Indications of Peripheral Pain, Dermal Hypersensitivity, and Neurogenic Inflammation in Patients with Lipedema
Abstract
:1. Introduction
2. Results
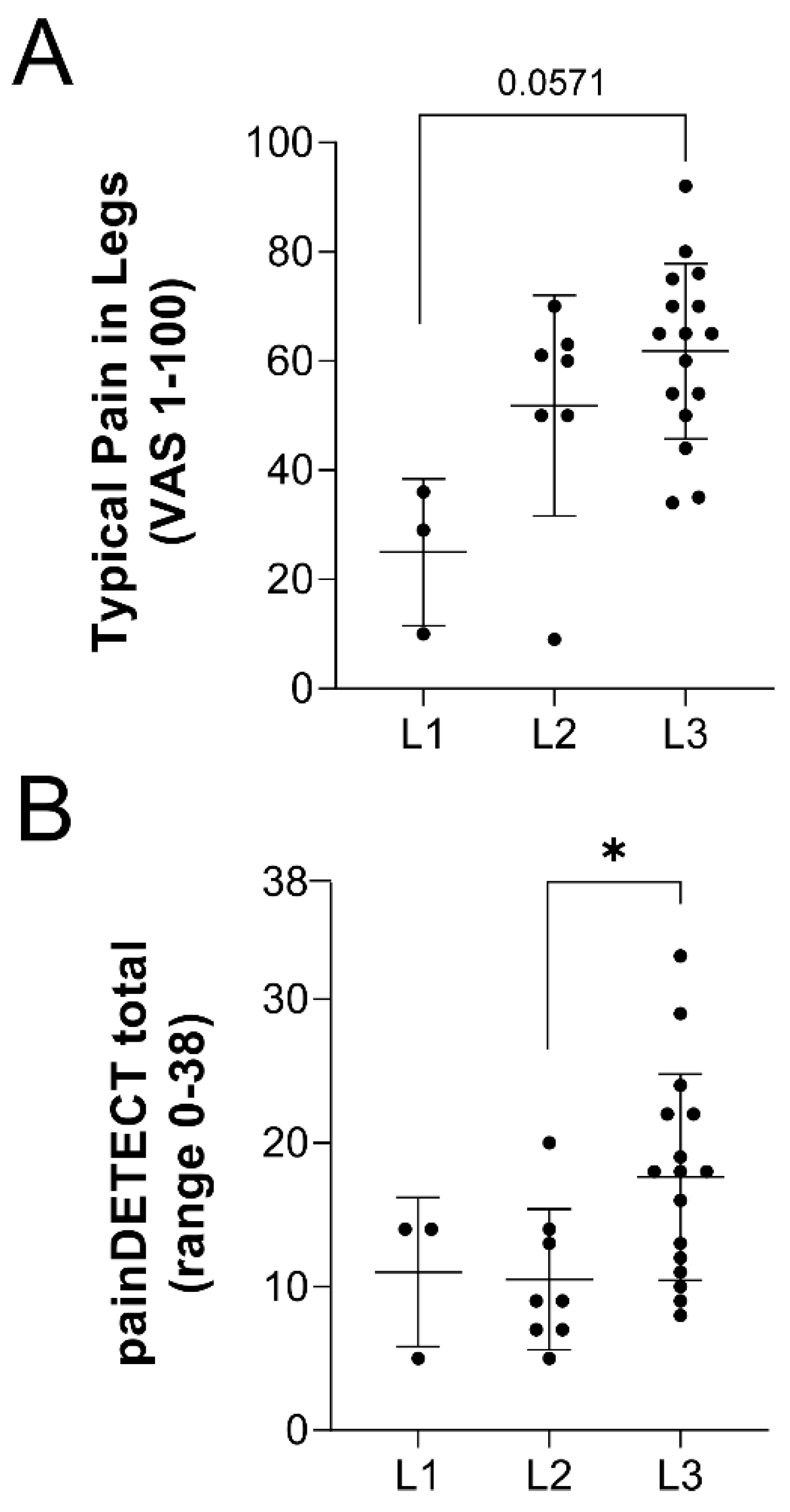
2.1. Lipedema Subjects Indicate Pain Symptoms as a Significant Factor in Their Disease
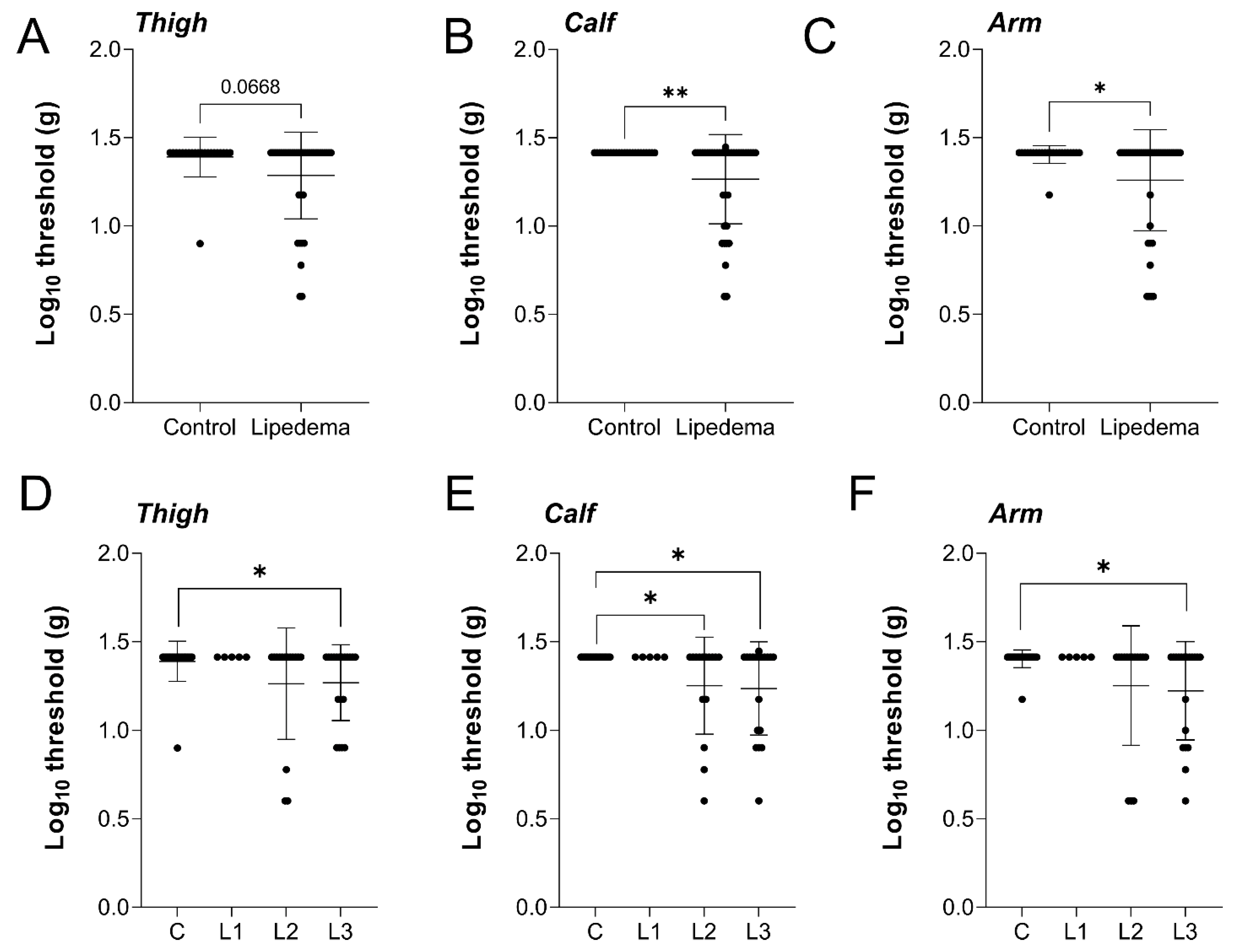
2.2. Cutaneous Mechanical Sensitivity Is Heightened in Lipedema
2.3. An Objective versus Subjective Comparison of Lipedema Pain
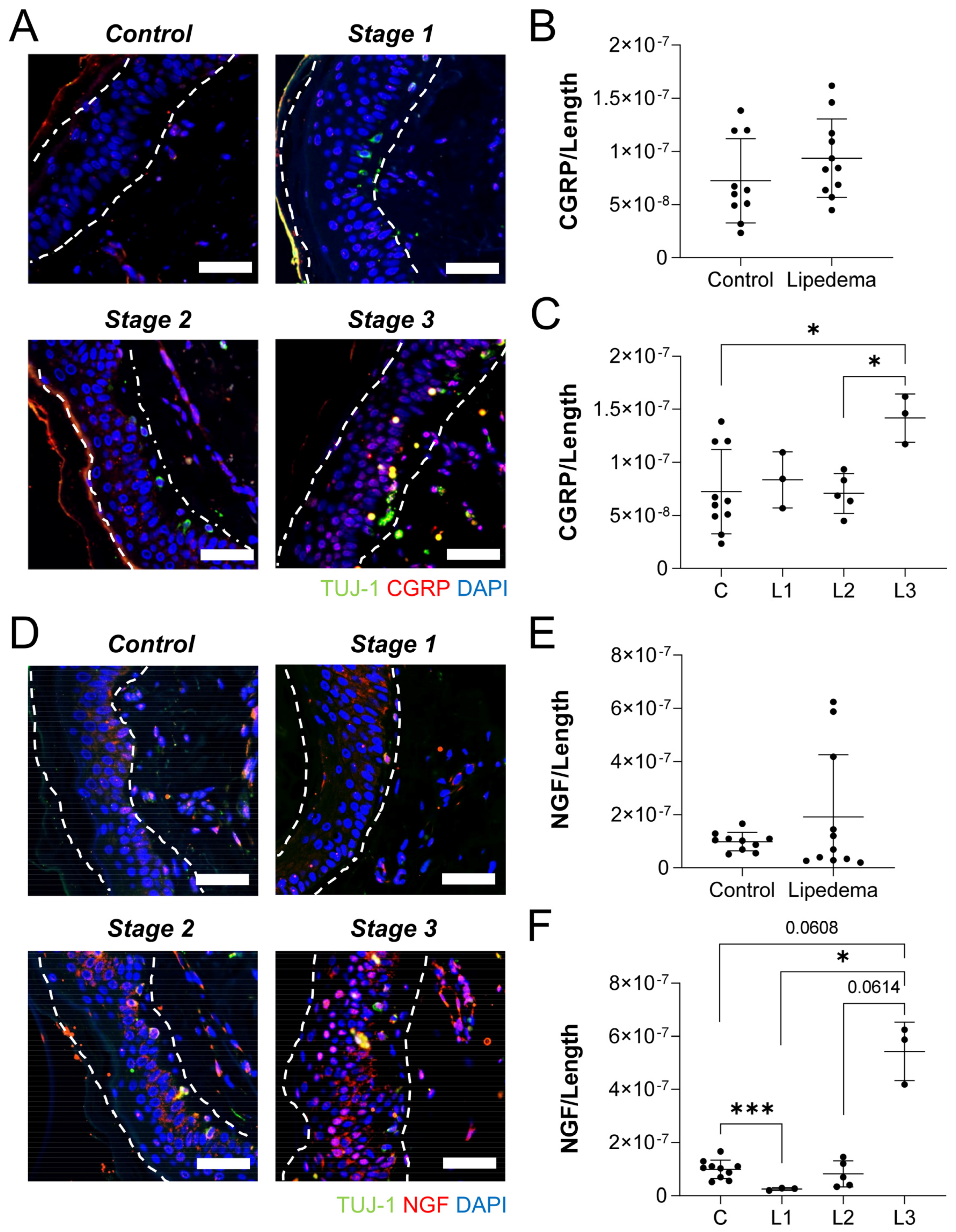
2.4. Neuronal Density Is Not Reduced in the Thigh but Is Reduced in the Abdominal Skin of Lipedema Patients
2.5. Lipedema Skin Exhibits Overexpression of Neuropeptides Assocatied with Neurogenic Inflammation
3. Discussion
4. Materials and Methods
4.1. Human Subject Recruitment
4.2. Clinical Examination and Pain Questionnaires
4.3. Von Frey Pain Assessment
4.4. Histology and Immunofluorescence
4.5. Image Analysis
4.6. Statistical Analysis and Data Presentation
5. Conclusions
6. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herbst, K.L.; Kahn, L.A.; Iker, E.; Ehrlich, C.; Wright, T.; McHutchison, L.; Schwartz, J.; Sleigh, M.; MC Donahue, P.; Lisson, K.H.; et al. Standard of care for lipedema in the United States. Phlebol. J. Venous Dis. 2021, 36, 779–796. [Google Scholar] [CrossRef]
- Herbst, K.; Mirkovskaya, L.; Bharhagava, A.; Chava, Y.; Te, H.T.C. Lipedema Fat and Signs and Symptoms of Illness, Increase with Advancing Stage. Arch. Med. 2015, 7, 410. [Google Scholar]
