Cell-Based and Selected Cell-Free Therapies for Myocardial Infarction: How Do They Compare to the Current Treatment Options?
Abstract
:1. Introduction
2. Myocardial Infarction (MI)—Pathology, Clinical Presentation, Diagnostics, and Types
2.1. Etiopathogenesis
2.2. Clinical Presentation and Diagnostics
2.3. Types of Myocardial Infarction (MI)
3. Current Therapies for MI
3.1. Drug Therapies
- Antithrombotic drugs,
- Β-receptor blockers,
- Angiotensin-converting enzyme inhibitors,
- Angiotensin receptor blockers and statins.
3.2. Thrombolytic Therapy
3.3. Percutaneous Coronary Intervention
3.4. Coronary Artery Bypass Grafting
4. Cell-Based Therapies for MI
4.1. Mesenchymal Stem Cell-Based Therapy for MI
4.2. Induced Pluripotent Stem Cell (iPSC)-Based Therapy for MI
4.3. iPSC-Derived Cardiomyocyte Cardiac Patches and Bioprinting
4.4. Potential of Telocytes in the Therapy of MI
5. Cell-Free Therapy for MI
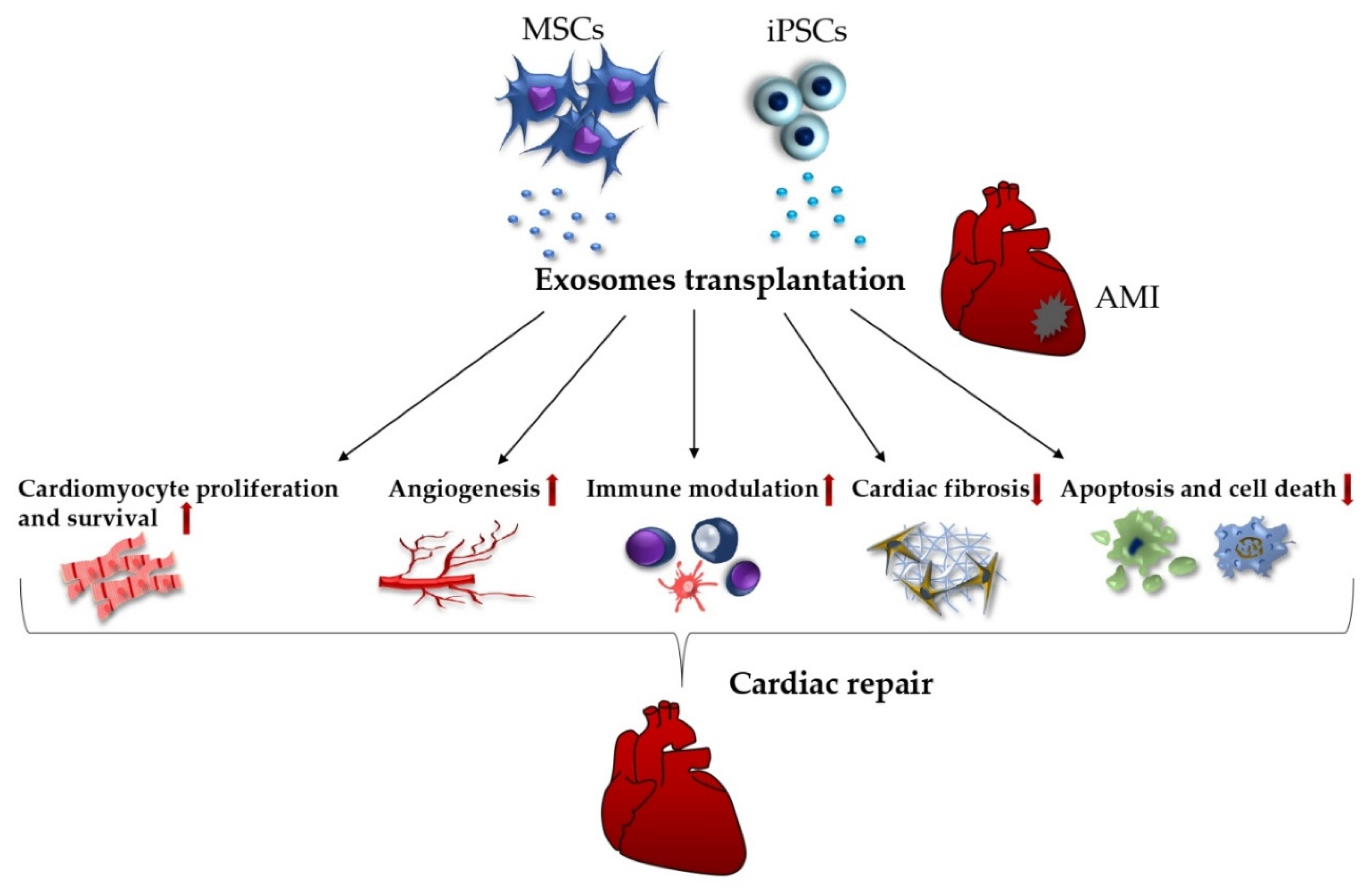
5.1. Exosomes
5.2. Non-Coding RNAs
5.3. Gene Therapy
5.4. Acellular Cardiac Patches
6. Summary and Possible Prospects
- Many years of experience with clinical application;
- Successful mitigation of mortality rates;
- Refined and elaborated guidelines available for all clinicians.
- Only moderate effect in addressing the post AMI complications;
- Impossibility to suppress the loss of functional heart muscle;
- Daily need of medications;
- High risk of recurrent development of the heart failure;
- Shortage of donor hearts.
- Patient specificity;
- High cardiac differentiation potential;
- Elimination of immune rejection;
- Development of large-scale cultivation systems;
- Substantial progress in understanding the molecular and cellular mechanism of MI;
- Encouraging results of iPSC-CMs regenerative capacity in animal models;
- Use of stem cells with additional 3D cardiac engineered biomaterials;
- Transplantation of iPSC-CMs as a patch or sheet resembling the structure and function of native myocardium, and restoring the lost function of damaged myocardium.
- Lack of uniform cardiac differentiation protocols;
- Incomplete stem cell differentiation into mature and functional CMs;
- Safety issues regarding the possible tumor formation caused by reprogramming factors, and epigenetic abnormalities;
- Heterogeneity of iPSCs populations;
- Costly and time-consuming process of iPSC-CMs generation;
- Lack of control over transplanted cell population;
- Poor graft survival rate;
- Difficulties in vascularized cardiac patches integration with host tissue regarding to electrical, mechanical, vascular, and biochemical compatibility;
- Possible toxicity of various biomaterials’ nanoparticles;
- Post-transplantation arrhythmia due to lack of electromechanical coupling with host cells;
- Clinically moderate benefits;
- Need for human clinical trials.
- Beneficial roles of EVs paracrine bioactive components in terms of protection of heart tissue from disease progression (promotion of angiogenesis, inhibition of ventricular remodeling, improvement of heart function, inhibition of local inflammation, regulation of immune responses);
- Reduction of above-mentioned challenges related to the use of cell-based therapy;
- Positive therapeutic effect demonstrated in animal studies;
- Possibility to generate bioengineered exosomes with enhanced targeting properties;
- Low/no risk of mutagenesis.
- Do not fulfil the medical need for heart regeneration in MI patients;
- Need for clear understanding of the paracrine signalling pathways;
- Issues such as lack of targeting, and low retention related to direct infusions of EVs paracrine agents;
- Need for establishment of standardized fabrication and purification methods;
- Challenging quality control of cell-free products;
- EV immunogenicity;
- Problems with biosafety, and biodegradation of bioengineered products;
- Significant reduction of retained growth factors within acellular cardiac patches;
- Need for human clinical trials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Ojha, N.; Dhamoon, A.S. Myocardial Infarction. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Daubert, M.A.; Jeremias, A. The utility of troponin measurement to detect myocardial infarction: Review of the current findings. Vasc. Health Risk Manag. 2010, 6, 691–699. [Google Scholar] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [PubMed]
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 20 June 2022).
