High-Precision Automatic Identification of Fentanyl-Related Drugs by Terahertz Spectroscopy with Molecular Dynamics Simulation and Spectral Similarity Mapping
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Geometric Configuration
2.2. Comparison of Experimental and Theoretical Spectra
2.3. Assignment of Absorption Peaks
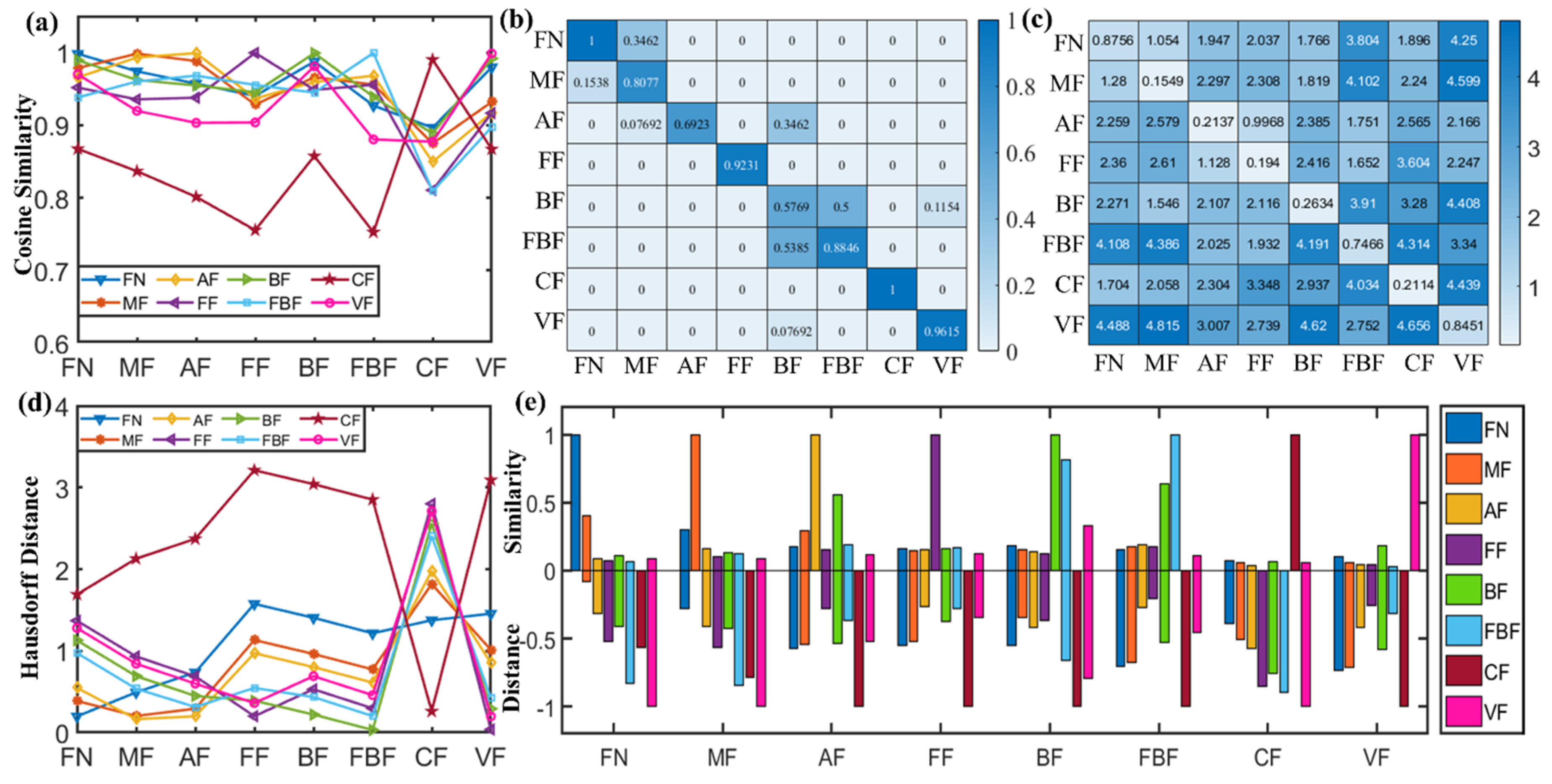
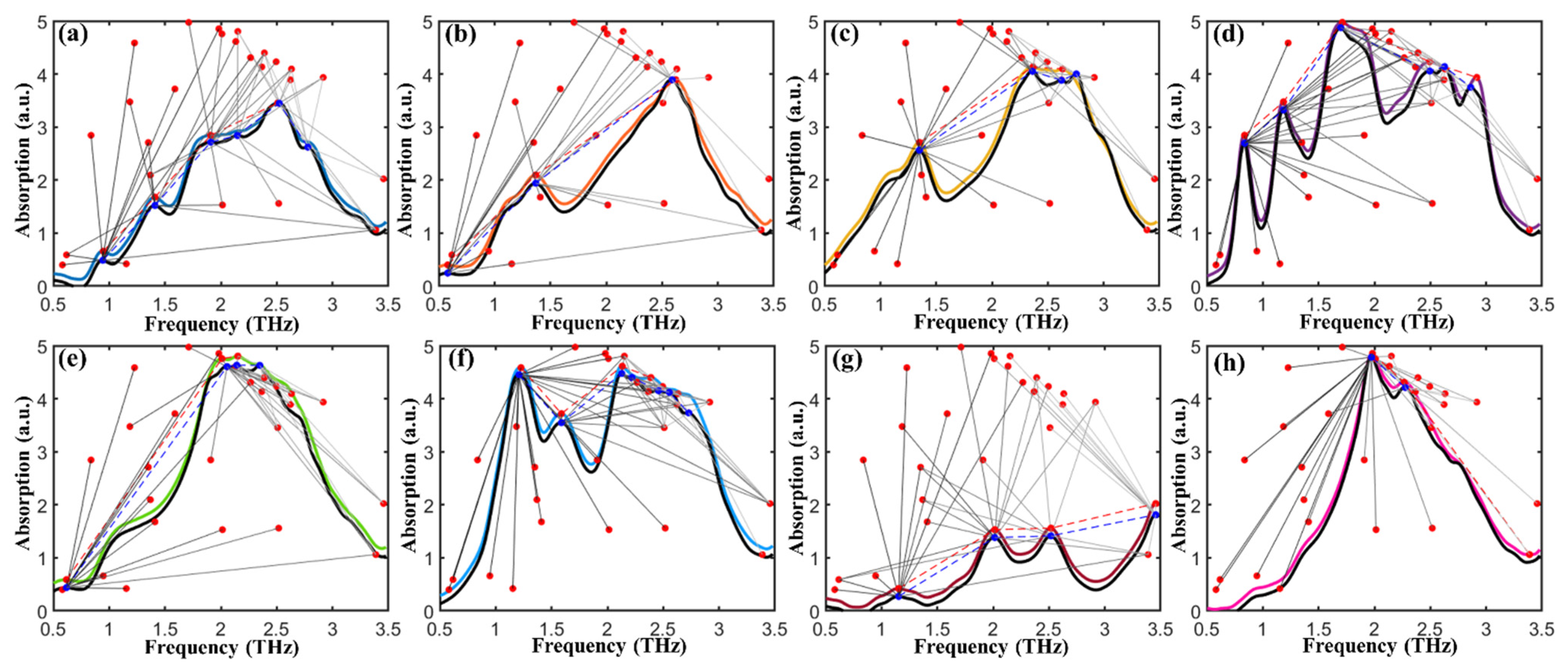
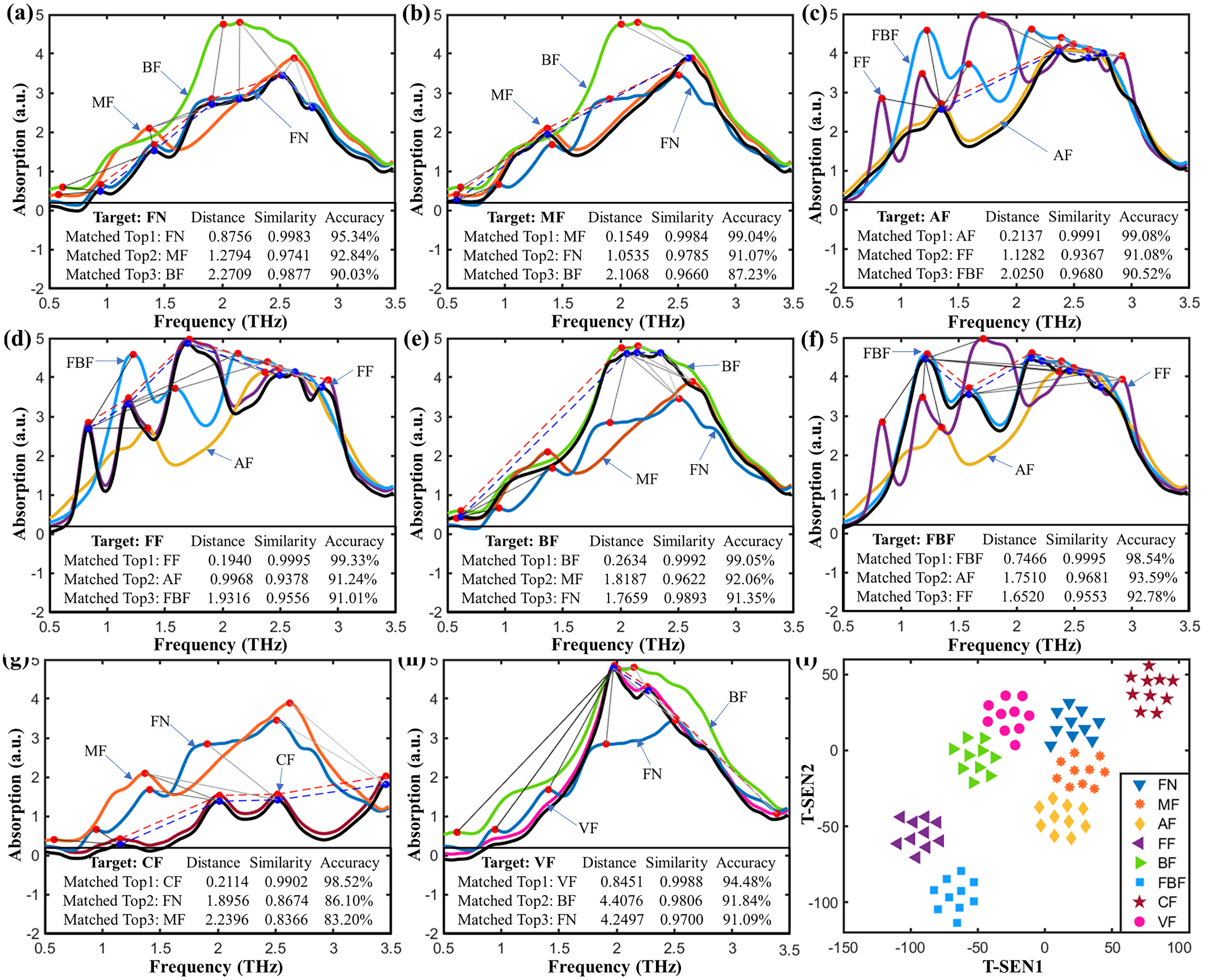
2.4. Identification of Fentanyl Based on Spectral Similarity
2.5. Application of Mass Spectral Similarity Mapping to Fentanyl Analogs
3. Materials and Methods
3.1. Preparation of Drug Pellets
3.2. THz Apparatus and Spectral Measurements
3.3. DFT Theoretical Simulations
3.4. Spectral Similarity Calculations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peeters, O.M.; Blaton, N.M.; Deranter, C.J.; Vanherk, A.M.; Goubitz, K. Crystal and Molecular-Structure of N-[1-(2-Phenylethyl)-4-Piperidinylium]-N-Phenylpropanamide (Fentanyl) Citrate-Toluene Solvate. J. Cryst. Mol. Struct. 1979, 9, 153–161. [Google Scholar] [CrossRef]
- Casy, A.F.; Huckstep, M.R. Structure-Activity Studies of Fentanyl. J. Pharm. Pharmacol. 1988, 40, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Faggiani, F.; Bolchi, C.; Pallavicini, M.; Dei Cas, M. Ten Years of Fentanyl-like Drugs: A Technical-analytical Review. Anal. Sci. 2019, 35, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, J.; Fiorica, F.; Sacchetto, A.; Franceschini, G.; Vaccari, F.; Bonetti, A. The role of fentanyl in the treatment of breakthrough cancer pain: Different biotechnologies, different results and different drug costs. J. Oncol. Pharm. Pract. 