Liquid Biopsy as a Tool for the Diagnosis, Treatment, and Monitoring of Breast Cancer
Abstract
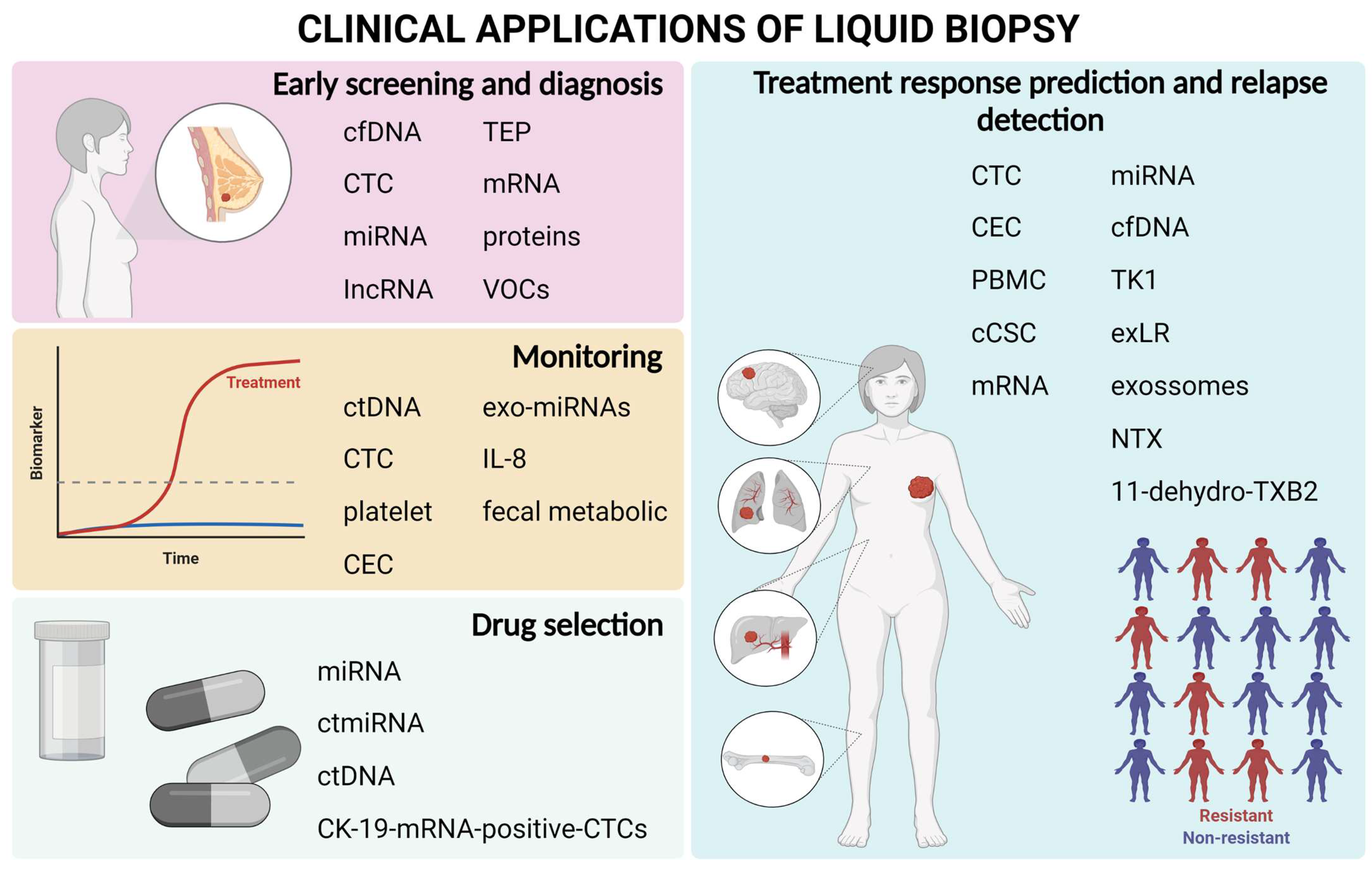
:1. Introduction
2. Breast Cancer Screening Using Liquid Biopsy
3. Use of Liquid Biopsy to Aid in Drug Selection
4. Monitoring Residual Disease Using Liquid Biopsy Biomarkers during Treatment
4.1. Circulating ctDNA
4.2. Platelets, CTC, and CEC
4.3. Exo-miRNAs, IL-8, and Fecal Metabolomics
5. Prediction of Treatment Response and Early Detection of Relapse
5.1. Circulating Cells as Biomarkers
5.2. Nucleic Acids as Biomarkers
5.3. Metabolic Biomarkers
6. Applications of Liquid Biopsy in Clinical Practice
6.1. Circulating Tumor Cells
| Test | Biomarkers | Method | Ref. |
|---|---|---|---|
| CellSearch | CTCs | CellSearch System | [108] |
| Guardant360 | ctDNA | NGS | [109] |
| FoundationOne Liquid | ctDNA | NGS | [110] |
6.2. Circulating Tumor DNA
6.3. Other Serum Markers
7. Final Considerations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomi, T.; Kosaka, T.; Endoh, H.; Horio, Y.; Hida, T.; Mori, S.; Hatooka, S.; Shinoda, M.; Takahashi, T.; Yatabe, Y. Mutations of the Epidermal Growth Factor Receptor Gene Predict Prolonged Survival After Gefitinib Treatment in Patients With Non–Small-Cell Lung Cancer With Postoperative Recurrence. JCO 2005, 23, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.D.; Stover, D.G.; Hai, T. Chemotherapy-Exacerbated Breast Cancer Metastasis: A Paradox Explainable by Dysregulated Adaptive-Response. Int. J. Mol. Sci. 2018, 19, 3333. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, G.S.; Pastoriza, J.M.; Wang, Y.; Harney, A.S.; Entenberg, D.; Pignatelli, J.; Sharma, V.P.; Xue, E.A.; Cheng, E.; D’Alfonso, T.M.; et al. Neoadjuvant Chemotherapy Induces Breast Cancer Metastasis through a TMEM-Mediated Mechanism. Sci. Transl. Med. 2017, 9, eaan0026. [Google Scholar] [CrossRef]
- Ming, H.; Li, B.; Zhou, L.; Goel, A.; Huang, C. Long Non-Coding RNAs and Cancer Metastasis: Molecular Basis and Therapeutic Implications. Biochim. Biophys. Acta (BBA) Rev. Cancer 2021, 1875, 188519. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, X.; Yang, J.-J.; Liu, X.; Zhao, X.; Wang, Q.; Han, L.; Song, X.; Zhu, Z.; Tian, W.; et al. AC1MMYR2 Impairs High Dose Paclitaxel-Induced Tumor Metastasis by Targeting MiR-21/CDK5 Axis. Cancer Lett. 2015, 362, 174–182. [Google Scholar] [CrossRef]
- Yu, K.-H.; Snyder, M. Omics Profiling in Precision Oncology. Mol. Cell Proteom. 2016, 15, 2525–2536. [Google Scholar] [CrossRef]
- Freitas, A.J.A.D.; Causin, R.L.; Varuzza, M.B.; Hidalgo Filho, C.M.T.; Silva, V.D.D.; Souza, C.D.P.; Marques, M.M.C. Molecular Biomarkers Predict Pathological Complete Response of Neoadjuvant Chemotherapy in Breast Cancer Patients: Review. Cancers 2021, 13, 5477. [Google Scholar] [CrossRef]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and Future Perspectives of Liquid Biopsies in Genomics-Driven Oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef]
- Alimirzaie, S.; Bagherzadeh, M.; Akbari, M.R. Liquid Biopsy in Breast Cancer: A Comprehensive Review. Clin. Genet. 2019, 95, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xu, T.; Wang, S.; Chang, H.; Yu, T.; Zhu, Y.; Chen, J. Liquid Biopsy Applications in the Clinic. Mol. Diagn. Ther. 2020, 24, 125–132. [Google Scholar] [CrossRef]
- Rubis, G.D.; Krishnan, S.R.; Bebawy, M. Liquid Biopsies in Cancer Diagnosis, Monitoring, and Prognosis. Trends Pharmacol. Sci. 2019, 40, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Preethi, K.A.; Selvakumar, S.C.; Ross, K.; Jayaraman, S.; Tusubira, D.; Sekar, D. Liquid Biopsy: Exosomal MicroRNAs as Novel Diagnostic and Prognostic Biomarkers in Cancer. Mol. Cancer 2022, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, H. Next-Generation Sequencing in Liquid Biopsy: Cancer Screening and Early Detection. Hum. Genom. 2019, 13, 34. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Circulating Tumor Cells: Liquid Biopsy of Cancer. Clin. Chem. 2013, 59, 110–118. [Google Scholar] [CrossRef]
- Thierry, A.R.; El Messaoudi, S.; Gahan, P.B.; Anker, P.; Stroun, M. Origins, Structures, and Functions of Circulating DNA in Oncology. Cancer Metastasis Rev. 2016, 35, 347–376. [Google Scholar] [CrossRef]
- Kopreski, M.S.; Benko, F.A.; Kwak, L.W.; Gocke, C.D. Detection of Tumor Messenger RNA in the Serum of Patients with Malignant Melanoma. Clin. Cancer Res. 1999, 5, 1961–1965. [Google Scholar]
- Lee, I.; Baxter, D.; Lee, M.Y.; Scherler, K.; Wang, K. The Importance of Standardization on Analyzing Circulating RNA. Mol. Diagn. Ther. 2017, 21, 259–268. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Causin, R.L.; de Freitas, A.J.A.; Trovo Hidalgo Filho, C.M.; dos Reis, R.; Reis, R.M.; Marques, M.M.C. A Systematic Review of MicroRNAs Involved in Cervical Cancer Progression. Cells 2021, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Risitano, A.; Beaulieu, L.M.; Vitseva, O.; Freedman, J.E. Platelets and Platelet-like Particles Mediate Intercellular RNA Transfer. Blood 2012, 119, 6288–6295. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; EL Andaloussi, S.; Wood, M.J.A. Exosomes and Microvesicles: Extracellular Vesicles for Genetic Information Transfer and Gene Therapy. Hum. Mol. Genet. 2012, 21, R125–R134. [Google Scholar] [CrossRef] [PubMed]
- Zubor, P.; Kubatka, P.; Kajo, K.; Dankova, Z.; Polacek, H.; Bielik, T.; Kudela, E.; Samec, M.; Liskova, A.; Vlcakova, D.; et al. Why the Gold Standard Approach by Mammography Demands Extension by Multiomics? Application of Liquid Biopsy MiRNA Profiles to Breast Cancer Disease Management. Int. J. Mol. Sci. 2019, 20, 2878. [Google Scholar] [CrossRef]
