Orlistat Mitigates Oxidative Stress-Linked Myocardial Damage via NF-κβ- and Caspase-Dependent Activities in Obese Rats
Abstract
:1. Introduction
2. Results
2.1. Orlistat Decreased Anthropometrical Measurement in Obese Rats
2.2. Orlistat Decreased Lipid Components and Cardiac Enzymes in Obese Rats
2.3. Orlistat Reduced Cardiac Oxidative Stress Markers in Obese Rats
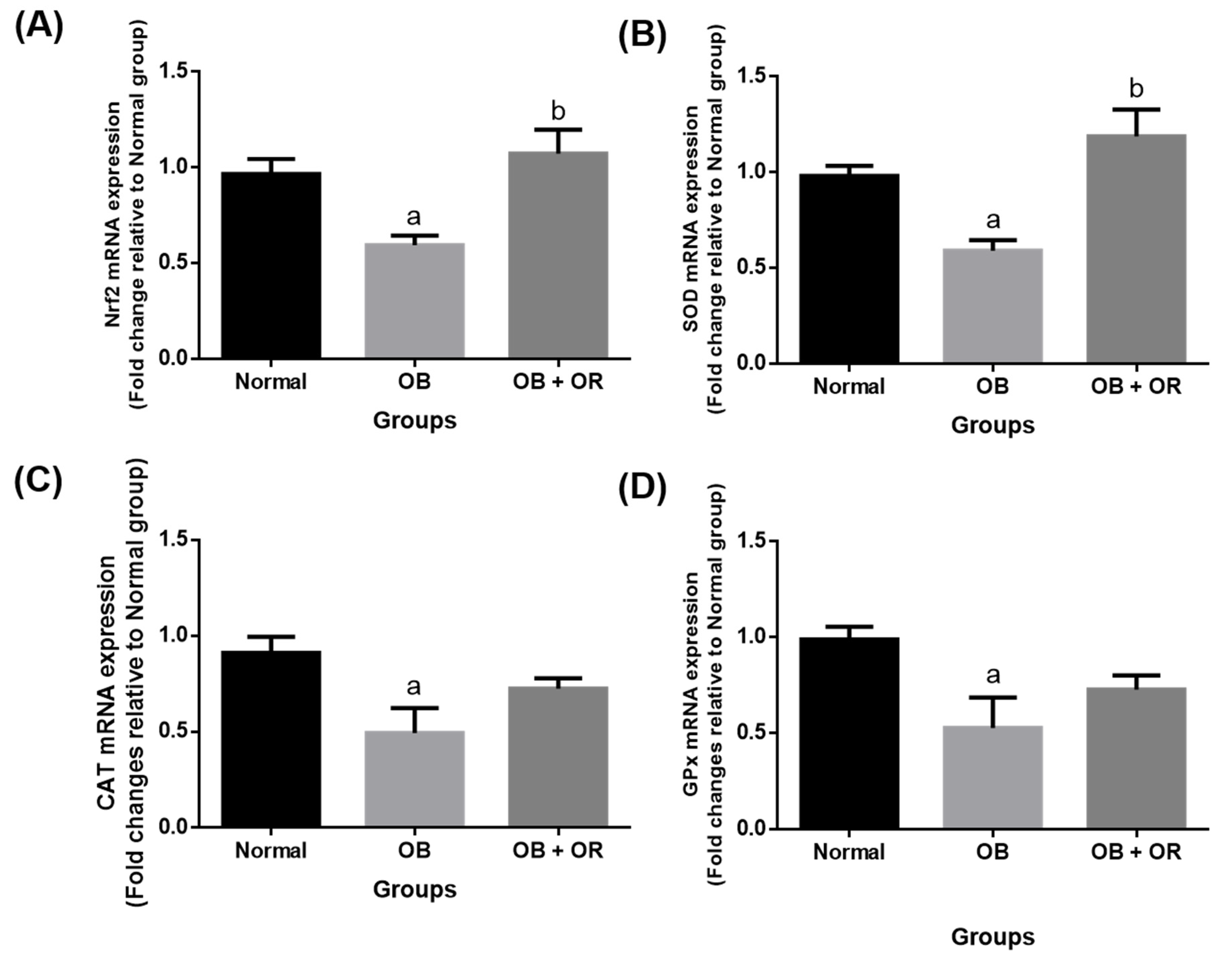
2.4. Orlistat Increased mRNA Levels of Cardiac Antioxidant Enzymes in Obese Rats
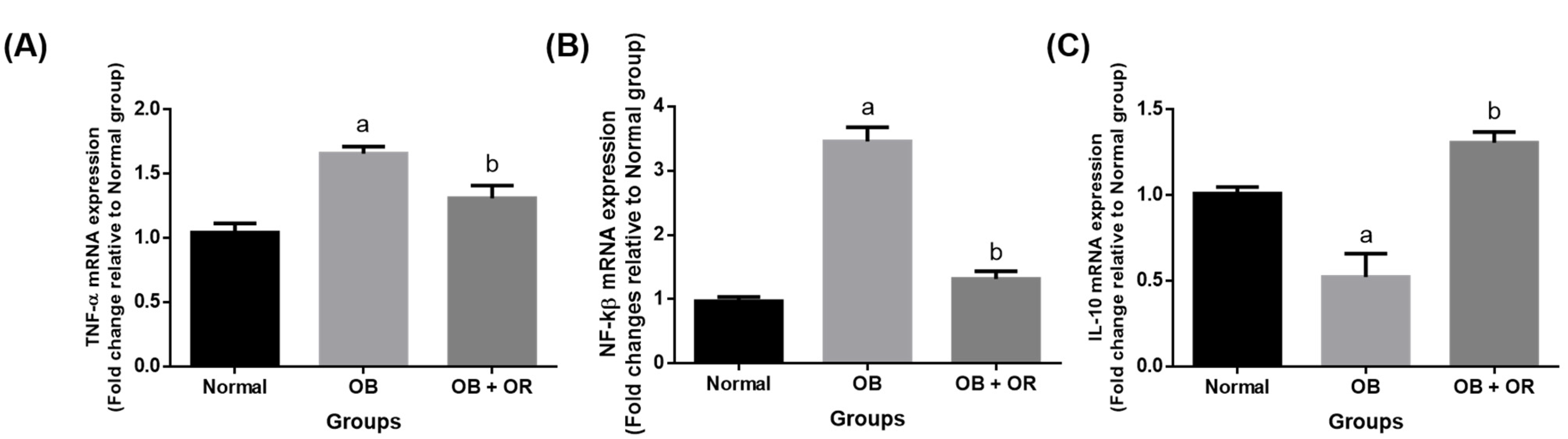
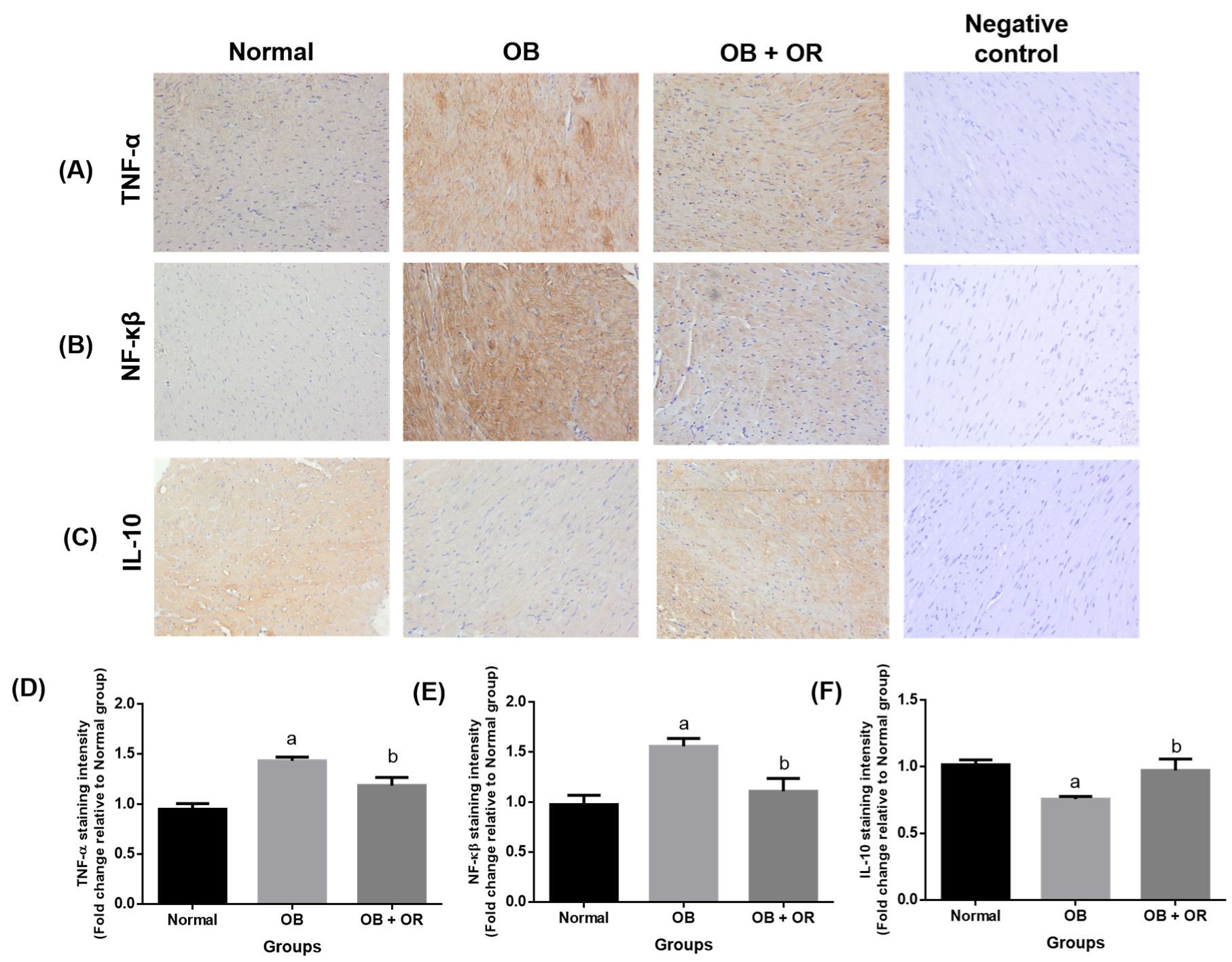
2.5. Orlistat Improved mRNA and Protein Levels of Cardiac Inflammation Markers in Obese Rats
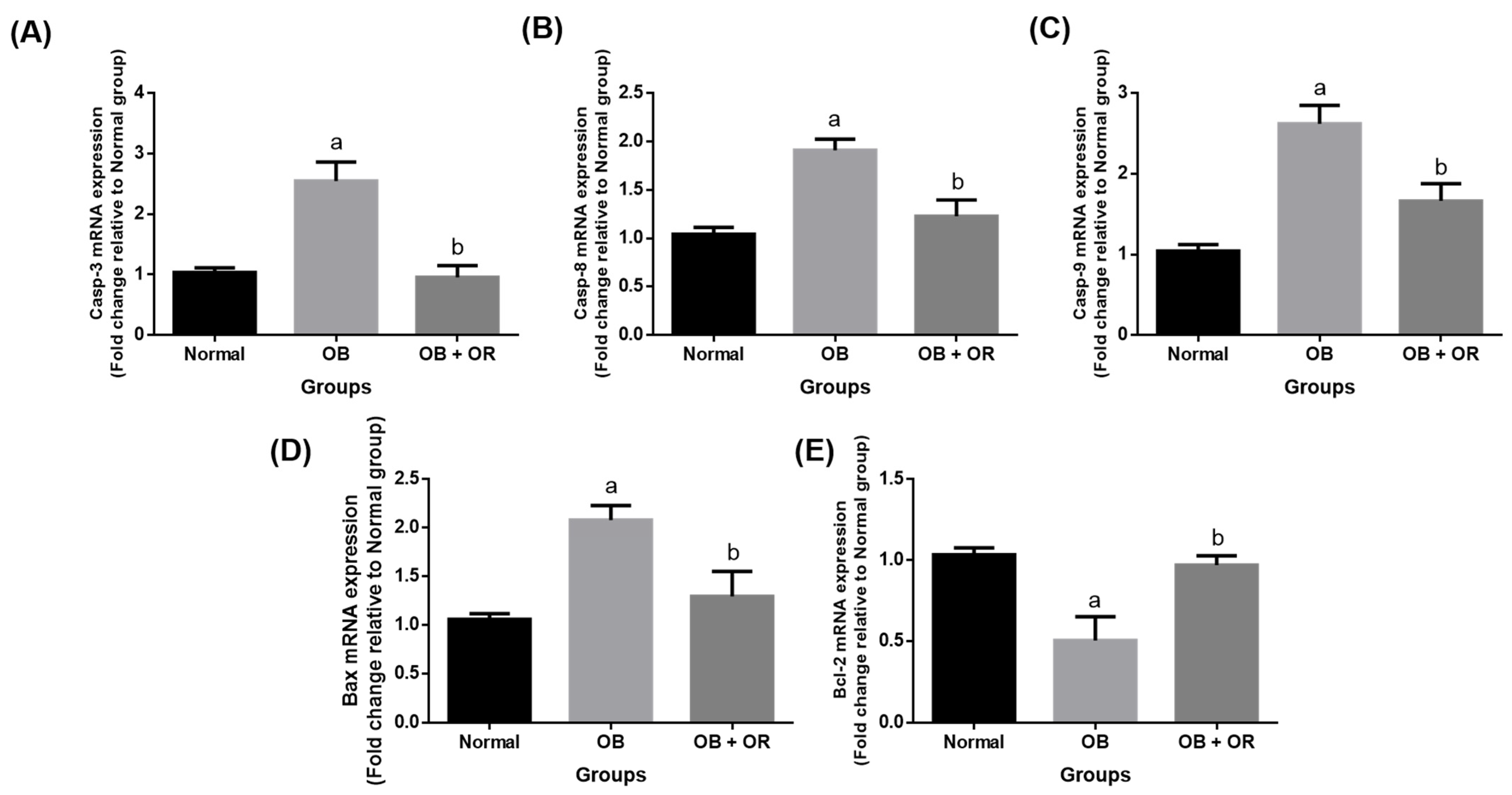
2.6. Orlistat Improved mRNA and Protein Levels of Cardiac Apoptotic Markers in Obese Rats
2.7. Effect of Orlistat on Cardiac Histology
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Orlistat Preparation
4.3. Animals and Diet
4.4. Animal Experimental Design
4.5. Anthropometrical Measurement
4.6. Sample Collection
4.7. Determination of Total Cholesterol and Triglyceride
4.8. Determination of Cardiac Enzymes
4.9. Determination of Cardiac Oxidant-Antioxidant Statuses
4.10. Immunohistochemical Analysis
4.11. Analysis of Gene Expression
4.11.1. RNA Extraction, Purity and Integrity Assessment
4.11.2. Real-Time Quantitative PCR
4.12. Cardiac Histology
4.13. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinlen, D.; Cody, D.; O’Shea, D. Complications of obesity. QJM Int. J. Med. 2018, 111, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.L.; Shibu, M.A.; Lii, C.K.; Viswanadha, V.P.; Lin, Y.L.; Lai, C.H.; Chen, Y.F.; Lin, K.H.; Kuo, W.W.; Huang, C.Y. Andrographis paniculata extract attenuates pathological cardiac hypertrophy and apoptosis in high-fat diet fed mice. J. Ethnopharmacol. 