Stem Cell Therapy for Sequestration of Traumatic Brain Injury-Induced Inflammation
Abstract
:1. Traumatic Brain Injury and Inflammation
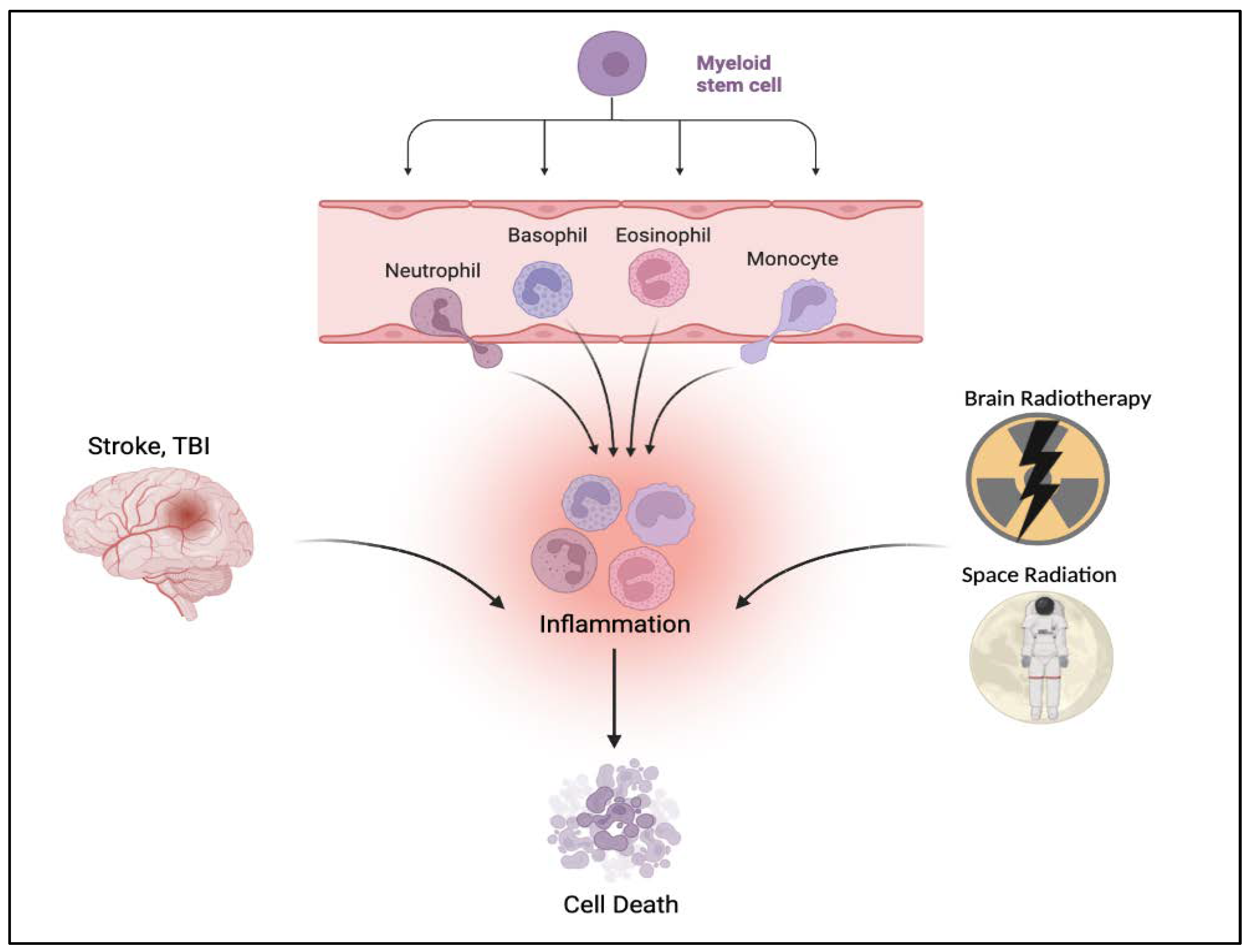
2. Inflammation-Plagued TBI-like Events
3. TBI-Induced Stem Cell Dysfunction: Potential of Stem Cell-Based Therapies
3.1. The Bone Marrow Niche and Hematopoiesis
3.2. TBI and the Hematopoietic Response
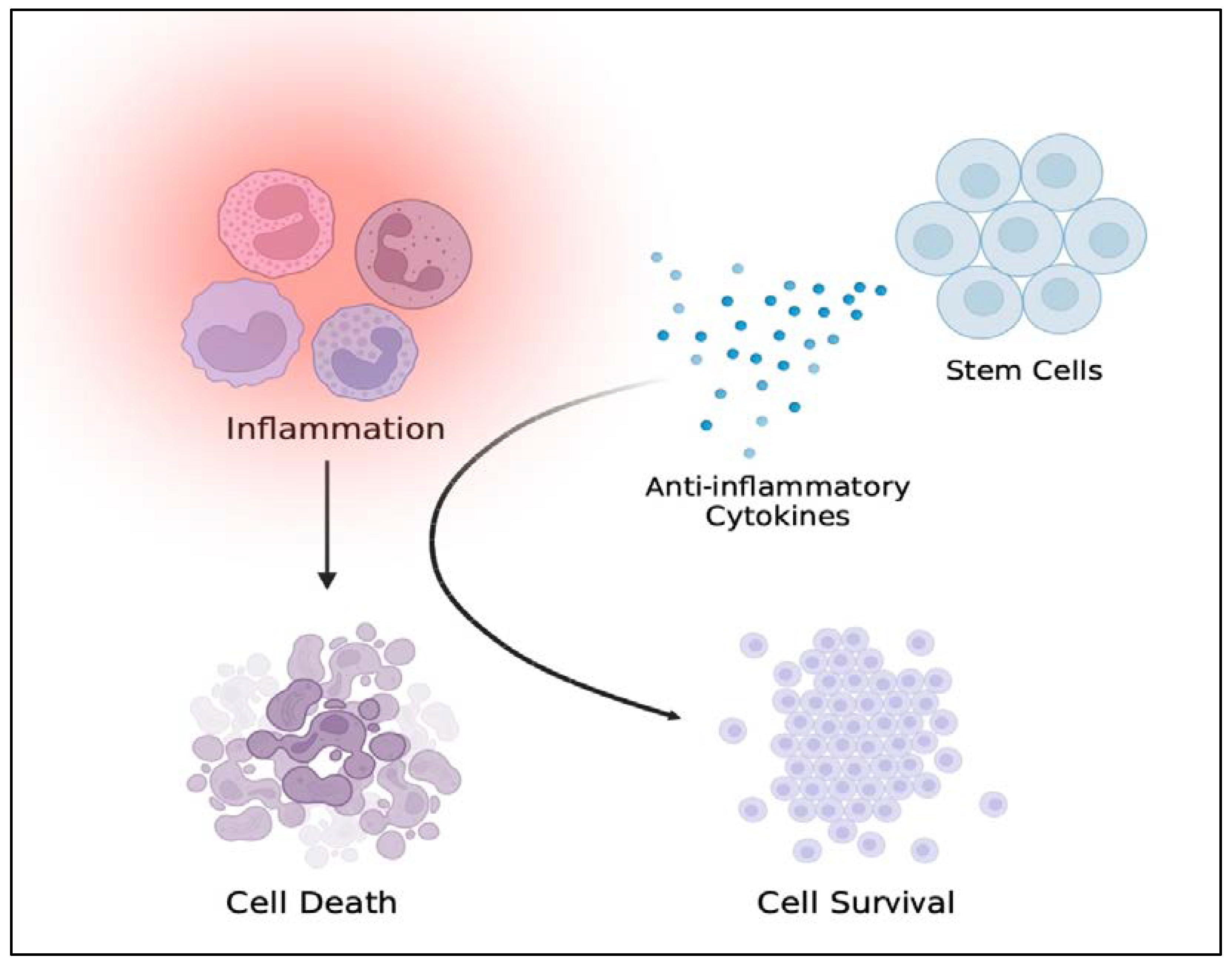
3.3. TBI-like Events and Inflammation
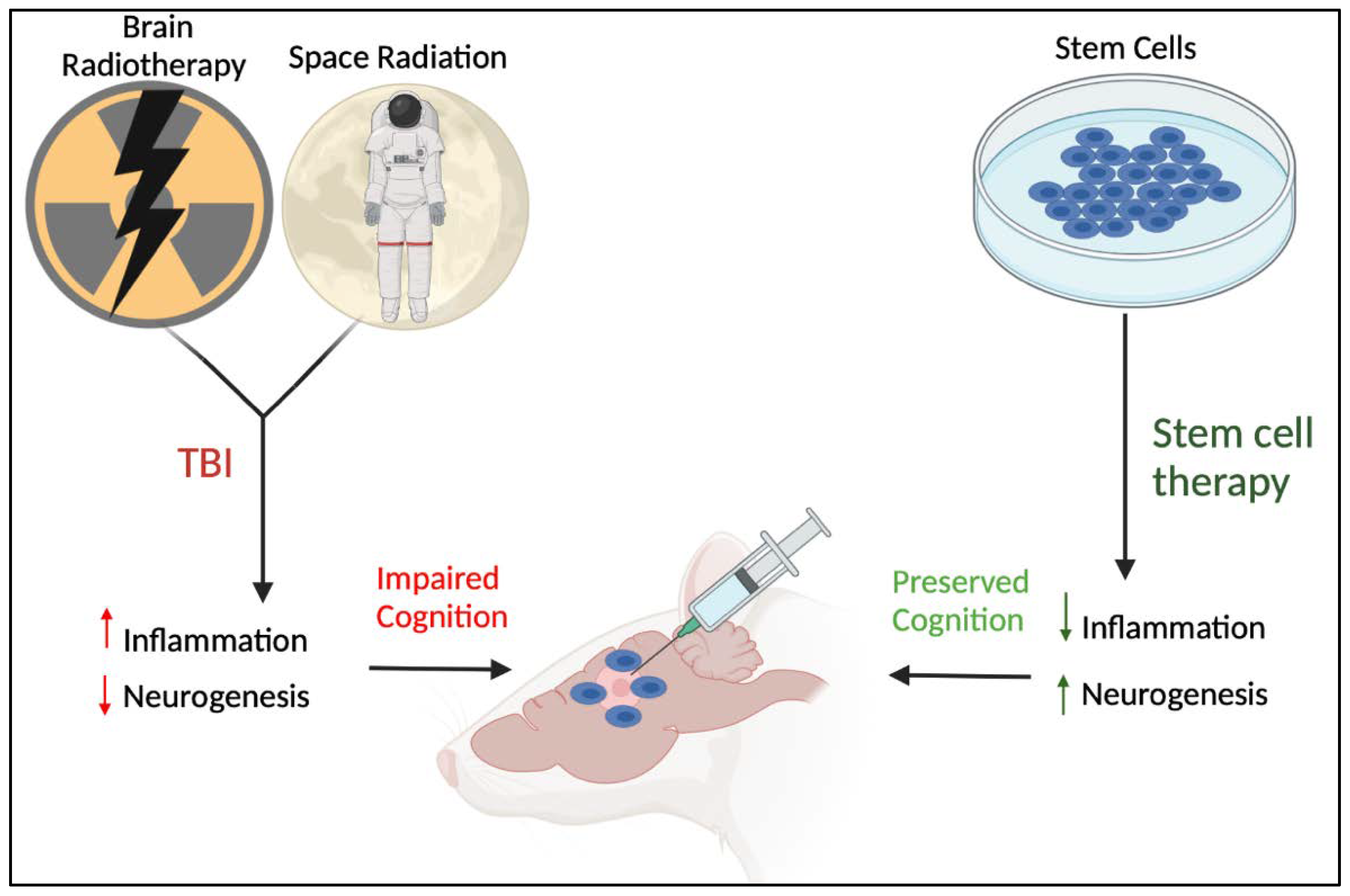
3.4. Stem Cell Therapy for Reducing Neural Injury-Induced Inflammation
4. Conclusions
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TBI | Traumatic brain injury |
| CSF | Cerebrospinal fluid |
| CCR2 | C-C chemokine receptor 2 |
| CX3CR1 | CX3C chemokine receptor 1 |
| GFP | Green fluorescent protein |
| RFP | Red fluorescent protein |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| C1q | Complement component 1q |
| C3 | Complement component 3 |
| CR3 | Complement component R3 |
| RNA | Ribonucleic acid |
| CNS | Central nervous system |
| IL-1β | Interleukin-1beta |
| IFN-γ | Interferon-gamma |
References
- Broglio, S.P.; Eckner, J.T.; Paulson, H.L.; Kutcher, J.S. Cognitive Decline and Aging: The role of concussive and subconcussive impacts. Exerc. Sport Sci. Rev. 2012, 40, 138–144. [Google Scholar] [CrossRef]
- Cole, J.H.; Leech, R.; Sharp, D.J.; Alzheimer’s Disease Neuroimaging Initiative. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann. Neurol. 2015, 77, 571–581. [Google Scholar] [CrossRef]
- Fleminger, S.; Oliver, D.L.; Lovestone, S.; Rabe-Hesketh, S.; Giora, A. Head injury as a risk factor for Alzheimer’s disease: The evidence 10 years on; A partial replication. J. Neurol. Neurosurg. Psychiatry 2003, 74, 841. [Google Scholar] [CrossRef]
- Masel, B.E.; DeWitt, D.S. Traumatic Brain Injury: A Disease Process, Not an Event. J. Neurotrauma 2010, 27, 1529–1540. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhang, S.; Zhao, J.; Zhu, X.; Tian, G. Head Injury as a Risk Factor for Dementia and Alzheimer’s Disease: A Systematic Review and Meta-Analysis of 32 Observational Studies. PLoS ONE 2017, 12, e0169650. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.R. Invited Commentary on “Centers for Disease Control and Prevention Report to Congress: Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation”. Arch. Phys. Med. Rehabil. 2015, 96, 1753–1755. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic Brain Injury–Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2007 and 2013. MMWR Surveill. Summ. 2017, 66, 1. [Google Scholar] [CrossRef]
- Coronado, V.G.; Xu, L.; Basavaraju, S.V.; McGuire, L.C.; Wald, M.M.; Faul, M.D.; Guzman, B.R.; Hemphill, J.D.; Centers for Disease Control and Prevention (CDC). Surveillance for traumatic brain injury-related deaths-United States, 1997–2007. Morbidity and mortality weekly report. Surveill. Summ. 2002, 60, 1–32. [Google Scholar]
- Coronado, V.G.; Haileyesus, T.; Cheng, T.A.; Bell, J.M.; Haarbauer-Krupa, J.; Lionbarger, M.R.; Flores-Herrera, J.; McGuire, L.C.; Gilchrist, J. Trends in Sports- and Recreation-Related Traumatic Brain Injuries Treated in US Emergency Departments: The National Electronic Injury Surveillance System-All Injury Program (NEISS-AIP) 2001-2012. J. Head Trauma Rehabil. 2015, 30, 185–197. [Google Scholar] [CrossRef]
