Cytokine Secretion Dynamics of Isolated PBMC after Cladribine Exposure in RRMS Patients
Abstract
:1. Introduction
2. Results
2.1. Secretory Profile of PBMCs Harvested from All RRMS Patients Compared to HCs
- IL-4 (Table 3)
- -
- IL-4 secretion was significantly increased in CLD− PBMCs from HCs on day 14 compared to day 7 (p = 0.0275), while the increase was not significant in CLD+ PBMCs (p = 0.3308).
- -
- IL-4 secretion was significantly increased in CLD− (p < 0.0001) and CLD+ (p < 0.0001) PBMCs from RRMS patients on day 14 compared to day 7.
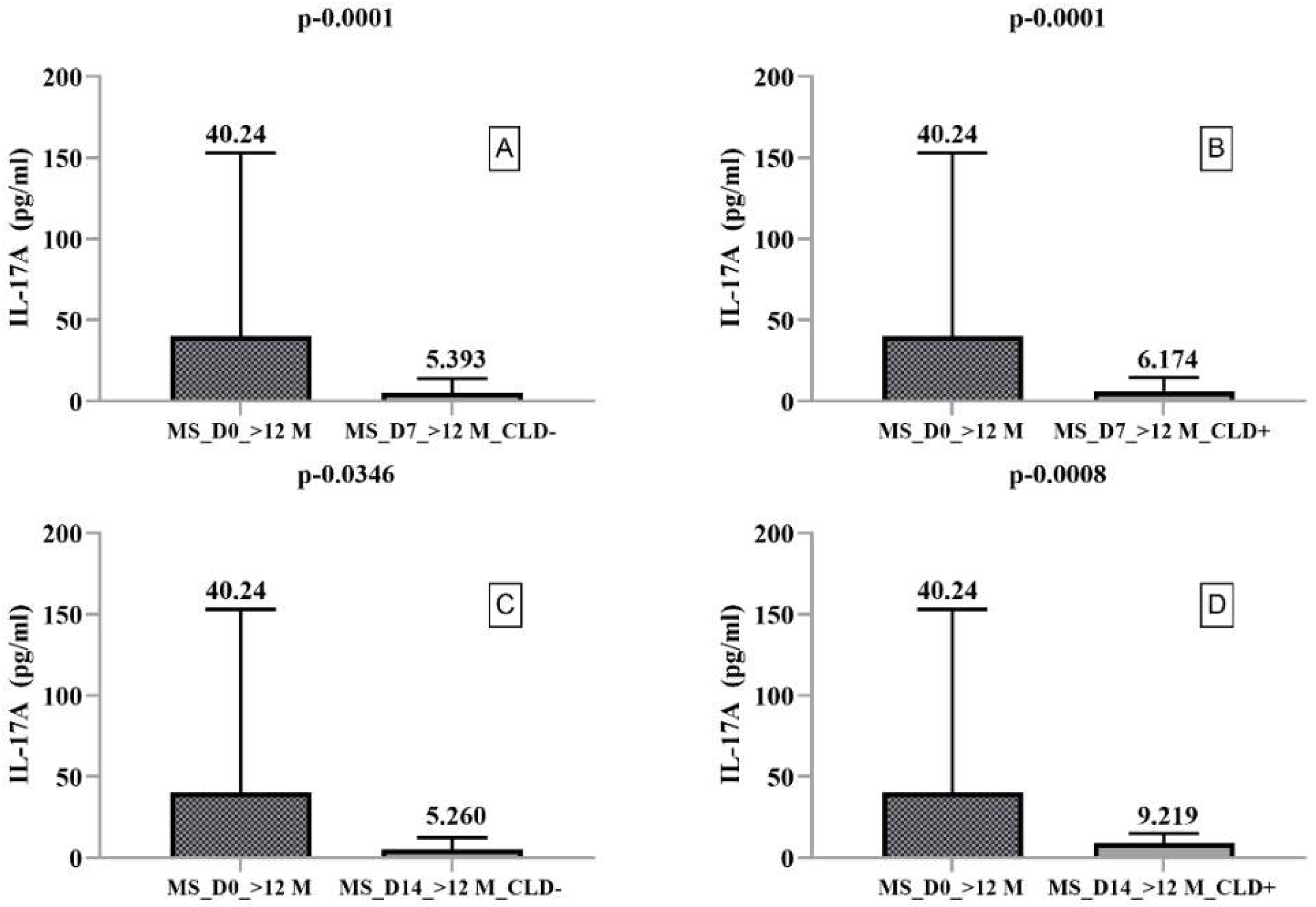
- IL-17A
- -
- IL-17A secretion was nonsignificantly decreased in CLD+ but nonsignificantly increased in CLD- PBMCs from HCs on day 14 compared to day 7.
- -
- IL-17A secretion was nonsignificantly increased in CLD− PBMCs from RRMS patients on day 14 compared to day 7 but significantly increased in CLD+ PBMCs on day 14 (9.20) compared to day 7 (5.99).
- TNF-α (Table 4)
- -
- TNF-α secretion was significantly increased in CLD+ (p = 0.0440) and CLD− (p = 0.0074) PBMCs from RRMS patients on day 14 compared to day 7, but not in HC-harvested cells (p > 0.3233).
- IL-10 and IFN-γ secretion and the inflammatory ratio did not differ significantly.