2016, 192, 170–177. [Google Scholar] [CrossRef]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D.; Faria, A.; Calhau, C. High-fat diet-induced obesity Rat model: A comparison between Wistar and Sprague-Dawley Rat. Adipocyte 2016, 5, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Jia, Z.; Nallasamy, P.; Liu, D.; Shah, H.; Li, J.Z.; Chitrakar, R.; Si, H.; McCormick, J.; Zhu, H.; Zhen, W.; et al. Luteolin protects against vascular inflammation in mice and TNF-alpha-induced monocyte adhesion to endothelial cells via suppressing IΚBα/NF-κB signaling pathway. J. Nutr. Biochem. 2015, 26, 293–302. [Google Scholar] [CrossRef]
- Li, W.; Fang, Q.; Zhong, P.; Chen, L.; Wang, L.; Zhang, Y.; Wang, J.; Li, X.; Wang, Y.; Wang, J.; et al. EGFR inhibition blocks palmitic acidinduced inflammation in cardiomyocytes and prevents hyperlipidemia-induced cardiac injury in mice. Sci. Rep. 2016, 6, 24580. [Google Scholar] [CrossRef]
- Bishopric, N.H.; Andreka, P.; Slepak, T.; Webster, K.A. Molecular mechanisms of apoptosis in the cardiac myocyte. Curr. Opin. Pharmacol. 2001, 1, 141–150. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I. Effect of orlistat alone or in combination with Garcinia cambogia on visceral adiposity index in obese patients. J. Intercult. Ethnopharmacol. 2016, 5, 408–414. [Google Scholar] [CrossRef]
- Kujawska-Łuczak, M.; Szulinska, M.; Skrypnik, D.; Musialik, K.; Swora-Cwynar, E.; Kregielska-Narozna, M.; Markuszewski, L.; Grzymisławski, M.; Bogdanski, P. The influence of orlistat, metformin and diet on serum levels of insulin-like growth factor-1 in obese women with and without insulin resistance. J. Pharm. Pharmacol. 2018, 69, 1–8. [Google Scholar]
- Othman, Z.A.; Noordin, L.; Omar, N.; Mohd. Yusof, N.A.; Mohamaed, M. Protective Effects of Orlistat on Lipid Profile, Cardiac Oxidative Stress Biomarkers and Histology in High-fat Diet-induced Obese Rats. IIUM Med. J. Malays. 2019, 18, 1–6. [Google Scholar]
