The Role of Ergosterol and Sphingolipids in the Localization and Activity of Candida albicans’ Multidrug Transporter Cdr1p and Plasma Membrane ATPase Pma1p
Abstract
:1. Introduction
2. Results
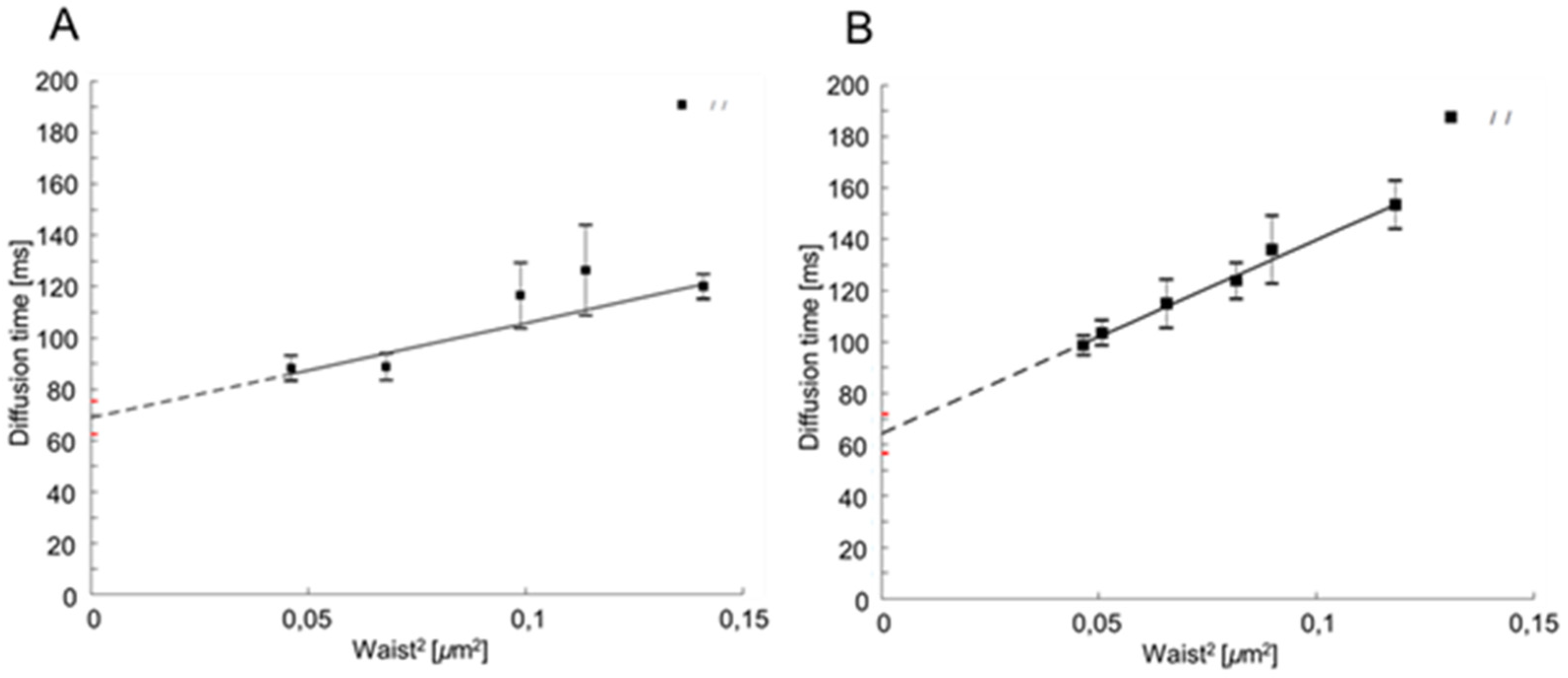
2.1. CaCdr1p Is Located in Nanodomains of PM of C. albicans
2.2. Low Sphingolipid Level in the PM Strongly Inhibits the Growth of C. albicans Mutant without Ergosterol (erg11∆/∆)
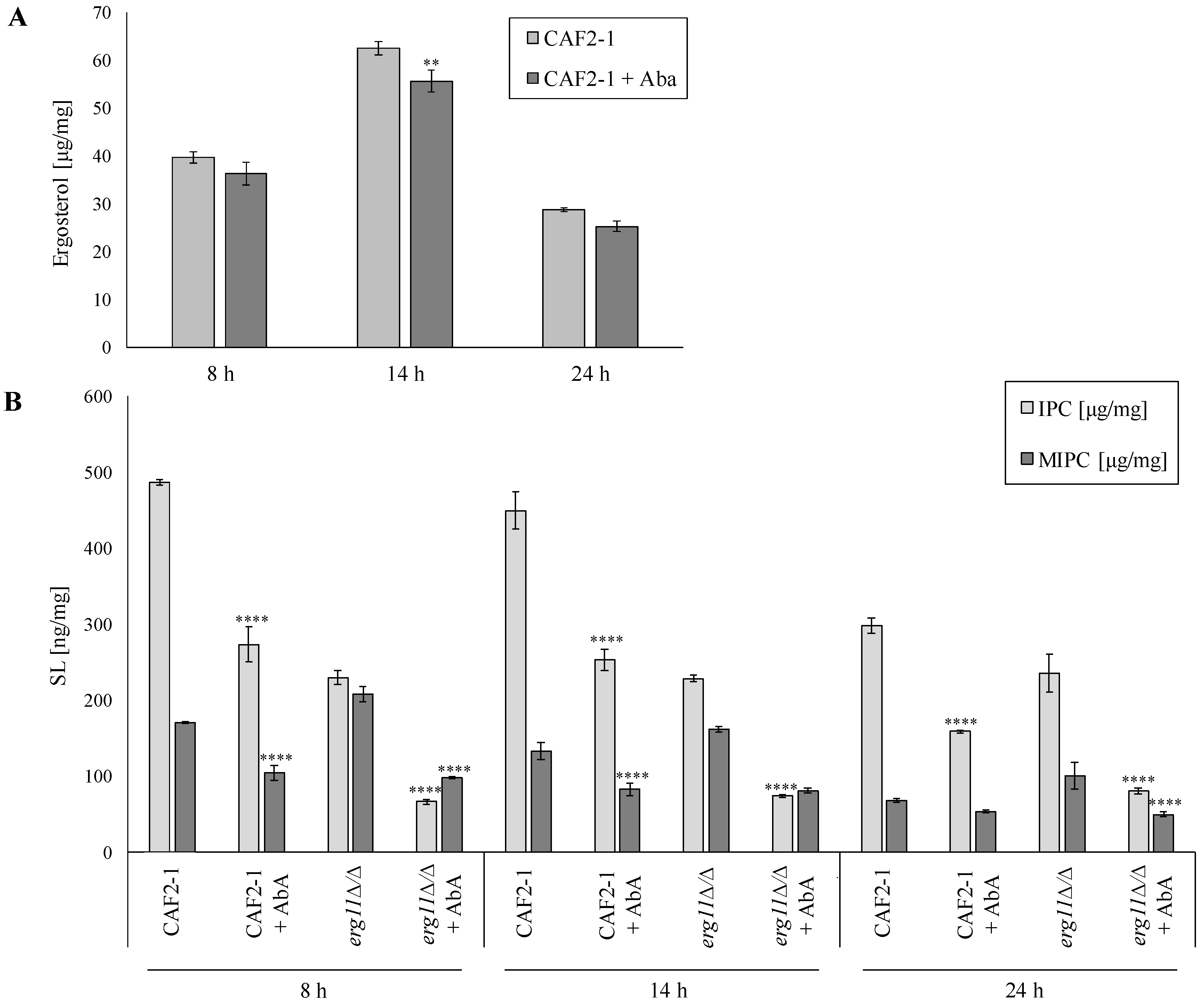
2.3. Aureobasidin a Changes the Lipid Composition in PM of C. albicans
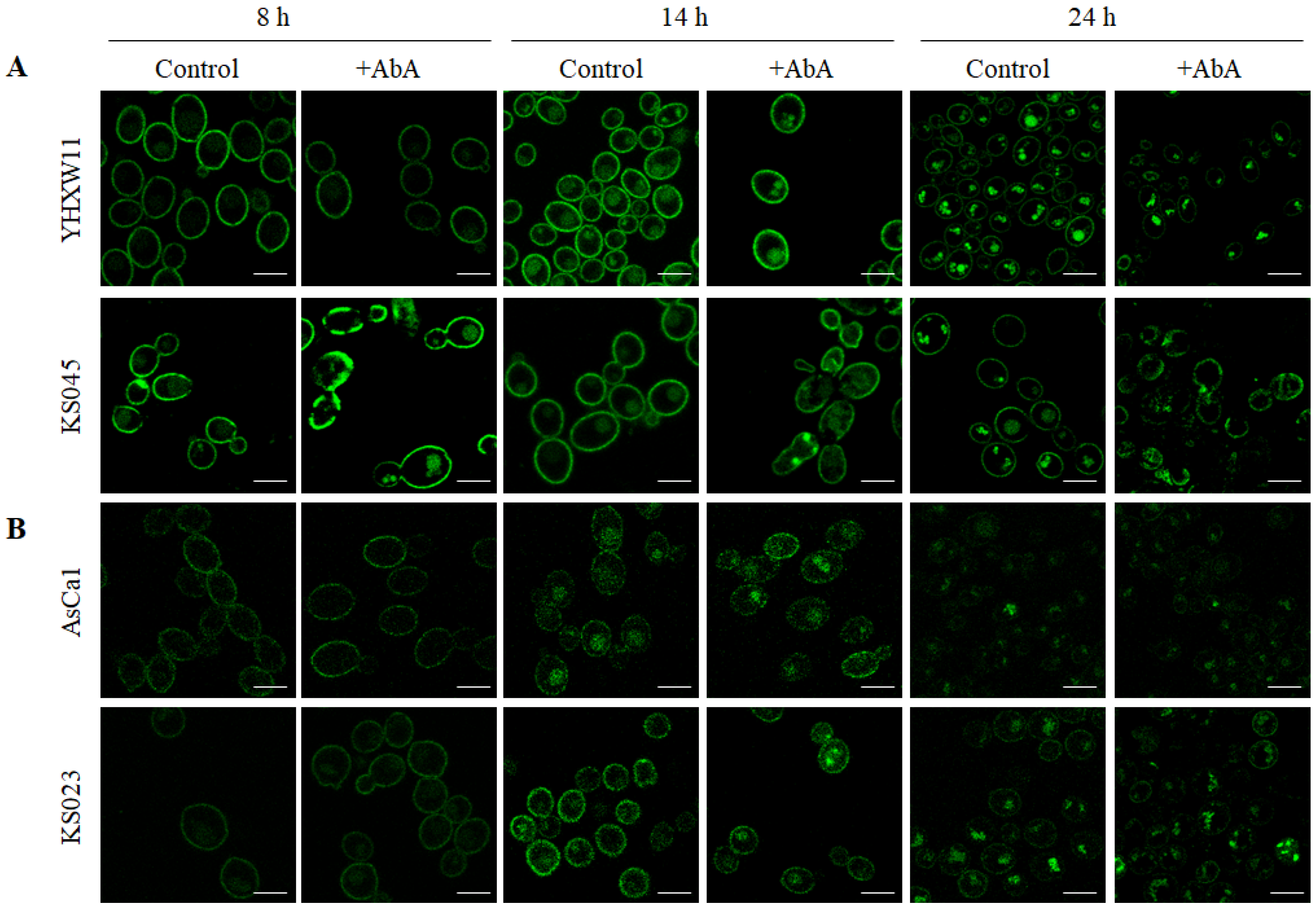
2.4. The Lack of Ergosterol but Not the Reduced Level of Sphingolipids in C. albicans’ PM Caused the Delocalization of CaCdr1p and CaPma1p to the Inside of the Cells at Different Times
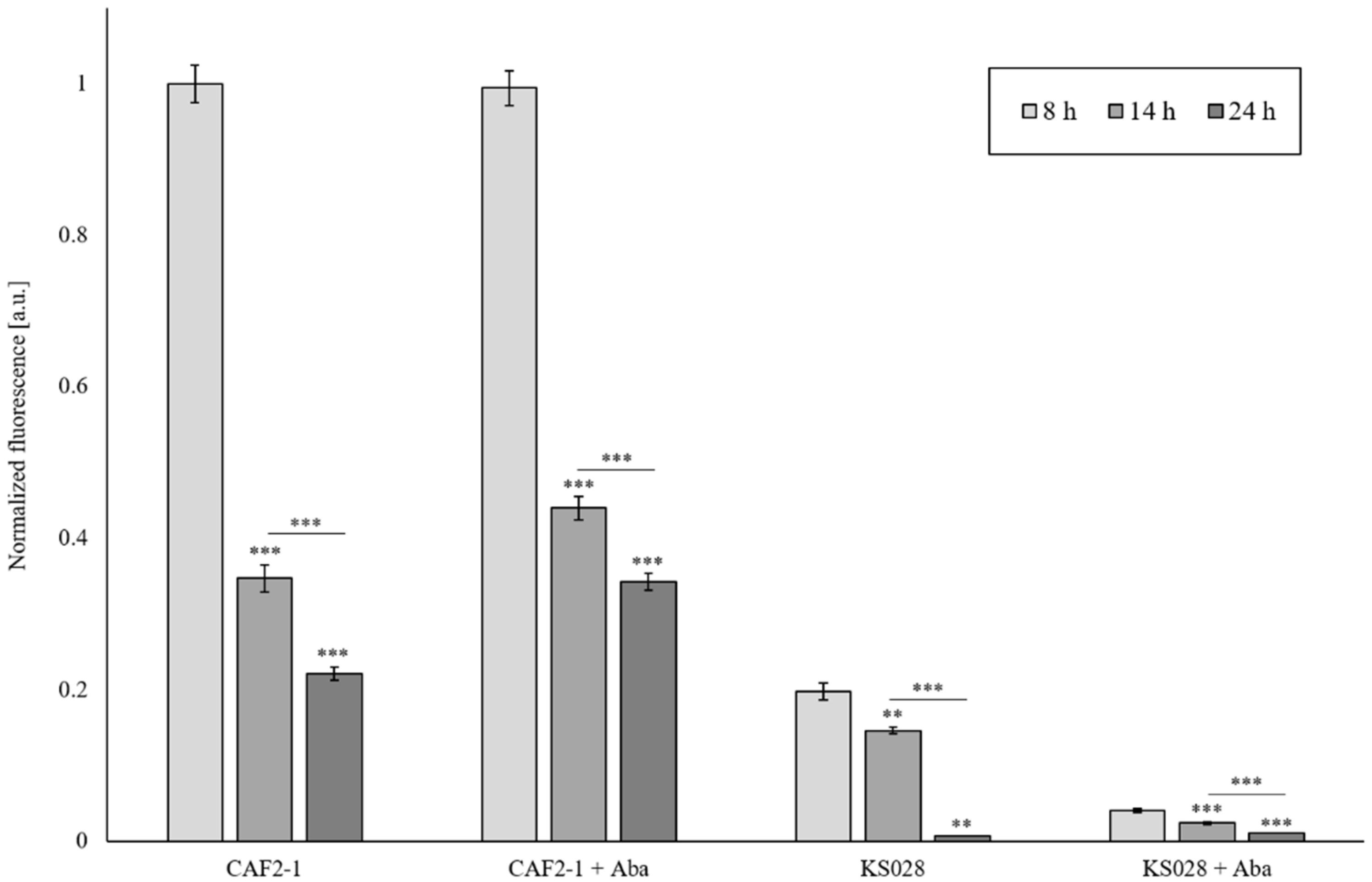
2.5. Lack of Ergosterol as Well as Reduced Sphingolipid Level in PM Reduce Cdr1p and H+-ATPase Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Strains and Growth Conditions
| Strain | Genotype | Reference |
|---|---|---|
| CAF2-1 | ura3∆::imm434/URA3 | [29] |
| KS023 | ura3∆::imm434/ura3∆::imm434 CDR1/CDR1-yEGFP-URA3 erg11∆::SAT1-FLIP/erg11∆::FRT | [19] |
| KS028 | ura3∆::imm434/URA3 erg11∆::SAT1-FLIP/erg11∆::FRT ura3∆::imm434/ura3∆::imm434 | [19] |
| AsCa1 | ura3∆::imm434/ura3∆::imm434 CDR1/CDR1-yEGFP-URA3 | [30] |
| YHXW11 | ura3∆::imm434/ura3∆::imm434 PMA1/PMA1-GFPγ-URA3 | [31] |
4.3. Determination of Minimal Inhibitory Concentration (MIC) and Spot Tests
4.4. Isolation of Plasma Membrane
4.5. Sphingolipids Determination
4.6. GC-MS Sterols Analysis in Plasma Membrane
4.7. Determination of Diffusion Time for Cdr1-GFP
4.8. Isolation of DRMs from C. albicans Cells
