Implication of Irisin in Different Types of Cancer: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Searching and Quality Assessment
2.1.1. Search Strategy and Selection Criteria
2.1.2. Risk of Bias Assessment
2.1.3. Data Extraction
2.2. Meta-Analyses
2.2.1. Differences in Irisin Protein Level and Cell Viability
2.2.2. Rating of Overall Study Effect
3. Results
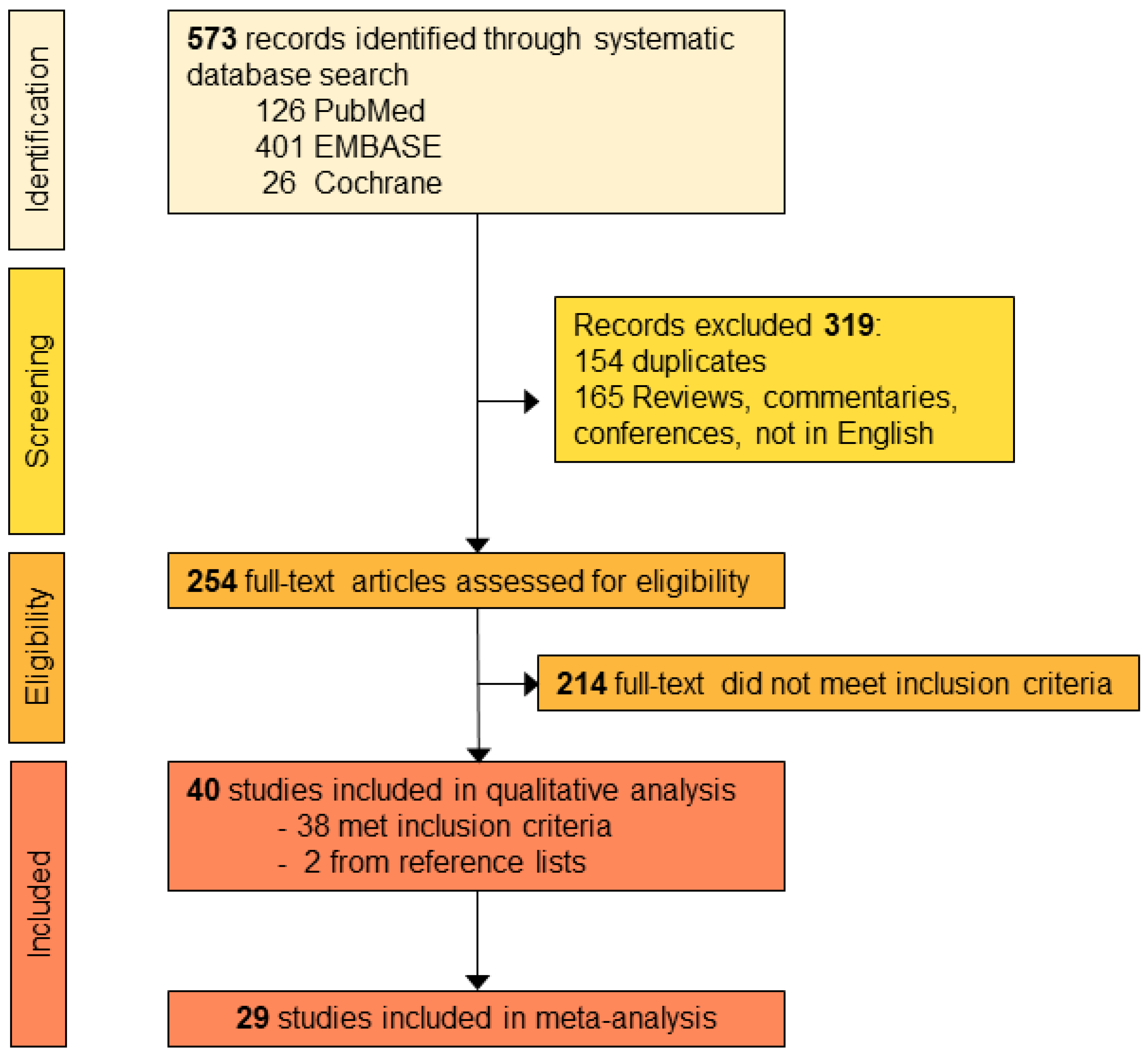
3.1. Screening of Publications
3.2. Risk of Bias Assessment
3.3. Data Extraction
3.3.1. In Vivo Studies
3.3.2. In Vitro Studies
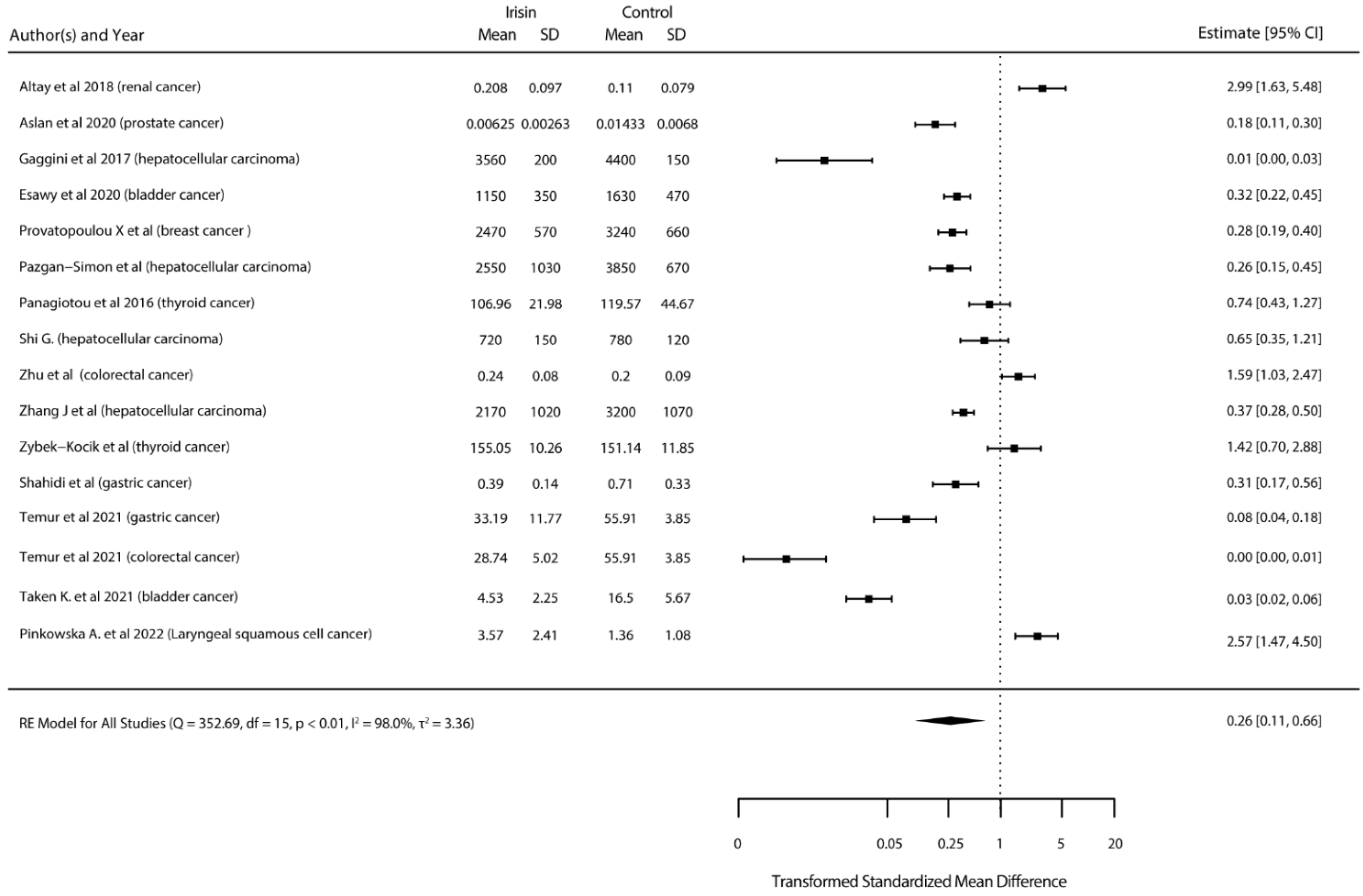
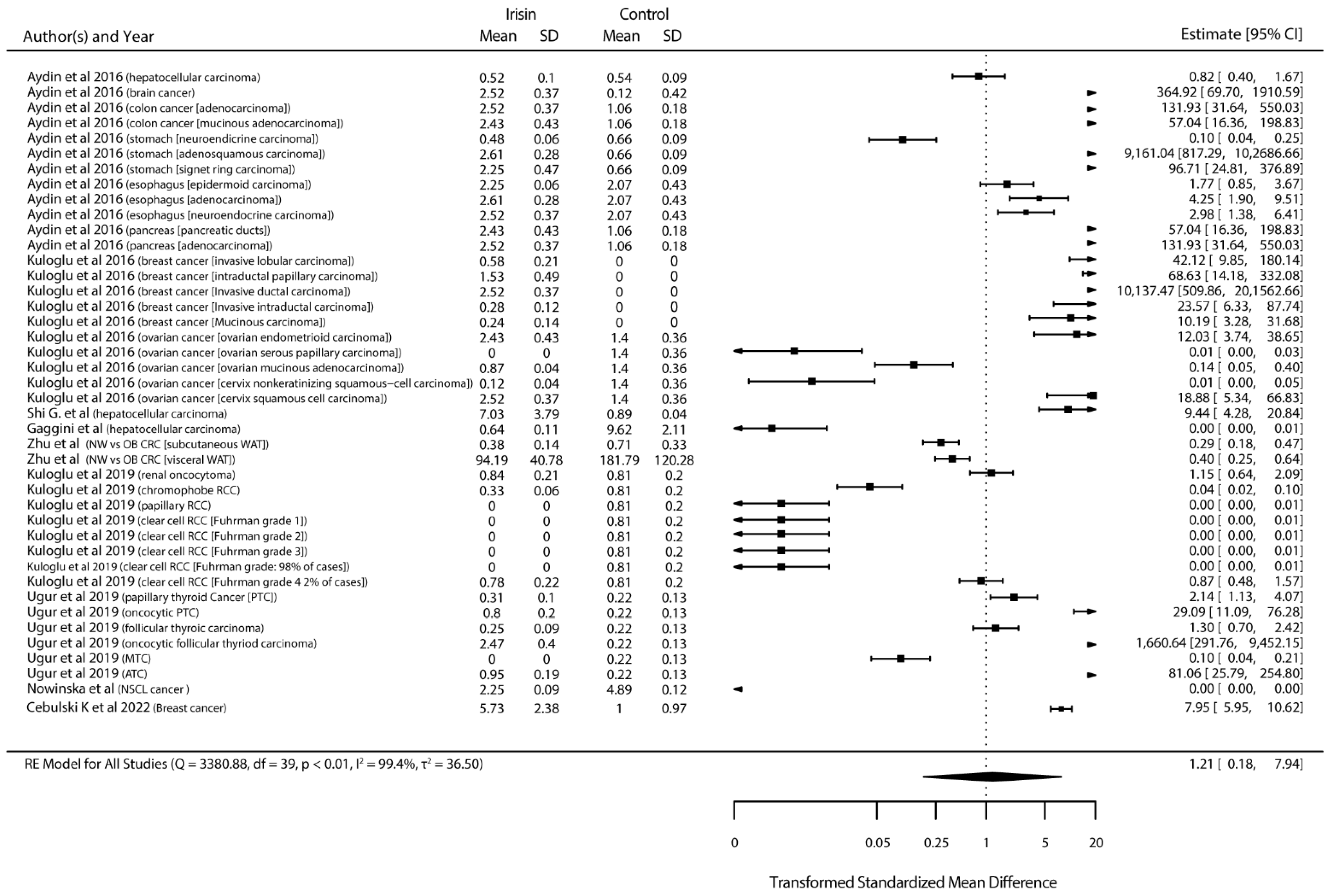
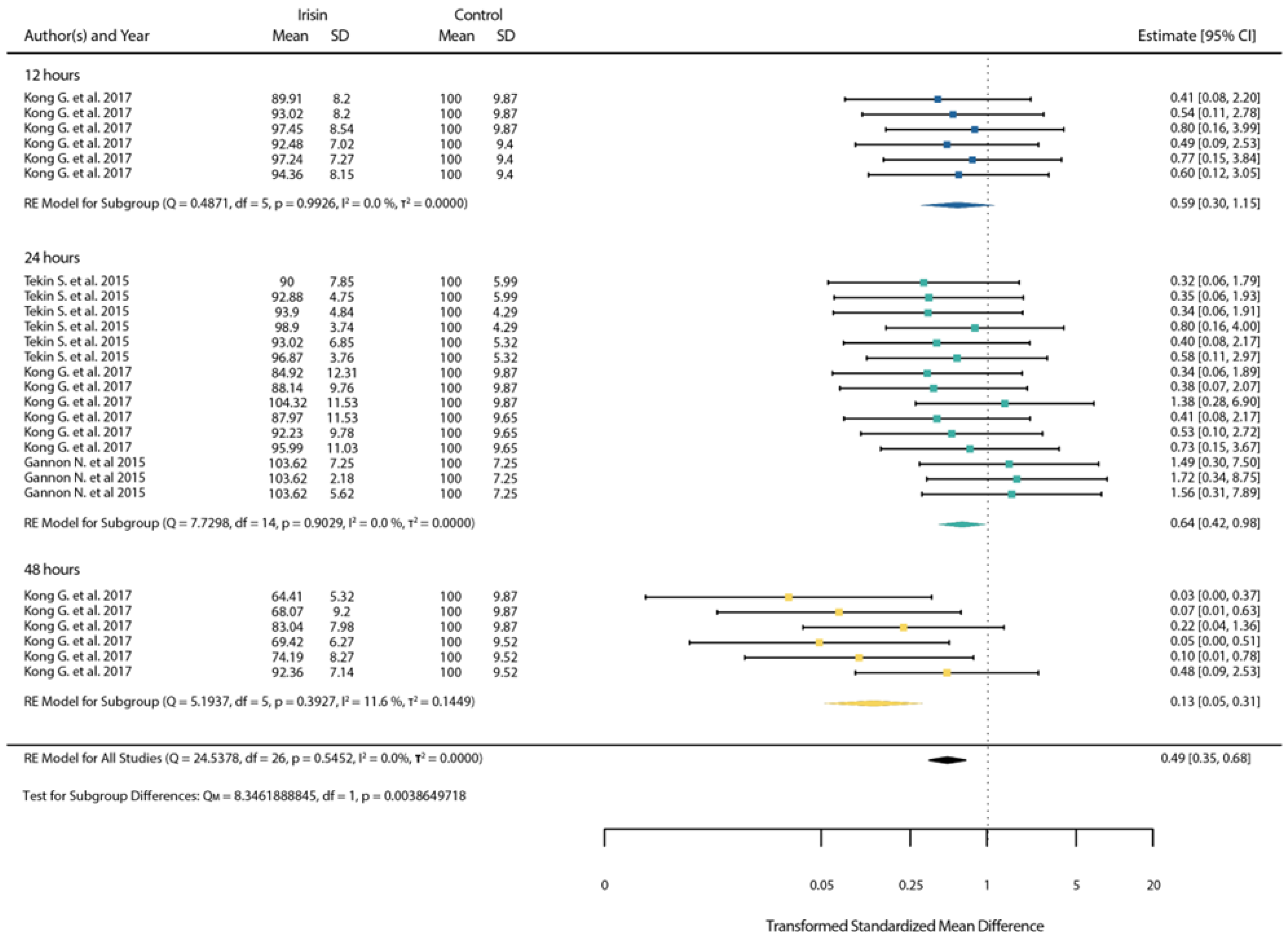
3.4. Meta-Analysis Results
3.4.1. Standardized Mean Differences
3.4.2. ROSE Meta-Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019. 2020. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 1 August 2022).
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Tsiani, E.; Tsakiridis, N.; Kouvelioti, R.; Jaglanian, A.; Klentrou, P. Current Evidence of the Role of the Myokine Irisin in Cancer. Cancers 2021, 13, 2628. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I.; Bilski, J.; Pochec, E.; Brzozowski, T. New insight into the direct anti-inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes. Implication for exercise in obesity. J. Physiol. Pharmacol. 2017, 68, 243–251. [Google Scholar] [PubMed]
- Mazur-Bialy, A.I.; Bilski, J.; Wojcik, D.; Brzozowski, B.; Surmiak, M.; Hubalewska-Mazgaj, M.; Chmura, A.; Magierowski, M.; Magierowska, K.; Mach, T.; et al. Beneficial Effect of Voluntary Exercise on Experimental Colitis in Mice Fed a High-Fat Diet: The Role of Irisin, Adiponectin and Proinflammatory Biomarkers. Nutrients 2017, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.U.; Galvão, D.A. Exercise in Preventionand Management of Cancer. Curr. Treat. Options Oncol. 2008, 9, 135–146. [Google Scholar] [CrossRef]
- Fatouros, I.G. Is irisin the new player in exercise-induced adaptations or not? A 2017 update. Clin. Chem. Lab. Med. 2018, 56, 525–548. [Google Scholar] [CrossRef]