- Tay, T.K.Y.; Tan, P.H. Liquid Biopsy in Breast Cancer: A Focused Review. Arch. Pathol. Lab. Med. 2020, 145, 678–686. [Google Scholar] [CrossRef]
- Kamel, A.M.; Teama, S.; Fawzy, A.; El Deftar, M. Plasma DNA Integrity Index as a Potential Molecular Diagnostic Marker for Breast Cancer. Tumour. Biol. 2016, 37, 7565–7572. [Google Scholar] [CrossRef]
- Li, Z.; Guo, X.; Tang, L.; Peng, L.; Chen, M.; Luo, X.; Wang, S.; Xiao, Z.; Deng, Z.; Dai, L.; et al. Methylation Analysis of Plasma Cell-Free DNA for Breast Cancer Early Detection Using Bisulfite next-Generation Sequencing. Tumour. Biol. 2016, 37, 13111–13119. [Google Scholar] [CrossRef]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and Localization of Surgically Resectable Cancers with a Multi-Analyte Blood Test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef]
- Beaver, J.A.; Jelovac, D.; Balukrishna, S.; Cochran, R.; Croessmann, S.; Zabransky, D.J.; Wong, H.Y.; Toro, P.V.; Cidado, J.; Blair, B.G.; et al. Detection of Cancer DNA in Plasma of Early Stage Breast Cancer Patients. Clin. Cancer Res. 2014, 20, 2643–2650. [Google Scholar] [CrossRef]
- Kruspe, S.; Dickey, D.D.; Urak, K.T.; Blanco, G.N.; Miller, M.J.; Clark, K.C.; Burghardt, E.; Gutierrez, W.R.; Phadke, S.D.; Kamboj, S.; et al. Rapid and Sensitive Detection of Breast Cancer Cells in Patient Blood with Nuclease-Activated Probe Technology. Mol. Ther. Nucleic. Acids 2017, 8, 542–557. [Google Scholar] [CrossRef]
- Shimomura, A.; Shiino, S.; Kawauchi, J.; Takizawa, S.; Sakamoto, H.; Matsuzaki, J.; Ono, M.; Takeshita, F.; Niida, S.; Shimizu, C.; et al. Novel Combination of Serum MicroRNA for Detecting Breast Cancer in the Early Stage. Cancer Sci. 2016, 107, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erbes, T.; Hirschfeld, M.; Rücker, G.; Jaeger, M.; Boas, J.; Iborra, S.; Mayer, S.; Gitsch, G.; Stickeler, E. Feasibility of Urinary MicroRNA Detection in Breast Cancer Patients and Its Potential as an Innovative Non-Invasive Biomarker. BMC Cancer 2015, 15, 193. [Google Scholar] [CrossRef]
- Hirschfeld, M.; Rücker, G.; Weiß, D.; Berner, K.; Ritter, A.; Jäger, M.; Erbes, T. Urinary Exosomal MicroRNAs as Potential Non-Invasive Biomarkers in Breast Cancer Detection. Mol. Diagn. Ther. 2020, 24, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Souza, K.C.B.; Evangelista, A.F.; Leal, L.F.; Souza, C.P.; Vieira, R.A.; Causin, R.L.; Neuber, A.C.; Pessoa, D.P.; Passos, G.A.S.; Reis, R.M.V.; et al. Identification of Cell-Free Circulating MicroRNAs for the Detection of Early Breast Cancer and Molecular Subtyping. J. Oncol. 2019, 2019, 8393769. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, X.; Gao, S.; Jing, F.; Yang, Y.; Du, L.; Zheng, G.; Li, P.; Li, C.; Wang, C. Exosomal Long Noncoding RNA CRNDE-h as a Novel Serum-Based Biomarker for Diagnosis and Prognosis of Colorectal Cancer. Oncotarget 2016, 7, 85551–85563. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, Y.; Zhang, X.; Yang, Y.; Zheng, X.; Li, X.; Liu, Y.; Zhang, Y. Exosomal Long Noncoding RNA HOTTIP as Potential Novel Diagnostic and Prognostic Biomarker Test for Gastric Cancer. Mol. Cancer 2018, 17, 68. [Google Scholar] [CrossRef]
- Zhong, G.; Wang, K.; Li, J.; Xiao, S.; Wei, W.; Liu, J. Determination of Serum Exosomal H19 as a Noninvasive Biomarker for Breast Cancer Diagnosis. Onco. Targets Ther. 2020, 13, 2563–2571. [Google Scholar] [CrossRef]
- Zhou, W.; Ye, X.-L.; Xu, J.; Cao, M.-G.; Fang, Z.-Y.; Li, L.-Y.; Guan, G.-H.; Liu, Q.; Qian, Y.-H.; Xie, D. The LncRNA H19 Mediates Breast Cancer Cell Plasticity during EMT and MET Plasticity by Differentially Sponging MiR-200b/c and Let-7b. Sci. Signal 2017, 10, eaak9557. [Google Scholar] [CrossRef]
- Best, M.G.; Sol, N.; Kooi, I.; Tannous, J.; Westerman, B.A.; Rustenburg, F.; Schellen, P.; Verschueren, H.; Post, E.; Koster, J.; et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell 2015, 28, 666–676. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, H.; Karlan, S.; Zhou, H.; Gross, J.; Elashoff, D.; Akin, D.; Yan, X.; Chia, D.; Karlan, B.; et al. Discovery and Preclinical Validation of Salivary Transcriptomic and Proteomic Biomarkers for the Non-Invasive Detection of Breast Cancer. PLoS ONE 2010, 5, e15573. [Google Scholar] [CrossRef]
- López-Jornet, P.; Aznar, C.; Ceron, J.; Asta, T. Salivary Biomarkers in Breast Cancer: A Cross-Sectional Study. Support Care Cancer 2021, 29, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Kure, S.; Satoi, S.; Kitayama, T.; Nagase, Y.; Nakano, N.; Yamada, M.; Uchiyama, N.; Miyashita, S.; Iida, S.; Takei, H.; et al. A Prediction Model Using 2-Propanol and 2-Butanone in Urine Distinguishes Breast Cancer. Sci. Rep. 2021, 11, 19801. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Di Cosimo, S.; Appierto, V.; Pizzamiglio, S.; Silvestri, M.; Baselga, J.; Piccart, M.; Huober, J.; Izquierdo, M.; de la Pena, L.; Hilbers, F.S.; et al. Early Modulation of Circulating MicroRNAs Levels in HER2-Positive Breast Cancer Patients Treated with Trastuzumab-Based Neoadjuvant Therapy. Int. J. Mol. Sci. 2020, 21, 1386. [Google Scholar] [CrossRef]
- Bovy, N.; Blomme, B.; Frères, P.; Dederen, S.; Nivelles, O.; Lion, M.; Carnet, O.; Martial, J.A.; Noël, A.; Thiry, M.; et al. Endothelial Exosomes Contribute to the Antitumor Response during Breast Cancer Neoadjuvant Chemotherapy via MicroRNA Transfer. Oncotarget 2015, 6, 10253–10266. [Google Scholar] [CrossRef]