- Bansal, A.B.; Al Khalili, Y. Orlistat. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Othman, Z.A.; Zakaria, Z.; Suleiman, J.B.; Wan Ghazali, W.S.; Mohamed, M. Anti-atherogenic effects of orlistat on obesity-induced vascular oxidative stress rat model. Antioxidants 2021, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Yalim, Z.; Yalim, S.A. The effect of orlistat treatment on cardiovascular novel inflammatory biomarkers. Med. Sci. 2020, 9, 427–432. [Google Scholar] [CrossRef]
- Azadbakht, L.; Jamali-Gojani, Z.; Heidari-Beni, M. Anti-obesity drug orlistat (xenical) is a novel antitumor medication. Shiraz E-Med. J. 2015, 16, e26242. [Google Scholar] [CrossRef]
- Galaly, S.R.; Hozayen, W.G.; Amin, K.A.; Ramadan, S.M. Effects of Orlistat and herbal mixture extract on brain, testes functions and oxidative stress biomarkers in a rat model of high fat diet. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 93–105. [Google Scholar] [CrossRef]
- Suleiman, J.B.; Nna, V.U.; Zakaria, Z.; Othman, Z.A.; Abu Bakar, A.; Mohamed, M. Obesity-induced testicular oxidative stress, inflammation and apoptosis: Protective and therapeutic effects of orlistat. Reprod. Toxicol. 2020, 95, 113–122. [Google Scholar] [CrossRef]
- Zakaria, Z.; Othman, Z.A.; Bagi Suleiman, J.; Che Jalil, N.A.; Wan Ghazali, W.S.; Mohamed, M. Protective and therapeutic effects of orlistat on metabolic syndrome and oxidative stress in high-fat diet-induced metabolic dysfunction-associated fatty liver disease (MAFLD) in rats: Role on Nrf2 activation. Vet. Sci. 2021, 8, 274. [Google Scholar] [CrossRef]
- Gomaa, A.A.; El-Sers, D.A.; Al-Zokeim, N.I.; Gomaa, M.A. Amelioration of experimental metabolic syndrome induced in rats by orlistat and Corchorus olitorius leaf extract; role of adipo/cytokines. J. Pharm. Pharmacol. 2018, 71, 281–291. [Google Scholar] [CrossRef]