- Aydin, S. Is irisin a decisive protein in cancer cachexia and death of cancer cells? Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3727–3729. [Google Scholar]
- Gaggini, M.; Cabiati, M.; Del Turco, S.; Navarra, T.; De Simone, P.; Filipponi, F.; Del Ry, S.; Gastaldelli, A.; Basta, G. Increased FNDC5/Irisin expression in human hepatocellular carcinoma. Peptides 2017, 88, 62–66. [Google Scholar] [CrossRef]
- Kuloğlu, T.; Artaş, G.; Yardim, M.; Sahin, I.; Aydin, Y.; Beyoğlu, N.; Özercan, I.H.; Yalcin, M.H.; Ugur, K.; Aydin, S. Immunostaining characteristics of irisin in benign and malignant renal cancers. Biotech. Histochem. 2019, 94, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Kuloglu, T.; Celik, O.; Aydin, S.; Hanifi Ozercan, I.; Acet, M.; Aydin, Y.; Artas, G.; Turk, A.; Yardim, M.; Ozan, G.; et al. Irisin immunostaining characteristics of breast and ovarian cancer cells. Cell. Mol. Biol. 2016, 62, 40–44. [Google Scholar] [PubMed]
- Kong, G.; Jiang, Y.; Sun, X.; Cao, Z.; Zhang, G.; Zhao, Z.; Zhao, Y.; Yu, Q.; Cheng, G. Irisin reverses the IL-6 induced epithelial-mesenchymal transition in osteosarcoma cell migration and invasion through the STAT3/Snail signaling pathway. Oncol. Rep. 2017, 38, 2647–2656. [Google Scholar] [CrossRef]
- Liu, J.; Song, N.; Huang, Y.; Chen, Y. Irisin inhibits pancreatic cancer cell growth via the AMPK-mTOR pathway. Sci. Rep. 2018, 8, 15247. [Google Scholar] [CrossRef]
- Shi, G.; Tang, N.; Qiu, J.; Zhang, D.; Huang, F.; Cheng, Y.; Ding, K.; Li, W.; Zhang, P.; Tan, X. Irisin stimulates cell proliferation and invasion by targeting the PI3K/AKT pathway in human hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2017, 493, 585–591. [Google Scholar] [CrossRef]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef]
- OfHaAS. Tool for Risk of Bias. Updated 2020. Available online: https://ntp.niehs.nih.gov/whatwestudy/assessments/noncancer/riskbias/index.html (accessed on 1 August 2022).
- Mellow, M.; Crozier, A.; Dumuid, D.; Wade, A.; Goldsworthy, M.; Dorrian, J.; Smith, A. How are combinations of physical activity, sedentary behaviour and sleep related to cognitive function in older adults? A systematic review. Exp. Gerontol. 2022, 159, 111698. [Google Scholar] [CrossRef]