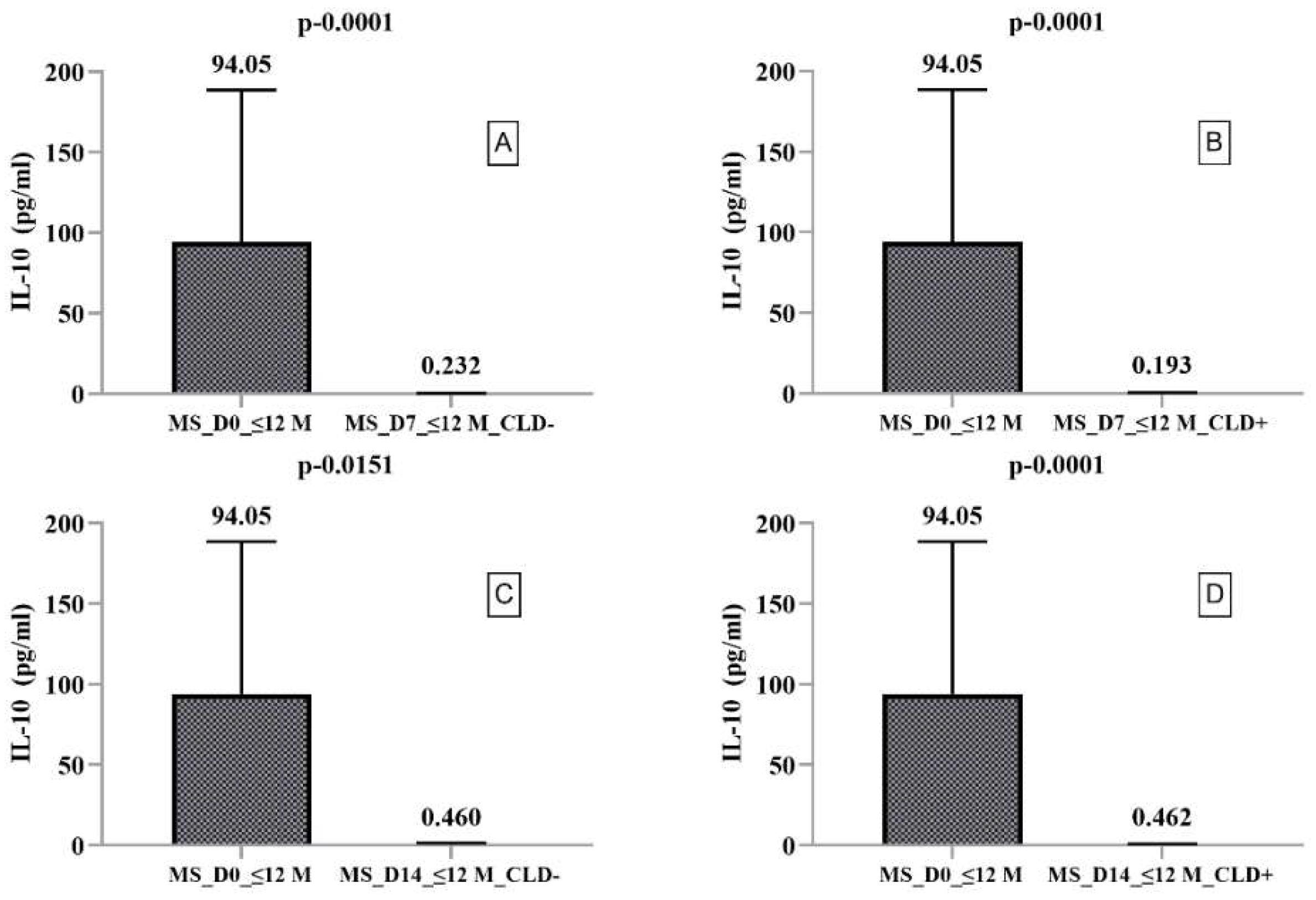
2.2. Secretory Profile of PBMCs Harvested from RRMS Patients with Disease Duration ≤ 12 Months
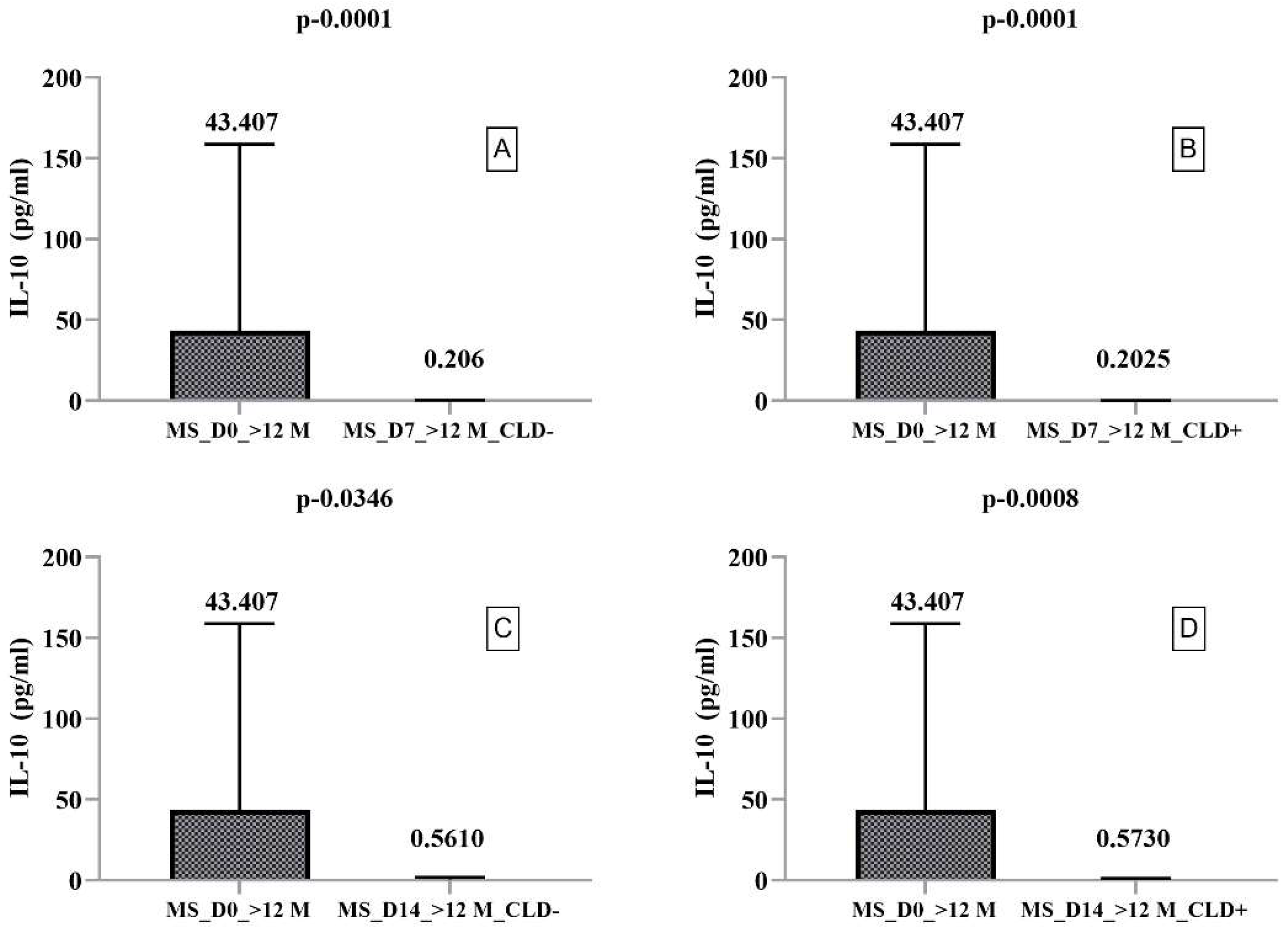
2.3. Secretory Profile of PMBCs Harvested from RRMS Patients with Disease Duration > 12 Months
3. Discussion
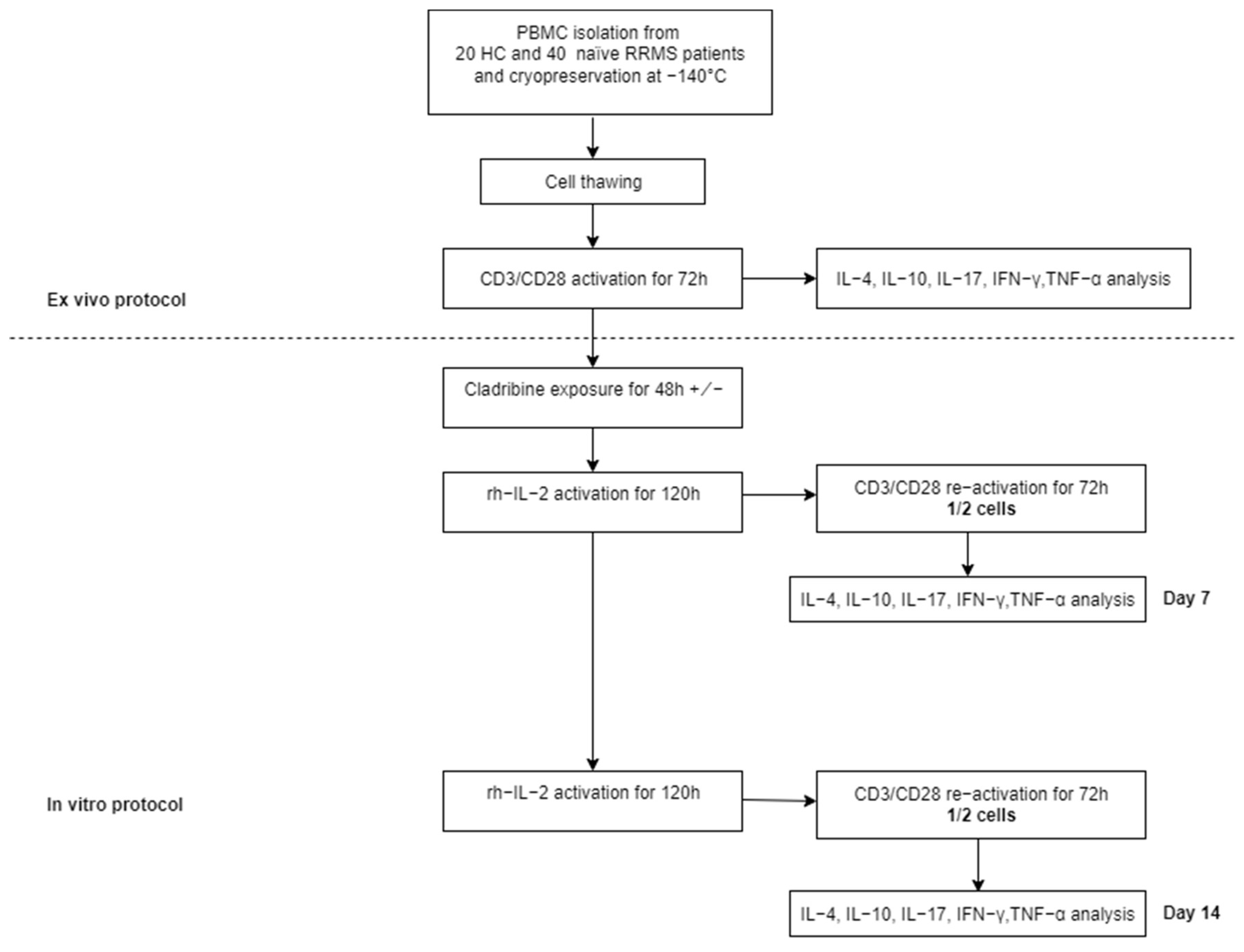
4. Materials and Methods
4.1. Patients Selection
4.2. Cell Isolation and Culture
4.3. Cytokine Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ehtesham, N.; Rafie, M.Z.; Mosallaei, M. The Global Prevalence of Familial Multiple Sclerosis: An Updated Systematic Review and Meta-Analysis. BMC Neurol. 2021, 21, 246. [Google Scholar] [CrossRef] [PubMed]
- Callegari, I.; Derfuss, T.; Galli, E. Update on Treatment in Multiple Sclerosis. Presse Médicale 2021, 50, 104068. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, T.; Prat, A. A Roadmap to Precision Medicine for Multiple Sclerosis. Mult. Scler. J. 2020, 26, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple Sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Van Langelaar, J.; Rijvers, L.; Smolders, J.; Van Luijn, M.M. B and T Cells Driving Multiple Sclerosis: Identity, Mechanisms and Potential Triggers. Front. Immunol. 2020, 11, 760. [Google Scholar] [CrossRef]
- Lünemann, J.D.; Ruck, T.; Muraro, P.A.; Bar-Or, A.; Wiendl, H. Immune Reconstitution Therapies: Concepts for Durable Remission in Multiple Sclerosis. Nat. Rev. Neurol. 2020, 16, 56–62. [Google Scholar] [CrossRef]
- Jacobs, B.M.; Ammoscato, F.; Giovannoni, G.; Baker, D.; Schmierer, K. Cladribine: Mechanisms and Mysteries in Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1266–1271. [Google Scholar] [CrossRef]
- Korsen, M.; Bragado Alonso, S.; Peix, L.; Bröker, B.M.; Dressel, A. Cladribine Exposure Results in a Sustained Modulation of the Cytokine Response in Human Peripheral Blood Mononuclear Cells. PLoS ONE 2015, 10, e0129182. [Google Scholar] [CrossRef]
- Stuve, O.; Soelberg Soerensen, P.; Leist, T.; Giovannoni, G.; Hyvert, Y.; Damian, D.; Dangond, F.; Boschert, U. Effects of Cladribine Tablets on Lymphocyte Subsets in Patients with Multiple Sclerosis: An Extended Analysis of Surface Markers. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419854986. [Google Scholar] [CrossRef]
- Giovannoni, G.; Mathews, J. Cladribine Tablets for Relapsing-Remitting Multiple Sclerosis: A Clinician’s Review. Neurol. Ther. 2022, 11, 571–595. [Google Scholar] [CrossRef]
- Giovannoni, G.; Soelberg Sorensen, P.; Cook, S.; Rammohan, K.; Rieckmann, P.; Comi, G.; Dangond, F.; Adeniji, A.K.; Vermersch, P. Safety and Efficacy of Cladribine Tablets in Patients with Relapsing-Remitting Multiple Sclerosis: Results from the Randomized Extension Trial of the CLARITY Study. Mult. Scler. J. 2018, 24, 1594–1604. [Google Scholar] [CrossRef]