- O’Leary, B.; Hrebien, S.; Morden, J.P.; Beaney, M.; Fribbens, C.; Huang, X.; Liu, Y.; Bartlett, C.H.; Koehler, M.; Cristofanilli, M.; et al. Early Circulating Tumor DNA Dynamics and Clonal Selection with Palbociclib and Fulvestrant for Breast Cancer. Nat. Commun. 2018, 9, 896. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Hancock, B.A.; Solzak, J.P.; Brinza, D.; Scafe, C.; Miller, K.D.; Radovich, M. Next-Generation Sequencing of Circulating Tumor DNA to Predict Recurrence in Triple-Negative Breast Cancer Patients with Residual Disease after Neoadjuvant Chemotherapy. Npj Breast Cancer 2017, 3, 24. [Google Scholar] [CrossRef]
- Ignatiadis, M.; Rack, B.; Rothé, F.; Riethdorf, S.; Decraene, C.; Bonnefoi, H.; Dittrich, C.; Messina, C.; Beauvois, M.; Trapp, E.; et al. Liquid Biopsy-Based Clinical Research in Early Breast Cancer: The EORTC 90091-10093 Treat CTC Trial. Eur. J. Cancer 2016, 63, 97–104. [Google Scholar] [CrossRef]
- Ignatiadis, M.; Xenidis, N.; Perraki, M.; Apostolaki, S.; Politaki, E.; Kafousi, M.; Stathopoulos, E.N.; Stathopoulou, A.; Lianidou, E.; Chlouverakis, G.; et al. Different Prognostic Value of Cytokeratin-19 MRNA–Positive Circulating Tumor Cells According to Estrogen Receptor and HER2 Status in Early-Stage Breast Cancer. JCO 2007, 25, 5194–5202. [Google Scholar] [CrossRef]
- Xenidis, N.; Ignatiadis, M.; Apostolaki, S.; Perraki, M.; Kalbakis, K.; Agelaki, S.; Stathopoulos, E.N.; Chlouverakis, G.; Lianidou, E.; Kakolyris, S.; et al. Cytokeratin-19 MRNA-Positive Circulating Tumor Cells After Adjuvant Chemotherapy in Patients With Early Breast Cancer. JCO 2009, 27, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Litière, S.; Rothe, F.; Riethdorf, S.; Proudhon, C.; Fehm, T.; Aalders, K.; Forstbauer, H.; Fasching, P.A.; Brain, E.; et al. Trastuzumab versus Observation for HER2 Nonamplified Early Breast Cancer with Circulating Tumor Cells (EORTC 90091-10093, BIG 1-12, Treat CTC): A Randomized Phase II Trial. Ann. Oncol. 2018, 29, 1777–1783. [Google Scholar] [CrossRef]
- Tellez-Gabriel, M.; Knutsen, E.; Perander, M. Current Status of Circulating Tumor Cells, Circulating Tumor DNA, and Exosomes in Breast Cancer Liquid Biopsies. Int. J. Mol. Sci 2020, 21, 9457. [Google Scholar] [CrossRef]
- Cullinane, C.; Fleming, C.; O’Leary, D.P.; Hassan, F.; Kelly, L.; O’Sullivan, M.J.; Corrigan, M.A.; Redmond, H.P. Association of Circulating Tumor DNA With Disease-Free Survival in Breast Cancer: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2020, 3, e2026921. [Google Scholar] [CrossRef]
- Kong, L.; Birkeland, A.C. Liquid Biopsies in Head and Neck Cancer: Current State and Future Challenges. Cancers 2021, 13, 1874. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabières, C. Liquid Biopsy and Minimal Residual Disease—Latest Advances and Implications for Cure. Nat. Rev. Clin. Oncol 2019, 16, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.K.; Strickler, J.H. Use of Circulating Cell-Free DNA to Guide Precision Medicine in Patients with Colorectal Cancer. Annu. Rev. Med. 2021, 72, 399–413. [Google Scholar] [CrossRef]
- van’t Erve, I.; Rovers, K.P.; Constantinides, A.; Bolhuis, K.; Wassenaar, E.C.; Lurvink, R.J.; Huysentruyt, C.J.; Snaebjornsson, P.; Boerma, D.; van den Broek, D.; et al. Detection of Tumor-Derived Cell-Free DNA from Colorectal Cancer Peritoneal Metastases in Plasma and Peritoneal Fluid. J. Pathol. Clin. Res. 2021, 7, 203–208. [Google Scholar] [CrossRef]
- Tran, L.; Xiao, J.-F.; Agarwal, N.; Duex, J.E.; Theodorescu, D. Advances in Bladder Cancer Biology and Therapy. Nat. Rev. Cancer 2021, 21, 104–121. [Google Scholar] [CrossRef]
- Garcia-Murillas, I.; Schiavon, G.; Weigelt, B.; Ng, C.; Hrebien, S.; Cutts, R.J.; Cheang, M.; Osin, P.; Nerurkar, A.; Kozarewa, I.; et al. Mutation Tracking in Circulating Tumor DNA Predicts Relapse in Early Breast Cancer. Sci. Transl. Med. 2015, 7, 302ra133. [Google Scholar] [CrossRef]
- Kodahl, A.R.; Ehmsen, S.; Pallisgaard, N.; Jylling, A.M.B.; Jensen, J.D.; Lænkholm, A.; Knoop, A.S.; Ditzel, H.J. Correlation between Circulating Cell-free PIK3CA Tumor DNA Levels and Treatment Response in Patients with PIK3CA-mutated Metastatic Breast Cancer. Mol. Oncol. 2018, 12, 925–935. [Google Scholar] [CrossRef] [Green Version]
- McDonald, B.R.; Contente-Cuomo, T.; Sammut, S.-J.; Odenheimer-Bergman, A.; Ernst, B.; Perdigones, N.; Chin, S.-F.; Farooq, M.; Mejia, R.; Cronin, P.A.; et al. Personalized Circulating Tumor DNA Analysis to Detect Residual Disease after Neoadjuvant Therapy in Breast Cancer. Sci. Transl. Med. 2019, 11, eaax7392. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Swigart, L.B.; Wu, H.-T.; Hirst, G.L.; Yau, C.; Wolf, D.M.; Tin, A.; Salari, R.; Shchegrova, S.; Pawar, H.; et al. Circulating Tumor DNA in Neoadjuvant-Treated Breast Cancer Reflects Response and Survival. Ann. Oncol. 2021, 32, 229–239. [Google Scholar] [CrossRef]
- Olsson, E.; Winter, C.; George, A.; Chen, Y.; Howlin, J.; Tang, M.-H.E.; Dahlgren, M.; Schulz, R.; Grabau, D.; van Westen, D.; et al. Serial Monitoring of Circulating Tumor DNA in Patients with Primary Breast Cancer for Detection of Occult Metastatic Disease. EMBO Mol. Med. 2015, 7, 1034–1047. [Google Scholar] [CrossRef]
- Darga, E.P.; Dolce, E.M.; Fang, F.; Kidwell, K.M.; Gersch, C.L.; Kregel, S.; Thomas, D.G.; Gill, A.; Brown, M.E.; Gross, S.; et al. PD-L1 Expression on Circulating Tumor Cells and Platelets in Patients with Metastatic Breast Cancer. PLoS ONE 2021, 16, e0260124. [Google Scholar] [CrossRef]
- Pierga, J.-Y.; Bidard, F.-C.; Autret, A.; Petit, T.; Andre, F.; Dalenc, F.; Levy, C.; Ferrero, J.-M.; Romieu, G.; Bonneterre, J.; et al. Circulating Tumour Cells and Pathological Complete Response: Independent Prognostic Factors in Inflammatory Breast Cancer in a Pooled Analysis of Two Multicentre Phase II Trials (BEVERLY-1 and -2) of Neoadjuvant Chemotherapy Combined with Bevacizumab. Ann. Oncol. 2017, 28, 103–109. [Google Scholar] [CrossRef]