4.9. Microscopic Study of Cdr1-GFP Localization
4.10. Propidium Iodide (PI) Staining of C. albicans Cells
4.11. Cdr1p Efflux Pump Assay
4.12. Proton Extrusion Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Henriques, M.; Williams, D. Pathogenesis and Virulence of Candida albicans and Candida glabrata. Pathogens 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, T.Y.; Friggeri, L.; Wawrzak, Z.; Qi, A.; Hoekstra, W.J.; Schotzinger, R.J.; York, J.D.; Guengerich, F.P.; Lepesheva, G.I. Structural analyses of Candida albicans sterol 14α-demethylase complexed with azole drugs address the molecular basis of azole-mediated inhibition of fungal sterol biosynthesis. J. Biol. Chem. 2017, 292, 6728–6743. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.V.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sobel, J.D.; White, T.C. A Combination Fluorescence Assay Demonstrates Increased Efflux Pump Activity as a Resistance Mechanism in Azole-Resistant Vaginal Candida albicans Isolates. Antimicrob. Agents Chemother. 2016, 60, 5858–5866. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, H.; Li, Z.; Wong, A.H.; Wang, Y.Z.; Guo, Y.; Lin, X.; Zeng, G.; Liu, H.; Wang, Y.; et al. Candida albicans gains azole resistance by altering sphingolipid composition. Nat. Commun. 2018, 9, 4495. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, K.; Prasad, T.; Saini, P.; Pucadyil, T.J.; Chattopadhyay, A.; Prasad, R. Membrane sphingolipid-ergosterol interactions are important determinants of multidrug resistance in Candida albicans. Antimicrob. Agents Chemother. 2004, 48, 1778–1787. [Google Scholar] [CrossRef]