- Rohatgi, A. WebPlotDigitizer. 4.5. Available online: https://automeris.io/WebPlotDigitizer/ (accessed on 1 August 2022).
- Moon, H.-S.; Dincer, F.; Mantzoros, C.S. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metab. Clin. Exp. 2013, 62, 1131–1136. [Google Scholar] [CrossRef]
- Aydin, S.; Kuloglu, T.; Ozercan, M.R.; Albayrak, S.; Aydin, S.; Bakal, U.; Yilmaz, M.; Kalayci, M.; Yardim, M.; Sarac, M.; et al. Irisin immunohistochemistry in gastrointestinal system cancers. Biotech. Histochem. 2016, 91, 242–250. [Google Scholar] [CrossRef]
- Altay, D.U.; Keha, E.E.; Karagüzel, E.; Menteşe, A.; Yaman, S.O.; Alver, A. The Diagnostic Value of FNDC5/Irisin in Renal Cell Cancer. Int. Braz. J. Urol. 2018, 44, 734–739. [Google Scholar] [CrossRef]
- Aslan, R.; Alp, H.H.; Eryılmaz, R.; Huyut, Z.; Sevim, M.; Araz, Ş.; Ertas, K.; Taken, K. Can the Irisin be a Biomarker for Prostate Cancer? A Case Control Study. Asian Pac. J. Cancer Prev. 2020, 21, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Xu, D.; Chu, K.; Cao, Z.; Sun, X.; Yang, Y. The Effects of MiR-214-3p and Irisin/FNDC5 on the Biological Behavior of Osteosarcoma Cells. Cancer Biother. Radiopharm. 2020, 35, 92–100. [Google Scholar] [CrossRef]
- Coletta, A.M.; Agha, N.H.; Baker, F.L.; Niemiro, G.M.; Mylabathula, P.L.; Brewster, A.M.; Bevers, T.B.; Fuentes-Mattei, E.; Basen-Engquist, K.; Gilchrist, S.C.; et al. The impact of high-intensity interval exercise training on NK-cell function and circulating myokines for breast cancer prevention among women at high risk for breast cancer. Breast Cancer Res. Treat. 2021, 187, 407–416. [Google Scholar] [CrossRef]
- de Castro, G.S.; Correia-Lima, J.; Simoes, E.; Orsso, C.E.; Xiao, J.; Gama, L.R.; Gomes, S.P.; Gonçalves, D.C.; Costa, R.G.F.; Radloff, K.; et al. Myokines in treatment-naïve patients with cancer-associated cachexia. Clin. Nutr. 2021, 40, 2443–2455. [Google Scholar] [CrossRef]