- Todorova, V.K.; Byrum, S.D.; Gies, A.J.; Haynie, C.; Smith, H.; Reyna, N.S.; Makhoul, I. Circulating Exosomal MicroRNAs as Predictive Biomarkers of Neoadjuvant Chemotherapy Response in Breast Cancer. Curr. Oncol. 2022, 29, 613–630. [Google Scholar] [CrossRef]
- Tiainen, L.; Hämäläinen, M.; Luukkaala, T.; Tanner, M.; Lahdenperä, O.; Vihinen, P.; Jukkola, A.; Karihtala, P.; Moilanen, E.; Kellokumpu-Lehtinen, P.-L. Low Plasma IL-8 Levels During Chemotherapy Are Predictive of Excellent Long-Term Survival in Metastatic Breast Cancer. Clin. Breast Cancer 2019, 19, e522–e533. [Google Scholar] [CrossRef]
- Zidi, O.; Souai, N.; Raies, H.; Ben Ayed, F.; Mezlini, A.; Mezrioui, S.; Tranchida, F.; Sabatier, J.-M.; Mosbah, A.; Cherif, A.; et al. Fecal Metabolic Profiling of Breast Cancer Patients during Neoadjuvant Chemotherapy Reveals Potential Biomarkers. Molecules 2021, 26, 2266. [Google Scholar] [CrossRef] [PubMed]
- Herberts, C.; Wyatt, A.W. Technical and Biological Constraints on CtDNA-Based Genotyping. Trends Cancer 2021, 7, 995–1009. [Google Scholar] [CrossRef]
- Valpione, S.; Campana, L. Detection of Circulating Tumor DNA (CtDNA) by Digital Droplet Polymerase Chain Reaction (Dd-PCR) in Liquid Biopsies. Methods Enzymol. 2019, 629, 1–15. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, A.; de Miguel-Pérez, D.; Ortega, F.G.; García-Puche, J.L.; Robles-Fernández, I.; Exposito, J.; Martorell-Marugan, J.; Carmona-Sáez, P.; María del Carmen, G.N.; Rolfo, C.; et al. Exosomal MiRNA Profile as Complementary Tool in the Diagnostic and Prediction of Treatment Response in Localized Breast Cancer under Neoadjuvant Chemotherapy. Breast Cancer Res. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Sempere, L.F.; Keto, J.; Fabbri, M. Exosomal MicroRNAs in Breast Cancer towards Diagnostic and Therapeutic Applications. Cancers 2017, 9, 71. [Google Scholar] [CrossRef]
- Li, X.-J.; Peng, L.-X.; Shao, J.-Y.; Lu, W.-H.; Zhang, J.-X.; Chen, S.; Chen, Z.-Y.; Xiang, Y.-Q.; Bao, Y.-N.; Zheng, F.-J.; et al. As an Independent Unfavorable Prognostic Factor, IL-8 Promotes Metastasis of Nasopharyngeal Carcinoma through Induction of Epithelial-Mesenchymal Transition and Activation of AKT Signaling. Carcinogenesis 2012, 33, 1302–1309. [Google Scholar] [CrossRef]
- Shao, N.; Chen, L.-H.; Ye, R.-Y.; Lin, Y.; Wang, S.-M. The Depletion of Interleukin-8 Causes Cell Cycle Arrest and Increases the Efficacy of Docetaxel in Breast Cancer Cells. Biochem. Biophys. Res. Commun. 2013, 431, 535–541. [Google Scholar] [CrossRef]
- Trethewey, R.N.; Krotzky, A.J.; Willmitzer, L. Metabolic Profiling: A Rosetta Stone for Genomics? Curr. Opin. Plant. Biol. 1999, 2, 83–85. [Google Scholar] [CrossRef]
- Corona, G.; Rizzolio, F.; Giordano, A.; Toffoli, G. Pharmaco-Metabolomics: An Emerging “Omics” Tool for the Personalization of Anticancer Treatments and Identification of New Valuable Therapeutic Targets. J. Cell Physiol. 2012, 227, 2827–2831. [Google Scholar] [CrossRef]
- Ma, G.; Jiang, Y.; Liang, M.; Li, J.; Wang, J.; Mao, X.; Veeramootoo, J.S.; Xia, T.; Liu, X.; Wang, S. Dynamic Monitoring of CD45-/CD31+/DAPI+ Circulating Endothelial Cells Aneuploid for Chromosome 8 during Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920918470. [Google Scholar] [CrossRef]
- Pierga, J.-Y.; Hajage, D.; Bachelot, T.; Delaloge, S.; Brain, E.; Campone, M.; Diéras, V.; Rolland, E.; Mignot, L.; Mathiot, C.; et al. High Independent Prognostic and Predictive Value of Circulating Tumor Cells Compared with Serum Tumor Markers in a Large Prospective Trial in First-Line Chemotherapy for Metastatic Breast Cancer Patients. Ann. Oncol. 2012, 23, 618–624. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef]
- Horimoto, Y.; Tokuda, E.; Murakami, F.; Uomori, T.; Himuro, T.; Nakai, K.; Orihata, G.; Iijima, K.; Togo, S.; Shimizu, H.; et al. Analysis of Circulating Tumour Cell and the Epithelial Mesenchymal Transition (EMT) Status during Eribulin-Based Treatment in 22 Patients with Metastatic Breast Cancer: A Pilot Study. J. Transl. Med. 2018, 16, 287. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Muinelo-Romay, L.; Cebey-López, V.; Pereira-Veiga, T.; Martínez-Pena, I.; Abreu, M.; Abalo, A.; Lago-Lestón, R.M.; Abuín, C.; Palacios, P.; et al. Analysis of a Real-World Cohort of Metastatic Breast Cancer Patients Shows Circulating Tumor Cell Clusters (CTC-Clusters) as Predictors of Patient Outcomes. Cancers 2020, 12, 1111. [Google Scholar] [CrossRef] [PubMed]
- Brisotto, G.; Biscontin, E.; Rossi, E.; Bulfoni, M.; Piruska, A.; Spazzapan, S.; Poggiana, C.; Vidotto, R.; Steffan, A.; Colombatti, A.; et al. Dysmetabolic Circulating Tumor Cells Are Prognostic in Metastatic Breast Cancer. Cancers 2020, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- Galardi, F.; De Luca, F.; Biagioni, C.; Migliaccio, I.; Curigliano, G.; Minisini, A.M.; Bonechi, M.; Moretti, E.; Risi, E.; McCartney, A.; et al. Circulating Tumor Cells and Palbociclib Treatment in Patients with ER-Positive, HER2-Negative Advanced Breast Cancer: Results from a Translational Sub-Study of the TREnd Trial. Breast Cancer Res. 2021, 23, 38. [Google Scholar] [CrossRef]
- Jakabova, A.; Bielcikova, Z.; Pospisilova, E.; Petruzelka, L.; Blasiak, P.; Bobek, V.; Kolostova, K. Characterization of Circulating Tumor Cells in Early Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. Ther. Adv. Med. Oncol. 2021, 13, 17588359211028492. [Google Scholar] [CrossRef]
- Chen, J.; Ye, C.; Dong, J.; Cao, S.; Hu, Y.; Situ, B.; Xi, X.; Qin, S.; Xu, J.; Cai, Z.; et al. Metabolic Classification of Circulating Tumor Cells as a Biomarker for Metastasis and Prognosis in Breast Cancer. J. Transl. Med. 2020, 18, 59. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, X.; Wu, S.; Guo, J.; Zhang, K.; Xu, C.; Chen, H.; Jin, Y.; Sun, Y.; Zheng, S.; et al. Epithelial-Mesenchymal Transition Status of Circulating Tumor Cells in Breast Cancer and Its Clinical Relevance. Cancer Biol. Med. 2020, 17, 169–180. [Google Scholar] [CrossRef]