- Marquês, J.T.; Marinho, H.S.; de Almeida, R.F.M. Sphingolipid hydroxylation in mammals, yeast and plants—An integrated view. Prog. Lipid Res. 2018, 71, 18–42. [Google Scholar] [CrossRef]
- Santos, F.C.; Marquês, J.T.; Bento-Oliveira, A.; de Almeida, R.F.M. Sphingolipid-enriched domains in fungi. FEBS Lett. 2020, 594, 3698–3718. [Google Scholar] [CrossRef]
- Mailfert, S.; Wojtowicz, K.; Brustlein, S.; Blaszczak, E.; Bertaux, N.; Łukaszewicz, M.; Marguet, D.; Trombik, T. Spot Variation Fluorescence Correlation Spectroscopy for Analysis of Molecular Diffusion at the Plasma Membrane of Living Cells. J. Vis. Exp. 2020, 165, e61823. [Google Scholar] [CrossRef]
- Rockwell, N.C.; Wolfger, H.; Kuchler, K.; Thorner, J. ABC transporter Pdr10 regulates the membrane microenvironment of Pdr12 in Saccharomyces cerevisiae. J. Membr. Biol. 2009, 229, 27–52. [Google Scholar] [CrossRef] [PubMed]
- Bagnat, M.; Kerānen, S.; Shevchenko, A.; Shevchenko, A.; Simons, K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA 2000, 97, 3254–3259. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, Y.; Yamaguchi, Y.; Tani, M. Overexpression of PDR16 confers resistance to complex sphingolipid biosynthesis inhibitor aureobasidin A in yeast Saccharomyces cerevisoae. FEMS Microbiol. Lett. 2018, 365, fnx255. [Google Scholar] [CrossRef]
- Eggeling, C.; Ringemann, C.; Medda, R.; Schwarzmann, G.; Sandhoff, K.; Polyakova, S.; Belov, V.N.; Hein, B.; von Middendorff, C.; Schönle, A.; et al. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 2009, 457, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Favard, C.; Wenger, J.; Lenne, P.F.; Rigneault, H. FCS diffusion laws in two-phase lipid membranes: Determination of domain mean size by experiments and Monte Carlo simulations. Biophys. J. 