- Corrigan, J.D.; Cuthbert, J.P.; Harrison-Felix, C.; Whiteneck, G.G.; Bell, J.M.; Miller, A.C.; Coronado, V.G.; Pretz, C.R. US Population Estimates of Health and Social Outcomes 5 Years After Rehabilitation for Traumatic Brain Injury. J. Head Trauma Rehabil. 2014, 29, E1–E9. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.F.; Daugherty, J.; Waltzman, D.; Sarmiento, K. Predictors of traumatic brain injury morbidity and mortality: Examination of data from the national trauma data bank: Predictors of TBI morbidity & mortality. Injury 2021, 52, 1138–1144. [Google Scholar] [CrossRef]
- Gupte, R.P.; Brooks, W.; Vukas, R.; Pierce, J.D.; Harris, J.L. Sex Differences in Traumatic Brain Injury: What We Know and What We Should Know. J. Neurotrauma 2019, 36, 3063–3091. [Google Scholar] [CrossRef] [PubMed]
- Blaya, M.O.; Raval, A.P.; Bramlett, H.M. Traumatic brain injury in women across lifespan. Neurobiol. Dis. 2022, 164, 105613. [Google Scholar] [CrossRef] [PubMed]
- Malec, J.F.; Brown, A.W.; Leibson, C.L.; Flaada, J.T.; Mandrekar, J.N.; Diehl, N.N.; Perkins, P.K. The Mayo Classification System for Traumatic Brain Injury Severity. J. Neurotrauma 2007, 24, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Dijkland, S.A.; Foks, K.A.; Polinder, S.; Dippel, D.W.; Maas, A.I.; Lingsma, H.F.; Steyerberg, E.W. Prognosis in Moderate and Severe Traumatic Brain Injury: A Systematic Review of Contemporary Models and Validation Studies. J. Neurotrauma 2020, 37, 1–13. [Google Scholar] [CrossRef]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.-R. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell Transplant. 2017, 26, 1118–1130. [Google Scholar] [CrossRef]
- Faden, A.I.; Loane, D. Chronic Neurodegeneration After Traumatic Brain Injury: Alzheimer Disease, Chronic Traumatic Encephalopathy, or Persistent Neuroinflammation? Neurotherapeutics 2015, 12, 143–150. [Google Scholar] [CrossRef]
- Washington, P.M.; Villapol, S.; Burns, M.P. Polypathology and dementia after brain trauma: Does brain injury trigger distinct neurodegenerative diseases, or should they be classified together as traumatic encephalopathy? Exp. Neurol. 2016, 275, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.R.; Miller, M.W.; Wolf, E.J.; Logue, M.W.; Robinson, M.E.; Fortier, C.B.; Fonda, J.R.; Wang, D.; Milberg, W.P.; McGlinchey, R.E.; et al. Cerebral perfusion is associated with blast exposure in military personnel without moderate or severe TBI. J. Cereb. Blood Flow Metab. 2020, 41, 886–900. [Google Scholar] [CrossRef]
- Wright, W.G.; Handy, J.D.; Haskell, M.A.; Servatius, M.L.; Servatius, R.J. History of Mild Traumatic Brain Injury Affects Static Balance under Complex Multisensory Manipulations. J. Neurotrauma 2022, 39, 821–828. [Google Scholar] [CrossRef]
- Lippa, S.M.; French, L.M.; Brickell, T.A.; Driscoll, M.A.; Glazer, M.E.; Tippett, C.E.; Sullivan, M.J.; Lange, R.T. Post-Traumatic Stress Disorder Symptoms Are Related to Cognition after Complicated Mild and Moderate Traumatic Brain Injury but Not Severe and Penetrating Traumatic Brain Injury. J. Neurotrauma 2021, 38, 3137–3145. [Google Scholar] [CrossRef]
- Corrigan, F.; Cernak, I.; McAteer, K.; Hellewell, S.C.; Rosenfeld, J.V.; Turner, R.J.; Vink, R. NK1 antagonists attenuate tau phosphorylation after blast and repeated concussive injury. Sci. Rep. 2021, 11, 8861. [Google Scholar] [CrossRef]
- Morin, A.; Mouzon, B.; Ferguson, S.; Paris, D.; Saltiel, N.; Lungmus, C.; Mullan, M.; Crawford, F. Treatment with Nilvadipine Mitigates Inflammatory Pathology and Improves Spatial Memory in Aged hTau Mice After Repetitive Mild TBI. Front. Aging Neurosci. 2018, 10, 292. [Google Scholar] [CrossRef]