- Comi, G.; Cook, S.; Giovannoni, G.; Rieckmann, P.; Sørensen, P.S.; Vermersch, P.; Galazka, A.; Nolting, A.; Hicking, C.; Dangond, F. Effect of Cladribine Tablets on Lymphocyte Reduction and Repopulation Dynamics in Patients with Relapsing Multiple Sclerosis. Mult. Scler. Relat. Disord. 2019, 29, 168–174. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef]
- Dobreanu, M.; Manu, D.R.; Mănescu, I.B.; Gabor, M.R.; Huţanu, A.; Bărcuţean, L.; Bălaşa, R. Treatment with Cladribine Selects IFNγ+ IL17+ T Cells in RRMS Patients—An In Vitro Study. Front. Immunol. 2021, 12, 743010. [Google Scholar] [CrossRef]
- Bălaşa, R.; Bajko, Z.; Huţanu, A. Serum Levels of IL-17A in Patients with Relapsing–Remitting Multiple Sclerosis Treated with Interferon-β. Mult. Scler. 2013, 19, 885–890. [Google Scholar] [CrossRef]
- Kraus, S.H.; Luessi, F.; Trinschek, B.; Lerch, S.; Hubo, M.; Poisa-Beiro, L.; Paterka, M.; Jonuleit, H.; Zipp, F.; Jolivel, V. Cladribine Exerts an Immunomodulatory Effect on Human and Murine Dendritic Cells. Int. Immunopharmacol. 2014, 18, 347–357. [Google Scholar] [CrossRef]
- Deeks, E.D. Cladribine Tablets: A Review in Relapsing MS. CNS Drugs 2018, 32, 785–796. [Google Scholar] [CrossRef]
- Balasa, R.; Barcutean, L.; Mosora, O.; Manu, D. Reviewing the Significance of Blood–Brain Barrier Disruption in Multiple Sclerosis Pathology and Treatment. Int. J. Mol. Sci. 2021, 22, 8370. [Google Scholar] [CrossRef]
- Giovannoni, G. Cladribine to Treat Relapsing Forms of Multiple Sclerosis. Neurotherapeutics 2017, 14, 874–887. [Google Scholar] [CrossRef]
- Cook, S.; Leist, T.; Comi, G.; Montalban, X.; Giovannoni, G.; Nolting, A.; Hicking, C.; Galazka, A.; Sylvester, E. Safety of Cladribine Tablets in the Treatment of Patients with Multiple Sclerosis: An Integrated Analysis. Mult. Scler. Relat. Disord. 2019, 29, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Leist, T.P.; Weissert, R. Cladribine: Mode of Action and Implications for Treatment of Multiple Sclerosis. Clin. Neuropharmacol. 2011, 34, 28–35. [Google Scholar] [CrossRef]
- Rammohan, K.; Coyle, P.K.; Sylvester, E.; Galazka, A.; Dangond, F.; Grosso, M.; Leist, T.P. The Development of Cladribine Tablets for the Treatment of Multiple Sclerosis: A Comprehensive Review. Drugs 2020, 80, 1901–1928. [Google Scholar] [CrossRef]
- Bose, G.; Rush, C.; Atkins, H.L.; Freedman, M.S. A Real-World Single-Centre Analysis of Alemtuzumab and Cladribine for Multiple Sclerosis. Mult. Scler. Relat. Disord. 2021, 52, 102945. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, P.S.; Sellebjerg, F. Pulsed Immune Reconstitution Therapy in Multiple Sclerosis. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419836913. [Google Scholar] [CrossRef]