- Papadaki, M.A.; Koutsopoulos, A.V.; Tsoulfas, P.G.; Lagoudaki, E.; Aggouraki, D.; Monastirioti, A.; Koutoulaki, C.; Apostolopoulou, C.A.; Merodoulaki, A.C.; Papadaki, C.; et al. Clinical Relevance of Immune Checkpoints on Circulating Tumor Cells in Breast Cancer. Cancers 2020, 12, 376. [Google Scholar] [CrossRef]
- Papadaki, M.A.; Monastirioti, A.; Apostolopoulou, C.A.; Aggouraki, D.; Papadaki, C.; Michaelidou, K.; Vassilakopoulou, M.; Alexakou, K.; Mavroudis, D.; Agelaki, S. TLR4 and PSTAT3 Expression on Circulating Tumor Cells (CTCs) and Immune Cells in the Peripheral Blood of Breast Cancer Patients: Prognostic Implications. Cancers 2022, 14, 1053. [Google Scholar] [CrossRef]
- Lee, C.-H.; Hsieh, J.C.-H.; Wu, T.M.-H.; Yeh, T.-S.; Wang, H.-M.; Lin, Y.-C.; Chen, J.-S.; Lee, C.-L.; Huang, W.-K.; Hung, T.-M.; et al. Baseline Circulating Stem-like Cells Predict Survival in Patients with Metastatic Breast Cancer. BMC Cancer 2019, 19, 1167. [Google Scholar] [CrossRef]
- Aaltonen, K.E.; Novosadová, V.; Bendahl, P.-O.; Graffman, C.; Larsson, A.-M.; Rydén, L. Molecular Characterization of Circulating Tumor Cells from Patients with Metastatic Breast Cancer Reflects Evolutionary Changes in Gene Expression under the Pressure of Systemic Therapy. Oncotarget 2017, 8, 45544–45565. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, D.; Hills, A.; Page, K.; Hastings, R.K.; Toghill, B.; Goddard, K.S.; Ion, C.; Ogle, O.; Boydell, A.R.; Gleason, K.; et al. Plasma Cell-Free DNA (CfDNA) as a Predictive and Prognostic Marker in Patients with Metastatic Breast Cancer. Breast Cancer Res. 2019, 21, 149. [Google Scholar] [CrossRef] [PubMed]
- Bonechi, M.; Galardi, F.; Biagioni, C.; De Luca, F.; Bergqvist, M.; Neumüller, M.; Guarducci, C.; Boccalini, G.; Gabellini, S.; Migliaccio, I.; et al. Plasma Thymidine Kinase-1 Activity Predicts Outcome in Patients with Hormone Receptor Positive and HER2 Negative Metastatic Breast Cancer Treated with Endocrine Therapy. Oncotarget 2018, 9, 16389–16399. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, T.; Yang, Z.; Zheng, Y.; Yu, R.; Wu, X.; Yan, J.; Shao, Y.W.; Shao, X.; Cao, W.; et al. Monitoring Treatment Efficacy and Resistance in Breast Cancer Patients via Circulating Tumor DNA Genomic Profiling. Mol. Genet. Genomic. Med. 2019, 8, e1079. [Google Scholar] [CrossRef]
- Raimondi, L.; Raimondi, F.M.; Pietranera, M.; Di Rocco, A.; Di Benedetto, L.; Miele, E.; Lazzeroni, R.; Cimino, G.; Spinelli, G.P. Assessment of Resistance Mechanisms and Clinical Implications in Patients with KRAS Mutated-Metastatic Breast Cancer and Resistance to CDK4/6 Inhibitors. Cancers 2021, 13, 1928. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.M.; Shibayama, T.; Chan, H.T.; Otaki, M.; Hara, F.; Kobayashi, T.; Kobayashi, K.; Hosonaga, M.; Fukada, I.; Inagaki, L.; et al. Serial Circulating Tumor DNA Monitoring of CDK4/6 Inhibitors Response in Metastatic Breast Cancer. Cancer Sci. 2022, 113, 1808–1820. [Google Scholar] [CrossRef]
- Gerratana, L.; Davis, A.A.; Zhang, Q.; Basile, D.; Rossi, G.; Strickland, K.; Franzoni, A.; Allegri, L.; Mu, Z.; Zhang, Y.; et al. Longitudinal Dynamics of Circulating Tumor Cells and Circulating Tumor DNA for Treatment Monitoring in Metastatic Breast Cancer. JCO Precis Oncol. 2021, 5, 943–952. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, L.; Li, L.; Wen, J.; Chi, Y.; Hao, R.; Dai, X.; Chen, Y.; Huang, D.; Zhou, Y.; et al. Genetic Landscape of Breast Cancer and Mutation Tracking with Circulating Tumor DNA in Chinese Women. Aging 2021, 13, 11860–11876. [Google Scholar] [CrossRef]
- Shivapurkar, N.; Vietsch, E.E.; Carney, E.; Isaacs, C.; Wellstein, A. Circulating MicroRNAs in Patients with Hormone Receptor-Positive, Metastatic Breast Cancer Treated with Dovitinib. Clin. Transl. Med. 2017, 6, 37. [Google Scholar] [CrossRef]
- Salvador-Coloma, C.; Santaballa, A.; Sanmartín, E.; Calvo, D.; García, A.; Hervás, D.; Cordón, L.; Quintas, G.; Ripoll, F.; Panadero, J.; et al. Immunosuppressive Profiles in Liquid Biopsy at Diagnosis Predict Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Eur. J. Cancer 2020, 139, 119–134. [Google Scholar] [CrossRef]
- Griñán-Lisón, C.; Olivares-Urbano, M.A.; Jiménez, G.; López-Ruiz, E.; del Val, C.; Morata-Tarifa, C.; Entrena, J.M.; González-Ramírez, A.R.; Boulaiz, H.; Zurita Herrera, M.; et al. MiRNAs as Radio-response Biomarkers for Breast Cancer Stem Cells. Mol. Oncol. 2020, 14, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, Y.; Guo, R.; Zhao, J.; Chi, W.; Lai, H.; Wang, J.; Wang, Z.; Li, L.; Sang, Y.; et al. Plasma Extracellular Vesicle Long RNA Profiles in the Diagnosis and Prediction of Treatment Response for Breast Cancer. NPJ Breast Cancer 2021, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Chanteloup, G.; Cordonnier, M.; Isambert, N.; Bertaut, A.; Hervieu, A.; Hennequin, A.; Luu, M.; Zanetta, S.; Coudert, B.; Bengrine, L.; et al. Monitoring HSP70 Exosomes in Cancer Patients’ Follow up: A Clinical Prospective Pilot Study. J. Extracell. Vesicles 2020, 9, 1766192. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.R.; Alho, I.; Shan, N.; Matias, M.; Faria, M.; Casimiro, S.; Leitzel, K.; Ali, S.; Lipton, A.; Costa, L. N-Telopeptide of Type I Collagen Long-Term Dynamics in Breast Cancer Patients With Bone Metastases: Clinical Outcomes and Influence of Extraskeletal Metastases. Oncologist 2016, 21, 1418–1426. [Google Scholar] [CrossRef]