- Jurick, S.M.; Crocker, L.D.; Merritt, V.C.; Sanderson-Cimino, M.E.; Keller, A.V.; Glassman, L.H.; Twamley, E.W.; Rodgers, C.S.; Schiehser, D.M.; Aupperle, R.L.; et al. Independent and Synergistic Associations Between TBI Characteristics and PTSD Symptom Clusters on Cognitive Performance and Postconcussive Symptoms in Iraq and Afghanistan Veterans. J. Neuropsychiatry Clin. Neurosci. 2021, 33, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Verboon, L.N.; Patel, H.C.; Greenhalgh, A.D. The Immune System’s Role in the Consequences of Mild Traumatic Brain Injury (Concussion). Front. Immunol. 2021, 12, 620698. [Google Scholar] [CrossRef] [PubMed]
- Chaban, V.; Clarke, G.J.; Skandsen, T.; Islam, R.; Einarsen, C.E.; Vik, A.; Damås, J.K.; Mollnes, T.E.; Håberg, A.K.; Pischke, S.E. Systemic Inflammation Persists the First Year after Mild Traumatic Brain Injury: Results from the Prospective Trondheim Mild Traumatic Brain Injury Study. J. Neurotrauma 2020, 37, 2120–2130. [Google Scholar] [CrossRef]
- Gill, J.; Latour, L.; Diaz-Arrastia, R.; Motamedi, V.; Turtzo, C.; Shahim, P.; Mondello, S.; DeVoto, C.; Veras, E.; Hanlon, D.; et al. Glial fibrillary acidic protein elevations relate to neuroimaging abnormalities after mild TBI. Neurology 2018, 91, e1385–e1389. [Google Scholar] [CrossRef]
- Vedantam, A.; Brennan, J.; Levin, H.S.; McCarthy, J.J.; Dash, P.K.; Redell, J.B.; Yamal, J.-M.; Robertson, C.S. Early versus Late Profiles of Inflammatory Cytokines after Mild Traumatic Brain Injury and Their Association with Neuropsychological Outcomes. J. Neurotrauma 2021, 38, 53–62. [Google Scholar] [CrossRef]
- Molina, I.S.M.; Salo, R.A.; Abdollahzadeh, A.; Tohka, J.; Gröhn, O.; Sierra, A. In Vivo Diffusion Tensor Imaging in Acute and Subacute Phases of Mild Traumatic Brain Injury in Rats. Eneuro 2020, 7, 0476-19. [Google Scholar] [CrossRef]
- Weiss, E.; Dhir, T.; Collett, A.; Reola, M.; Kaplan, M.; Minimo, C.; Omert, L.; Leung, P. Effect of complement C1-esterase inhibitor on brain edema and inflammation after mild traumatic brain injury in an animal model. Clin. Exp. Emerg. Med. 2020, 7, 87–94. [Google Scholar] [CrossRef]
- Tweedie, D.; Karnati, H.K.; Mullins, R.; Pick, C.G.; Hoffer, B.J.; Goetzl, E.J.; Kapogiannis, D.; Greig, N.H. Time-dependent cytokine and chemokine changes in mouse cerebral cortex following a mild traumatic brain injury. ELife 2020, 9, e55827. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.L.; Yue, J.K.; Korley, F.K.; Puccio, A.M.; Yuh, E.L.; Sun, M.X.; Rabinowitz, M.; Vassar, M.M.; Taylor, S.R.; Winkler, E.A.; et al. High-Sensitivity C-Reactive Protein is a Prognostic Biomarker of Six-Month Disability after Traumatic Brain Injury: Results from the TRACK-TBI Study. J. Neurotrauma 2021, 38, 918–927. [Google Scholar] [CrossRef]
- Glushakova, O.Y.; Glushakov, A.O.; Borlongan, C.V.; Valadka, A.B.; Hayes, R.L.; Glushakov, A.V. Role of Caspase-3-Mediated Apoptosis in Chronic Caspase-3-Cleaved Tau Accumulation and Blood–Brain Barrier Damage in the Corpus Callosum after Traumatic Brain Injury in Rats. J. Neurotrauma 2018, 35, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Shahim, P.; Politis, A.; van der Merwe, A.; Moore, B.; Ekanayake, V.; Lippa, S.M.; Chou, Y.-Y.; Pham, D.L.; Butman, J.A.; Diaz-Arrastia, R.; et al. Time course and diagnostic utility of NfL, tau, GFAP, and UCH-L1 in subacute and chronic TBI. Neurology 2020, 95, e623–e636. [Google Scholar] [CrossRef] [PubMed]
