Analysis of Microorganism Colonization, Biofilm Production, and Antibacterial Susceptibility in Recurrent Tonsillitis and Peritonsillar Abscess Patients
Abstract
:1. Introduction
2. Results
2.1. Patient Data
2.2. Diversity of Isolated Microorganisms
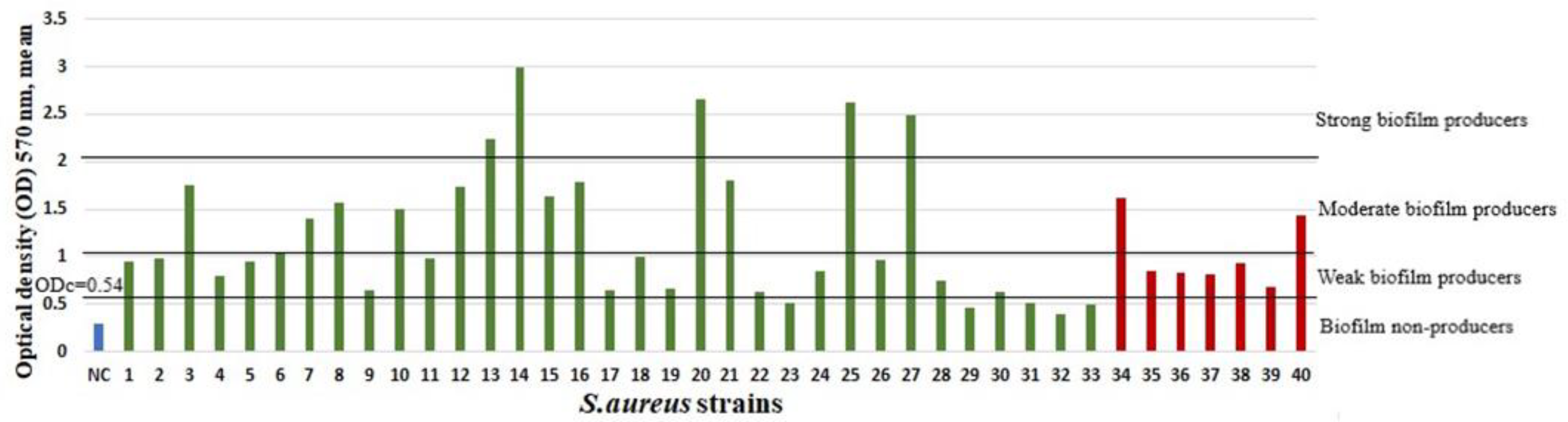
2.3. Biofilm Growth and Associations
2.4. Antibacterial Susceptibility
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Isolation of Microorganisms and Microbiological Investigation
4.3. Biofilm Growth Using Cristal Violet Assay
4.4. Biofilm Calculation
4.5. Statistical Analysis
5. Conclusions
5.1. Conclusions
5.2. Strengths and Limitations
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buname, G.; Kiwale, G.A.; Mushi, M.F.; Silago, V.; Rambau, P.; Mshana, S.E. Bacteria Patterns on Tonsillar Surface and Tonsillar Core Tissue among Patients Scheduled for Tonsillectomy at Bugando Medical Centre, Mwanza, Tanzania. Pathogens 2021, 10, 1560. [Google Scholar] [CrossRef] [PubMed]
- Zautner, A.E.; Krause, M.; Stropahl, G.; Holtfreter, S.; Frickmann, H.; Maletzki, C.; Kreikemeyer, B.; Pau, W.H.; Podbielski, A. Intracellular Persisting Staphylococcus aureus Is the Major Pathogen in Recurrent Tonsillitis. PLoS ONE 2010, 5, e9452. [Google Scholar] [CrossRef] [PubMed]
- Windfuhr, J.P.; Toepfner, N.; Steffen, G.; Waldfahrer, F.; Berner, R. Clinical practice guideline: Tonsillitis I. Diagnostics and nonsurgical management. Eur. Arch. Otorhinolaryngol. 2016, 273, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Glasziou, P.P.; Chong, L.Y.; Venekamp, R.P. Tonsillectomy or adenotonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitis. Cochrane Database Syst Rev. 2014, 2014, CD001802. [Google Scholar] [CrossRef] [PubMed]
- Katkowska, M.; Garbacz, K.; Stromkowski, J. Staphylococcus aureus isolated from tonsillectomized adult patients with recurrent tonsillitis. APMIS 2017, 125, 46–51. [Google Scholar] [CrossRef]
- Paradise, J.L.; Bluestone, C.D.; Bachman, R.Z.; Colborn, D.K.; Bernard, B.S.; Taylor, F.H.; Rogers, K.D.; Schwarzbach, R.H.; Stool, S.E.; Friday, G.A. Efficacy of Tonsillectomy for Recurrent Throat Infection in Severely Affected Children: Results of Parallel Randomized and Nonrandomized Clinical Trials. N. Engl. J. Med. 1984, 310, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Slouka, D.; Hanakova, J.; Kostlivy, T.; Skopek, P.; Kubec, V.; Babuska, V.; Pecen, L.; Topolcan, O.; Kucera, R. Epidemiological and Microbiological Aspects of the Peritonsillar Abscess. Int. J. Environ. Res. Public Health 2020, 17, 4020. [Google Scholar] [CrossRef]
- Windfuhr, J.P.; Toepfner, N.; Steffen, G.; Waldfahrer, F.; Berner, R. Clinical practice guideline: Tonsillitis II. Surgical management. Eur. Arch. Otorhinolaryngol. 2016, 273, 989–1009. [Google Scholar] [CrossRef]
- Pichichero, M.; Casey, J. Systematic review of factors contributing to penicillin treatment failure in Streptococcus pyogenes pharyngitis. Otolaryngol. Head Neck Surg. 2007, 137, 851–857. [Google Scholar] [CrossRef]