- Ferroni, P.; Santilli, F.; Cavaliere, F.; Simeone, P.; Costarelli, L.; Liani, R.; Tripaldi, R.; Riondino, S.; Roselli, M.; Davi, G.; et al. Oxidant Stress as a Major Determinant of Platelet Activation in Invasive Breast Cancer. Int. J. Cancer 2017, 140, 696–704. [Google Scholar] [CrossRef]
- Moon, D.H.; Lindsay, D.P.; Hong, S.; Wang, A.Z. Clinical Indications for, and the Future of, Circulating Tumor Cells. Adv. Drug. Deliv. Rev. 2018, 125, 143–150. [Google Scholar] [CrossRef]
- Onstenk, W.; Gratama, J.W.; Foekens, J.A.; Sleijfer, S. Towards a Personalized Breast Cancer Treatment Approach Guided by Circulating Tumor Cell (CTC) Characteristics. Cancer Treat. Rev. 2013, 39, 691–700. [Google Scholar] [CrossRef]
- Kraan, J.; Sleijfer, S.; Strijbos, M.H.; Ignatiadis, M.; Peeters, D.; Pierga, J.-Y.; Farace, F.; Riethdorf, S.; Fehm, T.; Zorzino, L.; et al. External Quality Assurance of Circulating Tumor Cell Enumeration Using the CellSearch® System: A Feasibility Study. Cytom. Part. B Clin. Cytometry 2011, 80B, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Jacob, S.; Gerratana, L.; Shah, A.N.; Wehbe, F.; Katam, N.; Zhang, Q.; Flaum, L.; Siziopikou, K.P.; Platanias, L.C.; et al. Landscape of Circulating Tumour DNA in Metastatic Breast Cancer. EBioMedicine 2020, 58, 102914. [Google Scholar] [CrossRef]
- Radovich, M.; Jiang, G.; Hancock, B.A.; Chitambar, C.; Nanda, R.; Falkson, C.; Lynce, F.C.; Gallagher, C.; Isaacs, C.; Blaya, M.; et al. Association of Circulating Tumor DNA and Circulating Tumor Cells After Neoadjuvant Chemotherapy With Disease Recurrence in Patients With Triple-Negative Breast Cancer: Preplanned Secondary Analysis of the BRE12-158 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1410–1415. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.M.M.; et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Mu, Z.; Wang, C.; Ye, Z.; Austin, L.; Civan, J.; Hyslop, T.; Palazzo, J.P.; Jaslow, R.; Li, B.; Myers, R.E.; et al. Prospective Assessment of the Prognostic Value of Circulating Tumor Cells and Their Clusters in Patients with Advanced-Stage Breast Cancer. Breast Cancer Res. Treat. 2015, 154, 563–571. [Google Scholar] [CrossRef]
- Jansson, S.; Bendahl, P.-O.; Larsson, A.-M.; Aaltonen, K.E.; Rydén, L. Prognostic Impact of Circulating Tumor Cell Apoptosis and Clusters in Serial Blood Samples from Patients with Metastatic Breast Cancer in a Prospective Observational Cohort. BMC Cancer 2016, 16, 433. [Google Scholar] [CrossRef]
- Larsson, A.-M.; Jansson, S.; Bendahl, P.-O.; Levin Tykjaer Jörgensen, C.; Loman, N.; Graffman, C.; Lundgren, L.; Aaltonen, K.; Rydén, L. Longitudinal Enumeration and Cluster Evaluation of Circulating Tumor Cells Improve Prognostication for Patients with Newly Diagnosed Metastatic Breast Cancer in a Prospective Observational Trial. Breast Cancer Res. 2018, 20, 48. [Google Scholar] [CrossRef]
- Kaldjian, E.P.; Ramirez, A.B.; Sun, Y.; Campton, D.E.; Werbin, J.L.; Varshavskaya, P.; Quarre, S.; George, T.; Madan, A.; Blau, C.A.; et al. The RareCyte® Platform for Next-Generation Analysis of Circulating Tumor Cells. Cytom. Part A 2018, 93, 1220–1225. [Google Scholar] [CrossRef]
- Dirix, L.; Buys, A.; Oeyen, S.; Peeters, D.; Liègeois, V.; Prové, A.; Rondas, D.; Vervoort, L.; Mariën, V.; Laere, S.V.; et al. Circulating Tumor Cell Detection: A Prospective Comparison between CellSearch® and RareCyte® Platforms in Patients with Progressive Metastatic Breast Cancer. Breast Cancer Res. Treat. 2022, 193, 437–444. [Google Scholar] [CrossRef]
- Reduzzi, C.; Di Cosimo, S.; Gerratana, L.; Motta, R.; Martinetti, A.; Vingiani, A.; D’Amico, P.; Zhang, Y.; Vismara, M.; Depretto, C.; et al. Circulating Tumor Cell Clusters Are Frequently Detected in Women with Early-Stage Breast Cancer. Cancers 2021, 13, 2356. [Google Scholar] [CrossRef]
- Dawson, S.-J.; Tsui, D.W.Y.; Murtaza, M.; Biggs, H.; Rueda, O.M.; Chin, S.-F.; Dunning, M.J.; Gale, D.; Forshew, T.; Mahler-Araujo, B.; et al. Analysis of Circulating Tumor DNA to Monitor Metastatic Breast Cancer. N. Engl. J. Med. 2013, 368, 1199–1209. [Google Scholar] [CrossRef]
- Tan, G.; Chu, C.; Gui, X.; Li, J.; Chen, Q. The Prognostic Value of Circulating Cell-Free DNA in Breast Cancer: A Meta-Analysis. Medicine 2018, 97, e0197. [Google Scholar] [CrossRef]
- Turner, N.C.; Kingston, B.; Kilburn, L.S.; Kernaghan, S.; Wardley, A.M.; Macpherson, I.R.; Baird, R.D.; Roylance, R.; Stephens, P.; Oikonomidou, O.; et al. Circulating Tumour DNA Analysis to Direct Therapy in Advanced Breast Cancer (PlasmaMATCH): A Multicentre, Multicohort, Phase 2a, Platform Trial. Lancet Oncol. 2020, 21, 1296–1308. [Google Scholar] [CrossRef]
- De Laurentiis, M.; Malorni, L.; Bianchini, G.; Caputo, R.; Giuliano, M.; Zambelli, A.; Puglisi, F.; Mastro, L.D.; Colleoni, M.; Montemurro, F.; et al. Abstract P5-01-07: Bioitalee—Biomarker Analysis on Liquid Biopsies of Patients Treated with Ribociclib and Letrozole as First-Line Therapy for Advanced Breast Cancer (ABC) (NCT03439046). Cancer Res. 2020, 80, P5-01-07. [Google Scholar] [CrossRef]
- Neven, P.; Petrakova, K.; Val Bianchi, G.; De la Cruz-Merino, L.; Jerusalem, G.; Sonke, G.; Nusch, A.; Beck, J.; Chia, S.; Solovieff, N.; et al. Abstract PD2-05: Biomarker Analysis by Baseline Circulating Tumor DNA Alterations in the MONALEESA-3 Study. Cancer Res. 2019, 79, PD2-05. [Google Scholar] [CrossRef]