- Reed, G.W.; Rossi, J.E.; Cannon, C.P. Acute myocardial infarction. Lancet 2017, 389, 197–210. [Google Scholar] [CrossRef]
- Alonaizan, R.; Carr, C. Cardiac regeneration following myocardial infarction: The need for regeneration and a review of cardiac stromal cell populations used for transplantation. Biochem. Soc. Trans. 2022, 50, 269–281. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- White, H.D.; van de Werf, F.J. Thrombolysis for acute myocardial infarction. Circulation 1998, 97, 1632–1646. [Google Scholar] [CrossRef]
- Ibáñez, B.; Heusch, G.; Ovize, M.; van de Werf, F. Evolving therapies for myocardial ischemia/reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1454–1471. [Google Scholar] [CrossRef] [PubMed]
- Sarkisian, L.; Saaby, L.; Poulsen, T.S.; Gerke, O.; Hosbond, S.; Jangaard, N.; Diederichsen, A.C.; Thygesen, K.; Mickley, H. Prognostic Impact of Myocardial Injury Related to Various Cardiac and Noncardiac Conditions. Am. J. Med. 2016, 129, 506–514.e501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyers, H.P.; Bracey, A.; Lee, D.; Lichtenheld, A.; Li, W.J.; Singer, D.D.; Kane, J.A.; Dodd, K.W.; Meyers, K.E.; Thode, H.C.; et al. Comparison of the ST-Elevation Myocardial Infarction (STEMI) vs. NSTEMI and Occlusion MI (OMI) vs. NOMI Paradigms of Acute MI. J. Emerg. Med. 2021, 60, 273–284. [Google Scholar] [CrossRef]
- Badescu, M.C.; Ciocoiu, M.; Rezus, E.; Badulescu, O.V.; Tanase, D.M.; Ouatu, A.; Dima, N.; Ganceanu-Rusu, A.R.; Popescu, D.; Seritean Isac, P.N.; et al. Current Therapeutic Approach to Acute Myocardial Infarction in Patients with Congenital Hemophilia. Life 2021, 11, 1072. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Bergmark, B.A.; Murphy, S.A.; O’Gara, P.T.; Smith, P.K.; Serruys, P.W.; Kappetein, A.P.; Park, S.J.; Park, D.W.; Christiansen, E.H.; et al. Percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass grafting in left main coronary artery disease: An individual patient data meta-analysis. Lancet 2021, 398, 2247–2257. [Google Scholar] [CrossRef]
- Yandrapalli, S.; Andries, G.; Gupta, S.; Dajani, A.R.; Aronow, W.S. Investigational drugs for the treatment of acute myocardial infarction: Focus on antiplatelet and anticoagulant agents. Expert Opin. Investig. Drugs 2019, 28, 223–234. [Google Scholar] [CrossRef]
- Kunadian, V.; Gibson, C.M. Thrombolytics and myocardial infarction. Cardiovasc. Ther. 2012, 30, e81–e88. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e21–e129. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Herrmann, J. Myocardial infarction due to percutaneous coronary intervention. N. Engl. J. Med. 2011, 364, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Morelli, M.B.; Matarese, A.; Sardu, C.; Santulli, G. Cardiomyocyte-derived exosomal microRNA-92a mediates post-ischemic myofibroblast activation both in vitro and ex vivo. ESC Heart Fail 2020, 7, 284–288. [Google Scholar] [CrossRef]
- Chioncel, V.; Andrei, C.L.; Brezeanu, R.; Sinescu, C.; Avram, A.; Tatu, A.L. Some Perspectives on Hypersensitivity to Coronary Stents. Int. J. Gen. Med. 2021, 14, 4327–4336. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Stone, G.W.; Holmes, D.R.; Gersh, B. Reperfusion injury, microvascular dysfunction, and cardioprotection: The “dark side” of reperfusion. Circulation 2009, 120, 2105–2112. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.H.; Smith, P.K. Coronary-Artery Bypass Grafting. N. Engl. J. Med. 2016, 374, 1954–1964. [Google Scholar] [CrossRef]
- Staritz, P.; de Moerloose, P.; Schutgens, R.; Dolan, G. Applicability of the European Society of Cardiology guidelines on management of acute coronary syndromes to people with haemophilia—An assessment by the ADVANCE Working Group. Haemophilia 2013, 19, 833–840. [Google Scholar] [CrossRef]
- Pan, Q.; Xu, J.; Wen, C.J.; Xiong, Y.Y.; Gong, Z.T.; Yang, Y.J. Nanoparticles: Promising Tools for the Treatment and Prevention of Myocardial Infarction. Int. J. Nanomed. 2021, 16, 6719–6747. [Google Scholar] [CrossRef] [PubMed]
- Burridge, P.W.; Keller, G.; Gold, J.D.; Wu, J.C. Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 2012, 10, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.C.; Ye, J.; Shukla, P.; Hua, G.; Chen, D.; Lin, Z.; Liu, J.; Chai, J.; Gold, J.; Wu, J.; et al. Development of a Scalable Suspension Culture for Cardiac Differentiation from Human Pluripotent Stem Cells. Stem Cell Res. 2015, 15, 365–375. [Google Scholar] [CrossRef]
- Ramesh, S.; Govarthanan, K.; Ostrovidov, S.; Zhang, H.; Hu, Q.; Camci-Unal, G.; Verma, R.S.; Ramalingam, M. Correction to: Cardiac Differentiation of Mesenchymal Stem Cells: Impact of Biological and Chemical Inducers. Stem Cell Rev. Rep. 2021, 17, 1515, Erratum in Stem Cell Rev. Rep. 2021, 17, 1343–1361. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Xu, W.; Zhang, H.; Wang, Q.; Yu, J.; Zhang, R.; Chen, Y.; Xia, Y.; Wang, J.; Wang, D. Transplantation of human induced pluripotent stem cell-derived cardiomyocytes improves myocardial function and reverses ventricular remodeling in infarcted rat hearts. Stem Cell Res. Ther. 2020, 11, 73. [Google Scholar] [CrossRef]
- Fang, Y.H.; Wang, S.P.H.; Gao, Z.H.; Wu, S.N.; Chang, H.Y.; Yang, P.J.; Liu, P.Y.; Liu, Y.W. Efficient Cardiac Differentiation of Human Amniotic Fluid-Derived Stem Cells into Induced Pluripotent Stem Cells and Their Potential Immune Privilege. Int. J. Mol. Sci. 2020, 21, 2359. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, S.H.; Youn, Y.J.; Ahn, M.S.; Kim, J.Y.; Yoo, B.S.; Yoon, J.; Kwon, W.; Hong, I.S.; Lee, K.; et al. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J. Korean Med. Sci. 2014, 29, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yu, J.; Zhang, N.; Li, W.; Wang, J.; Cai, G.; Chen, Y.; Yang, Y.; Liu, Z. Bone marrow mesenchymal stem cells transfer in patients with ST-segment elevation myocardial infarction: Single-blind, multicenter, randomized controlled trial. Stem Cell Res. Ther. 2021, 12, 33. [Google Scholar] [CrossRef]
- Higuchi, T.; Miyagawa, S.; Pearson, J.T.; Fukushima, S.; Saito, A.; Tsuchimochi, H.; Sonobe, T.; Fujii, Y.; Yagi, N.; Astolfo, A.; et al. Functional and Electrical Integration of Induced Pluripotent Stem Cell-Derived Cardiomyocytes in a Myocardial Infarction Rat Heart. Cell Transpl. 2015, 24, 2479–2489. [Google Scholar] [CrossRef]
- Ou, M.; Zhao, M.; Li, C.; Tang, D.; Xu, Y.; Dai, W.; Sui, W.; Zhang, Y.; Xiang, Z.; Mo, C.; et al. Single-cell sequencing reveals the potential oncogenic expression atlas of human iPSC-derived cardiomyocytes. Biol. Open 2021, 10, bio053348. [Google Scholar] [CrossRef] [PubMed]
- Traverse, J.H. Using biomaterials to improve the efficacy of cell therapy following acute myocardial infarction. J. Cardiovasc. Transl. Res. 2012, 5, 67–72. [Google Scholar] [CrossRef]
- Yeung, E.; Fukunishi, T.; Bai, Y.; Bedja, D.; Pitaktong, I.; Mattson, G.; Jeyaram, A.; Lui, C.; Ong, C.S.; Inoue, T.; et al. Cardiac regeneration using human-induced pluripotent stem cell-derived biomaterial-free 3D-bioprinted cardiac patch in vivo. J. Tissue Eng. Regen. Med. 2019, 13, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, M.; di Pasquale, E. Toward Cardiac Regeneration: Combination of Pluripotent Stem Cell-Based Therapies and Bioengineering Strategies. Front. Bioeng. Biotechnol. 2020, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.M.; Fisher, S.A.; Brunskill, S.J.; Doree, C.; Mathur, A.; Watt, S.; Martin-Rendon, E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst. Rev. 2012, CD006536. [Google Scholar] [CrossRef]
- Gao, L.R.; Chen, Y.; Zhang, N.K.; Yang, X.L.; Liu, H.L.; Wang, Z.G.; Yan, X.Y.; Wang, Y.; Zhu, Z.M.; Li, T.C.; et al. Intracoronary infusion of Wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: Double-blind, randomized controlled trial. BMC Med. 2015, 13, 162. [Google Scholar] [CrossRef]
- Lemcke, H.; Voronina, N.; Steinhoff, G.; David, R. Recent Progress in Stem Cell Modification for Cardiac Regeneration. Stem Cells Int. 2018, 2018, 1909346. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, R.; Li, W.; Lin, J.; Stamm, C.; Steinhoff, G.; Ma, N. Therapeutic potential of menstrual blood-derived endometrial stem cells in cardiac diseases. Cell. Mol. Life Sci. 2019, 76, 1681–1695. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Miyoshi, S.; Ikegami, Y.; Hida, N.; Asada, H.; Togashi, I.; Suzuki, J.; Satake, M.; Nakamizo, H.; Tanaka, M.; et al. Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ. Res. 2010, 106, 1613–1623. [Google Scholar] [CrossRef] [Green Version]