- Torre, Y.S.; Wadeea, R.; Rosas, V.; Herbst, K.L. Lipedema: Friend and foe. Horm. Mol. Biol. Clin. Investig. 2018, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Angst, F.; Lehmann, S.; Aeschlimann, A.; Sandòr, P.S.; Wagner, S. Cross-sectional validity and specificity of comprehensive measurement in lymphedema and lipedema of the lower extremity: A comparison of five outcome instruments. Health Qual. Life Outcomes 2020, 18, 245. [Google Scholar] [CrossRef] [PubMed]
- Buso, G.; Depairon, M.; Tomson, D.; Raffoul, W.; Vettor, R.; Mazzolai, L. Lipedema: A Call to Action! Obesity 2019, 27, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadban, S.; Cromer, W.; Allen, M.; Ussery, C.; Badowski, M.; Harris, D.; Herbst, K.L. Dilated Blood and Lymphatic Microvessels, Angiogenesis, Increased Macrophages, and Adipocyte Hypertrophy in Lipedema Thigh Skin and Fat Tissue. J. Obes. 2019, 2019, 8747461. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, R.; Donahue, P.M.; Petersen, K.J.; Garza, M.; Patel, N.; Lee, C.; Beckman, J.A.; Donahue, M.J. Upper and Lower Extremity Measurement of Tissue Sodium and Fat Content in Patients with Lipedema. Obesity 2020, 28, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Kruppa, P.; Georgiou, I.; Schmidt, J.; Ghods, M. Ten-Year Experience With the Surgical Treatment of Lipedema—Long-Term Follow-up after Multi-stage Liposuction. Plast. Reconstr. Surg. Glob. Open 2020, 8, 78. [Google Scholar] [CrossRef]
- Donahue, P.M.; Crescenzi, R.; Petersen, K.J.; Garza, M.; Patel, N.; Lee, C.; Chen, S.-C.; Donahue, M.J. Physical Therapy in Women with Early Stage Lipedema: Potential Impact of Multimodal Manual Therapy, Compression, Exercise, and Education Interventions. Lymphat. Res. Biol. 2022, 20, 382–390. [Google Scholar] [CrossRef]