2011, 100, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, K.; Czogalla, A.; Trombik, T.; Łukaszewicz, M. Surfactin cyclic lipopeptides change the plasma membrane composition and lateral organization in mammalian cells. Biochim. Biophys. Acta (BBA)–Biomembr. 2021, 1863, 183730. [Google Scholar] [CrossRef] [PubMed]
- Malínská, K.; Malínský, J.; Opekarová, M.; Tanner, W. Visualization of protein compartmentation within the plasma membrane of living yeast cells. Mol. Biol. Cell 2003, 14, 4427–4436. [Google Scholar] [CrossRef] [PubMed]
- Pasrija, R.; Panwar, S.L.; Prasad, R. Multidrug transporters CaCdr1p and CaMdr1p of Candida albicans display different lipid specificities: Both ergosterol and sphingolipids are essential for targeting of CaCdr1p to membrane rafts. Antimicrob. Agents Chemother. 2008, 52, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.; Muraszko, J.; Bernat, P.; Krasowska, A. A Crucial Role for Ergosterol in Plasma Membrane Composition, Localisation, and Activity of Cdr1p and H+-ATPase in Candida albicans. Microorganisms 2019, 7, 378. [Google Scholar] [CrossRef]
- Suchodolski, J.; Derkacz, D.; Bernat, P.; Krasowska, A. Capric acid secreted by Saccharomyces boulardii influences the susceptibility of Candida albicans to fluconazole and amphotericin B. Sci. Rep. 2021, 11, 6519. [Google Scholar] [CrossRef]
- Suchodolski, J.; Derkacz, D.; Muraszko, J.; Panek, J.; Jezierska, A.; Łukaszewicz, M.; Krasowska, A. Fluconazole and lipopeptide surfactin interplay during Candida albicans plasma membrane and cell wall remodeling increases fungal immune system exposure. Pharmaceutics 