- Ishikane, S.; Hosoda, H.; Yamahara, K.; Akitake, Y.; Kyoungsook, J.; Mishima, K.; Iwasaki, K.; Fujiwara, M.; Miyazato, M.; Kangawa, K.; et al. Allogeneic transplantation of fetal membrane-derived mesenchymal stem cell sheets increases neovascularization and improves cardiac function after myocardial infarction in rats. Transplantation 2013, 96, 697–706. [Google Scholar] [CrossRef]
- Van Linthout, S.; Stamm, C.; Schultheiss, H.P.; Tschöpe, C. Mesenchymal stem cells and inflammatory cardiomyopathy: Cardiac homing and beyond. Cardiol. Res. Pract. 2011, 2011, 757154. [Google Scholar] [CrossRef]
- Banerjee, M.N.; Bolli, R.; Hare, J.M. Clinical Studies of Cell Therapy in Cardiovascular Medicine: Recent Developments and Future Directions. Circ. Res. 2018, 123, 266–287. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Falguera, D.; Iborra-Egea, O.; Gálvez-Montón, C. iPSC Therapy for Myocardial Infarction in Large Animal Models: Land of Hope and Dreams. Biomedicines 2021, 9, 1836. [Google Scholar] [CrossRef] [PubMed]
- Siu, C.W.; Moore, J.C.; Li, R.A. Human embryonic stem cell-derived cardiomyocytes for heart therapies. Cardiovasc. Hematol. Disord. Drug Targets 2007, 7, 145–152. [Google Scholar] [CrossRef]
- Anderson, M.E.; Goldhaber, J.; Houser, S.R.; Puceat, M.; Sussman, M.A. Embryonic stem cell-derived cardiac myocytes are not ready for human trials. Circ. Res. 2014, 115, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.J.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.W.; Weyers, J.J.; Mahoney, W.M.; van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Cacciapuoti, I.; Parouchev, A.; Benhamouda, N.; Tachdjian, G.; Tosca, L.; et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: First clinical case report. Eur. Heart J. 2015, 36, 2011–2017. [Google Scholar] [CrossRef]
- Müller, P.; Lemcke, H.; David, R. Stem Cell Therapy in Heart Diseases—Cell Types, Mechanisms and Improvement Strategies. Cell Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Li, R.K.; Weisel, R.D.; Mickle, D.A.; Kim, E.J.; Sakai, T.; Jia, Z.Q. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 1999, 100, Ii247–Ii256. [Google Scholar] [CrossRef] [PubMed]
- Baikova, Y.P.; Fatkhudinov, T.; Bol’shakova, G.B.; Bukharova, T.B.; Slashcheva, G.A.; Khokhlova, O.V.; Murashev, A.N.; Gol’dshtein, D.V. Reparation of the myocardium after transplantation of mononuclear bone marrow cells. Bull Exp. Biol. Med. 2011, 150, 522–529. [Google Scholar] [CrossRef]
- Henning, R.J. Stem cells for cardiac repair: Problems and possibilities. Future Cardiol. 2013, 9, 875–884. [Google Scholar] [CrossRef]
- Luger, D.; Lipinski, M.J.; Westman, P.C.; Glover, D.K.; Dimastromatteo, J.; Frias, J.C.; Albelda, M.T.; Sikora, S.; Kharazi, A.; Vertelov, G.; et al. Intravenously Delivered Mesenchymal Stem Cells: Systemic Anti-Inflammatory Effects Improve Left Ventricular Dysfunction in Acute Myocardial Infarction and Ischemic Cardiomyopathy. Circ. Res. 2017, 120, 1598–1613. [Google Scholar] [CrossRef] [PubMed]
- Karpov, A.A.; Uspenskaya, Y.K.; Minasian, S.M.; Puzanov, M.V.; Dmitrieva, R.I.; Bilibina, A.A.; Anisimov, S.V.; Galagudza, M.M. The effect of bone marrow- and adipose tissue-derived mesenchymal stem cell transplantation on myocardial remodelling in the rat model of ischaemic heart failure. Int. J. Exp. Pathol. 2013, 94, 169–177. [Google Scholar] [CrossRef]
- Kir, D.; Patel, M.J.; Munagala, M.R. What Is the Status of Regenerative Therapy in Heart Failure? Curr. Cardiol. Rep. 2021, 23, 146. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Cho, D.I.; Jeong, H.Y.; Kang, H.J.; Kim, M.R.; Cho, M.; Kim, Y.S.; Ahn, Y. Adjuvant role of macrophages in stem cell-induced cardiac repair in rats. Exp. Mol. Med. 2018, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ben-Mordechai, T.; Holbova, R.; Landa-Rouben, N.; Harel-Adar, T.; Feinberg, M.S.; Abd Elrahman, I.; Blum, G.; Epstein, F.H.; Silman, Z.; Cohen, S.; et al. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J. Am. Coll. Cardiol. 2013, 62, 1890–1901. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, S.; Zhang, Y.; Yu, B.; Xu, Y.; Guan, Z. Paracrine action mediate the antifibrotic effect of transplanted mesenchymal stem cells in a rat model of global heart failure. Mol. Biol. Rep. 2009, 36, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Meng, Q.; Li, Y.; Deng, S.; Yu, Z.; Liu, Z.; Zhang, L.; Fan, H. C-Kit Positive Cardiac Stem Cells and Bone Marrow-Derived Mesenchymal Stem Cells Synergistically Enhance Angiogenesis and Improve Cardiac Function After Myocardial Infarction in a Paracrine Manner. J. Card. Fail 2017, 23, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. Cell-Based Therapy in Cardiac Regeneration: An Overview. Circ. Res. 2018, 123, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Choudry, F.; Hamshere, S.; Saunders, N.; Veerapen, J.; Bavnbek, K.; Knight, C.; Pellerin, D.; Locca, D.; Westwood, M.; Rakhit, R.; et al. A randomized double-blind control study of early intra-coronary autologous bone marrow cell infusion in acute myocardial infarction: The REGENERATE-AMI clinical trial. Eur. Heart J. 2016, 37, 256–263. [Google Scholar] [CrossRef]
- Mathur, A.; Sim, D.S.; Choudry, F.; Veerapen, J.; Colicchia, M.; Turlejski, T.; Hussain, M.; Hamshere, S.; Locca, D.; Rakhit, R.; et al. Five-year follow-up of intracoronary autologous cell therapy in acute myocardial infarction: The REGENERATE-AMI trial. ESC Heart Fail. 2022, 9, 1152–1159. [Google Scholar] [CrossRef]
- Kang, S.; Yang, Y.J.; Li, C.J.; Gao, R.L. Effects of intracoronary autologous bone marrow cells on left ventricular function in acute myocardial infarction: A systematic review and meta-analysis for randomized controlled trials. Coron. Artery Dis. 2008, 19, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.; Nijveldt, R.; van der Vleuten, P.A.; Tijssen, J.G.; van der Giessen, W.J.; Tio, R.A.; Waltenberger, J.; ten Berg, J.M.; Doevendans, P.A.; Aengevaeren, W.R.; et al. Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: Results of the randomized controlled HEBE trial. Eur. Heart J. 2011, 32, 1736–1747. [Google Scholar]
- Delewi, R.; Andriessen, A.; Tijssen, J.G.; Zijlstra, F.; Piek, J.J.; Hirsch, A. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: A meta-analysis of randomised controlled clinical trials. Heart 2013, 99, 225–232. [Google Scholar] [CrossRef]
- Povsic, T.J.; Gersh, B.J. Stem Cells in Cardiovascular Diseases: 30,000-Foot View. Cells 2021, 10, 600. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Afjeh-Dana, E.; Naserzadeh, P.; Moradi, E.; Hosseini, N.; Seifalian, A.M.; Ashtari, B. Stem Cell Differentiation into Cardiomyocytes: Current Methods and Emerging Approaches. Stem Cell Rev. Rep. 2022. [Google Scholar] [CrossRef]
- Terashvili, M.; Bosnjak, Z.J. Stem Cell Therapies in Cardiovascular Disease. J. Cardiothorac. Vasc. Anesth 2019, 33, 209–222. [Google Scholar] [CrossRef]
- Bartolucci, J.; Verdugo, F.J.; González, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients with Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ. Res. 2017, 121, 1192–1204. [Google Scholar]
- Hu, Z.P.; Bao, Y.; Chen, D.N.; Cheng, Y.; Song, B.; Liu, M.; Li, D.; Wang, B.N. Effects of recombinant adenovirus hepatocyte growth factor gene on myocardial remodeling in spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Ulus, A.T.; Mungan, C.; Kurtoglu, M.; Celikkan, F.T.; Akyol, M.; Sucu, M.; Toru, M.; Gul, S.S.; Cinar, O.; Can, A. Intramyocardial Transplantation of Umbilical Cord Mesenchymal Stromal Cells in Chronic Ischemic Cardiomyopathy: A Controlled, Randomized Clinical Trial (HUC-HEART Trial). Int. J. Stem. Cells. 2020, 13, 364–376. [Google Scholar] [CrossRef]
- Stem Cell in Acute Myocardial Infarction (AMI). Available online: https://clinicaltrials.gov/ct2/show/NCT04340609?term=stem+cells&cond=Myocardial+Infarction&draw=2&rank=5 (accessed on 21 July 2022).