- Morganti, J.M.; Jopson, T.D.; Liu, S.; Riparip, L.-K.; Guandique, C.K.; Gupta, N.; Ferguson, A.; Rosi, S. CCR2 Antagonism Alters Brain Macrophage Polarization and Ameliorates Cognitive Dysfunction Induced by Traumatic Brain Injury. J. Neurosci. 2015, 35, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Morganti, J.M.; Riparip, L.-K.; Rosi, S. Call Off the Dog(ma): M1/M2 Polarization Is Concurrent following Traumatic Brain Injury. PLoS ONE 2016, 11, e0148001. [Google Scholar] [CrossRef] [PubMed]
- Morganti, J.M.; Riparip, L.-K.; Chou, A.; Liu, S.; Gupta, N.; Rosi, S. Age exacerbates the CCR2/5-mediated neuroinflammatory response to traumatic brain injury. J. Neuroinflammation 2016, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.; Krukowski, K.; Morganti, J.M.; Riparip, L.-K.; Rosi, S. Persistent Infiltration and Impaired Response of Peripherally-Derived Monocytes after Traumatic Brain Injury in the Aged Brain. Int. J. Mol. Sci. 2018, 19, 1616. [Google Scholar] [CrossRef]
- Krukowski, K.; Chou, A.; Feng, X.; Tiret, B.; Paladini, M.-S.; Riparip, L.-K.; Chaumeil, M.M.; Lemere, C.; Rosi, S. Traumatic Brain Injury in Aged Mice Induces Chronic Microglia Activation, Synapse Loss, and Complement-Dependent Memory Deficits. Int. J. Mol. Sci. 2018, 19, 3753. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.R.; Eilertson, K.; Chakraborti, A.; Sharma, S.; Baure, J.; Habdank-Kolaczkowski, J.; Allen, B.; Rosi, S.; Raber, J.; Fike, J.R. Radiation exposure prior to traumatic brain injury induces responses that differ as a function of animal age. Int. J. Radiat. Biol. 2013, 90, 214–223. [Google Scholar] [CrossRef]
- Feng, X.; Frias, E.S.; Paladini, M.S.; Chen, D.; Boosalis, Z.; Becker, M.; Gupta, S.; Liu, S.; Gupta, N.; Rosi, S. Functional role of brain-engrafted macrophages against brain injuries. J. Neuroinflammation 2021, 18, 232. [Google Scholar] [CrossRef]
- Feng, X.; Jopson, T.D.; Paladini, M.S.; Liu, S.; West, B.L.; Gupta, N.; Rosi, S. Colony-stimulating factor 1 receptor blockade prevents fractionated whole-brain irradiation-induced memory deficits. J. Neuroinflammation 2016, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, S.; Chen, D.; Rosi, S.; Gupta, N. Rescue of cognitive function following fractionated brain irradiation in a novel preclinical glioma model. ELife 2018, 7, e38865. [Google Scholar] [CrossRef] [PubMed]
- Belarbi, K.; Jopson, T.; Arellano, C.; Fike, J.R.; Rosi, S. CCR2 Deficiency Prevents Neuronal Dysfunction and Cognitive Impairments Induced by Cranial Irradiation. Cancer Res. 2013, 73, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Hohsfield, L.A.; Najafi, A.R.; Ghorbanian, Y.; Soni, N.; Hingco, E.E.; Kim, S.J.; Jue, A.D.; Swarup, V.; Inlay, M.A.; Green, K.N. Effects of long-term and brain-wide colonization of peripheral bone marrow-derived myeloid cells in the CNS. J. Neuroinflammation 2020, 17, 279. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, K.; Grue, K.; Becker, M.; Elizarraras, E.; Frias, E.S.; Halvorsen, A.; Koenig-Zanoff, M.; Frattini, V.; Nimmagadda, H.; Feng, X.; et al. The impact of deep space radiation on cognitive performance: From biological sex to biomarkers to countermeasures. Sci. Adv. 2021, 7, eabg6702. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, K.; Grue, K.; Frias, E.S.; Pietrykowski, J.; Jones, T.; Nelson, G.; Rosi, S. Female mice are protected from space radiation-induced maladaptive responses. Brain Behav. Immun. 2018, 74, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.M.; Cheng-Campbell, M.; Blaber, E.A.; Anand, S.; Bhattacharya, S.; Zwart, S.R.; Crucian, B.E.; Smith, S.M.; Meller, R.; Grabham, P.; et al. Beyond Low-Earth Orbit: Characterizing Immune and microRNA Differentials following Simulated Deep Spaceflight Conditions in Mice. iScience 2020, 23, 101747. [Google Scholar] [CrossRef]