- Esawy, M.M.; Abdel-Samd, K.M. The diagnostic and prognostic roles of serum irisin in bladder cancer. Curr. Probl. Cancer 2020, 44, 100529. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.W.; Hwang, I.G.; Jang, J.S.; Hong, S.; Kim, T.Y.; Baek, J.Y.; Shin, S.; Sun, D.S.; Hong, D.S.; et al. Serum frailty biomarkers to predict overall survival in older patients with metastatic solid tumors: A substudy of prospective multi-center cohort study (KCSG PC13-09). J. Clin. Oncol. 2017, 35, 10041. [Google Scholar] [CrossRef]
- Nowinska, K.; Jablonska, K.; Pawelczyk, K.; Piotrowska, A.; Partynska, A.; Gomulkiewicz, A.; Ciesielska, U.; Katnik, E.; Grzegrzolka, J.; Glatzel-Plucinska, N.; et al. Expression of Irisin/FNDC5 in Cancer Cells and Stromal Fibroblasts of Non-small Cell Lung Cancer. Cancers 2019, 11, 1538. [Google Scholar] [CrossRef]
- Panagiotou, G.; Pazaitou-Panayiotou, K.; Paschou, S.A.; Komninou, D.; Kalogeris, N.; Vryonidou, A.; Mantzoros, C.S. Changes in Thyroid Hormone Levels Within the Normal and/or Subclinical Hyper- or Hypothyroid Range Do Not Affect Circulating Irisin Levels in Humans. Thyroid. Off. J. Am. Thyroid. Assoc. 2016, 26, 1039–1045. [Google Scholar] [CrossRef]
- Pazgan-Simon, M.; Zuwala-Jagiello, J.; Menzyk, T.; Bator, M.; Derra, A.; Lekstan, A.; Grzebyk, E.; Simon, K.; Kukla, M. Serum betatrophin and irisin levels in hepatocellular carcinoma. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2020, 71, 1. [Google Scholar] [CrossRef]
- Provatopoulou, X.; Georgiou, G.P.; Kalogera, E.; Kalles, V.; Matiatou, M.A.; Papapanagiotou, I.; Sagkriotis, A.; Zografos, G.C.; Gounaris, A. Serum irisin levels are lower in patients with breast cancer: Association with disease diagnosis and tumor characteristics. BMC Cancer 2015, 15, 898. [Google Scholar] [CrossRef]
- Sadim, M.; Xu, Y.; Selig, K.; Paulus, J.; Uthe, R.; Agarwl, S.; Dubin, I.; Oikonomopoulou, P.; Zaichenko, L.; McCandlish, S.A.; et al. A prospective evaluation of clinical and genetic predictors of weight changes in breast cancer survivors. Cancer 2017, 123, 2413–2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, S.; Hejazi, J.; Moghimi, M.; Borji, S.; Zabihian, S.; Fathi, M. Circulating Irisin Levels and Redox Status Markers in Patients with Gastric Cancer: A Case-Control Study. Asian Pac. J. Cancer Prev. 2020, 21, 2847–2851. [Google Scholar] [CrossRef] [PubMed]