- Gensior, M.H.L.; Cornely, M. Pain in lipoedema, fat in lipoedema and its consequences: Results of a patient survey based on a pain questionnaire. Handchir. Mikrochir. Plast. Chir. 2019, 51, 249–254. [Google Scholar] [PubMed]
- Aksoy, H.; Karadag, A.S.; Wollina, U. Cause and management of lipedema-associated pain. Dermatol. Ther. 2020, 34, e14364. [Google Scholar] [CrossRef]
- Malik, R.A. Pathology of human diabetic neuropathy. Handb. Clin. Neurol. 2014, 126, 249–259. [Google Scholar] [PubMed]
- Shillo, P.; Yiangou, Y.; Donatien, P.; Greig, M.; Selvarajah, D.; Wilkinson, I.D.; Anand, P.; Tesfaye, S. Nerve and Vascular Biomarkers in Skin Biopsies Differentiate Painful From Painless Peripheral Neuropathy in Type 2 Diabetes. Front. Pain Res. 2021, 2, 731658. [Google Scholar] [CrossRef] [PubMed]
- Siems, W.; Grune, T.; Voss, P.; Brenke, R. Anti-fibrosclerotic effects of shock wave therapy in lipedema and cellulite. BioFactors 2005, 24, 275–282. [Google Scholar] [CrossRef]
- Amann-Vesti, B.R.; Franzeck, U.K.; Bollinger, A. Microlymphatic aneurysms in patients with lipedema. Lymphology 2001, 34, 170–175. [Google Scholar]
- Zechner, U.; Kohlschmidt, N.; Kempf, O.; Gebauer, K.; Haug, K.; Engels, H.; Haaf, T.; Bartsch, O. Familial Sotos syndrome caused by a novel missense mutation, C2175S, in NSD1 and associated with normal intelligence, insulin dependent diabetes, bronchial asthma, and lipedema. Eur. J. Med. Genet. 