2020, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Valachovic, M.; Bareither, B.M.; Bhuiyan, M.S.A.; Eckstein, J.; Barbuch, R.; Balderes, D.; Wilcox, L.; Sturley, S.L.; Dickson, R.C.; Bard, M. Cumulative mutations affecting sterol biosynthesis in the yeast Saccharomyces cerevisiae result in synthetic lethality that is suppressed by alterations in sphingolipid profiles. Genetics 2006, 173, 1893–1908. [Google Scholar] [CrossRef]
- Gutarowska, B.; Zakowska, Z. Mathematical models of mycelium growth and ergosterol synthesis in stationary mould culture. Lett. Appl. Microbiol. 2008, 48, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; MacKenzie, A.; Girnun, G.; Del Poeta, M. Analysis of sphingolipids, sterols, and phospholipids in human pathogenic Cryptococcus strains. J. Lipid Res. 2017, 58, 2017–2036. [Google Scholar] [CrossRef] [PubMed]
- Rella, A.; Farnoud, A.M.; Del Poeta, M. Plasma membrane lipids and their role in fungal virulence. Prog. Lipid Res. 2016, 61, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Farnoud, A.M.; Toledo, A.M.; Konopka, J.B.; Del Poeta, M.; London, E. Chapter seven-raft like membrane domains in pathogenic microorganisms. Curr. Top Membr. 2015, 75, 233–268. [Google Scholar] [PubMed]
- Kohli, A.; Smriti, N.; Mukhopadhyay, K.; Rattan, A.; Prasad, R. In vitro low-level resistance to azoles in Candida albicans is associated with changes in membrane lipid fluidity and asymmetry. Antimicrob. Agents Chemother. 2002, 46, 1046–1052. [Google Scholar] [CrossRef]
- Szczepaniak, J.; Łukaszewicz, M.; Krasowska, A. Estimation of Candida albicans ABC Transporter Behavior in Real-Time via Fluorescence. Front Microbiol. 2015, 6, 1382. [Google Scholar] [CrossRef]