- Ugur, K.; Aydin, S.; Kuloglu, T.; Artas, G.; Kocdor, M.A.; Sahin, İ.; Yardim, M.; Ozercan, İ.H. Comparison of irisin hormone expression between thyroid cancer tissues and oncocytic variant cells. Cancer Manag. Res. 2019, 11, 2595–2603. [Google Scholar] [CrossRef]
- Us Altay, D.; Keha, E.E.; Ozer Yaman, S.; Ince, I.; Alver, A.; Erdogan, B.; Canpolat, S.; Cobanoglu, U.; Mentese, A. Investigation of the expression of irisin and some cachectic factors in mice with experimentally induced gastric cancer. QJM Int. J. Med. 2016, 109, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ke, M.; Ren, Y.; Bi, J.; Du, Z.; Zhang, M.; Wang, Y.; Zhang, L.; Wu, Z.; Lv, Y.; et al. Serum Irisin Predicts Posthepatectomy Complications in Patients with Hepatocellular Carcinoma. Dis Markers 2019, 2019, 9850191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Zhang, X.F.; Li, H.; Liu, T.J.; Zhao, Q.P.; Huang, L.H.; Cao, Z.J.; He, L.M.; Hao, D.J. Serum irisin associates with breast cancer to spinal metastasis. Medicine 2018, 97, e0524. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, M.; Zhang, N.; Pan, H.; Lin, G.; Li, N.; Wang, L.; Yang, H.; Yan, K.; Gong, F. Serum and Adipose Tissue mRNA Levels of ATF3 and FNDC5/Irisin in Colorectal Cancer Patients with or without Obesity. Front. Physiol. 2018, 9, 1125. [Google Scholar] [CrossRef]
- Zybek-Kocik, A.; Sawicka-Gutaj, N.; Szczepanek-Parulska, E.; Andrusiewicz, M.; Waligórska-Stachura, J.; Białas, P.; Krauze, T.; Guzik, P.; Skrobisz, J.; Ruchała, M. The association between irisin and muscle metabolism in different thyroid disorders. Clin. Endocrinol. 2018, 88, 460–467. [Google Scholar] [CrossRef]
- Cebulski, K.; Nowinska, K.; Jablonska, K.; Romanowicz, H.; Smolarz, B.; Dziegiel, P.; Podhorska-Okolow, M. Expression of Irisin/FNDC5 in Breast Cancer. Int. J. Mol. Sci. 2022, 23, 3530. [Google Scholar] [CrossRef]