- Aydin, B.; Onbasi, K. Lipase inhibitor orlistat: An old but still effective weapon. Med. Sci. 2021, 10, 1406–1411. [Google Scholar] [CrossRef]
- Díaz-Rúa, R.; Keijer, J.; Palou, A.; van Schothorst, E.M.; Oliver, P. Longterm intake of a high-protein diet increases liver triacylglycerol deposition pathways and hepatic signs of injury in rats. J. Nutr. Biochem. 2017, 46, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Chtourou, Y.; Slima, A.B.; Makni, M.; Gdoura, R.; Fetoui, H. Naringenin protects cardiac hypercholesterolemia-induced oxidative stress and subsequent necroptosis in rats. Pharmacol. Rep. 2015, 67, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Lopaschuk, G.D. Cardiac fatty acid oxidation in heart failure associated with obesity and diabetes. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2016, 1861, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Glatz, J.F.; Luiken, J.J.; Bonen, A. Membrane fatty acid transporters as regulators of lipid metabolism: Implications for metabolic disease. Physiol. Rev. 2010, 10, 367–417. [Google Scholar] [CrossRef]
- Wu, G.; Li, H.; Zhou, M.; Fang, Q.; Bao, Y.; Xu, A.; Jia, W. Mechanism and clinical evidence of lipocalin-2 and adipocyte fatty acid-binding protein linking obesity and atherosclerosis. Diabetes Metab. Res. Rev. 2014, 30, 447–456. [Google Scholar] [CrossRef]
- Liao, P.H.; Kuo, W.W.; Kuo, C.H.; Yeh, Y.L.; Shen, C.Y.; Chen, Y.H.; Chen, R.J.; Padma, V.V.; Chen, Y.H.; Huang, C.Y. Lactobacillus reuteri GMNL263 reduces hyperlipidaemia and the heart failure process in high-calorie diet-fed induced heart dysfunction in rats. J. Funct. Foods 2016, 20, 226–235. [Google Scholar] [CrossRef]
- Feron, O.; Dessy, C.; Moniotte, S.; Desager, J.; Balligand, J. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J. Clin. Investig. 1999, 103, 897–905. [Google Scholar] [CrossRef]
