 2007, 301, 126–130. [Google Scholar] [CrossRef]
- Medellín-Castillo, N.A.; Isaacs-Páez, E.D.; Rodríguez-Méndez, I.; González-García, R.; Labrada-Delgado, G.J.; Aragón-Piña, A.; García-Arreola, M.E. Formaldehyde and tripolyphosphate crosslinked chitosan hydrogels: Synthesis, characterization and modelling. Int. J. Biol. Macromol. 2021, 183, 2293–2304. [Google Scholar] [CrossRef]
- Chen, A.-H.; Liu, S.; Chen, C.; Chen, C.-Y. Comparative adsorption of Cu(II), Zn(II), and Pb(II) ions in aqueous solution on the crosslinked chitosan with epichlorohydrin. J. Hazard. Mater. 2008, 154, 184–191. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Yang, Q.; Dou, F.; Liang, B.; Shen, Q. Studies of cross-linking reaction on chitosan fiber with glyoxal. Carbohydr. Polym. 2005, 59, 205–210. [Google Scholar] [CrossRef]
- Stievano, M.; Elvassore, N. High-pressure density and vapor–liquid equilibrium for the binary systems carbon dioxide–ethanol, carbon dioxide–acetone and carbon dioxide–dichloromethane. J. Supercrit. Fluids 2005, 33, 7–14. [Google Scholar] [CrossRef]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohydr. Polym. 2020, 231, 115744. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.; García-González, C. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, J.; Feng, J.; Jiang, Y. Formation of enhanced gelatum using ethanol/water binary medium for fabricating chitosan aerogels with high specific surface area. Chem. Eng. J. 2017, 309, 700–707. [Google Scholar] [CrossRef]
- Takeshita, S.; Yoda, S. Chitosan aerogels: Transparent flexible thermal insulators. Chem. Mater. 2015, 27, 7569–7572. [Google Scholar] [CrossRef]
- Takeshita, S.; Akasaka, S.; Yoda, S. Structural and acoustic properties of transparent chitosan aerogel. Mater. Lett. 2019, 254, 258–261. [Google Scholar] [CrossRef]
- Obaidat, R.M.; Tashtoush, B.M.; Bayan, M.; Al Bustami, R.T.; Alnaief, M. Drying Using Supercritical Fluid Technology as a Potential Method for Preparation of Chitosan Aerogel Microparticles. Pharm. Sci. Tech. 2015, 16, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Konishi, A.; Takebayashi, Y.; Yoda, S.; Otake, K. Aldehyde Approach to Hydrophobic Modification of Chitosan Aerogels. Biomacromolecules 2017, 18, 2172–2178. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Zhao, S.; Malfait, W.J. Transparent, Aldehyde-Free Chitosan Aerogel. Carbohydr. Polym. 2021, 251, 117089. [Google Scholar] [CrossRef]
- Venault, A.; Chang, Y.; Wang, D.-M.; Bouyer, D. A Review on Polymeric Membranes and Hydrogels Prepared by Vapor-Induced Phase Separation Process. Polym. Rev. 2013, 53, 568–626. [Google Scholar] [CrossRef]
- Hołda, A.K.; Vankelecom, I.F.J. Understanding and guiding the phase inversion process for synthesis of solvent resistant nanofiltration membranes. J. Appl. Polym. Sci. 2015, 132, 42130. [Google Scholar] [CrossRef]
- Matsuyama, H.; Karkhanechi, H.; Rajabzadeh, S. Chapter 3—Polymeric membrane fabrication via thermally induced phase separation (TIPS) method. In Hollow Fiber Membranes; Chung, T.-S., Feng, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 57–83. [Google Scholar]
- Mi, F.-L.; Wu, Y.-B.; Shyu, S.-S.; Chao, A.-C.; Lai, J.-Y.; Su, C.-C. Asymmetric chitosan membranes prepared by dry/wet phase separation: A new type of wound dressing for controlled antibacterial release. J. Membr. Sci. 2003, 212, 237–254. [Google Scholar] [CrossRef]
- Hong, H.; Wei, J.; Liu, C. Development of asymmetric gradational-changed porous chitosan membrane for guided periodontal tissue regeneration. Compos. Part B 2007, 38, 311–316. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Xue, P.H.; Li, W.J. Preparation of the Porous Chitosan Membrane by Cryogenic Induced Phase Separation. Polym. Adv. Technol. 2001, 12, 665–669. [Google Scholar] [CrossRef]
- Qin, W.; Li, J.; Tu, J.; Yang, H.; Chen, Q.; Liu, H. Fabrication of porous chitosan membranes composed of nanofibers by low temperature thermally induced phase separation, and their adsorption behavior for Cu2+. Carbohydr. Polym. 2017, 178, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.-L.; Shyu, S.-S.; Chen, C.-T.; Schoung, J.-Y. Porous chitosan microsphere for controlling the antigen release of Newcastle disease vaccine: Preparation of antigen-adsorbed microsphere and in vitro release. Biomaterials 1999, 20, 1603–1612. [Google Scholar] [CrossRef]