- Makkar, R.R.; Kereiakes, D.J.; Aguirre, F.; Kowalchuk, G.; Chakravarty, T.; Malliaras, K.; Francis, G.S.; Povsic, T.J.; Schatz, R.; Traverse, J.H.; et al. Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): A randomized, placebo-controlled, double-blinded trial. Eur. Heart. J. 2020, 41, 3451–3458. [Google Scholar] [CrossRef]
- Bockeria, L.; Bogin, V.; Bockeria, O.; Le, T.; Alekyan, B.; Woods, E.J.; Brown, A.A.; Ichim, T.E.; Patel, A.N. Endometrial regenerative cells for treatment of heart failure: A new stem cell enters the clinic. J. Transl. Med. 2013, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Hida, N.; Nishiyama, N.; Miyoshi, S.; Kira, S.; Segawa, K.; Uyama, T.; Mori, T.; Miyado, K.; Ikegami, Y.; Cui, C.; et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells 2008, 26, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.W.; Huang, Q.M.; Wu, H.Y.; Zuo, G.S.; Gu, H.C.; Deng, K.Y.; Xin, H.B. Characteristics and Therapeutic Potential of Human Amnion-Derived Stem Cells. Int. J. Mol. Sci. 2021, 22, 970. [Google Scholar] [CrossRef]
- Nasseri Maleki, S.; Aboutaleb, N.; Nazarinia, D.; Allahverdi Beik, S.; Qolamian, A.; Nobakht, M. Conditioned medium obtained from human amniotic membrane-derived mesenchymal stem cell attenuates heart failure injury in rats. Iran J. Basic Med. Sci. 2019, 22, 1253–1258. [Google Scholar]
- Razavi Tousi, S.M.; Faghihi, M.; Nobakht, M.; Molazem, M.; Kalantari, E.; Darbandi Azar, A.; Aboutaleb, N. Improvement of Heart Failure by Human Amniotic Mesenchymal Stromal Cell Transplantation in Rats. J. Tehran Heart Cent. 2016, 11, 123–138. [Google Scholar] [PubMed]
- Takov, K.; He, Z.; Johnston, H.E.; Timms, J.F.; Guillot, P.V.; Yellon, D.M.; Davidson, S.M. Small extracellular vesicles secreted from human amniotic fluid mesenchymal stromal cells possess cardioprotective and promigratory potential. Basic Res. Cardiol. 2020, 115, 26. [Google Scholar] [CrossRef]
- Chen, S.L.; Fang, W.W.; Ye, F.; Liu, Y.H.; Qian, J.; Shan, S.J.; Zhang, J.J.; Chunhua, R.Z.; Liao, L.M.; Lin, S.; et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am. J. Cardiol. 2004, 94, 92–95. [Google Scholar] [CrossRef]
- Suncion, V.Y.; Ghersin, E.; Fishman, J.E.; Zambrano, J.P.; Karantalis, V.; Mandel, N.; Nelson, K.H.; Gerstenblith, G.; DiFede Velazquez, D.L.; Breton, E.; et al. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally? An analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial. Circ. Res. 2014, 114, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Guijarro, D.; Lebrin, M.; Lairez, O.; Bourin, P.; Piriou, N.; Pozzo, J.; Lande, G.; Berry, M.; le Tourneau, T.; Cussac, D.; et al. Intramyocardial transplantation of mesenchymal stromal cells for chronic myocardial ischemia and impaired left ventricular function: Results of the MESAMI 1 pilot trial. Int. J. Cardiol. 2016, 209, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Bone Marrow Transfer to Enhance ST-Elevation Infarct Regeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT00224536 (accessed on 21 July 2022).
- Bone Marrow-Derived Stem Cell Transfer in Acute Myocardial Infarctions. Available online: https://clinicaltrials.gov/ct2/show/NCT00264316?term=stem+cells&cond=Myocardial+Infarction&draw=4&rank=23 (accessed on 21 July 2022).
- Myocardial Stem Cell Administration after Acute Myocardial Infarction (MYSTAR) Study (MYSTAR). Available online: https://clinicaltrials.gov/ct2/show/NCT00384982?term=stem+cells&cond=Myocardial+Infarction&draw=2&rank=2 (accessed on 21 July 2022).
- Stem Cell Therapy to Improve Myocardial Function in Patients with Acute Myocardial Infarction. Available online: https://clinicaltrials.gov/ct2/show/NCT00316381 (accessed on 21 July 2022).
- Intracoronary Autologous Stem Cell Transplantation in ST Elevation Myocardial Infarction: TRACIA STUDY (TRACIA). Available online: https://clinicaltrials.gov/ct2/show/NCT00725738?term=stem+cells&cond=Myocardial+Infarction&draw=2&rank=1 (accessed on 21 July 2022).
- Safety and Efficacy of Intracoronary Adult Human Mesenchymal Stem Cells after Acute Myocardial Infarction (SEED-MSC). Available online: https://clinicaltrials.gov/ct2/show/NCT01392105?term=stem+cells&cond=Myocardial+Infarction&draw=2&rank=4 (accessed on 21 July 2022).
- Bone Marrow Mesenchymal Stem Cells Transfer in Patients with ST-Segment Elevation Myocardial Infarction (Bmmsct). Available online: https://clinicaltrials.gov/ct2/show/NCT04421274?term=stem+cells&cond=Myocardial+Infarction&draw=2&rank=6 (accessed on 21 July 2022).
- Use of Adult Autologous Stem Cells in Treating People 2 to 3 Weeks After Having a Heart Attack (The Late TIME Study). Available online: https://clinicaltrials.gov/ct2/show/NCT00684060?term=stem+cells&cond=Myocardial+Infarction&draw=5&rank=63 (accessed on 21 July 2022).
- Intracoronary Human Wharton’s Jelly- Derived Mesenchymal Stem Cells (WJ-MSCs) Transfer in Patients with Acute Myocardial Infarction (AMI) (WJ-MSC-AMI). Available online: https://clinicaltrials.gov/ct2/show/NCT01291329?term=stem+cells&cond=Myocardial+Infarction&draw=3&rank=14 (accessed on 21 July 2022).
- Cell Therapy in Myocardial Infarction (EMRTCC). Available online: https://clinicaltrials.gov/ct2/show/NCT00350766?term=stem+cells&cond=Myocardial+Infarction&draw=2&rank=49 (accessed on 21 July 2022).
- Human Umbilical Cord Stroma MSC in Myocardial Infarction (HUC-HEART). Available online: https://clinicaltrials.gov/ct2/show/NCT02323477?term=stem+cells&cond=Myocardial+Infarction&draw=5&rank=69 (accessed on 21 July 2022).
- Bone Marrow Derived Adult Stem Cells for Acute Anterior Myocardial Infarction (REGEN-AMI). Available online: https://clinicaltrials.gov/ct2/show/NCT00765453 (accessed on 21 July 2022).
- Allogeneic Heart Stem Cells to Achieve Myocardial Regeneration (ALLSTAR). Available online: https://clinicaltrials.gov/ct2/show/NCT01458405 (accessed on 21 July 2022).
- Heart Patch for Myocardial Infarction COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04728906?term=stem+cells&cond=Myocardial+Infarction&draw=5&rank=33 (accessed on 21 July 2022).
- A Study of iPS Cell-derived Cardiomyocyte Spheroids (HS-001) in Patients with Heart Failure (LAPiS Study) (LAPiS). Available online: https://clinicaltrials.gov/ct2/show/NCT04945018 (accessed on 21 July 2022).