- Francisqueti, F.V.; Chiaverini, L.C.T.; Santos, K.C.D.; Minatel, I.O.; Ronchi, C.B.; Ferron, A.J.T.; Ferreira, A.L.A.; Corrêa, C.R. The role of oxidative stress on the pathophysiology of metabolic syndrome. Rev. Assoc. Med. Bras. 2017, 63, 85–91. [Google Scholar] [CrossRef]
- Amen, O.M.; Sarker, S.D.; Ghildyal, R.; Arya, A. Endoplasmic reticulum stress activates unfolded protein response signaling and mediates inflammation, obesity, and cardiac dysfunction: Therapeutic and molecular approach. Front. Pharmacol. 2019, 10, 977. [Google Scholar] [CrossRef]
- Lavie, C.J.; Sharma, A.; Alpert, M.A.; De Schutter, A.; Lopez-Jimenez, F.; Milani, R.V.; Ventura, H.O. Update on obesity and obesity paradox in heart failure. Prog. Cardiovasc. Dis. 2016, 58, 393–400. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, J.; Li, D.; Li, P.; Zhou, X.; Li, Y.; He, Z.; Qin, L.; Liang, L.; Luo, X. Interleukin-10 inhibits interleukin-1β production and inflammasome activation of microglia in epileptic seizures. J. Neuroinflamm. 2019, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Eid, B.G.; El-Shitany, N.A. Captopril downregulates expression of Bax/cytochrome C/caspase-3 apoptotic pathway, reduces inflammation, and oxidative stress in cisplatin-induced acute hepatic injury. Biomed. Pharmacother. 2021, 139, 111670. [Google Scholar] [CrossRef]
- Lee, S.D.; Tzang, B.S.; Kuo, W.W.; Lin, Y.M.; Yang, A.L.; Chen, S.H.; Tsai, F.J.; Wu, F.L.; Lu, M.C.; Huang, C.Y. Cardiac Fas receptor-dependent apoptotic pathway in obese Zucker rats. Obesity 2007, 15, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, L.L.; Bernardis, L.L. Effect of dorsomedial hypothalamic nuclei knife cuts on ingestive behavior. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1999, 276, R1772–R1779. [Google Scholar] [CrossRef]