- Salerno, S.; de Santo, M.P.; Drioli, E.; de Bartolo, L. Nano-and Micro-Porous Chitosan Membranes for Human Epidermal Stratification and Differentiation. Membranes 2021, 11, 394. [Google Scholar] [CrossRef]
- Biswas, D.P.; Tran, P.A.; Tallon, C.; O’Connor, A.J. Combining mechanical foaming and thermally induced phase separation to generate chitosan scaffolds for soft tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 207–226. [Google Scholar] [CrossRef]
- Mansour, F.R.; Waheed, S.; Paull, B.; Maya, F. Porogens and porogen selection in the preparation of porouspolymer monoliths. J. Sep. Sci. 2020, 43, 56–69. [Google Scholar] [CrossRef]
- Chevalier, E.; Chulia, D.; Pouget, C.; Viana, M. Fabrication of Porous Substrates: A Review of Processes Using Pore Forming Agents in the Biomaterial Field. J. Pharm. Sci. 2008, 97, 1135–1154. [Google Scholar] [CrossRef]
- Janik, H.; Marzec, M. A review: Fabrication of porous polyurethane scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 586–591. [Google Scholar] [CrossRef]
- Zhou, M.; Shen, L.; Lin, X.; Hong, Y.; Feng, Y. Design and pharmaceutical applications of porous particles. RSC Adv. 2017, 7, 39490–39501. [Google Scholar] [CrossRef]
- Chao, A.-C.; Yu, S.-H.; Chuang, G.-S. Using NaCl particles as porogen to prepare a highly adsorbent chitosan membranes. J. Membr. Sci. 2006, 280, 163–174. [Google Scholar] [CrossRef]
- Santos, D.E.S.; Neto, C.G.T.; Fonseca, J.L.C.; Pereira, M.R. Chitosan macroporous asymmetric membranes—Preparation, characterization and transport of drugs. J. Membr. Sci. 2008, 325, 362–370. [Google Scholar] [CrossRef]
- Zeng, M.; Fang, Z.; Xu, C. Novel method of preparing microporous membrane by selective dissolution of chitosan/polyethylene glycol blend membrane. J. Appl. Polym. Sci. 2004, 91, 2840–2847. [Google Scholar] [CrossRef]
- Lim, J.I.; Lee, Y.-K.; Shin, J.-S.; Lim, K.-J. Preparation of interconnected porous chitosan scaffolds by sodium acetate particulate leaching. J. Biomater. Sci. Polym. Ed. 2011, 22, 1319–1329. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Z.; Ma, M.; Sheng, L.; Huang, X. Effect of eggshell membrane as porogen on the physicochemical structure and protease immobilization of chitosan-based macroparticles. Carbohydr. Polym. 2020, 242, 116387. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Lv, C.; Wu, L.; Hou, X. Porous chitosan/hydroxyapatite composite membrane for dyes static and dynamic removal from aqueous solution. J. Hazard. Mater. 2017, 338, 241–249. [Google Scholar] [CrossRef]
- Rekik, S.B.; Gassara, S.; Bouaziz, J.; Deratani, A.; Baklouti, S. Enhancing hydrophilicity and permeation flux of chitosan/kaolin composite membranes by using polyethylene glycol as porogen. Appl. Clay Sci. 2019, 168, 312–323. [Google Scholar] [CrossRef]
- Alias, S.S.; Ariff, Z.M.; Mohamad, A.A. Porous membrane based on chitosan–SiO2 for coin cell proton battery. Ceram. Int. 2015, 41, 5484–5491. [Google Scholar] [CrossRef]







| Type of Material | Pore Characteristics | Porosity (%)/Pore Size (μm) | Application | Reference(s) |
|---|---|---|---|---|
| Membranes | Open-pore structure; oblong pores; slice building of the membrane | 70–80/- | Catalysis (catalyst site for Heck coupling reaction) | [60] |
| Membranes | Interconnected macropores; uniform pore structure; fewer pores on the surface of the membrane | -/- | Drug release, inhibition of cancer cell growth | [59] |
| Scaffolds | Relatively small, interconnected pores; sponge-like scaffold structure | -/10–20 | Drug delivery, tissue engineering | [57] |
| Scaffolds | Polygonal and interconnected pores; sheet-like scaffold structure; randomly-located pores | 88–97/60–90 | Biomedicine, cell proliferation | [5] |
| Scaffolds | Uniform, spherical pores; open-pore structure; pore size depended on initial chitosan concentration | -/40–250 | Tissue engineering | [61] |
| Scaffolds | Small oblong pores that were heterogeneously distributed; specific surface area calculated using BHJ desorption isotherms (2.19 m2/g) | -/- | Bioengineering | [62] |
| Scaffolds | Medium-sized pores; uniform sponge-like scaffold structure | -/50–200 | Tissue engineering (nerve regeneration) | [63] |
| Scaffolds | Interconnected pores with thin walls; pores on the scaffold surface and inside the structure | 75–85/60–80 | Cell proliferation, tissue engineering | [64] |
| Scaffolds | Spherical, interconnected pores; uniform scaffold structure; narrow range of pore sizes | 30–80/105–138 | Tissue engineering | [65] |
