Cordycepin Inhibits Growth and Metastasis Formation of MDA-MB-231 Xenografts in Nude Mice by Modulating the Hedgehog Pathway
Abstract
1. Introduction
2. Results
2.1. Cordycepin Inhibits the Growth of MDA-MB-231 Xenografts in Nude Mice
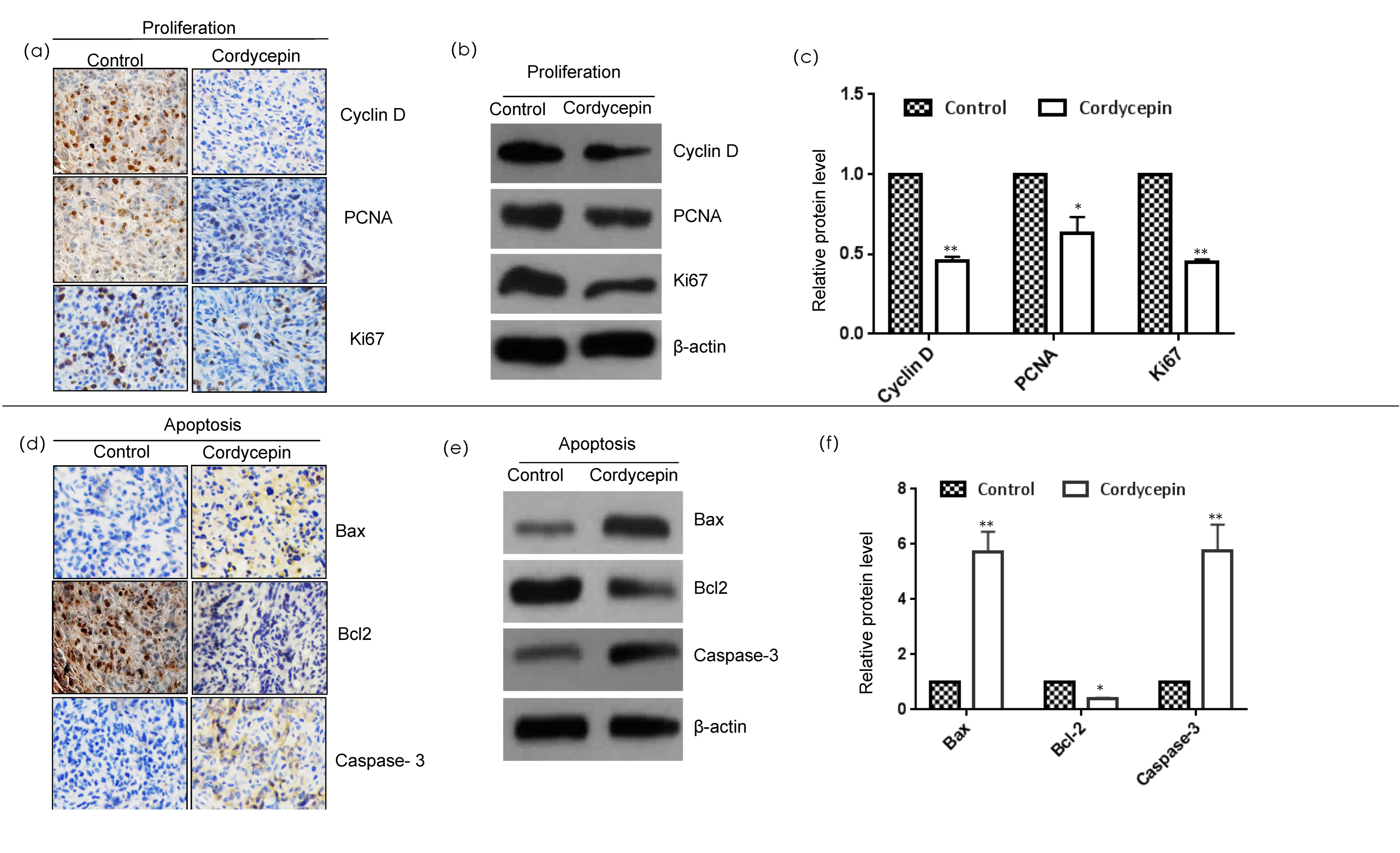