- Rejdak, K.; Stelmasiak, Z.; Grieb, P. Cladribine Induces Long Lasting Oligoclonal Bands Disappearance in Relapsing Multiple Sclerosis Patients: 10-Year Observational Study. Mult. Scler. Relat. Disord. 2019, 27, 117–120. [Google Scholar] [CrossRef]
- Singh, V.; Voss, E.V.; Bénardais, K.; Stangel, M. Effects of 2-Chlorodeoxyadenosine (Cladribine) on Primary Rat Microglia. J. Neuroimmune Pharmacol. 2012, 7, 939–950. [Google Scholar] [CrossRef]
- Fissolo, N.; Calvo-Barreiro, L.; Eixarch, H.; Boschert, U.; Espejo, C.; Montalban, X.; Comabella, M. Immunomodulatory Effects Associated with Cladribine Treatment. Cells 2021, 10, 3488. [Google Scholar] [CrossRef]
- Kopadze, T.; Döbert, M.; Leussink, V.; Dehmel, T.; Kieseier, B. Cladribine Impedes in Vitro Migration of Mononuclear Cells: A Possible Implication for Treating Multiple Sclerosis. Eur. J. Neurol. 2009, 16, 409–412. [Google Scholar] [CrossRef]
- Leist, T.; Cook, S.; Comi, G.; Montalban, X.; Giovannoni, G.; Nolting, A.; Damian, D.; Syed, S.; Galazka, A. Long-Term Safety Data from the Cladribine Tablets Clinical Development Program in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2020, 46, 102572. [Google Scholar] [CrossRef]
- Ceronie, B.; Jacobs, B.M.; Baker, D.; Dubuisson, N.; Mao, Z.; Ammoscato, F.; Lock, H.; Longhurst, H.J.; Giovannoni, G.; Schmierer, K. Cladribine Treatment of Multiple Sclerosis Is Associated with Depletion of Memory B Cells. J. Neurol. 2018, 265, 1199–1209. [Google Scholar] [CrossRef] [Green Version]
- Dooley, J.; Pauwels, I.; Franckaert, D.; Smets, I.; Garcia-Perez, J.E.; Hilven, K.; Danso-Abeam, D.; Terbeek, J.; Nguyen, A.T.; De Muynck, L. Immunologic Profiles of Multiple Sclerosis Treatments Reveal Shared Early B Cell Alterations. Neurol.-Neuroimmunol. Neuroinflamm. 2016, 3, e240. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Herrod, S.S.; Alvarez-Gonzalez, C.; Zalewski, L.; Albor, C.; Schmierer, K. Both Cladribine and Alemtuzumab May Effect MS via B-Cell Depletion. Neurol.-Neuroimmunol. Neuroinflamm. 2017, 4, e360. [Google Scholar] [CrossRef] [PubMed]
- Bălaşa, R.; Huţanu, A.; Bajko, Z.; Feier, C.; Pascu, I. Does the Serum IL-17 Titer Influence the Efficacy of Interferon-β Treatment in Multiple Sclerosis Patients? Rev. Romana Med. Lab. 2011, 19, 381–389. [Google Scholar]
- Hu, D.; Notarbartolo, S.; Croonenborghs, T.; Patel, B.; Cialic, R.; Yang, T.-H.; Aschenbrenner, D.; Andersson, K.M.; Gattorno, M.; Pham, M. Transcriptional Signature of Human Pro-Inflammatory TH17 Cells Identifies Reduced IL10 Gene Expression in Multiple Sclerosis. Nat. Commun. 2017, 8, 1600. [Google Scholar] [CrossRef]
- De Jesús Guerrero-García, J.; Castañeda-Moreno, V.A.; Torres-Carrillo, N.; Muñoz-Valle, J.F.; Bitzer-Quintero, O.K.; Ponce-Regalado, M.D.; Mireles-Ramírez, M.A.; Valle, Y.; Ortuño-Sahagún, D. Interleukin-17A Levels Vary in Relapsing-Remitting Multiple Sclerosis Patients in Association with Their Age, Treatment and the Time of Evolution of the Disease. Neuroimmunomodulation 2016, 23, 8–17. [Google Scholar] [CrossRef]