2021, 27, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.P.; Kennedy, D.J.; Lau, E.Y.; Valdez, C.A. Solution-State Structure and Affinities of Cyclodextrin:Fentanyl Complexes by Nuclear Magnetic Resonance Spectroscopy and Molecular Dynamics Simulation. J. Phys. Chem. B 2016, 120, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Vardanyan, R.S.; Hruby, V.J. Fentanyl-related compounds and derivatives: Current status and future prospects for pharmaceutical applications. Future Med. Chem. 2014, 6, 385–412. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; El-Haddad, S. A review: Fentanyl and non-pharmaceutical fentanyls. Drug Alcohol Depen. 2017, 171, 107–116. [Google Scholar] [CrossRef]
- Hok, S.; Leif, R.; Mayer, B.; Valdez, C. Improved and optimized syntheses of fentanyl and related analogs. Abstr. Pap. Am. Chem. Soc. 2015, 250, 1155. [Google Scholar]
- Krausz, R.M.; Nikoo, M.; Jang, K.; Choi, F. The North American Overdose Crisis and the European-American “Fentanyl and Treatment Gap”. Eur. Addict. Res. 2021, 27, 304–310. [Google Scholar] [CrossRef]
- Zawadzki, M.; Nowak, K. Fentanyl and its derivatives as a group of new psychoactive substances (designer drugs). Adv. Hyg. Exp. Med. 2018, 72, 547–556. [Google Scholar] [CrossRef]
- Dai, Z.; Abate, M.A.; Smith, G.S.; Kraner, J.C.; Mock, A.R. Fentanyl and fentanyl-analog involvement in drug-related deaths. Drug Alcohol Depen. 2019, 196, 1–8. [Google Scholar] [CrossRef]