- Laiakis, E.C.; Shuryak, I.; Deziel, A.; Wang, Y.W.; Barnette, B.L.; Yu, Y.; Ullrich, R.L.; Fornace, A.J., Jr.; Emmett, M.R. Effects of low dose space radiation exposures on the splenic metabolome. Int. J. Mol. Sci. 2021, 22, 3070. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Acosta, S.; Tuazon, J.P.; Xu, K.; Nguyen, H.; Lippert, T.; Liska, M.G.; Semechkin, A.; Garitaonandia, I.; Gonzalez, R.; et al. Human parthenogenetic neural stem cell grafts promote multiple regenerative processes in a traumatic brain injury model. Theranostics 2019, 9, 1029–1046. [Google Scholar] [CrossRef] [PubMed]
- Marcet, P.; Santos, N.; Borlongan, C.V. When friend turns foe: Central and peripheral neuroinflammation in central nervous system injury. Neuroimmunol. Neuroinflammation 2017, 4, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Toyoshima, A.; Yasuhara, T.; Kameda, M.; Morimoto, J.; Takeuchi, H.; Wang, F.; Sasaki, T.; Sasada, S.; Shinko, A.; Wakamori, T.; et al. Intra-Arterial Transplantation of Allogeneic Mesenchymal Stem Cells Mounts Neuroprotective Effects in a Transient Ischemic Stroke Model in Rats: Analyses of Therapeutic Time Window and Its Mechanisms. PLoS ONE 2015, 10, e0127302. [Google Scholar] [CrossRef]
- Amaroli, A.; Pasquale, C.; Zekiy, A.; Benedicenti, S.; Marchegiani, A.; Sabbieti, M.G.; Agas, D. Steering the multipotent mesenchymal cells towards an anti-inflammatory and osteogenic bias via photobiomodulation therapy: How to kill two birds with one stone. J. Tissue Eng. 2022, 13, 20417314221110192. [Google Scholar] [CrossRef]
- Granata, V.; Crisafulli, L.; Nastasi, C.; Ficara, F.; Sobacchi, C. Bone Marrow Niches and Tumour Cells: Lights and Shadows of a Mutual Relationship. Front. Immunol. 2022, 13, 884024. [Google Scholar] [CrossRef]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef]
- Fröbel, J.; Landspersky, T.; Percin, G.; Schreck, C.; Rahmig, S.; Ori, A.; Nowak, D.; Essers, M.; Waskow, C.; Oostendorp, R.A.J. The Hematopoietic Bone Marrow Niche Ecosystem. Front. Cell Dev. Biol. 2021, 9, 705410. [Google Scholar] [CrossRef]
- Bartl, M.; Xylaki, M.; Bähr, M.; Weber, S.; Trenkwalder, C.; Mollenhauer, B. Evidence for immune system alterations in peripheral biological fluids in Parkinson’s disease. Neurobiol. Dis. 2022, 170, 105744. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, H.C. Chronic inflammation as a promotor of mutagenesis in essential thrombocythemia, polycythemia vera and myelofibrosis. A human inflammation model for cancer development? Leuk. Res. 2013, 37, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Waclawiczek, A.; Hamilton, A.; Rouault-Pierre, K.; Abarrategi, A.; Garcia-Albornoz, M.; Miraki-Moud, F.; Bah, N.; Gribben, J.; FitzGibbon, J.; Taussig, D.; et al. Mesenchymal niche remodeling impairs hematopoiesis via stanniocalcin 1 in acute myeloid leukemia. J. Clin. Investig. 2020, 130, 3038–3050. [Google Scholar] [CrossRef] [PubMed]