- Angeloco, L.R.N.; Deminice, R.; Leme, I.d.A.; Lataro, R.C.; Jordao, A.A. Bioelectrical impedance analysis and anthropometry for the determination of body composition in rats: Effects of high-fat and high-sucrose diets. Rev. Nutr. 2012, 25, 331–339. [Google Scholar] [CrossRef]
- Chatterjee, P.; Cuzzocrea, S.; Brown, P.; Zacharowski, K.; Stewart, K.; Thiemermann, C.; Mota-Filipe, H. Tempol, a membrane permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000, 58, 658–673. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.; Lyras, L.; Halliwell, B. Measurement of protein carbonyls in human brain tissue. Meth. Enzymol. 1999, 300, 145–156. [Google Scholar]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Dogan, P.; Tanrikulu, G.; Soyuer, U.; Kose, K. Oxidative enzymes of polymorphonuclear leucocytes and plasma fibrinogen, ceruloplasmin, and copper levels in Behcet’s disease. Clin. Biochem. 1994, 27, 413–418. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Glutathione reductase. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1985; pp. 484–490. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Ali, S.S.; Mohamed, S.F.A.; Rozalei, N.H.; Boon, Y.W.; Zainalabidin, S. Anti-fibrotic actions of roselle extract in rat model of myocardial infarction. Cardiovasc. Toxicol. 2019, 19, 72–81. [Google Scholar] [CrossRef] [PubMed]


| Anthropometrical Measurements | Normal | OB | OB + OR |
|---|---|---|---|
| Initial weight (g) | 216.3 (7.16) | 228.5 (6.91) | 228.4 (9.05) |
| Final weight (g) | 335.1 (8.85) | 466.5 (11.66) a | 407.0 (12.63) a,b |
| Weight changes (g) | 106.7 (5.61) | 228.6 (13.46) a | 176.2 (9.97) a,b |
| Lee Obesity index | 303.3 (1.79) | 327.7 (2.50) a | 319.2 (1.72) a,b |
| BMI (g/cm2) | 0.67 (0.02) | 0.86 (0.03) a | 0.77 (0.02) a,b |
| Parameters | Normal | OB | OB + OR |
|---|---|---|---|
| Serum TC (mmol/L) | 1.54 (0.06) | 2.81 (0.31) a | 2.00 (0.09) b |