- Latkin, C.A.; Dayton, L.; Davey-Rothwell, M.A.; Tobin, K.E. Fentanyl and Drug Overdose: Perceptions of Fentanyl Risk, Overdose Risk Behaviors, and Opportunities for Intervention among People who use Opioids in Baltimore, USA. Subst. Use Misuse 2019, 54, 998–1006. [Google Scholar] [CrossRef]
- Mahonski, S.; Leonard, J.; Gatz, D.; Seung, H.; Zhang, M.; Kim, H. Bystander naloxone administration for undifferentiated opioid overdose in the era of non-pharmaceutical fentanyl: A retrospective study of a regional poison center data. Clin. Toxicol. 2018, 56, 924, WOS:000449536500026. [Google Scholar]
- Schifano, F.; Chiappini, S.; Corkery, J.M.; Guirguis, A. Assessing the 2004–2018 Fentanyl Misusing Issues Reported to an International Range of Adverse Reporting Systems. Front Pharm. 2019, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, M.J.; Chronister, C.W.; Stanley, C.; Ogilvie, L.M.; Goldberger, B.A. Analysis of Acetyl Fentanyl in Postmortem Specimens by Gas Chromatography-Mass Spectrometry (GC-MS): Method Validation and Case Report. J. Anal. Toxicol. 2019, 43, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Valdez, C.A. Gas Chromatography-Mass Spectrometry Analysis of Synthetic Opioids Belonging to the Fentanyl Class: A Review. Crit. Rev. Anal. Chem. 2021, 1–31. [Google Scholar] [CrossRef]