- Cao, Y.; Goods, B.A.; Raddassi, K.; Nepom, G.T.; Kwok, W.W.; Love, J.C.; Hafler, D.A. Functional Inflammatory Profiles Distinguish Myelin-Reactive T Cells from Patients with Multiple Sclerosis. Sci. Transl. Med. 2015, 7, 287ra74. [Google Scholar] [CrossRef]
- Balasa, R.; Barcutean, L.; Balasa, A.; Motataianu, A.; Roman-Filip, C.; Manu, D. The Action of TH17 Cells on Blood Brain Barrier in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Hum. Immunol. 2020, 81, 237–243. [Google Scholar] [CrossRef]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human TH17 Lymphocytes Promote Blood-Brain Barrier Disruption and Central Nervous System Inflammation. Nat. Med. 2007, 13, 1173–1175. [Google Scholar] [CrossRef]
- Moser, T.; Schwenker, K.; Seiberl, M.; Feige, J.; Akgün, K.; Haschke-Becher, E.; Ziemssen, T.; Sellner, J. Long-term Peripheral Immune Cell Profiling Reveals Further Targets of Oral Cladribine in MS. Ann. Clin. Transl. Neurol. 2020, 7, 2199–2212. [Google Scholar] [CrossRef]
- Moser, T.; Hoepner, L.; Schwenker, K.; Seiberl, M.; Feige, J.; Akgün, K.; Haschke-Becher, E.; Ziemssen, T.; Sellner, J. Cladribine Alters Immune Cell Surface Molecules for Adhesion and Costimulation: Further Insights to the Mode of Action in Multiple Sclerosis. Cells 2021, 10, 3116. [Google Scholar] [CrossRef]
- Xu, W.; Li, R.; Sun, Y. Increased IFN-γ-Producing Th17/Th1 Cells and Their Association with Lung Function and Current Smoking Status in Patients with Chronic Obstructive Pulmonary Disease. BMC Pulm. Med. 2019, 19, 137. [Google Scholar] [CrossRef] [Green Version]
- Passos, G.R.D.; Sato, D.K.; Becker, J.; Fujihara, K. Th17 Cells Pathways in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders: Pathophysiological and Therapeutic Implications. Mediat. Inflamm. 2016, 2016, 5314541. [Google Scholar] [CrossRef]
- Tahani, S.; Dehghani, L.; Jahanbani-Ardakani, H.; Shaygannejad, V.; Fazli, A.; Hamidavi, A.; Eskandari, N. Elevated Serum Level of IL-4 in Neuromyelitis Optica and Multiple Sclerosis Patients. J. Immunoass. Immunochem. 2019, 40, 555–563. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNFα and the TNF Receptor Superfamily: Structure-function Relationship (s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Fresegna, D.; Bullitta, S.; Musella, A.; Rizzo, F.R.; De Vito, F.; Guadalupi, L.; Caioli, S.; Balletta, S.; Sanna, K.; Dolcetti, E. Re-Examining the Role of TNF in MS Pathogenesis and Therapy. Cells 2020, 9, 2290. [Google Scholar] [CrossRef]
- Mathiesen, C.B.; Rudjord-Levann, A.M.; Gad, M.; Larsen, J.; Sellebjerg, F.; Pedersen, A.E. Cladribine Inhibits Secretion of Pro-Inflammatory Cytokines and Phagocytosis in Human Monocyte-Derived M1 Macrophages in-Vitro. Int. Immunopharmacol. 2021, 91, 107270. [Google Scholar] [CrossRef]