2009, 52, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Angst, F.; Benz, T.; Lehmann, S.; Sandor, P.; Wagner, S. Common and Contrasting Characteristics of the Chronic Soft-Tissue Pain Conditions Fibromyalgia and Lipedema. J. Pain Res. 2021, 14, 2931–2941. [Google Scholar] [CrossRef]
- Beltran, K.; Herbst, K.L. Differentiating lipedema and Dercum’s disease. Int. J. Obes. 2016, 41, 240–245. [Google Scholar] [CrossRef]
- Dudek, J.E.; Białaszek, W.; Ostaszewski, P. Quality of life in women with lipoedema: A contextual behavioral approach. Qual. Life Res. 2015, 25, 401–408. [Google Scholar] [CrossRef]
- Felmerer, G.; Stylianaki, A.; Hollmén, M.; Ströbel, P.; Stepniewski, A.; Wang, A.; Frueh, F.S.; Kim, B.-S.; Giovanoli, P.; Lindenblatt, N.; et al. Increased levels of VEGF-C and macrophage infiltration in lipedema patients without changes in lymphatic vascular morphology. Sci. Rep. 2020, 10, 10947. [Google Scholar] [CrossRef]
- Michelini, S.; Chiurazzi, P.; Marino, V.; Dell’Orco, D.; Manara, E.; Baglivo, M.; Fiorentino, A.; Maltese, P.E.; Pinelli, M.; Herbst, K.L.; et al. Aldo-Keto Reductase 1C1 (AKR1C1) as the First Mutated Gene in a Family with Nonsyndromic Primary Lipedema. Int. J. Mol. Sci. 2020, 21, 6264. [Google Scholar] [CrossRef] [PubMed]
- Seidel, M.F.; Hügle, T.; Morlion, B.; Koltzenburg, M.; Chapman, V.; MaassenVanDenBrink, A.; Lane, N.E.; Perrot, S.; Zieglgänsberger, W. Neurogenic inflammation as a novel treatment target for chronic pain syndromes. Exp. Neurol. 2022, 356, 114108. [Google Scholar] [CrossRef] [PubMed]