- Fonzi, W.A.; Irwin, M.Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics 1993, 134, 717–728. [Google Scholar] [CrossRef]
- Szczepaniak, J.; Łukaszewicz, M.; Krasowska, A. Detection of inhibitors of Candida albicans Cdr transporters using a diS-C3 fluorescence. Front Microbiol. 2015, 6, 176. [Google Scholar] [CrossRef] [Green Version]
- Douglas, L.M.; Wang, H.X.; Keppler-Ross, S.; Dean, N.; Konopka, J.B. Sur7 promotes plasma membrane organization and is needed for resistance to stressful conditions and to the invasive growth and virulence of Candida albicans. mBio 2011, 3, e00254-11. [Google Scholar] [CrossRef] [PubMed]
- Derkacz, D.; Bernat, P.; Krasowska, A. K143R Amino Acid Substitution in 14-α-demethylase (Erg11p) Changes Plasma Membrane and Cell Wall Structure of Candida albicans. Int. J. Mol. Sci. 2022, 23, 1631. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.; Muraszko, J.; Korba, A.; Bernat, P.; Krasowska, A. Lipid composition and cell surface hydrophobicity of Candida albicans influence the efficacy of fluconazole-gentamicin treatment. Yeast 2020, 37, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Insenser, M.; Nombela, C.; Molero, G.; Gil, C. Proteomic analysis of detergent-resistant membranes from Candida albicans. Proteomics 2006, 6 (Suppl. S1), S74–S81. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, J.; Cieslik, W.; Romanowicz, A.; Musioł, R.; Krasowska, A. Blocking and dislocation of Candida albicans Cdr1p transporter by styrylquinolines. Int. J. Antimicrob. Agents 2017, 50, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, T.M.; Krasowska, A.; Łuczyński, J.; Witek, S. Plasma membrane H + ATPase activity in wildetype and mutants of yeast Saccharomyces cerevisiae treated by some lysosomotropic drugs. Folia Microbiol. 1998, 43, 203–205. [Google Scholar] [CrossRef]