- Thomasy, S.M.; Mama, K.R.; Stanley, S.D. Comparison of Liquid Chromatography-Mass Spectrometry and Radioimmunoassay for Measurement of Fentanyl and Determination of Pharmacokinetics in Equine Plasma. J. Anal. Toxicol. 2008, 32, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Thevis, M.; Geyer, H.; Bahr, D.; Schanzer, W. Identification of fentanyl, alfentanil, sufentanil, remifentanil and their major metabolites in human urine by liquid chromatography/tandem mass spectrometry for doping control purposes. Eur. J. Mass. Spectrom. 2005, 11, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Larabi, I.A.; Martin, M.; Etting, I.; Pfau, G.; Edel, Y.; Alvarez, J.C. Development and validation of liquid chromatography-tandem mass spectrometry targeted screening of 16 fentanyl analogs and U-47700 in hair: Application to 137 authentic samples. Drug Test Anal. 2020, 12, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Mannocchi, G.; Pirani, F.; Gottardi, M.; Sirignano, A.; Busardo, F.P.; Ricci, G. Determination of nine new fentanyl analogues and metabolites in consumers’ urine by ultra-high-performance liquid chromatography-tandem mass spectrometry. Eur. Rev. Med. Pharmacol. 2021, 25, 4394–4399. [Google Scholar] [CrossRef]
- Verkouteren, J.R.; Lawrence, J.; Verkouteren, R.M.; Sisco, E. Method for evaluating ion mobility spectrometers for trace detection of fentanyl and fentanyl-related substances. Anal. Methods 2019, 11, 6043–6052. [Google Scholar] [CrossRef]
- Schackmuth, M.; Kerrigan, S. Immunoassay-based detection of fentanyl analogs in forensic toxicology. Forensic Toxicol. 2019, 37, 231–237. [Google Scholar] [CrossRef]