| Cardiac TC (ng/mL) | 174.8 (13.65) | 265.4 (12.23) a | 217.7 (8.14) b |
| Serum TG (mmol/L) | 0.49 (0.03) | 0.93 (0.03) a | 0.70 (0.05) a,b |
| Cardiac TG (ng/mL) | 233.00 (7.49) | 313.9 (15.93) a | 256.8 (7.99) b |
| Serum CKMB (ng/mL) | 0.47 (0.03) | 0.67 (0.06) a | 0.52 (0.02) b |
| Serum LDH (ng/mL) | 0.12 (0.00) | 0.20 (0.01) a | 0.14 (0.00) b |
| Oxidant-Antioxidant Statuses | Normal | OB | OB + OR |
|---|---|---|---|
| TBARs (nmol/mg protein) | 1.74 (0.14) | 19.47 (0.56) a | 8.03 (1.03) a,b |
| PCO (nmol/mg protein) | 1.02 (0.18) | 2.31 (0.31) a | 1.18 (0.29) b |
| SOD (U/mg protein) | 100.7 (4.85) | 70.29 (2.74) a | 119.2 (5.88) a,b |
| CAT (U/mg protein) | 81.05 (6.55) | 44.81 (4.13) a | 57.79 (3.31) a |
| GPx (U/mg protein) | 1.96 (0.18) | 0.74 (0.14) a | 1.15 (0.15) a |
| GR (U/mg protein) | 0.70 (0.07) | 0.21 (0.05) a | 1.00 (0.15) b |
| GST (U/mg protein) | 9.52 (0.71) | 6.00 (0.66) a | 10.44 (0.98) b |
| Primary | Reference Number | Concentration | Incubation Temperature |
|---|---|---|---|
| TNF-α | PAA133Ra01 | 1:120 | RT |
| NF-κβ | PAB824Ra01 | 1:100 | 4 °C |
| IL-10 | PAA056Ra01 | 1:100 | 4 °C |
| Casp-3 | PAA626Ra01 | 1:150 | 4 °C |
| Bax | PAB343Ra01 | 1:100 | 4 °C |
| Bcl-2 | PAA778Ra01 | 1:50 | 4 °C |
| Gene | Accession Number | Forward (5′-3′) | Reverse (3′-5′) | Size (bp) |
|---|---|---|---|---|
| SOD | X05634.1 | CGAGCATGGGTTCCATGTC | CTGGACCGCCATGTTTCTTAG | 101 |
| CAT | NM_012520.2 | ACAACTCCCAGAAGCCTAAGAATG | GCTTTTCCCTTGGCAGCTATG | 76 |
| GPx | NM_030826.4 | GGAGAATGGCAAGAATGAAGA | CCGCAGGAAGGTAAAGAG | 139 |
| Nrf2 | NM_031789.1 | CAGGTTGCCCACATTCCCAA | ATATCCAGGGCAAGCGACTCAT | 110 |
| TNF-α | NM_012675.3 | ACTGAACTTCGGGGTGATCG | GCTTGGTGGTTTGCTACGAC | 153 |
| IL-10 | NM_012854.2 | TTGAACCACCCGGCATCTAC | CCAAGGAGTTGCTCCCGTTA | 91 |
| NF-κβ | NM_199267.2 | CGCGGGGACTATGACTTGAA | AGTTCCGGTTTACTCGGCAG | 163 |