| Strain/Time of Culture | 8 h | 14 h | 24 h | |||
|---|---|---|---|---|---|---|
| CAF2-1 | control | +AbA | control | +AbA | control | +AbA |
| 1.5 | 1.81 | 2.02 | 1.81 | 2.44 | 1.45 | |
| pH range | 7.495–5.995 | 7.495–5.685 | 7.495–5.475 | 7.495–5.405 | 7.495–5.055 | 7.495–6.045 |
| pH change (%) | 21% | −10.4% | −40.6% | |||
| KS028 | 2.01 | 1.88 | 1.94 | 1.31 | 1.79 | 1.58 |
| pH range | 7.495–5.485 | 7.495–5.615 | 7.495–5.555 | 7.495–6.185 | 7.495–5.705 | 7.495–5.915 |
| pH change (%) | −6.46% | −32.5% | −12% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanek, A.K.; Muraszko, J.; Derkacz, D.; Łukaszewicz, M.; Bernat, P.; Krasowska, A. The Role of Ergosterol and Sphingolipids in the Localization and Activity of Candida albicans’ Multidrug Transporter Cdr1p and Plasma Membrane ATPase Pma1p. Int. J. Mol. Sci. 2022, 23, 9975. https://doi.org/10.3390/ijms23179975
Urbanek AK, Muraszko J, Derkacz D, Łukaszewicz M, Bernat P, Krasowska A. The Role of Ergosterol and Sphingolipids in the Localization and Activity of Candida albicans’ Multidrug Transporter Cdr1p and Plasma Membrane ATPase Pma1p. International Journal of Molecular Sciences. 2022; 23(17):9975. https://doi.org/10.3390/ijms23179975
Chicago/Turabian StyleUrbanek, Aneta K., Jakub Muraszko, Daria Derkacz, Marcin Łukaszewicz, Przemysław Bernat, and Anna Krasowska. 2022. "The Role of Ergosterol and Sphingolipids in the Localization and Activity of Candida albicans’ Multidrug Transporter Cdr1p and Plasma Membrane ATPase Pma1p" International Journal of Molecular Sciences 23, no. 17: 9975. https://doi.org/10.3390/ijms23179975