- Girard, D.; Torossian, F.; Oberlin, E.; Alexander, K.A.; Gueguen, J.; Tseng, H.-W.; Genêt, F.; Lataillade, J.-J.; Salga, M.; Levesque, J.-P.; et al. Neurogenic Heterotopic Ossifications Recapitulate Hematopoietic Stem Cell Niche Development Within an Adult Osteogenic Muscle Environment. Front. Cell Dev. Biol. 2021, 9, 611842. [Google Scholar] [CrossRef]
- Foertsch, S.; Reber, S.O. The role of physical trauma in social stress-induced immune activation. Neurosci. Biobehav. Rev. 2020, 113, 169–178. [Google Scholar] [CrossRef]
- Hawthorne, A.L.; Popovich, P.G. Emerging Concepts in Myeloid Cell Biology after Spinal Cord Injury. Neurotherapeutics 2011, 8, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.M.; Barman, P.K.; Thiruppathi, M.; Mirza, R.E.; McKinney, R.D.; Deng, J.; Christman, J.W.; Du, X.; Fukai, T.; Ennis, W.J.; et al. Oxidant Signaling Mediated by Nox2 in Neutrophils Promotes Regenerative Myelopoiesis and Tissue Recovery following Ischemic Damage. J. Immunol. 2018, 201, 2414–2426. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Ley, K. Waking Up the Stem Cell Niche: How hematopoietic stem cells generate inflammatory monocytes after stroke. Circ. Res. 2015, 116, 389–392. [Google Scholar] [CrossRef]
- Mazzitelli, J.A.; Smyth, L.C.D.; Cross, K.A.; Dykstra, T.; Sun, J.; Du, S.; Mamuladze, T.; Smirnov, I.; Rustenhoven, J.; Kipnis, J. Cerebrospinal fluid regulates skull bone marrow niches via direct access through dural channels. Nat. Neurosci. 2022, 25, 555–560. [Google Scholar] [CrossRef]
- Shi, S.X.; Shi, K.; Liu, Q. Brain injury instructs bone marrow cellular lineage destination to reduce neuroinflammation. Sci. Transl. Med. 2021, 13, eabc7029. [Google Scholar] [CrossRef]
- Huin-Schohn, C.; Guéguinou, N.; Schenten, V.; Bascove, M.; Koch, G.G.; Baatout, S.; Tschirhart, E.; Frippiat, J. Gravity changes during animal development affect IgM heavy-chain transcription and probably lymphopoiesis. FASEB J. 2012, 27, 333–341. [Google Scholar] [CrossRef]
- Rostami, T.; Mousavi, S.A.; Kiumarsi, A.; Kasaeian, A.; Rad, S.; Yaghmaie, M.; Ghavamzadeh, A.; Mousavi, S.A. Radiation-free reduced-intensity hematopoietic stem cell transplantation with in vivo T-cell depletion from matched related and unrelated donors for Fanconi anemia: Prognostic factor analysis. Exp. Hematol. 2022, 109, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.A.; Tajiri, N.; Shinozuka, K.; Ishikawa, H.; Sanberg, P.R.; Sanchez-Ramos, J.; Song, S.; Kaneko, Y.; Borlongan, C.V. Combination Therapy of Human Umbilical Cord Blood Cells and Granulocyte Colony Stimulating Factor Reduces Histopathological and Motor Impairments in an Experimental Model of Chronic Traumatic Brain Injury. PLoS ONE 2014, 9, e90953. [Google Scholar] [CrossRef]
- Acosta, S.A.; Tajiri, N.; Shinozuka, K.; Ishikawa, H.; Grimmig, B.; Diamond, D.; Sanberg, P.R.; Bickford, P.; Kaneko, Y.; Borlongan, C.V. Long-Term Upregulation of Inflammation and Suppression of Cell Proliferation in the Brain of Adult Rats Exposed to Traumatic Brain Injury Using the Controlled Cortical Impact Model. PLoS ONE 2013, 8, e53376. [Google Scholar] [CrossRef]