- Kebir, H.; Ifergan, I.; Alvarez, J.I.; Bernard, M.; Poirier, J.; Arbour, N.; Duquette, P.; Prat, A. Preferential Recruitment of Interferon-γ–Expressing TH17 Cells in Multiple Sclerosis. Ann. Neurol. 2009, 66, 390–402. [Google Scholar] [CrossRef]
- Ottum, P.A.; Arellano, G.; Reyes, L.I.; Iruretagoyena, M.; Naves, R. Opposing Roles of Interferon-Gamma on Cells of the Central Nervous System in Autoimmune Neuroinflammation. Front. Immunol. 2015, 6, 539. [Google Scholar] [CrossRef]
- Olsson, T. Cytokines in Neuroinflammatory Disease: Role of Myelin Autoreactive T Cell Production of Interferon-Gamma. J. Neuroimmunol. 1992, 40, 211–218. [Google Scholar] [CrossRef]
- Fletcher, J.M.; Lalor, S.; Sweeney, C.; Tubridy, N.; Mills, K. T Cells in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Clin. Exp. Immunol. 2010, 162, 1–11. [Google Scholar] [CrossRef]
- Smith, B.C.; Sinyuk, M.; Jenkins, J.E.; Psenicka, M.W.; Williams, J.L. The Impact of Regional Astrocyte Interferon-γ Signaling during Chronic Autoimmunity: A Novel Role for the Immunoproteasome. J. Neuroinflamm. 2020, 17, 184. [Google Scholar] [CrossRef] [PubMed]
- Arellano, G.; Ottum, P.A.; Reyes, L.I.; Burgos, P.I.; Naves, R. Stage-Specific Role of Interferon-Gamma in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. Front. Immunol. 2015, 6, 492. [Google Scholar] [CrossRef] [PubMed]
- Bever, C.; Panitch, H.; Levy, H.; McFarlin, D.; Johnson, K. Gamma-interferon Induction in Patients with Chronic Progressive MS. Neurology 1991, 41, 1124. [Google Scholar] [CrossRef] [PubMed]
- Posová, H.; Horáková, D.; Čapek, V.; Uher, T.; Hrušková, Z.; Havrdová, E. Peripheral Blood Lymphocytes Immunophenotyping Predicts Disease Activity in Clinically Isolated Syndrome Patients. BMC Neurol. 2017, 17, 145. [Google Scholar] [CrossRef]
- Tintore, M.; Rovira, À.; Río, J.; Otero-Romero, S.; Arrambide, G.; Tur, C.; Comabella, M.; Nos, C.; Arévalo, M.J.; Negrotto, L. Defining High, Medium and Low Impact Prognostic Factors for Developing Multiple Sclerosis. Brain 2015, 138, 1863–1874. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]

| RRMS (n = 40) | RRMS ≤ 12 Months (n = 19) | RRMS > 12 Months (n = 21) | HC (n = 20) | |
|---|---|---|---|---|
| Age | 36.08 ± 9.59 | 33.85 ± 9.18 | 38.30 ± 9.25 | 35.82 ± 8.71 |
| Age at onset | 34.05 ± 8.85 | 32.55 ± 9.07 | 35.55 ± 8.59 | |
| Sex (F/M) | 27/13 | 14/5 | 13/8 | 13/7 |
| Disease duration (months) | 21.8 ± 23.5 | 5.3 ± 3.0 | 38.3 ± 23.5 | |
| Number of relapses in the last year | 1.38 ± 1.00 | 1.30 ± 0.73 | 1.45 ± 1.20 | |
| Total number of relapses after disease onset | 2.75 ± 0.50 | 1.40 ± 0.75 | 3.05 ± 2.06 | |
| EDSS | 2.5 ± 0.7 | 1.3 ± 1.0 | 2.5 ± 1.3 |