- Gnecchi, M.; He, H.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005, 11, 367–368. [Google Scholar] [CrossRef]
- Fischer, K.M.; Cottage, C.T.; Wu, W.; Din, S.; Gude, N.A.; Avitabile, D.; Quijada, P.; Collins, B.L.; Fransioli, J.; Sussman, M.A. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation 2009, 120, 2077–2087. [Google Scholar] [CrossRef]
- Mohsin, S.; Khan, M.; Toko, H.; Bailey, B.; Cottage, C.T.; Wallach, K.; Nag, D.; Lee, A.; Siddiqi, S.; Lan, F.; et al. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J. Am. Coll. Cardiol. 2012, 60, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Quijada, P.; Toko, H.; Fischer, K.M.; Bailey, B.; Reilly, P.; Hunt, K.D.; Gude, N.A.; Avitabile, D.; Sussman, M.A. Preservation of myocardial structure is enhanced by pim-1 engineering of bone marrow cells. Circ. Res. 2012, 111, 77–86. [Google Scholar] [CrossRef]
- Ebeid, D.E.; Firouzi, F.; Esquer, C.Y.; Navarrete, J.M.; Wang, B.J.; Gude, N.A.; Sussman, M.A. PIM1 Promotes Survival of Cardiomyocytes by Upregulating c-Kit Protein Expression. Cells 2020, 9, 2001. [Google Scholar] [CrossRef]
- Beliën, H.; Evens, L.; Hendrikx, M.; Bito, V.; Bronckaers, A. Combining stem cells in myocardial infarction: The road to superior repair? Med. Res. Rev. 2022, 42, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Pushp, P.; Nogueira, D.E.S.; Rodrigues, C.A.V.; Ferreira, F.C.; Cabral, J.M.S.; Gupta, M.K. A Concise Review on Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Personalized Regenerative Medicine. Stem Cell Rev. Rep. 2021, 17, 748–776. [Google Scholar] [CrossRef]
- Budniatzky, I.; Gepstein, L. Concise review: Reprogramming strategies for cardiovascular regenerative medicine: From induced pluripotent stem cells to direct reprogramming. Stem Cells Transl. Med. 2014, 3, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, M.A.; Chen, K.Y.; Naumova, A.V.; Muskheli, V.; Fugate, J.A.; Dupras, S.K.; Reinecke, H.; Xu, C.; Hassanipour, M.; Police, S.; et al. Cardiomyocytes Derived from Human Embryonic Stem Cells in Pro-Survival Factors Enhance Function of Infarcted Rat Hearts. Nat. Biotechnol. 2007, 25, 1015–1024. [Google Scholar] [CrossRef]
- Paige, S.L.; Osugi, T.; Afanasiev, O.K.; Pabon, L.; Reinecke, H.; Murry, C.E. Endogenous Wnt/β-Catenin Signaling Is Required for Cardiac Differentiation in Human Embryonic Stem Cells. PLoS ONE 2010, 5, e11134. [Google Scholar] [CrossRef]
- Burridge, P.W.; Holmström, A.; Wu, J.C. Chemically Defined Culture and Cardiomyocyte Differentiation of Human Pluripotent Stem Cells. Curr. Protoc. Hum. Genet. 2015, 87, 21.3.1–21.3.15. [Google Scholar] [CrossRef]
- Csöbönyeiová, M.; Danišovič, Ľ.; Polák, Š. Recent advances in iPSC technologies involving cardiovascular and neurodegenerative disease modeling. Gen. Physiol. Biophys. 2016, 35, 1–12. [Google Scholar]
- Ahmed, R.P.; Ashraf, M.; Buccini, S.; Shujia, J.; Haider, H. Cardiac tumorigenic potential of induced pluripotent stem cells in an immunocompetent host with myocardial infarction. Regen. Med. 2011, 6, 171–178. [Google Scholar] [CrossRef]
- Hattori, F.; Chen, H.; Yamashita, H.; Tohyama, S.; Satoh, Y.S.; Yuasa, S.; Li, W.; Yamakawa, H.; Tanaka, T.; Onitsuka, T.; et al. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat. Methods 2010, 7, 61–66. [Google Scholar] [CrossRef]
- Horikoshi, Y.; Yan, Y.; Terashvili, M.; Wells, C.; Horikoshi, H.; Fujita, S.; Bosnjak, Z.J.; Bai, X. Fatty Acid-Treated Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes Exhibit Adult Cardiomyocyte-Like Energy Metabolism Phenotypes. Cells 2019, 8, 1095. [Google Scholar] [CrossRef] [PubMed]
- Mattapally, S.; Zhu, W.; Fast, V.G.; Gao, L.; Worley, C.; Kannappan, R.; Borovjagin, A.V.; Zhang, J. Spheroids of cardiomyocytes derived from human-induced pluripotent stem cells improve recovery from myocardial injury in mice. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H327–H339. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Z.; Dong, M. Cardiac repair in a murine model of myocardial infarction with human induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2020, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Biagi, D.; Fantozzi, E.T.; Campos-Oliveira, J.C.; Naghetini, M.V.; Ribeiro, A.F., Jr.; Rodrigues, S.; Ogusuku, I.; Vanderlinde, R.; Christie, M.L.A.; Mello, D.B.; et al. In Situ Maturated Early-Stage Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Improve Cardiac Function by Enhancing Segmental Contraction in Infarcted Rats. J. Pers. Med. 2021, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Soma, Y.; Nakajima, K.; Kanazawa, H.; Tohyama, S.; Tabei, R.; Hirano, A.; Handa, N.; Yamada, Y.; Okuda, S.; et al. Intramyocardial Transplantation of Human iPS Cell-Derived Cardiac Spheroids Improves Cardiac Function in Heart Failure Animals. JACC Basic Transl. Sci. 2021, 6, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chang, Y.H.; Xiong, Q.; Zhang, P.; Zhang, L.; Somasundaram, P.; Lepley, M.; Swingen, C.; Su, L.; Wendel, J.S.; et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 2014, 15, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Miyagawa, S.; Saito, A.; Fukushima, S.; Harada, A.; Ito, E.; Ohashi, F.; Watabe, T.; Hatazawa, J.; Matsuura, K.; et al. Transplantation of Human-induced Pluripotent Stem Cell-derived Cardiomyocytes Is Superior to Somatic Stem Cell Therapy for Restoring Cardiac Function and Oxygen Consumption in a Porcine Model of Myocardial Infarction. Transplantation 2019, 103, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K.; et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388–391. [Google Scholar] [CrossRef]
- Cyranoski, D. ‘Reprogrammed’ stem cells approved to mend human hearts for the first time. Nature 2018, 557, 619–620. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, Y.; Pan, T.; Zhu, X.; Chong, H.; Xu, C.; Fan, F.; Cao, H.; Zhang, B.; Pan, J.; et al. Epicardial injection of allogeneic human-induced-pluripotent stem cell-derived cardiomyocytes in patients with advanced heart failure: Protocol for a phase I/IIa dose-escalation clinical trial. BMJ Open 2022, 12, e056264. [Google Scholar] [CrossRef] [PubMed]
- Mallapaty, S. Revealed: Two men in China were first to receive pioneering stem-cell treatment for heart disease. Nature 2020, 581, 249–250. [Google Scholar] [CrossRef]
- Tani, H.; Tohyama, S.; Kishino, Y.; Kanazawa, H.; Fukuda, K. Production of functional cardiomyocytes and cardiac tissue from human induced pluripotent stem cells for regenerative therapy. J. Mol. Cell. Cardiol. 2022, 164, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Chingale, M.; Zhu, D.; Cheng, K.; Huang, K. Bioengineering Technologies for Cardiac Regenerative Medicine. Front. Bioeng. Biotechnol. 2021, 9, 681705. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E.; et al. Large Cardiac Muscle Patches Engineered from Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery from Myocardial Infarction in Swine. Circulation 2018, 137, 1712–1730. [Google Scholar] [CrossRef] [PubMed]
- Navaei, A.; Saini, H.; Christenson, W.; Sullivan, R.T.; Ros, R.; Nikkhah, M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016, 41, 133–146. [Google Scholar] [CrossRef]
- Walker, B.W.; Lara, R.P.; Yu, C.H.; Sani, E.S.; Kimball, W.; Joyce, S.; Annabi, N. Engineering a naturally-derived adhesive and conductive cardiopatch. Biomaterials 2019, 207, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Baei, P.; Hosseini, M.; Baharvand, H.; Pahlavan, S. Electrically conductive materials for in vitro cardiac microtissue engineering. J. Biomed. Mater. Res. A 2020, 108, 1203–1213. [Google Scholar] [CrossRef]
- Cui, H.; Liu, C.; Esworthy, T.; Huang, Y.; Yu, Z.X.; Zhou, X.; San, H.; Lee, S.J.; Hann, S.Y.; Boehm, M.; et al. 4D physiologically adaptable cardiac patch: A 4-month in vivo study for the treatment of myocardial infarction. Sci. Adv. 2020, 6, eabb5067. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, S.J.; Zhen, Z.; Wei, R.; Zhang, N.; Liao, S.Y.; Tse, H.F. Myocardial repair of bioengineered cardiac patches with decellularized placental scaffold and human-induced pluripotent stem cells in a rat model of myocardial infarction. Stem Cell Res. Ther. 2021, 12, 13. [Google Scholar] [CrossRef]