- Lozano, D.; Gonzales-Portillo, G.S.; Acosta, S.; de la Pena, I.; Tajiri, N.; Kaneko, Y.; Borlongan, C.V. Neuroinflammatory responses to traumatic brain injury: Etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr. Dis. Treat. 2015, 11, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Pabón, M.M.; Acosta, S.; Guedes, V.A.; Tajiri, N.; Kaneko, Y.; Borlongan, C.V. Brain Region-Specific Histopathological Effects of Varying Trajectories of Controlled Cortical Impact Injury Model of Traumatic Brain Injury. CNS Neurosci. Ther. 2016, 22, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Rola, R.; Fishman, K.; Baure, J.; Rosi, S.; Lamborn, K.R.; Obenaus, A.; Nelson, G.A.; Fike, J.R. Hippocampal Neurogenesis and Neuroinflammation after Cranial Irradiation with 56Fe Particles. Radiat. Res. 2008, 169, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, K.; Wei, D.; Tian, Y.; Gao, Y.; Chen, Z.; Qian, A. The Impact of Spaceflight and Simulated Microgravity on Cell Adhesion. Int. J. Mol. Sci. 2020, 21, 3031. [Google Scholar] [CrossRef]
- Wnorowski, A.; Sharma, A.; Chen, H.; Wu, H.; Shao, N.-Y.; Sayed, N.; Liu, C.; Countryman, S.; Stodieck, L.S.; Rubins, K.H.; et al. Effects of Spaceflight on Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Structure and Function. Stem Cell Rep. 2019, 13, 960–969. [Google Scholar] [CrossRef]
- Cozene, B.; Sadanandan, N.; Farooq, J.; Kingsbury, C.; Park, Y.J.; Wang, Z.-J.; Moscatello, A.; Saft, M.; Cho, J.; Gonzales-Portillo, B.; et al. Mesenchymal Stem Cell-Induced Anti-Neuroinflammation Against Traumatic Brain Injury. Cell Transplant. 2021, 30, 9636897211035715. [Google Scholar] [CrossRef]
- Lee, S.; Mattingly, A.; Lin, A.; Sacramento, J.; Mannent, L.; Castel, M.-N.; Canolle, B.; Delbary-Gossart, S.; Ferzaz, B.; Morganti, J.M.; et al. A novel antagonist of p75NTR reduces peripheral expansion and CNS trafficking of pro-inflammatory monocytes and spares function after traumatic brain injury. J. Neuroinflammation 2016, 13, 88. [Google Scholar] [CrossRef]
- Neal, E.; Liska, M.G.; Lippert, T.; Lin, R.; Gonzalez, M.; Russo, E.; Xu, K.; Ji, X.; Vale, F.L.; Van Loveren, H.; et al. An update on intracerebral stem cell grafts. Expert Rev. Neurother. 2018, 18, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Grill, R.J.; Dunn, T.J.; Bedi, S.; Labastida, J.A.; Hetz, R.A.; Xue, H.; Thonhoff, J.R.; DeWitt, D.S.; Prough, D.S.; et al. Human Neural Stem Cell Transplantation-Mediated Alteration of Microglial/Macrophage Phenotypes after Traumatic Brain Injury. Cell Transplant. 2016, 25, 1863–1877. [Google Scholar] [CrossRef]
- Barretto, M.T.; Park, E.; Telliyan, M.T.; Liu, E.; Gallagher, D.; Librach, C.; Baker, A. Vascular Dysfunction after Modeled Traumatic Brain Injury Is Preserved with Administration of Umbilical Cord Derived Mesenchymal Stromal Cells and Is Associated with Modulation of the Angiogenic Response. J. Neurotrauma 2021, 38, 2747–2762. [Google Scholar] [CrossRef]
- Caplan, H.W.; Prabhakara, K.S.; Furman, N.E.T.; Zorofchian, S.; Kumar, A.; Martin, C.; Xue, H.; Olson, S.D.; Cox, C.S., Jr. Combination therapy with Treg and mesenchymal stromal cells enhances potency and attenuation of inflammation after traumatic brain injury compared to monotherapy. Stem Cells 2021, 39, 358–370. [Google Scholar] [CrossRef]
- Peña, I.D.; Borlongan, C.V. Translating G-CSF as an Adjunct Therapy to Stem Cell Transplantation for Stroke. Transl. Stroke Res. 2015, 6, 421–429. [Google Scholar] [CrossRef]
- Song, S.; Kong, X.; Borlongan, C.; Sava, V.; Sanchez-Ramos, J. Granulocyte Colony-Stimulating Factor Enhances Brain Repair Following Traumatic Brain Injury Without Requiring Activation of Cannabinoid Receptors. Cannabis Cannabinoid Res. 2021, 6, 48–57. [Google Scholar] [CrossRef]
- Qiu, X.; Ping, S.; Kyle, M.; Chin, L.; Zhao, L.-R. Long-term beneficial effects of hematopoietic growth factors on brain repair in the chronic phase of severe traumatic brain injury. Exp. Neurol. 2020, 330, 113335. [Google Scholar] [CrossRef]