- Abu Bakar, M.; McKimm, J.; Haque, S.Z.; Majumder, A.A.; Haque, M. Chronic tonsillitis and biofilms: A brief overview of treatment modalities. J. Inflamm. Res. 2018, 11, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, A.; Kankaanpää, H.; Silén, S.; Meri, S.; Haapaniemi, A.; Ylikoski, J.; Mäkitie, A. Tonsillar surface swab bacterial culture results differ from those of the tonsillar core in recurrent tonsillitis. Laryngoscope 2020, 130, E791–E794. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.H.; Pfaller, M.A.; Carroll, K.C. Manual of Clinical Microbiology; ASM Press: New York, NY, USA, 2015; p. 2892. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Vaikjärv, R.; Kasenõmm, P.; Jaanimäe, L.; Kivisild, A.; Rööp, T.; Sepp, E.; Mändar, R. Microbiology of peritonsillar abscess in the South Estonian population. Microb. Ecol. Health Dis. 2016, 27, 27787. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.N.; Ayub, Z.; Ahmed, A. Tonsillar Surface Micro Flora: Does it Truly Represent Pathological Tonsillar Flora? J. Coll. Physicians Surg.—Pak. JCPSP 2017, 27, 23–25. [Google Scholar] [PubMed]
- Khadilkar, M.N.; Ankle, N.R. Anaerobic Bacteriological Microbiota in Surface and Core of Tonsils in Chronic Tonsillitis. J. Clin. Diagn. Res. JCDR. 2016, 10, MC01–MC03. [Google Scholar] [CrossRef]
- Klagisa, R.; Kroica, J.; Kise, L.S. aureus and K. pneumoniae on the Surface and within Core of Tonsils in Adults with Recurrent Tonsillitis. Medicina 2021, 57, 1002. [Google Scholar] [CrossRef]
- Stjernquist-Desatnik, A.; Holst, E. Tonsillar Microbial Flora: Comparison of Recurrent Tonsillitis and Normal Tonsils. Acta Otolaryngol. 1999, 119, 102–106. [Google Scholar] [CrossRef]
- Van Staaij, B.K.; Van Den Akker, E.H.; De Haas Van Dorsser, E.H.M.; Fleer, A.; Hoes, A.W.; Schilder, A.G.M. Does the Tonsillar Surface Flora Differ in Children with and without Tonsillar Disease? Acta Otolaryngol. 2003, 123, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Skoulakis, C.; Tigiroglou, E.; Gkarelis, K.; Klapsa, D.; Damani, A.; Papadakis, C.; Petinaki, E. Level of Streptococcus pyogenes in patients with recurrent tonsillitis and tonsillar hypertrophy. Scand. J. Infect. Dis. 2008, 40, 899–903. [Google Scholar] [CrossRef]
- Jeong, J.H.; Lee, D.W.; Ryu, R.A.; Lee, Y.S.; Lee, S.H.; Kang, J.O.; Tae, K. Bacteriologic Comparison of Tonsil Core in Recurrent Tonsillitis and Tonsillar Hypertrophy. Laryngoscope 2007, 117, 2146–2151. [Google Scholar] [CrossRef]
- Klug, T.E. Peritonsillar abscess: Clinical aspects of microbiology, risk factors, and the association with parapharyngeal abscess. Dan. Med. J. 2017, 64, B5333. [Google Scholar]
- Ali, S.A.; Kovatch, K.J.; Smith, J.; Bellile, E.L.; Hanks, J.E.; Hoff, P.T. Implication of Fusobacterium necrophorum in recurrence of peritonsillar abscess: Fusobacterium necrophorum in Peritonsillar Abscess. Laryngoscope 2019, 129, 1567–1571. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Lee, Y.C.; Shin, S.Y.; Eun, Y.G. Risk factors for recurrence of peritonsillar abscess. J. Laryngol. Otol. 2014, 128, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.W.; Liu, Y.H.; Su, H.H. Bacteriology of peritonsillar abscess: The changing trend and predisposing factors. Braz. J. Otorhinolaryngol. 2018, 84, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Brook, I.; Foote, P.A. Isolation of methicillin resistant Staphylococcus aureus from the surface and core of tonsils in children. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 2099–2102. [Google Scholar] [CrossRef]