- Dotsikas, Y.; Loukas, Y.L.; Siafaka, I. Determination of umbilical cord and maternal plasma concentrations of fentanyl by using novel spectrophotometric and chemiluminescence enzyme immunoassays. Anal. Chim. Acta 2002, 459, 177–185. [Google Scholar] [CrossRef]
- Makowski, G.S.; Richter, J.J.; Moore, R.E.; Eisma, R.; Ostheimer, D.; Onoroski, M.; Wu, A.H.B. An Enzyme-Linked-Immunosorbent-Assay for Urinary Screening of Fentanyl Citrate Abuse. Ann. Clin. Lab. Sci. 1995, 25, 169–178. [Google Scholar]
- Hiolski, E. Electrochemical method for field detection of fentanyl. Chem. Eng. News 2019, 97, 15. [Google Scholar] [CrossRef]
- Angelini, D.J.; Biggs, T.D.; Prugh, A.M.; Smith, J.A.; Hanburger, J.A.; Llano, B.; Avelar, R.; Ellis, A.; Lusk, B.; Naanaa, A.; et al. Detection of fentanyl and derivatives using a lateral flow immunoassay. Forensic Chem. 2021, 23, 100309. [Google Scholar] [CrossRef]
- Wang, C.H.; Terracciano, A.C.; Masunov, A.E.; Xu, M.Y.; Vasu, S.S. Accurate prediction of terahertz spectra of molecular crystals of fentanyl and its analogs. Sci. Rep. 2021, 11, 4062. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.F.; Lin, L.; Cai, C.Y.; Chu, B.Q.; Wang, Y.; He, Y.; Nie, P.C. Terahertz fingerprint characterization of 2,4-dichlorophenoxyacetic acid and its enhanced detection in food matrices combined with spectral baseline correction. Food Chem. 2020, 334, 127474. [Google Scholar] [CrossRef]
- Chen, J.W.; Guo, Z.W.; Hu, J.L. Ring-Regularized Cosine Similarity Learning for Fine-Grained Face Verification. Pattern Recogn. Lett. 2021, 148, 68–74. [Google Scholar] [CrossRef]