- Song, S.; Kong, X.; Acosta, S.; Sava, V.; Borlongan, C.; Sanchez-Ramos, J. Granulocyte colony-stimulating factor promotes behavioral recovery in a mouse model of traumatic brain injury. J. Neurosci. Res. 2016, 94, 409–423. [Google Scholar] [CrossRef]
- Zhao, Y.; Mu, H.; Huang, Y.; Li, S.; Wang, Y.; Stetler, R.A.; Bennett, M.; Dixon, C.E.; Chen, J.; Shi, Y. Microglia-specific deletion of histone deacetylase 3 promotes inflammation resolution, white matter integrity, and functional recovery in a mouse model of traumatic brain injury. J. Neuroinflammation 2022, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Lippert, T.; Borlongan, C.V. Prophylactic treatment of hyperbaric oxygen treatment mitigates inflammatory response via mitochondria transfer. CNS Neurosci. Ther. 2019, 25, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Mason, H.D.; Johnson, A.M.; Mihelson, N.A.; Mastorakos, P.; McGavern, D.B. Glia limitans superficialis oxidation and breakdown promote cortical cell death after repetitive head injury. JCI Insight 2021, 6, e149229. [Google Scholar] [CrossRef]
- Newell-Rogers, M.K.; Rogers, S.K.; Tobin, R.P.; Mukherjee, S.; Shapiro, L.A. Antagonism of Macrophage Migration Inhibitory Factory (MIF) after Traumatic Brain Injury Ameliorates Astrocytosis and Peripheral Lymphocyte Activation and Expansion. Int. J. Mol. Sci. 2020, 21, 7448. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Wang, X.; Wang, X.; Wang, L.; Wang, X.; Wu, S.; Wan, Z. Autologous Bone Marrow Mesenchymal Stem Cell Therapy in the Subacute Stage of Traumatic Brain Injury by Lumbar Puncture. Exp. Clin. Transplant. 2013, 11, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.S., Jr.; Hetz, R.A.; Liao, G.P.; Aertker, B.M.; Ewing-Cobbs, L.; Juranek, J.; Savitz, S.I.; Jackson, M.L.; Romanowska-Pawliczek, A.M.; Triolo, F.; et al. Treatment of Severe Adult Traumatic Brain Injury Using Bone Marrow Mononuclear Cells. Stem Cells 2017, 35, 1065–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, J.; Li, X.; Shu, K. Cell-Derived Exosomes as Therapeutic Strategies and Exosome-Derived microRNAs as Biomarkers for Traumatic Brain Injury. J. Clin. Med. 2022, 11, 3223. [Google Scholar] [CrossRef] [PubMed]
- Alizada, M.; Lin, S.; Gao, H. Recent advances in the treatment of traumatic brain injury with autologous and non-autologous multipotent stem and progenitor cells: Preclinical models and clinical trials. Folia Neuropathol. 2021, 59, 298–316. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borlongan, M.C.; Rosi, S. Stem Cell Therapy for Sequestration of Traumatic Brain Injury-Induced Inflammation. Int. J. Mol. Sci. 2022, 23, 10286. https://doi.org/10.3390/ijms231810286
Borlongan MC, Rosi S. Stem Cell Therapy for Sequestration of Traumatic Brain Injury-Induced Inflammation. International Journal of Molecular Sciences. 2022; 23(18):10286. https://doi.org/10.3390/ijms231810286
Chicago/Turabian StyleBorlongan, Mia C., and Susanna Rosi. 2022. "Stem Cell Therapy for Sequestration of Traumatic Brain Injury-Induced Inflammation" International Journal of Molecular Sciences 23, no. 18: 10286. https://doi.org/10.3390/ijms231810286
APA StyleBorlongan, M. C., & Rosi, S. (2022). Stem Cell Therapy for Sequestration of Traumatic Brain Injury-Induced Inflammation. International Journal of Molecular Sciences, 23(18), 10286. https://doi.org/10.3390/ijms231810286