- Woo, J.H.; Kim, S.T.; Kang, I.G.; Lee, J.H.; Cha, H.E.; Kim, D.Y. Comparison of tonsillar biofilms between patients with recurrent tonsillitis and a control group. Acta Otolaryngol. 2012, 132, 1115–1120. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, G.; Chao, X.; Xie, L.; Wang, H. The Characteristic of Virulence, Biofilm and Antibiotic Resistance of Klebsiella pneumoniae. Int. J. Environ. Res Public Health 2020, 17, 6278. [Google Scholar] [CrossRef]
- Alasil, S.M.; Omar, R.; Ismail, S.; Yusof, M.Y.; Dhabaan, G.N.; Abdulla, M.A. Evidence of Bacterial Biofilms among Infected and Hypertrophied Tonsils in Correlation with the Microbiology, Histopathology, and Clinical Symptoms of Tonsillar Diseases. Int. J. Otolaryngol. 2013, 2013, 408238. [Google Scholar] [CrossRef]
- Babaiwa, U.F.; Onyeagwara, N.C.; Akerele, J.O. Bacterial tonsillar microbiota and antibiogram in recurrent tonsillitis. Biomed. Res. 2013, 24, 298–302. [Google Scholar]
- Syryło, A.; Wojdas, A.; Jurkiewicz, D. Flora bakteryjna występująca na powierzchni i w miąższu migdałków w przewlekłym zapaleniu migdałków podniebiennych. Otolaryngol. Pol. 2007, 61, 598–601. [Google Scholar] [CrossRef]
- Lindroos, R. Bacteriology of the tonsil core in recurrent tonsillitis and tonsillar hyperplasia--a short review. Acta Oto-Laryngol. Suppl. 2000, 543, 206–208. [Google Scholar] [CrossRef]
- Wikstén, J.; Kaltiainen, E.; Pitkäranta, A.; Blomgren, K. Renewal of peritonsillar abscess: Impact of the bacterial species of the infection and clinical features of the patient-A prospective comparative aetiological study. Clin. Otolaryngol. 2017, 42, 1358–1362. [Google Scholar] [CrossRef]
- Jokinen, K.; Pajarre, S.; Palva, A.; Sipilä, P. Mycotic flora in tonsils and adenoids: A microbiolgical and histological eveluation. J. Laryngol. Otol. 1976, 90, 945–952. [Google Scholar] [CrossRef]
- Fukui, Y.; Aoki, K.; Ishii, Y.; Tateda, K. The palatine tonsil bacteriome, but not the mycobiome, is altered in HIV infection. BMC Microbiol. 2018, 18, 127. [Google Scholar] [CrossRef]
- Hahn, J.; Barth, I.; Wigand, M.C.; Mayer, B.; Hoffmann, T.K.; Greve, J. The Surgical Treatment of Peritonsillar Abscess: A Retrospective Analysis in 584 Patients. Laryngoscope 2021, 131, 2706–2712. [Google Scholar] [CrossRef]
- Lepelletier, D.; Pinaud, V.; Le Conte, P.; Bourigault, C.; Asseray, N.; Ballereau, F.; Caillon, J.; Ferron, C.; Righini, C.; Batard, E.; et al. Peritonsillar abscess (PTA): Clinical characteristics, microbiology, drug exposures and outcomes of a large multicenter cohort survey of 412 patients hospitalized in 13 French university hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 867–873. [Google Scholar] [CrossRef]
- Gavriel, H.; Golan, Y.; Lazarovitch, T.; Eviatar, E. Bacteriology of peritonsillar abscess in patients over 40 years—a neglected age group. Eur. Arch. Otorhinolaryngol. 2015, 272, 981–984. [Google Scholar] [CrossRef]
- Yenigun, A. The efficacy of tonsillectomy in chronic tonsillitis patients as demonstrated by the neutrophil-to-lymphocyte ratio. J. Laryngol. Otol. 2015, 129, 386–391. [Google Scholar] [CrossRef]