- O’Leary, B.; Cutts, R.J.; Huang, X.; Hrebien, S.; Liu, Y.; André, F.; Loibl, S.; Loi, S.; Garcia-Murillas, I.; Cristofanilli, M.; et al. Circulating Tumor DNA Markers for Early Progression on Fulvestrant With or Without Palbociclib in ER+ Advanced Breast Cancer. JNCI J. Natl. Cancer Inst. 2021, 113, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, I.; Leo, A.; Galardi, F.; Guarducci, C.; Fusco, G.M.; Benelli, M.; Di Leo, A.; Biganzoli, L.; Malorni, L. Circulating Biomarkers of CDK4/6 Inhibitors Response in Hormone Receptor Positive and HER2 Negative Breast Cancer. Cancers 2021, 13, 2640. [Google Scholar] [CrossRef]
- Papakonstantinou, A.; Gonzalez, N.S.; Pimentel, I.; Suñol, A.; Zamora, E.; Ortiz, C.; Espinosa-Bravo, M.; Peg, V.; Vivancos, A.; Saura, C.; et al. Prognostic Value of CtDNA Detection in Patients with Early Breast Cancer Undergoing Neoadjuvant Therapy: A Systematic Review and Meta-Analysis. Cancer Treat. Rev. 2022, 104, 102362. [Google Scholar] [CrossRef]
- Laessig, D.; Nagel, D.; Heinemann, V.; Untch, M.; Kahlert, S.; Bauerfeind, I.; Stieber, P. Importance of CEA and CA 15-3 during Disease Progression in Metastatic Breast Cancer Patients. Anticancer. Res. 2007, 27, 1963–1968. [Google Scholar]
- Yang, Y.; Zhang, H.; Zhang, M.; Meng, Q.; Cai, L.; Zhang, Q. Elevation of Serum CEA and CA15-3 Levels during Antitumor Therapy Predicts Poor Therapeutic Response in Advanced Breast Cancer Patients. Oncol. Lett. 2017, 14, 7549–7556. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network—Home. Available online: https://www.nccn.org (accessed on 10 May 2022).
- Lennon, A.M.; Buchanan, A.H.; Kinde, I.; Warren, A.; Honushefsky, A.; Cohain, A.T.; Ledbetter, D.H.; Sanfilippo, F.; Sheridan, K.; Rosica, D.; et al. Feasibility of Blood Testing Combined with PET-CT to Screen for Cancer and Guide Intervention. Science 2020, 369, eabb9601. [Google Scholar] [CrossRef]
- Yoo, T.-K. Liquid Biopsy in Breast Cancer: Circulating Tumor Cells and Circulating Tumor DNA. Adv. Exp. Med. Biol. 2021, 1187, 337–361. [Google Scholar] [CrossRef]
- Gerratana, L.; Davis, A.A.; Polano, M.; Zhang, Q.; Shah, A.N.; Lin, C.; Basile, D.; Toffoli, G.; Wehbe, F.; Puglisi, F.; et al. Understanding the Organ Tropism of Metastatic Breast Cancer through the Combination of Liquid Biopsy Tools. Eur. J. Cancer 2021, 143, 147–157. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Jacot, W.; Kiavue, N.; Dureau, S.; Kadi, A.; Brain, E.; Bachelot, T.; Bourgeois, H.; Gonçalves, A.; Ladoire, S.; et al. Efficacy of Circulating Tumor Cell Count-Driven vs Clinician-Driven First-Line Therapy Choice in Hormone Receptor-Positive, ERBB2-Negative Metastatic Breast Cancer: The STIC CTC Randomized Clinical Trial. JAMA Oncol. 2021, 7, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Cabel, L.; Berger, F.; Cottu, P.; Loirat, D.; Rampanou, A.; Brain, E.; Cyrille, S.; Bourgeois, H.; Kiavue, N.; Deluche, E.; et al. Clinical Utility of Circulating Tumour Cell-Based Monitoring of Late-Line Chemotherapy for Metastatic Breast Cancer: The Randomised CirCe01 Trial. Br. J. Cancer 2021, 124, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Sanches, S.M.; Braun, A.C.; Calsavara, V.F.; Barbosa, P.N.V.P.; Chinen, L.T.D. Comparison of Hormonal Receptor Expression and HER2 Status between Circulating Tumor Cells and Breast Cancer Metastases. Clinics 2021, 76, e2971. [Google Scholar] [CrossRef] [PubMed]
- Urso, L.; Vernaci, G.; Carlet, J.; Lo Mele, M.; Fassan, M.; Zulato, E.; Faggioni, G.; Menichetti, A.; Di Liso, E.; Griguolo, G.; et al. ESR1 Gene Mutation in Hormone Receptor-Positive HER2-Negative Metastatic Breast Cancer Patients: Concordance Between Tumor Tissue and Circulating Tumor DNA Analysis. Front. Oncol. 2021, 11, 625636. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid Biopsy Enters the Clinic—Implementation Issues and Future Challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef] [PubMed]

| Study, Year | Sample | N | Stage of Disease | Biomarker | Sensitivity (%) | Specificity (%) | Accuracy (%) | Detection Method | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Kamel et al., 2016 | Plasma | 95 | I–IV | cf-DNA | 85.3 | 100 | - | RT-qPCR | [26] |
| Li et al., 2016 | Plasma | 86 | I–II | cf-DNA | 75.6–94.2 | 30.4–53.3 | 66–75 | Microfluidic PCR and Bisulfite Sequencing Technology | [27] |
| Cohen et al., 2018 | Plasma | 54 | I–III | ct-DNA | 33 | 99 | 73 | Multiplex-PCR, NGS and CancerSEEK | [28] |
| Beaver et al., 2014 | Plasma | 29 | I–III | ct-DNA | 93.3 | 100 | 96.7 | ddPCR | [29] |
| Kruspe et al., 2017 | Plasma | 29 | IV | CTCs | - | - | - | RT-qPCR | [30] |
| Shimomura et al., 2016 | Serum | 1206 | I–IV | miRNA | 97.3 | 82.9 | 89.7 | Microarray and RT-qPCR | [31] |
| Erbes et al., 2015 | Serum and urine | 24 | Early | miRNA | 83.3 | 87.5 | 88.7 | RT-qPCR | [32] |
| Hirschfeld et al., 2020 | Urine | 69 | Early | miRNA | 98.6 | 100 | 99.9 | RT-qPCR | [33] |
| Zhong et al., 2020 | Serum | 50 | I–IV | lncRNA | 87 | 70.6 | 87 | RT-qPCR | [37] |
| Best et al., 2015 | Blood | 39 | I–IV | TEPs | - | - | 71 | mRNA sequencing | [39] |
| Zhang et al., 2010 | Saliva | 40 | I–IV | mRNA and proteins | 83 | 97 | 92 | Microarray, RT-qPCR, and immunoblot | [40] |
| López-Jornet et al., 2021 | Saliva | 91 | I–IV | Proteins | 67.5 | 66.7 | - | Biochemical analyses | [41] |
| Kure et al., 2021 | Urine | 110 | I–II | VOCs | 93.3 | 83.3 | 88.3 | GCMS | [42] |
| Study, Year | Sample | Subtype | Role | Drug | Biomarker | Ref. |
|---|---|---|---|---|---|---|
| Cosimo el al., 2020 | Blood | HER2+ | Predictive biomarker | Trastuzumab | miRNA and ct-miRNA | [45] |
| Boyy et al., 2015 | Plasma | NA | Therapeutic target/prognostic indicator | Paclitaxel and epirubicin | miRNA | [46] |
| O’Leary B et al., 2018 | Plasma | ER+, HER2- | Predictive biomarker | Palbociclib and fulvestrant | ctDNA | [47] |
| Chen Y et al., 2017 | Plasma | TN | Predictive biomarker | Cisplatin and rucaparib | ctDNA | [48] |