- Shen, Y.C.; Lin, G.T.R.; Lin, J.R.; Wang, C.H. A Cross-Database Comparison to Discover Potential Product Opportunities Using Text Mining and Cosine Similarity. J. Sci. Ind. Res. India 2017, 76, 11–16. [Google Scholar]
- Qiu, Z.Y.; Zhao, H. A fuzzy rough set approach to hierarchical feature selection based on Hausdorff distance. Appl. Intell. 2022, 52, 11089–11102. [Google Scholar] [CrossRef]
- Bringmann, K.; Kunnemann, M.; Nusser, A. Discrete Frechet Distance under Translation: Conditional Hardness and an Improved Algorithm. ACM Trans. Algorithms 2021, 17, 25. [Google Scholar] [CrossRef]

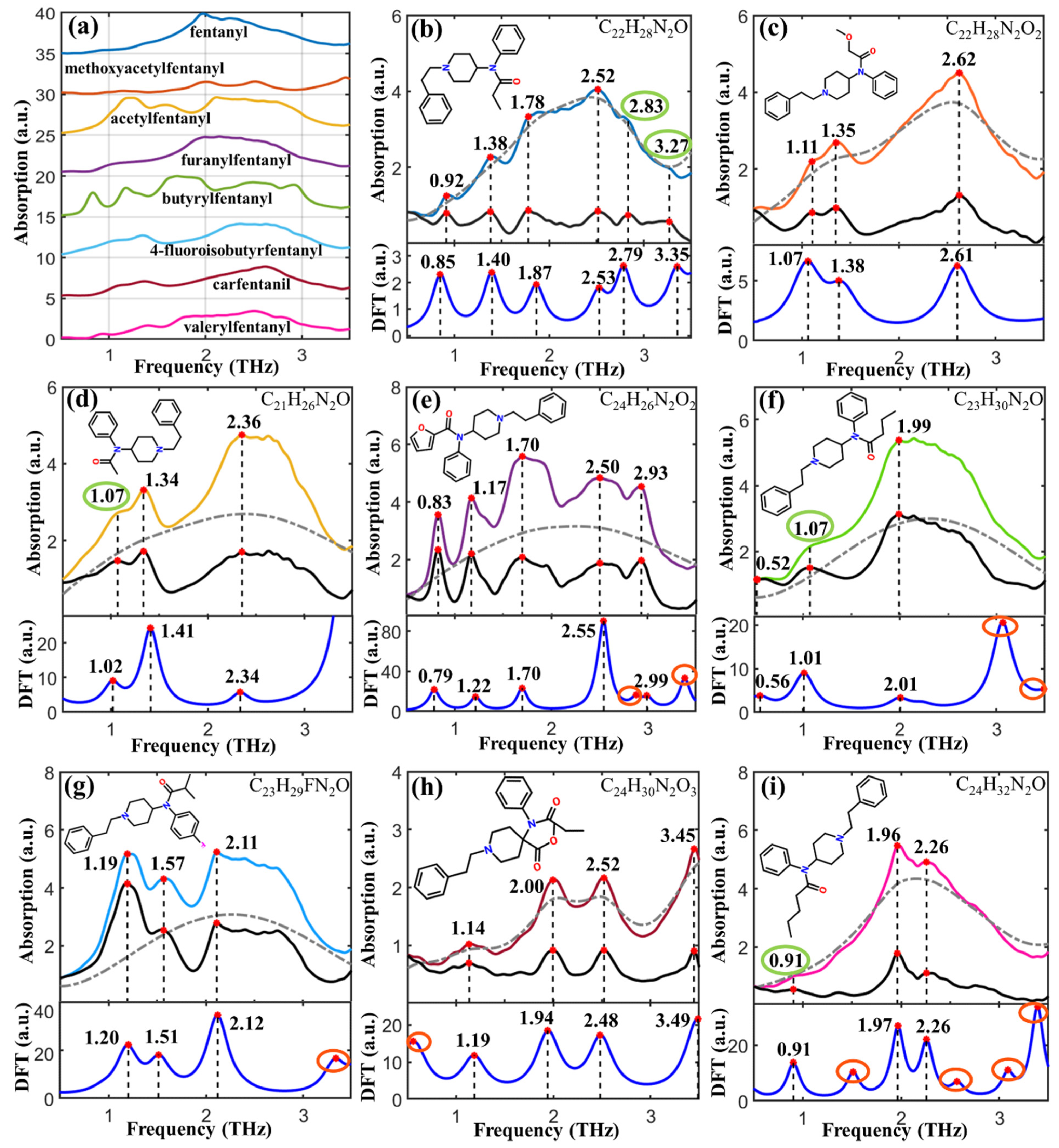
| DFT Peaks (THz) | THz Peaks (THz) | Shift (THz) | Vibration Modes |
|---|---|---|---|
| Fentanyl | |||
| 0.85 (w) | 0.92 | −0.07 | δring |
| 1.40 (w) | 1.38 | 0.02 | δring |
| 1.87 (w) | 1.78 | 0.09 | δ(C-C) |
| 2.53 (w) | 2.52 | 0.01 | δring |
| 2.79 (w) | 2.83 | −0.04 | δring |
| 3.35 (w) | 3.27 | 0.08 | δring |
| Methoxyacetylfentanyl | |||
| 1.07 (w) | 1.11 | −0.04 | δ(C-C) oop |
| 1.38 (w) | 1.35 | 0.03 | δ(C-C-C) oop |
| 2.61 (w) | 2.62 | −0.01 | δring |
| Acetylfentanyl | |||
| 1.02 (w) | 1.07 | −0.05 | δring |
| 1.41 (m) | 1.34 | 0.07 | δ(C-C)opp |
| 2.34 (w) | 2.36 | −0.02 | δring |
| Furanylfentanyl | |||
| 0.79 (s) | 0.83 | −0.04 | δ(C-C)ip |
| 1.22 (m) | 1.17 | 0.05 | δ(C-C)opp |
| 1.70 (m) | 1.70 | 0 | δring |
| 2.55 (vs) | 2.50 | 0.05 | δring |
| 2.99 (w) | 2.93 | 0.06 | δring |
| Butyrylfentanyl | |||