- Pinkowska, A.; Nowinska, K.; Ciesielska, U.; Podhorska-Okolow, M. Irisin association with ki-67, mcm3 and mt-i/ii in squamous cell carcinomas of the larynx. Biomolecules 2022, 12, 52. [Google Scholar] [CrossRef]
- Taken, K.; Aslan, R.; Eryilmaz, R.; Alp, H.H.; Huyut, Z.; Dönmez, M. Serum irisin is a novel biomarker for bladder cancer detection. Int. Urol. Nephrol. 2022, 54, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Abd Temur, A.; Aqeel Rashid, F. Irisin and Carcinoembryonic Antigen (CEA) as Potential Diagnostic Biomarkers in Gastric and Colorectal Cancers. Rep. Biochem. Mol. Biol. 2021, 10, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, S.; Nowinska, K.; Chabowski, M.; Dziegiel, P. Significance of Irisin (FNDC5) Expression in Colorectal Cancer. In Vivo 2022, 36, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Du, J.; Wang, M.H.; Li, J.M.; Yang, B.; Chen, Y.; Dai, J.C.; Zhang, C.; Zhou, J. Irisin Contributes to the Hepatoprotection of Dexmedetomidine during Intestinal Ischemia/Reperfusion. Oxid. Med. Cell Longev. 2019, 2019, 7857082. [Google Scholar] [CrossRef]
- Gannon, N.P.; Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Trujillo, K.A. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int. J. Cancer 2015, 136, E197–E202. [Google Scholar] [CrossRef]
- Huang, C.W.; Chang, Y.H.; Lee, H.H.; Wu, J.Y.; Huang, J.X.; Chung, Y.H.; Hsu, S.T.; Chow, L.P.; Wei, K.C.; Huang, F.T. Irisin, an exercise myokine, potently suppresses tumor proliferation, invasion, and growth in glioma. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 9678–9693. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Liu, Y.; Chen, Y. Irisin Enhances Doxorubicin-Induced Cell Apoptosis in Pancreatic Cancer by Inhibiting the PI3K/AKT/NF-κB Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 6085–6096. [Google Scholar] [CrossRef]
- Moon, H.S.; Mantzoros, C.S. Regulation of cell proliferation and malignant potential by irisin in endometrial, colon, thyroid and esophageal cancer cell lines. Metabolism 2014, 63, 188–193. [Google Scholar] [CrossRef]