| Sample | IL-4 | IL-10 | IL-17A | IFN-γ | TNF-α | Inflamatory Ratio | |
|---|---|---|---|---|---|---|---|
| pg/mL | pg/mL | pg/mL | pg/mL | pg/mL | |||
| HC_D0 | Median | 6.784 | 48.390 | 109.854 | 1304.469 | 156.642 | 21.011 |
| Minimum | 1.114 | 0.401 | 5.773 | 26.637 | 5.136 | 2.532 | |
| Maximum | 15.881 | 795.472 | 519.820 | 3671.328 | 870.336 | 166.842 | |
| HC_D7_CLD− | Median | 1.185 | 0.205 | 2.890 | 15.337 | 1.970 | 17.231 |
| Minimum | 0.477 | 0.016 | 0.763 | 1.732 | 0.049 | 3.644 | |
| Maximum | 455.557 | 631.851 | 199.197 | 975.272 | 2787.694 | 62.140 | |
| HC_D7_CLD+ | Median | 2.076 | 0.190 | 5.757 | 52.742 | 3.103 | 14.651 |
| Minimum | 0.614 | 0.016 | 0.234 | 2.297 | 0.049 | 2.680 | |
| Maximum | 1150.319 | 681.029 | 557.426 | 1187.327 | 3162.460 | 63.784 | |
| HC_D14_CLD− | Median | 7.822 | 0.284 | 4.643 | 64.914 | 10.721 | 9.448 |
| Minimum | 0.587 | 0.016 | 0.155 | 2.547 | 0.095 | 1.881 | |
| Maximum | 126.552 | 9.646 | 48.838 | 799.315 | 174.443 | 80.124 | |
| HC_D14_CLD+ | Median | 15.977 | 0.246 | 4.131 | 91.888 | 12.897 | 8.353 |
| Minimum | 0.306 | 0.016 | 0.329 | 1.915 | 0.049 | 3.115 | |
| Maximum | 166.812 | 7.330 | 178.369 | 634.519 | 446.454 | 75.199 | |
| MS_D0_≤12M | Median | 4.255 | 94.056 | 64.862 | 1033.515 | 310.297 | 15.340 |
| Minimum | 0.748 | 0.096 | 2.306 | 0.850 | 18.500 | 3.521 | |
| Maximum | 12.843 | 407.926 | 554.707 | 5250.620 | 1473.799 | 97.354 | |
| MS_D0_>12M | Median | 7.135 | 43.407 | 40.240 | 580.137 | 103.813 | 16.727 |
| Minimum | 0.748 | 0.124 | 0.793 | 4.139 | 0.049 | 1.226 | |
| Maximum | 23.987 | 291.273 | 346.410 | 4490.099 | 1078.030 | 115.659 | |
| MS_D7_≤12M_CLD− | Median | 1.432 | 0.232 | 4.189 | 16.059 | 1.0367 | 20.920 |
| Minimum | 0.123 | 0.011 | 0.598 | 4.945 | 0.049 | 5.326 | |
| Maximum | 31.854 | 93.441 | 177.527 | 1186.227 | 2454.743 | 114.248 | |
| MS_D7_>12M_CLD− | Median | 1.513 | 0.206 | 5.393 | 14.616 | 2.270 | 20.926 |
| Minimum | 0.123 | 0.025 | 0.162 | 1.230 | 0.049 | 3.673 | |
| Maximum | 639.767 | 265.128 | 389.493 | 1046.216 | 3283.021 | 45.340 | |
| MS_D7_≤12M_CLD+ | Median | 1.680 | 0.193 | 5.134 | 35.0514 | 1.695 | 20.166 |
| Minimum | 0.123 | 0.007 | 0.427 | 3.054 | 0.049 | 1.488 | |
| Maximum | 48.682 | 22.019 | 107.006 | 1022.714 | 898.379 | 290.501 | |
| MS_D7_>12M_CLD+ | Median | 1.102 | 0.202 | 6.173 | 13.002 | 0.947 | 18.850 |
| Minimum | 0.123 | 0.007 | 0.069 | 1.704 | 0.049 | 2.218 | |
| Maximum | 1245.248 | 286.989 | 418.538 | 1399.948 | 1580.564 | 78.493 | |
| MS_D14_≤12M_CLD− | Median | 7.161 | 0.460 | 6.245 | 79.069 | 10.721 | 11.645 |
| Minimum | 2.089 | 0.016 | 0.234 | 4.828 | 0.303 | 0.905 | |
| Maximum | 153.917 | 2.841 | 30.329 | 1046.216 | 259.611 | 56.422 | |
| MS_D14_>12M_CLD− | Median | 14.014 | 0.561 | 5.260 | 114.436 | 19.124 | 6.215 |