- Lauria, G.; Bakkers, M.; Schmitz, C.; Lombardi, R.; Penza, P.; Devigili, G.; Smith, A.G.; Hsieh, S.-T.; Mellgren, S.I.; Umapathi, T.; et al. Intraepidermal nerve fiber density at the distal leg: A worldwide normative reference study. J. Peripher. Nerv. Syst. 2010, 15, 202–207. [Google Scholar] [CrossRef]
- Anand, P. Nerve growth factor regulates nociception in human health and disease. Br. J. Anaesth. 1995, 75, 201–208. [Google Scholar] [CrossRef]
- Kilo, S.; Harding-Rose, C.; Hargreaves, K.M.; Flores, C.M. Peripheral CGRP release as a marker for neurogenic inflammation: A model system for the study of neuropeptide secretion in rat paw skin. Pain 1997, 73, 201–207. [Google Scholar] [CrossRef]
- Schou, W.S.; Ashina, S.; Amin, F.M.; Goadsby, P.; Ashina, M. Calcitonin gene-related peptide and pain: A systematic review. J. Headache Pain 2017, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Mizumura, K.; Murase, S. Role of Nerve Growth Factor in Pain. Handb. Exp. Pharmacol. 2015, 227, 57–77. [Google Scholar] [CrossRef]
- Halk, A.B.; Damstra, R.J. First Dutch guidelines on lipedema using the international classification of functioning, disability and health. Phlebol. J. Venous Dis. 2016, 32, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.J.; Garza, M.; Donahue, P.M.C.; Harkins, K.D.; Marton, A.; Titze, J.; Donahue, M.J.; Crescenzi, R. Neuroimaging of Cerebral Blood Flow and Sodium in Women with Lipedema. Obesity 2020, 28, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ResearchMatch.org.