- Shao, L.; Li, H.; Chen, J.; Song, H.; Zhang, Y.; Wu, F.; Wang, W.; Zhang, W.; Wang, F.; Li, H.; et al. Irisin suppresses the migration, proliferation, and invasion of lung cancer cells via inhibition of epithelial-to-mesenchymal transition. Biochem. Biophys. Res. Commun. 2017, 485, 598–605. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, P.; Li, L.; Tang, N.; Huang, F.; Kong, X.; Tan, X.; Shi, G. Irisin functions to inhibit malignant growth of human pancreatic cancer cells via downregulation of the PI3K/AKT signaling pathway. Onco Targets Ther. 2019, 12, 7243–7249. [Google Scholar] [CrossRef]
- Pinkowska, A.; Podhorska-Okolow, M.; Dziegiel, P.; Nowinska, K. The role of irisin in cancer disease. Cells 2021, 10, 1479. [Google Scholar] [CrossRef] [PubMed]
- Tekin, S.; Erden, Y.; Sandal, S.; Yilmaz, B. Is Irisin an Anticarcinogenic Peptide? Med. Sci. 2014, 4, 2172–2180. [Google Scholar] [CrossRef]
- Fan, G.H.; Zhu, T.Y.; Huang, J. FNDC5 promotes paclitaxel sensitivity of non-small cell lung cancers via inhibiting MDR1. Cell. Signal. 2020, 72, 109665. [Google Scholar] [CrossRef] [PubMed]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 2011, 11, 886–895. [Google Scholar] [CrossRef]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer—Mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Hudis, C.A.; Jones, L. Promoting exercise after a cancer diagnosis: Easier said than done. Br. J. Cancer 2014, 110, 829–830. [Google Scholar] [CrossRef]
- Hojman, P.; Gehl, J.; Christensen, J.F.; Pedersen, B.K. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2018, 27, 10–21. [Google Scholar] [CrossRef]
- Kim, J.S.; Galvão, D.A.; Newton, R.U.; Gray, E.; Taaffe, D.R. Exercise-induced myokines and their effect on prostate cancer. Nat. Rev. Urol. 2021, 18, 519–542. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, X.; Tang, N.; Huang, F.; Chen, Z.; Shi, G. Review of Research on the Role of Irisin in Tumors. Onco Targets Ther. 2020, 13, 4423–4430. [Google Scholar] [CrossRef]
- Schwab, M. (Ed.) Encyclopedia of Cancer; Springer: New York, NY, USA, 2008. [Google Scholar]