- Esmaeili, H.; Patino-Guerrero, A.; Hasany, M.; Ansari, M.O.; Memic, A.; Dolatshahi-Pirouz, A.; Nikkhah, M. Electroconductive biomaterials for cardiac tissue engineering. Acta Biomater. 2022, 139, 118–140. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, F.; Breckwoldt, K.; Pecha, S.; Kelly, A.; Geertz, B.; Starbatty, J.; Yorgan, T.; Cheng, K.H.; Lessmann, K.; Stolen, T.; et al. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci. Transl. Med. 2016, 8, 363ra148. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.; Geertz, B.; Reinsch, M.; Aksehirlioglu, B.; Hansen, A.; Eschenhagen, T.; Reichenspurner, H.; Weinberger, F.; Pecha, S. Implantation of hiPSC-derived Cardiac-muscle Patches after Myocardial Injury in a Guinea Pig Model. J. Vis. Exp. 2019, 145, e58810. [Google Scholar] [CrossRef] [PubMed]
- Wendel, J.S.; Ye, L.; Tao, R.; Zhang, J.; Zhang, J.; Kamp, T.J.; Tranquillo, R.T. Functional Effects of a Tissue-Engineered Cardiac Patch from Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in a Rat Infarct Model. Stem Cells Transl. Med. 2015, 4, 1324–1332. [Google Scholar] [CrossRef]
- Li, H.; Bao, M.; Nie, Y. Extracellular matrix-based biomaterials for cardiac regeneration and repair. Heart Fail. Rev. 2021, 26, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Häneke, T.; Sahara, M. Progress in Bioengineering Strategies for Heart Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 3482. [Google Scholar] [CrossRef]
- Traverse, J.H.; Henry, T.D.; Dib, N.; Patel, A.N.; Pepine, C.; Schaer, G.L.; DeQuach, J.A.; Kinsey, A.M.; Chamberlin, P.; Christman, K.L. First-in-Man Study of a Cardiac Extracellular Matrix Hydrogel in Early and Late Myocardial Infarction Patients. JACC Basic Transl. Sci. 2019, 4, 659–669. [Google Scholar] [CrossRef]
- Lu, T.Y.; Lin, B.; Kim, J.; Sullivan, M.; Tobita, K.; Salama, G.; Yang, L. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat. Commun. 2013, 4, 2307. [Google Scholar] [CrossRef] [PubMed]
- Guyette, J.P.; Charest, J.M.; Mills, R.W.; Jank, B.J.; Moser, P.T.; Gilpin, S.E.; Gershlak, J.R.; Okamoto, T.; Gonzalez, G.; Milan, D.J.; et al. Bioengineering Human Myocardium on Native Extracellular Matrix. Circ. Res. 2016, 118, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, H.; Bai, A.; Jiang, W.; Li, X.; Wang, X.; Mao, Y.; Lu, C.; Qian, R.; Guo, F.; et al. Functional engineered human cardiac patches prepared from nature’s platform improve heart function after acute myocardial infarction. Biomaterials 2016, 105, 52–65. [Google Scholar] [CrossRef]
- Ong, C.S.; Fukunishi, T.; Zhang, H.; Huang, C.Y.; Nashed, A.; Blazeski, A.; DiSilvestre, D.; Vricella, L.; Conte, J.; Tung, L.; et al. Biomaterial-Free Three-Dimensional Bioprinting of Cardiac Tissue using Human Induced Pluripotent Stem Cell Derived Cardiomyocytes. Sci. Rep. 2017, 7, 4566. [Google Scholar] [CrossRef] [PubMed]
- AnilKumar, S.; Allen, S.C.; Tasnim, N.; Akter, T.; Park, S.; Kumar, A.; Chattopadhyay, M.; Ito, Y.; Suggs, L.J.; Joddar, B. The applicability of furfuryl-gelatin as a novel bioink for tissue engineering applications. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 314–323. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef]
- Jang, J.; Kim, T.G.; Kim, B.S.; Kim, S.W.; Kwon, S.M.; Cho, D.W. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. 2016, 33, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Ma, Z.; Loskill, P.; Jeeawoody, S.; Healy, K.E. In vitro cardiac tissue models: Current status and future prospects. Adv. Drug Deliv. Rev. 2016, 96, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kim, S.W.; Choi, Y.J.; Lee, S.; Lee, S.H.; Kong, J.S.; Park, H.J.; Cho, D.W.; Jang, J. Decellularized extracellular matrix bioinks and the external stimuli to enhance cardiac tissue development in vitro. Acta Biomater. 2019, 95, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Yadid, M.; Oved, H.; Silberman, E.; Dvir, T. Bioengineering approaches to treat the failing heart: From cell biology to 3D printing. Nat. Rev. Cardiol. 2022, 19, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Zhou, Q.; Sun, Q.; Xiao, J. Telocytes in cardiac regeneration and repair. Semin. Cell Dev. Biol. 2016, 55, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Danisovic, L.; Kyselovic, J.; Gazova, A.; Musil, P.; Miko, M.; Polak, S. The functional morphology and role of cardiac telocytes in myocardium regeneration. Can. J. Physiol. Pharmacol. 2016, 94, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Zhaofu, L.; Dongqing, C. Cardiac Telocytes in Regeneration of Myocardium after Myocardial Infarction. Adv. Exp. Med. Biol. 2016, 913, 229–239. [Google Scholar] [PubMed]
- Zhao, B.; Liao, Z.; Chen, S.; Yuan, Z.; Yilin, C.; Lee, K.K.; Qi, X.; Shen, X.; Zheng, X.; Quinn, T.; et al. Intramyocardial transplantation of cardiac telocytes decreases myocardial infarction and improves post-infarcted cardiac function in rats. J. Cell. Mol. Med. 2014, 18, 780–789. [Google Scholar] [CrossRef]
- Lv, L.; Liao, Z.; Luo, J.; Chen, H.; Guo, H.; Yang, J.; Huang, R.; Pu, Q.; Zhao, H.; Yuan, Z.; et al. Cardiac telocytes exist in the adult Xenopus tropicalis heart. J. Cell. Mol. Med. 2020, 24, 2531–2541. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Lazana, I.; Anagnostopoulos, C. A Novel, Cell-Free Therapy to Enter Our Hearts: The Potential Role of Small EVs in Prevention and Treatment of CVD. Int. J. Mol. Sci. 2022, 23, 3662. [Google Scholar] [CrossRef]
- Khan, M.; Nickoloff, E.; Abramova, T.; Johnson, J.; Verma, S.K.; Krishnamurthy, P.; Mackie, A.R.; Vaughan, E.; Garikipati, V.N.; Benedict, C.; et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 2015, 117, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, J.; Yan, W.; Li, Y.; Shen, Z.; Asahara, T. Pretreatment of Cardiac Stem Cells with Exosomes Derived from Mesenchymal Stem Cells Enhances Myocardial Repair. J. Am. Heart Assoc. 2016, 5, e002856. [Google Scholar] [CrossRef]
- Zhu, L.P.; Tian, T.; Wang, J.Y.; He, J.N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.X.; Qiu, X.T.; Li, C.C.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, C.; Benedict, C.; Huang, G.; Truongcao, M.; Roy, R.; Cimini, M.; Garikipati, V.N.S.; Cheng, Z.; Koch, W.J.; et al. Interleukin-10 Deficiency Alters Endothelial Progenitor Cell-Derived Exosome Reparative Effect on Myocardial Repair via Integrin-Linked Kinase Enrichment. Circ. Res. 2020, 126, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Mol, E.A.; Goumans, M.J.; Sluijter, J.P.G. Cardiac Progenitor-Cell Derived Exosomes as Cell-Free Therapeutic for Cardiac Repair. Adv. Exp. Med. Biol. 2017, 998, 207–219. [Google Scholar]
- Loyer, X.; Zlatanova, I.; Devue, C.; Yin, M.; Howangyin, K.Y.; Klaihmon, P.; Guerin, C.L.; Kheloufi, M.; Vilar, J.; Zannis, K.; et al. Intra-Cardiac Release of Extracellular Vesicles Shapes Inflammation Following Myocardial Infarction. Circ. Res. 2018, 123, 100–106. [Google Scholar] [CrossRef]
- Gallet, R.; Dawkins, J.; Valle, J.; Simsolo, E.; de Couto, G.; Middleton, R.; Tseliou, E.; Luthringer, D.; Kreke, M.; Smith, R.R.; et al. Exosomes Secreted by Cardiosphere-Derived Cells Reduce Scarring, Attenuate Adverse Remodelling, and Improve Function in Acute and Chronic Porcine Myocardial Infarction. Eur. Heart J. 2017, 38, 201–211. [Google Scholar] [CrossRef]
- Adamiak, M.; Sahoo, S. Exosomes in Myocardial Repair: Advances and Challenges in the Development of Next-Generation Therapeutics. Mol. Ther. 2018, 26, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; Teng, H.; Qin, Y.; Luo, X.; Yang, P.; Zhang, W.; Chen, W.; Lv, D.; Tang, H. Extracellular vesicles derived from microRNA-150-5p-overexpressing mesenchymal stem cells protect rat hearts against ischemia/reperfusion. Aging 2020, 12, 12669–12683. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Chen, Y.; Chen, Y.; Meng, Q.; Sun, J.; Shao, L.; Yu, Y.; Huang, H.; Hu, Y.; Yang, Z.; et al. MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction. Stem Cells Int. 2018, 2018, 3290372. [Google Scholar] [CrossRef]
- Wang, N.; Chen, C.; Yang, D.; Liao, Q.; Luo, H.; Wang, X.; Zhou, F.; Yang, X.; Yang, J.; Zeng, C.; et al. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.S.; Afshari, J.T.; Esmaeili, S.A.; Saburi, E.; Joneidi, Z.; Momtazi-Borojeni, A.A. Cardioprotective microRNAs: Lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis 2019, 285, 1–9. [Google Scholar] [CrossRef]
- Shen, D.; He, Z. Mesenchymal stem cell-derived exosomes regulate the polarization and inflammatory response of macrophages via miR-21-5p to promote repair after myocardial reperfusion injury. Ann. Transl. Med. 2021, 9, 1323. [Google Scholar] [CrossRef]