| Scaffolds | Interconnected pores; organized structure throughout material | -/- | Protein coating, drug delivery | [66] |
| Scaffolds | Interconnected pores; highly-porous structure; wide distribution of pore sizes | 85–95/50–150 | Cell proliferation | [67] |
| Scaffolds | Interconnected pores; sponge-like scaffold structure | -/100–200 | Tissue engineering (bone regeneration) | [68] |
| Scaffolds | Structure with open, interconnected pores; round, uniformly-shaped pores | -/100–200 | Cell proliferation (chondrocytes growth) | [69] |
| Scaffolds (dried hydrogels) | Uniform, homogenous pores with thin walls; pore size depended on the lyophilization conditions | -/9–400 | - | [70] |
| Particles | Small, randomly dispersed pores within particles; pores on the particle surface were larger than interior pores | -/1.5–15 | Cell proliferation | [71] |
| Nanofibers | Irregularly-shaped pores crucial for the structure; pore volume between 13.89–33.32 mL/g depending on initial chitosan concentration | -/20–180 | Ion adsorption (copper ion removal) | [72] |
| Microspheres (microcarriers) | Pores distributed evenly on the surface and within microspheres; pores collapsed with higher initial concentrations of chitosan | 90/15–20 | Cell proliferation (hepatocyte growth) | [73] |
| Microspheres | Shape and structure of pores depended on chitosan concentration; uniformly distributed pores with spherical or oblong shapes | 23.14–63.15/- | Ion adsorption (hexavalent chromium removal) | [58] |
| Microspheres | Shape and structure of pores depended on chitosan concentration, freezing temperature, and time; sponge-like interior structure and porosity >90% for 1 wt.% chitosan; uniform distribution of pores and porosity ~50% for 2.0–3.5 wt.% chitosan | 45–95/- | Ion adsorption (hexavalent chromium removal) | [74] |
| Gelation | Structural Characteristics | Porosity (%)/Pore Size (nm) | Application | Reference(s) |
|---|---|---|---|---|
| Aqueous solution of NaOH | Structure comprising numerous fibers with meso- and macropores; fibers distributed uniformly generating a structure with high specific surface area (257–479 m2/g) and specific pore volume (1.01–1.70 cm3/g) | 96.7–97.2/ 2–50 and >50 | Chronic wound healing | [106] |
| Glutaraldehyde | Chitosan aerogel comprising entangled nanofibers with diameters ~40 nm; interconnected fibers create structure with large specific surface (658–973 m2/g) | -/ 50–120 | Adsorption of methyl orange | [107] |
| Glutaraldehyde | Chitosan aerogel structure comprising short nanofibers with diameters 5–10 nm; high specific surface area (545 m2/g) and thermal conductivity coefficient (0.022 W/mK) | 86–97/ 10–50 | House windows, glass walls of buildings, car windows | [108] |
| Formaldehyde | Aerogel structure comprising nanofibers with diameters ~15 nm and nanoparticulate aggregates; highly transparent, yellow aerogel with high specific surface area (737–872 m2/g) | 89.3–96.0/ 21.3–43.6 | Sound absorption | [109] |
| Sodium tripolyphosphate | Low-porosity aerogels with low specific surface area (73–103 m2/g); uniform aerogel structure with homogeneous pore shapes | 20–29/- | Drug delivery | [110] |
| Formaldehyde and alkyl aldehyde | Aerogel with uniform, interconnected pores; structure comprising nanofibers with diameters ~50 nm; high specific surface area (581–672 m2/g) | -/- | Thermal insulation | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzybek, P.; Jakubski, Ł.; Dudek, G. Neat Chitosan Porous Materials: A Review of Preparation, Structure Characterization and Application. Int. J. Mol. Sci. 2022, 23, 9932. https://doi.org/10.3390/ijms23179932
Grzybek P, Jakubski Ł, Dudek G. Neat Chitosan Porous Materials: A Review of Preparation, Structure Characterization and Application. International Journal of Molecular Sciences. 2022; 23(17):9932. https://doi.org/10.3390/ijms23179932
Chicago/Turabian StyleGrzybek, Paweł, Łukasz Jakubski, and Gabriela Dudek. 2022. "Neat Chitosan Porous Materials: A Review of Preparation, Structure Characterization and Application" International Journal of Molecular Sciences 23, no. 17: 9932. https://doi.org/10.3390/ijms23179932
APA StyleGrzybek, P., Jakubski, Ł., & Dudek, G. (2022). Neat Chitosan Porous Materials: A Review of Preparation, Structure Characterization and Application. International Journal of Molecular Sciences, 23(17), 9932. https://doi.org/10.3390/ijms23179932