| Minimum | 1.162 | 0.132 | 0.314 | 15.505 | 2.631 | 0.865 | |
| Maximum | 454.291 | 61.854 | 86.417 | 454.274 | 87.586 | 107.052 | |
| MS_D14_≤12M_CLD+ | Median | 8.673 | 0.462 | 9.004 | 93.323 | 13.166 | 8.154 |
| Minimum | 0.801 | 0.058 | 0.046 | 8.278 | 0.095 | 0.751 | |
| Maximum | 210.267 | 1.470 | 34.125 | 852.127 | 116.855 | 56.842 | |
| MS_D14_>12M_CLD+ | Median | 15.396 | 0.57278 | 9.21902 | 76.225 | 17.704 | 5.434 |
| Minimum | 0.801 | 0.073 | 0.046 | 0.439 | 0.049 | 0.611 | |
| Maximum | 86.561 | 2.867 | 173.672 | 824.659 | 203.954 | 74.150 | |
| Dunn’s Multiple Comparison Tests | Mean Rank Diff. | Significant? | Summary | Adjusted p |
|---|---|---|---|---|
| HC_D7_CLD− vs. HC_D14_CLD− | −94.00 | Yes | * | 0.0275 |
| HC_D7_CLD+ vs. HC_D14_CLD+ | −73.53 | No | ns | 0.3308 |
| MS_D7_CLD− vs. MS_D14_CLD− | −98.26 | Yes | **** | <0.0001 |
| MS_D7_CLD+ vs. MS_D14_CLD+ | −100.10 | Yes | **** | <0.0001 |
| Dunn’s Multiple Comparison Tests | Mean Rank diff. | Significant? | Summary | Adjusted p |
|---|---|---|---|---|
| HC_D7_CLD− vs. HC_D14_CLD− | −73.73 | No | ns | 0.3233 |
| HC_D7_CLD+ vs. HC_D14_CLD+ | −41.93 | No | ns | >0.9999 |
| MS_D7_CLD− vs. MS_D14_CLD− | −73.06 | Yes | ** | 0.0074 |
| MS_D7_CLD+ vs. MS_D14_CLD+ | −63.94 | Yes | * | 0.0440 |
| Dunn’s Multiple Comparison Tests | Mean Rank diff. | Significant? | Summary | Adjusted p |
|---|---|---|---|---|
| MS_D0 vs. MS_D7_CLD− | 44.23 | Yes | **** | <0.0001 |
| MS_D0 vs. MS_D7_CLD+ | 46.35 | Yes | **** | <0.0001 |
| MS_D0 vs. MS_D14_CLD− | 22.45 | No | ns | 0.1434 |
| MS_D0 vs. MS_D14_CLD+ | 26.98 | Yes | * | 0.0326 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balasa, R.; Maier, S.; Hutanu, A.; Voidazan, S.; Andone, S.; Oiaga, M.; Manu, D. Cytokine Secretion Dynamics of Isolated PBMC after Cladribine Exposure in RRMS Patients. Int. J. Mol. Sci. 2022, 23, 10262. https://doi.org/10.3390/ijms231810262
Balasa R, Maier S, Hutanu A, Voidazan S, Andone S, Oiaga M, Manu D. Cytokine Secretion Dynamics of Isolated PBMC after Cladribine Exposure in RRMS Patients. International Journal of Molecular Sciences. 2022; 23(18):10262. https://doi.org/10.3390/ijms231810262
Chicago/Turabian StyleBalasa, Rodica, Smaranda Maier, Adina Hutanu, Septimiu Voidazan, Sebastian Andone, Mirela Oiaga, and Doina Manu. 2022. "Cytokine Secretion Dynamics of Isolated PBMC after Cladribine Exposure in RRMS Patients" International Journal of Molecular Sciences 23, no. 18: 10262. https://doi.org/10.3390/ijms231810262
APA StyleBalasa, R., Maier, S., Hutanu, A., Voidazan, S., Andone, S., Oiaga, M., & Manu, D. (2022). Cytokine Secretion Dynamics of Isolated PBMC after Cladribine Exposure in RRMS Patients. International Journal of Molecular Sciences, 23(18), 10262. https://doi.org/10.3390/ijms231810262