- Herbst, K.L. Rare adipose disorders (RADs) masquerading as obesity. Acta Pharmacol. Sin. 2012, 33, 155–172. [Google Scholar] [CrossRef]
- Duhon, B.H.; Phan, T.T.; Taylor, S.L.; Crescenzi, R.L.; Rutkowski, J.M. Current Mechanistic Understandings of Lymphedema and Lipedema: Tales of Fluid, Fat, and Fibrosis. Int. J. Mol. Sci. 2022, 23, 6621. [Google Scholar] [CrossRef] [PubMed]
- Au-Kostek, M.; Polaski, A.; Kolber, B.; Ramsey, A.; Kranjec, A.; Szucs, K. A Protocol of Manual Tests to Measure Sensation and Pain in Humans. JoVE 2016, 118, e54130. [Google Scholar]
- Paling, I.; MacIntyre, L. Survey of lipoedema symptoms and experience with compression garments. Br. J. Community Nurs. 2020, 25, S17–S22. [Google Scholar] [CrossRef]
- Fink, J.M.; Schreiner, L.; Marjanovic, G.; Erbacher, G.; Seifert, G.J.; Foeldi, M.; Bertsch, T. Leg Volume in Patients with Lipoedema following Bariatric Surgery. Visc. Med. 2020, 37, 206–211. [Google Scholar] [CrossRef] [PubMed]



| Controls (n = 23) | Stage 1 (n = 5) | Stage 2 (n = 13) | Stage 3 (n = 19) | ANOVA p-Value | |
|---|---|---|---|---|---|
| Age (years) | 39.7 ± 16.8 | 41.4 ± 11.0 | 50.5 ± 10.1 | 57.5 ± 8.5 a,b | 0.0002 |
| BMI (kg/m2) | 26.6 ± 5.4 | 29.3 ± 7.0 | 32.1 ± 7.6 | 42.2 ± 6.7 a,b,c | <0.0001 |
| Shoulder circumference (cm) | 108.3 ± 12.7 | 110.3 ± 11.6 | 110.3 ± 11.1 | 118.8 ± 11.5 | 0.0456 |
| Waist circumference (cm) | 84.7 ± 14.7 | 88.4 ± 17.6 | 91.4 ± 16.4 | 109.7 ± 14.1 a,c | 0.0005 |
| Hip circumference (cm) | 106.0 ± 12.7 | 110.7 ± 14.7 | 121.8 ± 18.3 a | 141.5 ± 18.4 a,b,c | <0.0001 |
| WHR | 0.80 ± 0.08 | 0.79 ± 0.06 | 0.75 ± 0.10 | 0.78 ± 0.09 | 0.5559 |
| Diabetes | 4.3% (n = 1/23) | 0% | 23% (n = 3/13) | 0% | n/a |
| Stage 1 | Stage 2 | Stage 3 | ANOVA p-Value | |
|---|---|---|---|---|
| 1. Pain in legs (%yes, n = number of positive responders/total subjects) | 60.0% (n = 3/5) | 61.5% (n = 8/13) | 100% (n = 16/16) | n/a |
| 2. Typical pain in legs (VAS, 0–100) | 25.0 ± 13.5 | 51.9 ± 20.2 | 61.8 ± 16.0 | 0.0105 |
| 3. Pain now in legs (VAS, 0–100) | 19.7 ± 16.2 | 38.1 ± 25.4 | 53.4 ± 25.6 | 0.0580 |
| 4. Worst pain in legs in past 4 weeks (VAS, 0–100) | 55.7 ± 20.8 | 69.1 ± 21.9 | 79.2 ± 12.1 | 0.2050 |
| 5. painDETECT: cumulative score (range 0–38) | 11.0 ± 5.2 | 10.5 ± 4.9 | 17.6 ± 7.2 b | 0.0271 |
| 6. painDETECT: Does your pain radiate in your legs or to other areas of your body? (%yes) | 33.3% | 37.5% | 62.5% | n/a |
| 7. painDETECT: pain behavior patterns rated never (0) to very strongly (5) (mean ± standard deviation) |  never (0) very strongly (5) | |||
| a. Do you suffer from a burning sensation (e.g., stinging needles) in the marked areas? | 1.7 ± 1.5 | 1.6 ± 1.5 | 2.4 ± 1.3 | 0.9693 |