- Chen, X.; Luo, Q. Potential clinical applications of exosomes in the diagnosis, treatment, and prognosis of cardiovascular diseases: A narrative review. Ann. Transl. Med. 2022, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Kurtzwald-Josefson, E.; Zeevi-Levin, N.; Rubchevsky, V.; Bechar Erdman, N.; Schwartz Rohaker, O.; Nahum, O.; Hochhauser, E.; Ben-Avraham, B.; Itskovitz-Eldor, J.; Aravot, D.; et al. Cardiac Fibroblast-Induced Pluripotent Stem Cell-Derived Exosomes as a Potential Therapeutic Mean for Heart Failure. Int. J. Mol. Sci. 2020, 21, 7215. [Google Scholar] [CrossRef]
- Zhu, D.; Cheng, K. Cardiac Cell Therapy for Heart Repair: Should the Cells Be Left Out? Cells 2021, 10, 641. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Xiong, X.; Liu, Y.; Wang, J. MiRNA-1: Functional Roles and Dysregulation in Heart Disease. Mol. BioSyst. 2014, 10, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Vilimova, M.; Pfeffer, S. Post-transcriptional Regulation of Polycistronic microRNAs. WIREs RNA 2022, e1749. [Google Scholar] [CrossRef]
- Kabekkodu, S.P.; Shukla, V.; Varghese, V.K.; D’Souza, J.; Chakrabarty, S.; Satyamoorthy, K. Clustered MiRNAs and Their Role in Biological Functions and Diseases: Biological Regulation by MiRNA Clusters. Biol. Rev. 2018, 93, 1955–1986. [Google Scholar] [CrossRef] [PubMed]
- Colpaert, R.M.W.; Calore, M. MicroRNAs in Cardiac Diseases. Cells 2019, 8, 737. [Google Scholar] [CrossRef]
- Jiang, W.; Agrawal, D.K.; Boosani, C.S. Non-Coding RNAs as Epigenetic Gene Regulators in Cardiovascular Diseases. In Non-Coding RNAs in Cardiovascular Diseases; Xiao, J., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2020; Volume 1229, pp. 133–148. ISBN 9789811516702. [Google Scholar]
- Wu, C.; Liu, B.; Wang, R.; Li, G. The Regulation Mechanisms and Clinical Application of MicroRNAs in Myocardial Infarction: A Review of the Recent 5 Years. Front. Cardiovasc. Med. 2022, 8, 809580. [Google Scholar] [CrossRef]
- Sun, C.; Liu, H.; Guo, J.; Yu, Y.; Yang, D.; He, F.; Du, Z. MicroRNA-98 Negatively Regulates Myocardial Infarction-Induced Apoptosis by down-Regulating Fas and Caspase-3. Sci. Rep. 2017, 7, 7460. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Choong, O.K.; Liu, L.-W.; Cheng, Y.-C.; Li, S.-C.; Yen, C.Y.T.; Wu, M.-R.; Chiang, M.-H.; Tsang, T.-J.; Wu, Y.-W.; et al. MicroRNA Let-7-TGFBR3 Signalling Regulates Cardiomyocyte Apoptosis after Infarction. EBioMedicine 2019, 46, 236–247. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, X.; Zhang, S.; Lin, Y.; Yang, J.; Zhang, C. MicroRNA-21 Protects against the H2O2-Induced Injury on Cardiac Myocytes via Its Target Gene PDCD4. J. Mol. Cell. Cardiol. 2009, 47, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Chiang, M.; Liang, C.; Lin, L.; Yang, Y.; Huang, C.; Chen, Y.; Kao, H.; Chen, Y.; Ke, S.; Lee, C.; et al. MiR-26a Attenuates Cardiac Apoptosis and Fibrosis by Targeting Ataxia–Telangiectasia Mutated in Myocardial Infarction. J. Cell. Physiol. 2020, 235, 6085–6102. [Google Scholar] [CrossRef]
- He, F.; Liu, H.; Guo, J.; Yang, D.; Yu, Y.; Yu, J.; Yan, X.; Hu, J.; Du, Z. Inhibition of MicroRNA-124 Reduces Cardiomyocyte Apoptosis Following Myocardial Infarction via Targeting STAT3. Cell. Physiol. Biochem. 2018, 51, 186–200. [Google Scholar] [CrossRef]
- Diez-Cuñado, M.; Wei, K.; Bushway, P.J.; Maurya, M.R.; Perera, R.; Subramaniam, S.; Ruiz-Lozano, P.; Mercola, M. MiRNAs That Induce Human Cardiomyocyte Proliferation Converge on the Hippo Pathway. Cell Rep. 2018, 23, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Gabisonia, K.; Prosdocimo, G.; Aquaro, G.D.; Carlucci, L.; Zentilin, L.; Secco, I.; Ali, H.; Braga, L.; Gorgodze, N.; Bernini, F.; et al. MicroRNA Therapy Stimulates Uncontrolled Cardiac Repair after Myocardial Infarction in Pigs. Nature 2019, 569, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Z.-P.; Seok, H.Y.; Ding, J.; Kataoka, M.; Zhang, Z.; Hu, X.; Wang, G.; Lin, Z.; Wang, S.; et al. Mir-17–92 Cluster Is Required for and Sufficient to Induce Cardiomyocyte Proliferation in Postnatal and Adult Hearts. Circ. Res. 2013, 112, 1557–1566. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Wang, T.; Zhou, N.; Kong, J.; Chen, L.; Snitow, M.; Morley, M.; Li, D.; Petrenko, N.; et al. A MicroRNA-Hippo Pathway That Promotes Cardiomyocyte Proliferation and Cardiac Regeneration in Mice. Sci. Transl. Med. 2015, 7, 279ra38. [Google Scholar] [CrossRef]
- Huang, W.; Feng, Y.; Liang, J.; Yu, H.; Wang, C.; Wang, B.; Wang, M.; Jiang, L.; Meng, W.; Cai, W.; et al. Loss of MicroRNA-128 Promotes Cardiomyocyte Proliferation and Heart Regeneration. Nat. Commun. 2018, 9, 700. [Google Scholar] [CrossRef]
- Ge, Z.-W.; Zhu, X.-L.; Wang, B.-C.; Hu, J.-L.; Sun, J.-J.; Wang, S.; Chen, X.-J.; Meng, S.-P.; Liu, L.; Cheng, Z.-Y. MicroRNA-26b Relieves Inflammatory Response and Myocardial Remodeling of Mice with Myocardial Infarction by Suppression of MAPK Pathway through Binding to PTGS2. Int. J. Cardiol. 2019, 280, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Icli, B.; Dorbala, P.; Feinberg, M.W. An Emerging Role for the MiR-26 Family in Cardiovascular Disease. Trends Cardiovasc. Med. 2014, 24, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Garikipati, V.N.S.; Verma, S.K.; Jolardarashi, D.; Cheng, Z.; Ibetti, J.; Cimini, M.; Tang, Y.; Khan, M.; Yue, Y.; Benedict, C.; et al. Therapeutic Inhibition of MiR-375 Attenuates Post-Myocardial Infarction Inflammatory Response and Left Ventricular Dysfunction via PDK-1-AKT Signalling Axis. Cardiovasc. Res. 2017, 113, 938–949. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.-D.; Dai, L.-F.; Yang, L.; Wang, Y.-S.; Hao, X.-H.; Liu, Z.-C.; Chen, P.-L. Upregulation of MiR-335 Reduces Myocardial Injury Following Myocardial Infarction via Targeting MAP3K2. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 344–352. [Google Scholar]
- Qiao, L.; Hu, S.; Liu, S.; Zhang, H.; Ma, H.; Huang, K.; Li, Z.; Su, T.; Vandergriff, A.; Tang, J.; et al. MicroRNA-21-5p Dysregulation in Exosomes Derived from Heart Failure Patients Impairs Regenerative Potential. J. Clin. Investig. 2019, 129, 2237–2250. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-C.; Qiu, X.-T.; Sun, Q.; Zhou, J.-P.; Yang, H.-J.; Wu, W.-Z.; He, L.-F.; Tang, C.-E.; Zhang, G.-G.; Bai, Y.-P. Endogenous Reduction of MiR-185 Accelerates Cardiac Function Recovery in Mice Following Myocardial Infarction via Targeting of Cathepsin K. J. Cell. Mol. Med. 2019, 23, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Jazbutyte, V.; Fiedler, J.; Kneitz, S.; Galuppo, P.; Just, A.; Holzmann, A.; Bauersachs, J.; Thum, T. MicroRNA-22 Increases Senescence and Activates Cardiac Fibroblasts in the Aging Heart. Age 2013, 35, 747–762. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, H.; Ge, D.; Xu, Y.; Xu, H.; Yang, Y.; Gu, M.; Zhou, Y.; Zhu, J.; Ge, T.; et al. Mir-21 Promotes Cardiac Fibrosis After Myocardial Infarction Via Targeting Smad7. Cell Physiol. Biochem. 2017, 42, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Foinquinos, A.; Thum, S.; Remke, J.; Zimmer, K.; Bauters, C.; de Groote, P.; Boon, R.A.; de Windt, L.J.; Preissl, S.; et al. Preclinical Development of a MicroRNA-Based Therapy for Elderly Patients With Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 68, 1557–1571. [Google Scholar] [CrossRef]
- Lu, C.; Wang, X.; Ha, T.; Hu, Y.; Liu, L.; Zhang, X.; Yu, H.; Miao, J.; Kao, R.; Kalbfleisch, J.; et al. Attenuation of Cardiac Dysfunction and Remodeling of Myocardial Infarction by MicroRNA-130a Are Mediated by Suppression of PTEN and Activation of PI3K Dependent Signaling. J. Mol. Cell. Cardiol. 2015, 89, 87–97. [Google Scholar] [CrossRef]
- Li, J.; Cai, S.X.; He, Q.; Zhang, H.; Friedberg, D.; Wang, F.; Redington, A.N. Intravenous MiR-144 Reduces Left Ventricular Remodeling after Myocardial Infarction. Basic Res. Cardiol. 2018, 113, 36. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Foinquinos, A.; Jung, M.; Janssen-Peters, H.; Biss, S.; Bauersachs, J.; Gupta, S.K.; Thum, T. MiRNA-181a Is a Novel Regulator of Aldosterone–Mineralocorticoid Receptor-mediated Cardiac Remodelling. Eur. J. Heart Fail. 2020, 22, 1366–1377. [Google Scholar] [CrossRef]
- Liang, H.; Su, X.; Wu, Q.; Shan, H.; Lv, L.; Yu, T.; Zhao, X.; Sun, J.; Yang, R.; Zhang, L.; et al. LncRNA 2810403D21Rik/Mirf Promotes Ischemic Myocardial Injury by Regulating Autophagy through Targeting Mir26a. Autophagy 2020, 16, 1077–1091. [Google Scholar] [CrossRef]