| Ignatiadis et al., 2007 | Blood | ER+, ER-, TN, HER2+, and ER+/HER2- | Predictive biomarker | Fluorouracil, epirubicin, cyclophosphamide, docetaxel, methotrexate | CK-19 mRNA-positive CTCs | [50] |
| Xenidis et al., 2009 | Blood | ER+, ER-, TN, HER2+, and ER+/HER2- | Predictive biomarker | Fluorouracil, epirubicin, cyclophosphamide, docetaxel, methotrexate | CK-19 mRNA-positive CTCs | [51] |
| Study, Year | Sample | N | Stage of Disease | Biomarker | Detection Method | Ref. |
|---|---|---|---|---|---|---|
| Garcia-Murillas et al., 2015 | Plasma | 55 | Early | ctDNA | ddPCR | [60] |
| Kodahl el al., 2018 | Serum | 66 | Advanced disease | ctDNA | ddPCR | [61] |
| McDonald et al., 2019 | Plasma | 33 | Early and locally advanced disease | ctDNA | TARDIS | [62] |
| Magbanua et al., 2021 | Plasma | 291 | Early | ctDNA | WGS | [63] |
| Olsson et al., 2015 | Plasma | 20 | Early | ctDNA | WGS e ddPCR | [64] |
| Darga et al., 2021 | Blood and platelet | 124 | Advanced disease | CTC sand platelet PD-L1 | CellSearch System® | [65] |
| Pierga et al., 2017 | Blood | 137 | Locally advanced disease | CTCs and CECs | CellSearch System® | [66] |
| Todorova et al., 2022 | Plasma | 20 | Early and advanced disease | exo-miRNAs | NGS | [67] |
| Tiainen et al., 2019 | Plasma | 58 | Advanced disease | IL-8 | ELISA | [68] |
| Zidi et al., 2021 | Stool | 8 | Early | Fecal Metabolic | NMR Spectroscopy | [69] |
| Study, Year | Sample | N | Stage of Disease | Biomarker | Detection method | Ref. |
|---|---|---|---|---|---|---|
| Rodriguéz-Martínez et al., 2019 | Blood | 53 | Not available | CTCs/miRNA | Immunocytochemistry/RT-qPCR | [72] |
| Ma et al., 2020 | Blood | 41 | Locally advanced disease | CECs | SE-iFISH | [78] |
| Pierga et al., 2012 | Blood | 267 | Metastatic disease | CTCs | CellSearch System® | [79] |
| Yu et al., 2013 | Blood | 41 | Metastatic disease | CTCs | Microfluidic HB chip/NGS | [80] |
| Horimoto et al., 2018 | Blood | 22 | IV | CTCs | Microfluidic chip | [81] |
| Costa et al., 2020 | Blood | 54 | Metastatic disease | CTCs | CellSearch System® | [82] |
| Brisotto et al., 2020 | Blood | 31 | Metastatic disease | CTCs | MBA/CellSearch System® | [83] |
| Galardi et al., 2021 | Blood | 46 | Not available | CTCs | CellSearch System®/ddPCR | [84] |
| Jakabova et al., 2021 | Blood | 20 | Early and locally advanced disease | CTCs | MetaCell/q-PCR | [85] |
| Chen et al., 2020 | Blood | 64 | I–IV | CTCs | RNA-ISH | [86] |
| Zhou et al., 2020 | Blood | 89 | I–IV | CTCs | Flow cytometry/immunofluorescence/RT-qPCR | [87] |
| Papadaki et al., 2020 | Blood | 198 | Early and metastatic disease | CTCs/PBMC | Ficoll–Hypaque density-gradient centrifugation/Immunofluorescence | [88] |
| Papadaki et al., 2022 | Blood | 199 | Early and metastatic disease | CTCs/PBMC | Ficoll–Hypaque density-gradient centrifugation/immunofluorescence | [89] |
| Lee et al., 2019 | Blood | 48 | IV | CTCs/cCSCs | Flow cytometry | [90] |
| Aaltonen et al., 2017 | Plasma | 36 | Metastatic disease | CTCs/mRNA | CellSearch System/Multiplex q-PCR | [91] |
| Fernandez-Garcia et al., 2019 | Plasma | 194 | Metastatic disease | CTCs/cfDNA | CellSearch System/RT-qPCR | [92] |
| Bonechi et al., 2018 | Plasma | 32 | Metastatic disease | CTCs/ctDNA/TK1 | CellSearch System/ddPCR | [93] |
| Chen et al., 2020 | Plasma | 31 | I–IV | ctDNA | NGS | [94] |
| Raimondi et al., 2021 | Plasma | 106 | Metastatic disease | ctDNA | ddPCR | [95] |
| Chin et al., 2022 | Plasma | 33 | Metastatic disease | ctDNA | NGS/ddPCR | [96] |
| Gerratana et al., 2021 | Plasma | 107/48 | IV | ctDNA | NGS/ddPCR | [97] |
| Wang et al., 2021 | Plasma | 273 | Not available | ctDNA | NGS | [98] |
| Shivapurkar et al., 2017 | Plasma | 12 | Metastatic disease | miRNA | RT-qPCR | [99] |
| Salvador-Coloma et al., 2020 | Plasma | 34 | Early or locally advanced disease | miRNA | Microarray | [100] |
| Griñán-Lisón et al., 2021 | Blood | 60 | Not available | miRNA | q-PCR | [101] |
| Su et al., 2021 | Plasma | 172 | I–IV | exLR | NGS | [102] |
| Chanteloup et al., 2020 | Plasma/urine | 20 | Not available | Exosomes/CTCs | BLI/ELISA/NTA/CellSearch System | [103] |
| Ferreira et al., 2016 | Urine | 71 | Metastatic disease | NTX | ELISA | [104] |
| Ferroni et al., 2017 | Urine | 115 | I–III | 11-dehydro-TXB2 | Radioimmunoassay | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, A.J.A.d.; Causin, R.L.; Varuzza, M.B.; Calfa, S.; Hidalgo Filho, C.M.T.; Komoto, T.T.; Souza, C.d.P.; Marques, M.M.C. Liquid Biopsy as a Tool for the Diagnosis, Treatment, and Monitoring of Breast Cancer. Int. J. Mol. Sci. 2022, 23, 9952. https://doi.org/10.3390/ijms23179952
Freitas AJAd, Causin RL, Varuzza MB, Calfa S, Hidalgo Filho CMT, Komoto TT, Souza CdP, Marques MMC. Liquid Biopsy as a Tool for the Diagnosis, Treatment, and Monitoring of Breast Cancer. International Journal of Molecular Sciences. 2022; 23(17):9952. https://doi.org/10.3390/ijms23179952
Chicago/Turabian StyleFreitas, Ana Julia Aguiar de, Rhafaela Lima Causin, Muriele Bertagna Varuzza, Stéphanie Calfa, Cassio Murilo Trovo Hidalgo Filho, Tatiana Takahasi Komoto, Cristiano de Pádua Souza, and Márcia Maria Chiquitelli Marques. 2022. "Liquid Biopsy as a Tool for the Diagnosis, Treatment, and Monitoring of Breast Cancer" International Journal of Molecular Sciences 23, no. 17: 9952. https://doi.org/10.3390/ijms23179952
APA StyleFreitas, A. J. A. d., Causin, R. L., Varuzza, M. B., Calfa, S., Hidalgo Filho, C. M. T., Komoto, T. T., Souza, C. d. P., & Marques, M. M. C. (2022). Liquid Biopsy as a Tool for the Diagnosis, Treatment, and Monitoring of Breast Cancer. International Journal of Molecular Sciences, 23(17), 9952. https://doi.org/10.3390/ijms23179952