- Dhanapal, R.; Saraswathi, T.; Govind, R.N. Cancer cachexia. J. Oral Maxillofac. Pathol. JOMFP 2011, 15, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Bonewald, L.F.; Bonetto, A. Role of myokines and osteokines in cancer cachexia. Exp. Biol. Med. 2021, 246, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Vliora, M.; Grillo, E.; Corsini, M.; Ravelli, C.; Nintou, E.; Karligiotou, E.; Flouris, A.D.; Mitola, S. Irisin regulates thermogenesis and lipolysis in 3T3-L1 adipocytes. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2022, 1866, 130085. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liu, T.; Ma, P.; Mitteer, R.A., Jr.; Zhang, Z.; Kim, H.J.; Yeo, E.; Zhang, D.; Cai, P.; Li, C.; et al. c-Met-mediated endothelial plasticity drives aberrant vascularization and chemoresistance in glioblastoma. J. Clin. Investig. 2016, 126, 1801–1814. [Google Scholar] [CrossRef]
- Rodríguez-Carmona, A.; Pérez Fontán, M.; Sangiao Alvarellos, S.; García Falcón, T.; Pena Bello, M.L.; López Muñiz, A.; Cordido, F. Serum levels of the adipomyokine irisin in patients with chronic kidney disease. Nefrol. Publ. Off. Soc. Esp. Nefrol. 2016, 36, 496–502. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vliora, M.; Nintou, E.; Karligiotou, E.; Ioannou, L.G.; Grillo, E.; Mitola, S.; Flouris, A.D. Implication of Irisin in Different Types of Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 9971. https://doi.org/10.3390/ijms23179971
Vliora M, Nintou E, Karligiotou E, Ioannou LG, Grillo E, Mitola S, Flouris AD. Implication of Irisin in Different Types of Cancer: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2022; 23(17):9971. https://doi.org/10.3390/ijms23179971
Chicago/Turabian StyleVliora, Maria, Eleni Nintou, Eleni Karligiotou, Leonidas G. Ioannou, Elisabetta Grillo, Stefania Mitola, and Andreas D. Flouris. 2022. "Implication of Irisin in Different Types of Cancer: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 23, no. 17: 9971. https://doi.org/10.3390/ijms23179971
APA StyleVliora, M., Nintou, E., Karligiotou, E., Ioannou, L. G., Grillo, E., Mitola, S., & Flouris, A. D. (2022). Implication of Irisin in Different Types of Cancer: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 23(17), 9971. https://doi.org/10.3390/ijms23179971