| b. Do you have a tingling or prickling sensation in the area of your pain (like crawling ants or electrical tingling)? | 2.0 ± 1.0 | 1.8 ± 1.9 | 2.1 ± 1.6 | 0.8908 |
| c. Is light touching (clothing, a blanket) in this area painful? | 0.33 ± 0.1 | 0.6 ± 0.9 | 1.4 ± 1.3 a | 0.0122 |
| d. Do you have sudden pain attacks in the area of your pain, like electric shocks? | 1.0 ± 1.0 | 1.1 ± 1.2 | 2.4 ± 1.5 | 0.0532 |
| e. Is cold or heat (bath water) in this area occasionally painful? | 0.33 ± 0.1 | 0.8 ± 0.9 | 1.9 ± 1.4 a | 0.0011 |
| f. Do you suffer from a sensation of numbness in the painful areas? | 2.0 ± 2.0 | 1.8 ± 0.7 | 2.3 ± 1.6 | 0.7753 |
| g. Does slight pressure in this area trigger pain? | 2.7 ± 0.6 | 1.9 ± 0.6 | 3.6 ± 1.7 b | 0.0015 |
| Characteristic | Control | Lipedema |
|---|---|---|
| Number of subjects | 10 | 11 |
| Age (years) | 41.3 ± 10.6 | 43.8 ± 10.6 |
| BMI (kg/m2) | 26.0 ± 5.7 | 31.8 ± 4.3 * |
| Sex (% female) | 100 % | 100% |
| Race (% White) | 90% (n = 9/10) | 100% |
| Ethnicity (% Hispanic) | 30% (n = 3/10) | 0% |
| Menopausal status (% pre-menopausal) | 70% (n = 7/10) | 64% (n = 7/11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, A.; Crescenzi, R.; Usman, T.A.; Reyna, A.J.; Garza, M.E.; Al-Ghadban, S.; Herbst, K.L.; Donahue, P.M.C.; Rutkowski, J.M. Indications of Peripheral Pain, Dermal Hypersensitivity, and Neurogenic Inflammation in Patients with Lipedema. Int. J. Mol. Sci. 2022, 23, 10313. https://doi.org/10.3390/ijms231810313
Chakraborty A, Crescenzi R, Usman TA, Reyna AJ, Garza ME, Al-Ghadban S, Herbst KL, Donahue PMC, Rutkowski JM. Indications of Peripheral Pain, Dermal Hypersensitivity, and Neurogenic Inflammation in Patients with Lipedema. International Journal of Molecular Sciences. 2022; 23(18):10313. https://doi.org/10.3390/ijms231810313
Chicago/Turabian StyleChakraborty, Adri, Rachelle Crescenzi, Timaj A. Usman, Andrea J. Reyna, Maria E. Garza, Sara Al-Ghadban, Karen L. Herbst, Paula M. C. Donahue, and Joseph M. Rutkowski. 2022. "Indications of Peripheral Pain, Dermal Hypersensitivity, and Neurogenic Inflammation in Patients with Lipedema" International Journal of Molecular Sciences 23, no. 18: 10313. https://doi.org/10.3390/ijms231810313
APA StyleChakraborty, A., Crescenzi, R., Usman, T. A., Reyna, A. J., Garza, M. E., Al-Ghadban, S., Herbst, K. L., Donahue, P. M. C., & Rutkowski, J. M. (2022). Indications of Peripheral Pain, Dermal Hypersensitivity, and Neurogenic Inflammation in Patients with Lipedema. International Journal of Molecular Sciences, 23(18), 10313. https://doi.org/10.3390/ijms231810313