- Geißler, K.; Weigel, C.; Schubert, K.; Rubio, I.; Guntinas-Lichius, O. Cytokine production in patients with recurrent acute tonsillitis: Analysis of tonsil samples and blood. Sci. Rep. 2020, 10, 13006. [Google Scholar] [CrossRef]
- Ma, D.; Mandell, J.B.; Donegan, N.P.; Cheung, A.L.; Ma, W.; Rothenberger, S.; Shanks, R.; Richardson, A.R.; Urish, K.L. The Toxin-Antitoxin MazEF Drives Staphylococcus aureus Biofilm Formation, Antibiotic Tolerance, and Chronic Infection. mBio 2019, 24, e01658-19. [Google Scholar] [CrossRef]
- Forson, A.M.; van der Mei, H.C.; Sjollema, J. Impact of solid surface hydrophobicity and micrococcal nuclease production on Staphylococcus aureus Newman biofilms. Sci. Rep. 2020, 10, 12093. [Google Scholar] [CrossRef]
- Schnurr, E.; Paqué, P.N.; Attin, T.; Nanni, P.; Grossmann, J.; Holtfreter, S.; Bröker, B.M.; Kohler, C.; Diep, B.A.; Ribeiro, A.A.; et al. Staphylococcus aureus Interferes with Streptococci Spatial Distribution and with Protein Expression of Species within a Polymicrobial Oral Biofilm. Antibiotics 2021, 10, 116. [Google Scholar] [CrossRef]
- Adam, B.; Baillie, G.S.; Douglas, L.J. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J. Med. Microbiol. 2002, 51, 344–349. [Google Scholar] [CrossRef]
- Krause, J.; Geginat, G.; Tammer, I. Prostaglandin E2 from Candida albicans Stimulates the Growth of Staphylococcus aureus in Mixed Biofilms. PLoS ONE 2015, 10, e0135404. [Google Scholar] [CrossRef]
- Klagisa, R.; Kroica, J.; Kise, L. Punch Biopsy Needle. Latvia Patent Aplication No. LVP2020000055; Izgudrojumi, Preču Zīmes un Dizainparaugi; Patent Office of the Republic of Latvia: Riga, Latvia, 2021; Volume 5, p. 315, 20 May 2021. [Google Scholar]
- Cornaglia, G.; Courcol, R.; Herrmann, J.L. European manual of clinical microbiology. Société Franaise Microbiol. 2012, 262, 145–152. [Google Scholar]
- Anonymous; The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0. 2020, Volume 34–38, pp. 84–87. Available online: https://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/ (accessed on 12 February 2022).
- Reisner, A.; Krogfelt, K.A.; Klein, B.M.; Zechner, E.L.; Molin, S. In Vitro Biofilm Formation of Commensal and Pathogenic Escherichia coli Strains: Impact of Environmental and Genetic Factors. J. Bacteriol. 2006, 188, 3572–3581. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | RT | PTA | p-Value | |
|---|---|---|---|---|
| Gender | Male, n (%) | 26 (26%) | 15 (52%) | p = 0.061 |
| Female, n (%) | 73 (74%) | 14 (48%) | p = 0.061 | |
| Age | Age (between, mean ± SD), years | 20–72, 32.94 ± 11.19 | 18–58, 32.4 ± 12.2 | p = 0.279 |
| Age (median, IQR), years | 31, 10 | 31, 16 | ||
| Laboratory findings | CRP, median, mg/L | 1.17 | 85.5 | p < 0.001 |
| WBC, median, ×109/L | 6.52 | 12.97 | p < 0.001 | |
| Comorbidities | Primary arterial hypertension (n) | 9 | 1 | p = 0.454 |
| Cardiologic diseases (n) | 5 | 0 | p = 0.587 | |
| Type 2 diabetes mellitus (n) | 1 | 0 | p > 0.999 | |
| Bronchial asthma (n) | 5 | 1 | p > 0.999 | |
| Chronic gastritis or gastroesophageal reflux disease (n) | 17 | 1 | p = 0.188 | |
| Patients’ Microbiological Data | RT Group | PTA Group | p-Values | |
|---|---|---|---|---|
| Isolation rate | S. aureus, n (%) | 33/99 (33.33%) | 7/29 (24.14%) | p = 0.347 |
| K. pneumoniae, n (%) | 10/99 (10.10%) | 4/29 (13.79%) | p = 0.519 | |