| Casp-3 | NM_012922 | AAGATACCAGTGGAGGCCGACTTC | GGGAGAAGGACTCAAATTCCGTGG | 199 |
| Casp-8 | NM_022277.1 | GTTCTCTCAGTTGCCTTTCTCC | GGCCAGTCCGCCAAAGTTTA | 90 |
| Casp-9 | NM_031632 | CTGAGCCAGATGCTGTCCCATA | CCAAGGTCTCGATGTACCAGGAA | 168 |
| Bax | U49729.1 | CGCGTGGTTGCCCTCTTCTACTTT | CAAGCAGCCGCTCACGGAGGA | 124 |
| Bcl-2 | NM_016993.1 | ATCGCTCTGTGGATGACTGAGTAC | AGAGACAGCCAGGAGAAATCAAAC | 134 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, Z.A.; Zakaria, Z.; Suleiman, J.B.; Mustaffa, K.M.F.; Jalil, N.A.C.; Wan Ghazali, W.S.; Zulkipli, N.N.; Mohamed, M. Orlistat Mitigates Oxidative Stress-Linked Myocardial Damage via NF-κβ- and Caspase-Dependent Activities in Obese Rats. Int. J. Mol. Sci. 2022, 23, 10266. https://doi.org/10.3390/ijms231810266
Othman ZA, Zakaria Z, Suleiman JB, Mustaffa KMF, Jalil NAC, Wan Ghazali WS, Zulkipli NN, Mohamed M. Orlistat Mitigates Oxidative Stress-Linked Myocardial Damage via NF-κβ- and Caspase-Dependent Activities in Obese Rats. International Journal of Molecular Sciences. 2022; 23(18):10266. https://doi.org/10.3390/ijms231810266
Chicago/Turabian StyleOthman, Zaidatul Akmal, Zaida Zakaria, Joseph Bagi Suleiman, Khairul Mohd Fadzli Mustaffa, Nur Asyilla Che Jalil, Wan Syaheedah Wan Ghazali, Ninie Nadia Zulkipli, and Mahaneem Mohamed. 2022. "Orlistat Mitigates Oxidative Stress-Linked Myocardial Damage via NF-κβ- and Caspase-Dependent Activities in Obese Rats" International Journal of Molecular Sciences 23, no. 18: 10266. https://doi.org/10.3390/ijms231810266
APA StyleOthman, Z. A., Zakaria, Z., Suleiman, J. B., Mustaffa, K. M. F., Jalil, N. A. C., Wan Ghazali, W. S., Zulkipli, N. N., & Mohamed, M. (2022). Orlistat Mitigates Oxidative Stress-Linked Myocardial Damage via NF-κβ- and Caspase-Dependent Activities in Obese Rats. International Journal of Molecular Sciences, 23(18), 10266. https://doi.org/10.3390/ijms231810266