- Wang, Q.-S.; Zhou, J.; Li, X. LncRNA UCA1 Protects Cardiomyocytes against Hypoxia/Reoxygenation Induced Apoptosis through Inhibiting MiR-143/MDM2/P53 Axis. Genomics 2020, 112, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, Q.; Mao, J.; Zhang, J.; Li, L. The Roles of LncRNA in Myocardial Infarction: Molecular Mechanisms, Diagnosis Biomarkers, and Therapeutic Perspectives. Front. Cell Dev. Biol. 2021, 9, 680713. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, L.; Deng, W.; Jiang, M. CircRNA MFACR Is Upregulated in Myocardial Infarction and Downregulates MiR-125b to Promote Cardiomyocyte Apoptosis Induced by Hypoxia. J. Cardiovasc. Pharmacol. 2021, 78, 802–808. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Y.; Zhang, J.; Wang, J.; He, J.; Zhang, Z.; Liu, F. CircRNA ACAP2 Is Overexpressed in Myocardial Infarction and Promotes the Maturation of MiR-532 to Induce the Apoptosis of Cardiomyocyte. J. Cardiovasc. Pharmacol. 2021, 78, 247–252. [Google Scholar] [CrossRef]
- Chamberlain, K.; Riyad, J.M.; Weber, T. Cardiac Gene Therapy with Adeno-Associated Virus-Based Vectors. Curr. Opin. Cardiol. 2017, 32, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Scimia, M.C.; Gumpert, A.M.; Koch, W.J. Cardiovascular Gene Therapy for Myocardial Infarction. Expert Opin. Biol. Ther. 2014, 14, 183–195. [Google Scholar] [CrossRef]
- Yamada, K.P.; Tharakan, S.; Ishikawa, K. Consideration of Clinical Translation of Cardiac AAV Gene Therapy. Cell Gene Ther. Insights 2020, 6, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Jessup, M.; Greenberg, B.; Mancini, D.; Cappola, T.; Pauly, D.F.; Jaski, B.; Yaroshinsky, A.; Zsebo, K.M.; Dittrich, H.; Hajjar, R.J. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): A Phase 2 Trial of Intracoronary Gene Therapy of Sarcoplasmic Reticulum Ca2+-ATPase in Patients With Advanced Heart Failure. Circulation 2011, 124, 304–313. [Google Scholar] [CrossRef]
- Kawase, Y.; Hajjar, R.J. The Cardiac Sarcoplasmic/Endoplasmic Reticulum Calcium ATPase: A Potent Target for Cardiovascular Diseases. Nat. Rev. Cardiol. 2008, 5, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Domenech, M.; Polo-Corrales, L.; Ramirez-Vick, J.E.; Freytes, D.O. Tissue Engineering Strategies for Myocardial Regeneration: Acellular Versus Cellular Scaffolds? Tissue Eng. Part B Rev. 2016, 22, 438–458. [Google Scholar] [CrossRef]
- Serpooshan, V.; Zhao, M.; Metzler, S.A.; Wei, K.; Shah, P.B.; Wang, A.; Mahmoudi, M.; Malkovskiy, A.V.; Rajadas, J.; Butte, M.J.; et al. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials 2013, 34, 9048–9055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, M.; Kc, P.; Zhang, G. In Vivo Assessment of Decellularized Porcine Myocardial Slice as an Acellular Cardiac Patch. ACS Appl. Mater. Interfaces 2019, 11, 23893–23900. [Google Scholar] [CrossRef]
- Huang, K.; Ozpinar, E.W.; Su, T.; Tang, J.; Shen, D.; Qiao, L.; Hu, S.; Li, Z.; Liang, H.; Mathews, K.; et al. An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci. Transl. Med. 2020, 12, eaat9683. [Google Scholar] [CrossRef]
- Li, N.; Rochette, L.; Wu, Y.; Rosenblatt-Velin, N. New Insights into the Role of Exosomes in the Heart after Myocardial Infarction. J. Cardiovasc. Transl. Res. 2019, 12, 18–27. [Google Scholar] [CrossRef]
- Li, Z.; Hu, S.; Huang, K.; Su, T.; Cores, J.; Cheng, K. Targeted anti-IL-1β platelet microparticles for cardiac detoxing and repair. Sci. Adv. 2020, 6, eaay0589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Clinical Trial | Phase | Type of Stem Cell | Rout of Delivery | Status | Primary Outcome Measures | Results |
|---|---|---|---|---|---|---|
| BOOST (NCT00224536) [87] | 1 | (Bone marrow-derived mesenchymal stem cells (BM-MSCs) | Intracoronary injection | Completed 2003 | - change in global (left ventricular ejection fraction) LVEF | - no long-term benefit on left ventricular (LV) systolic function - acceleration of LVEF recovery |
| BM-MSCs transfer in AMI (NCT00264316) [88] | 2 | BM-MSCs | Intracoronary injection | Completed 2005 | - increase in global LVEF | - no augmentation of LVEF recovery |
| MYSTAR Study (NCT00384982) [89] | 2 | BM-MSCs | Intracoronary/or combination of intracoronary with percutaneous intramyocardial | Completed 2008 | - changes in resting myocardial perfusion defect size - Changes in global LVEF | - improvement of cardiac function - significant increase in right ventricular ejection fraction (RVEF) |
| Stem Cell Therapy to Improve Myocardial Function in Patients with AMI (NCT00316381) [90] | N/A | CD34+/CXCR4+/C-met stem cells | Intracoronary injection | Completed 2008 | - LVEF and volumes measured by echocardiography and angiography | - positive correlation between the mobilization of CD34+/CXCR4+/C-met stem cells into peripheral blood and LVEF |
| TRACIA study (NCT00725738) [91] | 2/3 | BM-MSCs | Intracoronary injection | Completed 2009 | - evaluation of LVEF increase between stem cell group and control group | No effect on LV function |
| SEED-MSC (NCT01392105) [92] | 2/3 | BM-MSCs | Intracoronary injection | Completed 2010 | - absolute changes in global LVEF | - improvement in the LVEF |
| Bmmsct (NCT04421274) [93] | 2/3 | BM-MSCs | Percutaneous coronary injection | Completed 2011 | - changes in myocardial metabolic activity - change in LVEF | - no effect on LVEF and myocardial viability |
| The late TIME Study (NCT00684060) [94] | 2 | Bone marrow (BM)- mononuclear stem cells | Intracoronary injection | Completed 2012 | - change in global LVEF - regional LVEF | - no improvement of LVEF |
| WJ-MSC-AMI (NCT01291329) [95] | 2 | WJ-MSCs | Intracoronary injection | Completed 2012 | - quantitative myocardial metabolic and perfusion - safety and efficacy | - significantly greater absolute increase in the myocardial viability and perfusion |
| EMRTCC (NCT00350766) [96] | 2/3 | BM- mononuclear stem cells | Intracoronary injection | Completed 2014 | - change in global LVEF | - no improvement of the echocardiographic parameters of systolic function |
| HUC-HEART (NCT02323477) [97] | 1/2 | (Umbilical cord stroma) UCS- MSCs | Intramyocardial injection | Completed 2018 | - ventricular remodeling | - possible positive effect in scar tissue reduction and restoration of ventricular wall function |
| REGEN-AMI (NCT00765453) [98] | - | BM- progenitor cells | Intracoronary injection | Completed 2018 | - longitudinal change in LVEF | - slight non-significant improvement in LVEF |
| ALLSTAR (NCT01458405) [99] | 1/2 | Allogeneic Cardiosphere-Derived Cells | Intracoronary injection | Completed 2019 | - safety and effective decreasing of infarct size | - no scar size reduction - significant reduction in LV end-systolic volume |
| Stem cells in acute myocardial infection (AMI) (NCT04340609) [76] | 1/2 | Umbilical cord (UC)-MSCs | intravenous injection and intracoronary injection | Completed 2022 | - major adverse cardiac events endpoints of mortality - re-infarction - target vessel revascularization - heart failure hospitalization | - final data collection |
| Heart Patch for MI COVID-19 (NCT04728906) [100] | N/A | - patch seeded with amnion epithelial stem cells and autologous cardiomyocytes (CMs) | - patch transplantation during coronary artery bypass grafting surgery | Recruiting | - change of the ischemic burden - Change in the regional heart wall motion abnormality | - expected completion date is September 2022 |
| LAPiS Study (NCT04945018) [101] | 1/2 | Induced pluripotent stem cells (iPSCs) | HS-001-D needle transplantation | ongoing | Evaluation of safety and tolerability of iPSC-derived CM spheroids | - expected completion date is March 2024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csöbönyeiová, M.; Beerová, N.; Klein, M.; Debreová-Čeháková, M.; Danišovič, Ľ. Cell-Based and Selected Cell-Free Therapies for Myocardial Infarction: How Do They Compare to the Current Treatment Options? Int. J. Mol. Sci. 2022, 23, 10314. https://doi.org/10.3390/ijms231810314
Csöbönyeiová M, Beerová N, Klein M, Debreová-Čeháková M, Danišovič Ľ. Cell-Based and Selected Cell-Free Therapies for Myocardial Infarction: How Do They Compare to the Current Treatment Options? International Journal of Molecular Sciences. 2022; 23(18):10314. https://doi.org/10.3390/ijms231810314
Chicago/Turabian StyleCsöbönyeiová, Mária, Nikoleta Beerová, Martin Klein, Michaela Debreová-Čeháková, and Ľuboš Danišovič. 2022. "Cell-Based and Selected Cell-Free Therapies for Myocardial Infarction: How Do They Compare to the Current Treatment Options?" International Journal of Molecular Sciences 23, no. 18: 10314. https://doi.org/10.3390/ijms231810314
APA StyleCsöbönyeiová, M., Beerová, N., Klein, M., Debreová-Čeháková, M., & Danišovič, Ľ. (2022). Cell-Based and Selected Cell-Free Therapies for Myocardial Infarction: How Do They Compare to the Current Treatment Options? International Journal of Molecular Sciences, 23(18), 10314. https://doi.org/10.3390/ijms231810314