| Candida spp., n (%) | 8/99 (8.08%) | 14/29 (48.28%) | p < 0.001 | |
| Biofilms, mean OD | S. aureus biofilms, mean OD | 1.24 | 1.02 | p = 0.929 |
| K. pneumoniae biofilms, mean OD | 0.44 | 0.39 | p = 0.322 | |
| Biofilm-producing strains | Biofilm-producing strains, n | 37 | 8 | p = 0.111 |
| S. aureus biofilm-producing strains, n | 28 | 7 | p = 0.642 | |
| S. aureus moderate and strong biofilm producers, n (%) | 13/33 (39.39) | 2/7 (28.57%) | p = 0.691 | |
| K. pneumoniae moderate and strong biofilm producers, n | 0 | 0 | ||
| Associations between variables by study groups | Gram-positive microbe and biofilm-producing strain | p < 0.001 | p < 0.001 | |
| Gram-negative microbe and biofilm-producing strain | p = 0.227 | p > 0.999 | ||
| Candida spp. and biofilm-producing strain | p > 0.999 | p = 0.215 | ||
| Comorbidities and biofilm-producing strain | p = 0.759 | p = 0.540 | ||
| Episodes of tonsillitis and biofilm-producing strain | p = 0.313 | p = 0.738 | ||
| PTA in medical history and biofilm-producing strain | p = 0.091 | p = 0.640 | ||
| Antibiotics | S. aureus Strains (n = 33) of Patients with RT | S. aureus Strains (n = 7) of Patients with a PTA | ||||
|---|---|---|---|---|---|---|
| Resistant Strains (n) | Non- and Weak Biofilm Producers (n) | Moderate and Strong Biofilm Producers (n) | Resistant Strains (n) | Non- and Weak Biofilm Producers | Moderate and Strong Biofilm Producers | |
| P, AMP, CIP * | 20/33 | 12/20 | 8/20 | 5/7 | 4/5 | 1/5 |
| P, AMP, CIP *, CD * | 1/33 | 1 | ||||
| CIP * | 9/33 | 5/9 | 4/9 | 2/7 | 1/2 | 1/2 |
| P, AMP, CIP *, E | 1/33 | 1 | ||||
| CIP *, E | 1/33 | 1 | ||||
| FOX, CRO, P, AMP, AMS, AUG, CIP * | 1 **/33 | 1 | ||||
| Patient Group | Biofilm Formation | Antibiotic Resistance (P, AMP) | No Antibiotic Resistance (or Antibiotic Resistance to One Antibiotic) | p-Value |
|---|---|---|---|---|
| RT group | Non- or weak biofilm producer | 14 | 6 | p = 0.590 |
| Moderate/strong biofilm producer | 9 | 4 | p > 0.999 | |
| PTA group | Non- or weak biofilm producer | 4 | 1 | p > 0.999 |
| Moderate/strong biofilm producer | 1 | 1 | p > 0.999 | |
| PTA + RT group | Non- or weak biofilm producer | 18 | 7 | p = 0.590 |
| Moderate/strong biofilm producer | 10 | 5 | p > 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klagisa, R.; Racenis, K.; Broks, R.; Balode, A.O.; Kise, L.; Kroica, J. Analysis of Microorganism Colonization, Biofilm Production, and Antibacterial Susceptibility in Recurrent Tonsillitis and Peritonsillar Abscess Patients. Int. J. Mol. Sci. 2022, 23, 10273. https://doi.org/10.3390/ijms231810273
Klagisa R, Racenis K, Broks R, Balode AO, Kise L, Kroica J. Analysis of Microorganism Colonization, Biofilm Production, and Antibacterial Susceptibility in Recurrent Tonsillitis and Peritonsillar Abscess Patients. International Journal of Molecular Sciences. 2022; 23(18):10273. https://doi.org/10.3390/ijms231810273
Chicago/Turabian StyleKlagisa, Renata, Karlis Racenis, Renars Broks, Arta Olga Balode, Ligija Kise, and Juta Kroica. 2022. "Analysis of Microorganism Colonization, Biofilm Production, and Antibacterial Susceptibility in Recurrent Tonsillitis and Peritonsillar Abscess Patients" International Journal of Molecular Sciences 23, no. 18: 10273. https://doi.org/10.3390/ijms231810273