| 0.56 (w) | 0.52 | 0.01 | δring |
| 1.01 (vs) | 1.07 | −0.06 | δ(C-C)opp |
| 2.01 (w) | 1.99 | 0.02 | δring |
| 4-Fluoroisobutyrfentanyl | |||
| 1.20 (m) | 1.19 | 0.01 | δ(C-C)opp |
| 1.51 (m) | 1.57 | −0.06 | δ(C-N)ip |
| 2.12 (s) | 2.11 | 0.01 | δ(C-C)opp |
| Carfentanil | |||
| 1.19 (w) | 1.14 | 0.05 | δring |
| 1.94 (m) | 2.00 | −0.06 | δring |
| 2.48 (m) | 2.52 | −0.04 | δring |
| 3.49 (m) | 3.45 | 0.04 | δ(C-C)opp |
| Valerylfentanyl | |||
| 0.91 (m) | 0.91 | 0 | δ(C-N)opp |
| 1.97 (m) | 1.96 | 0.01 | δ(C-C)opp |
| 2.26 (m) | 2.26 | 0 | δ(C-C-C)opp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, F.; Lin, L.; Nie, P.; Xia, Z. High-Precision Automatic Identification of Fentanyl-Related Drugs by Terahertz Spectroscopy with Molecular Dynamics Simulation and Spectral Similarity Mapping. Int. J. Mol. Sci. 2022, 23, 10321. https://doi.org/10.3390/ijms231810321
Qu F, Lin L, Nie P, Xia Z. High-Precision Automatic Identification of Fentanyl-Related Drugs by Terahertz Spectroscopy with Molecular Dynamics Simulation and Spectral Similarity Mapping. International Journal of Molecular Sciences. 2022; 23(18):10321. https://doi.org/10.3390/ijms231810321
Chicago/Turabian StyleQu, Fangfang, Lei Lin, Pengcheng Nie, and Zhengyan Xia. 2022. "High-Precision Automatic Identification of Fentanyl-Related Drugs by Terahertz Spectroscopy with Molecular Dynamics Simulation and Spectral Similarity Mapping" International Journal of Molecular Sciences 23, no. 18: 10321. https://doi.org/10.3390/ijms231810321