2.2. Cordycepin Inhibits Proliferation and Induces Apoptosis in MDA-MB-231 Xenografts
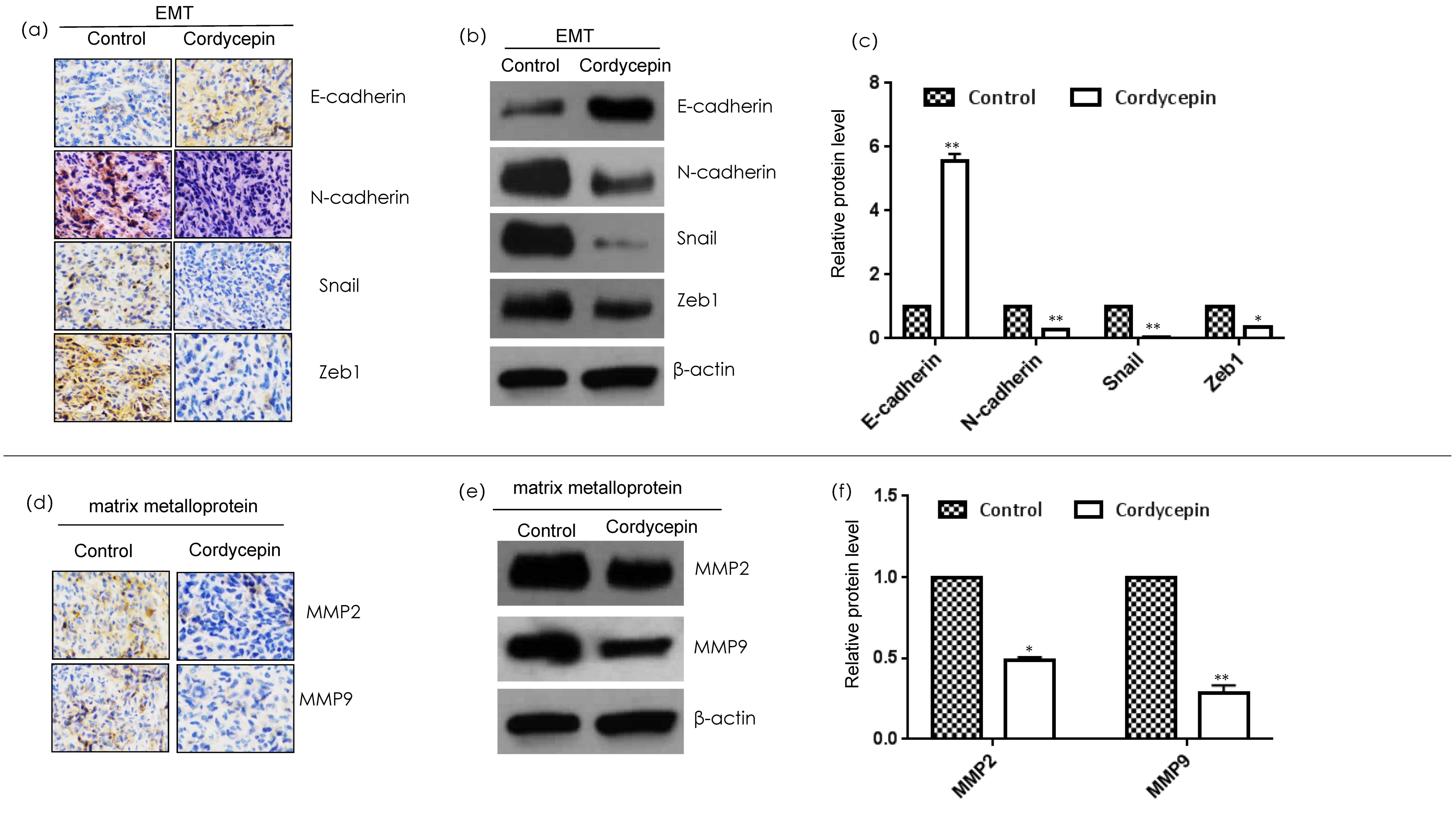
2.3. Cordycepin Inhibits Invasion and Metastasis of MDA-MB-231 Xenograft
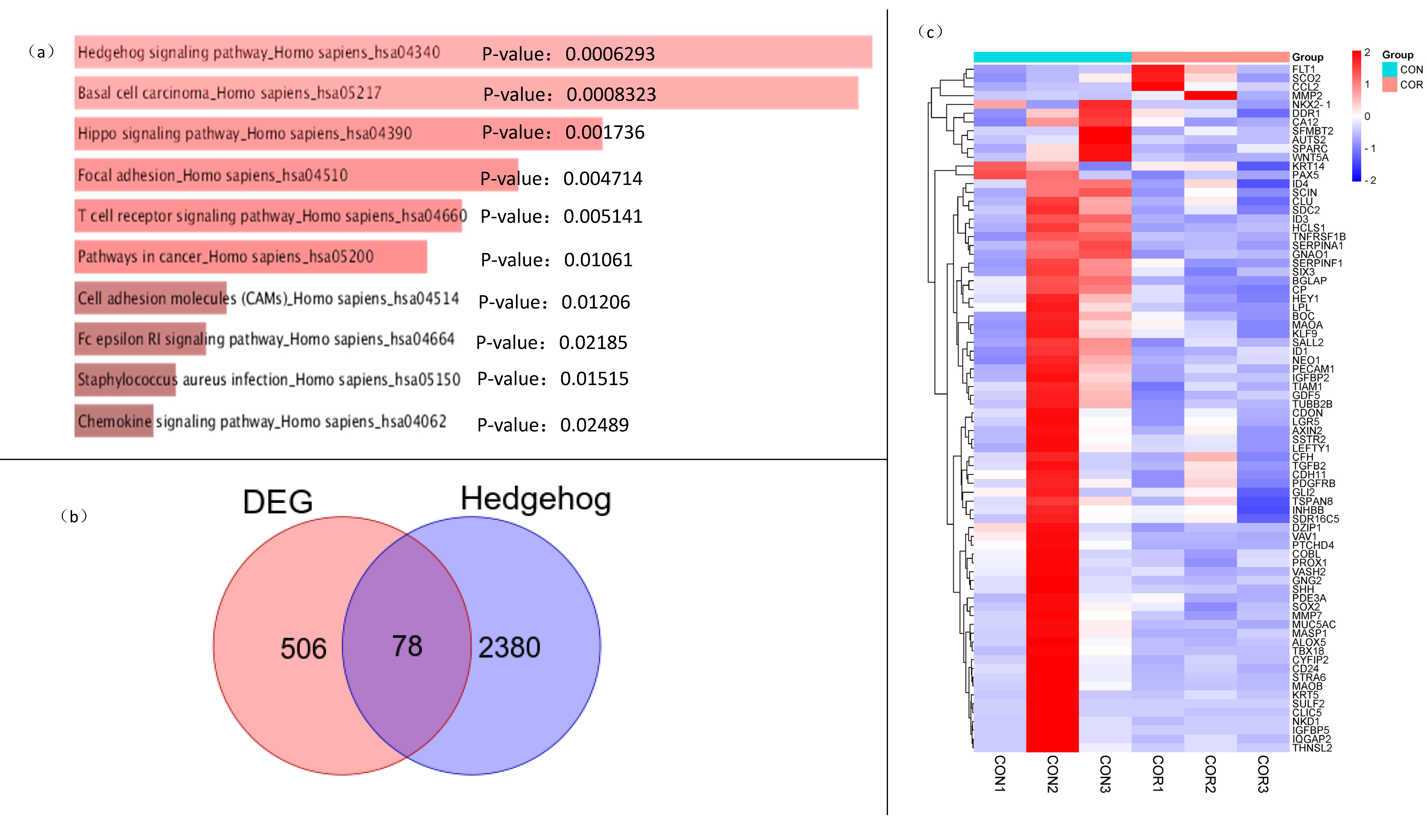
2.4. Cordycepin Inhibition of Breast Cancer Is Related to the Hedgehog Pathway
2.5. Analysis of Key Genes in the Protein–Protein Interaction Network
2.6. Breast Cancer Tissue and Normal Breast Tissue Differently Express Hedgehog Pathway Markers
2.7. Hedgehog Pathway Markers Are Associated with Prognosis in Breast Cancer Patients
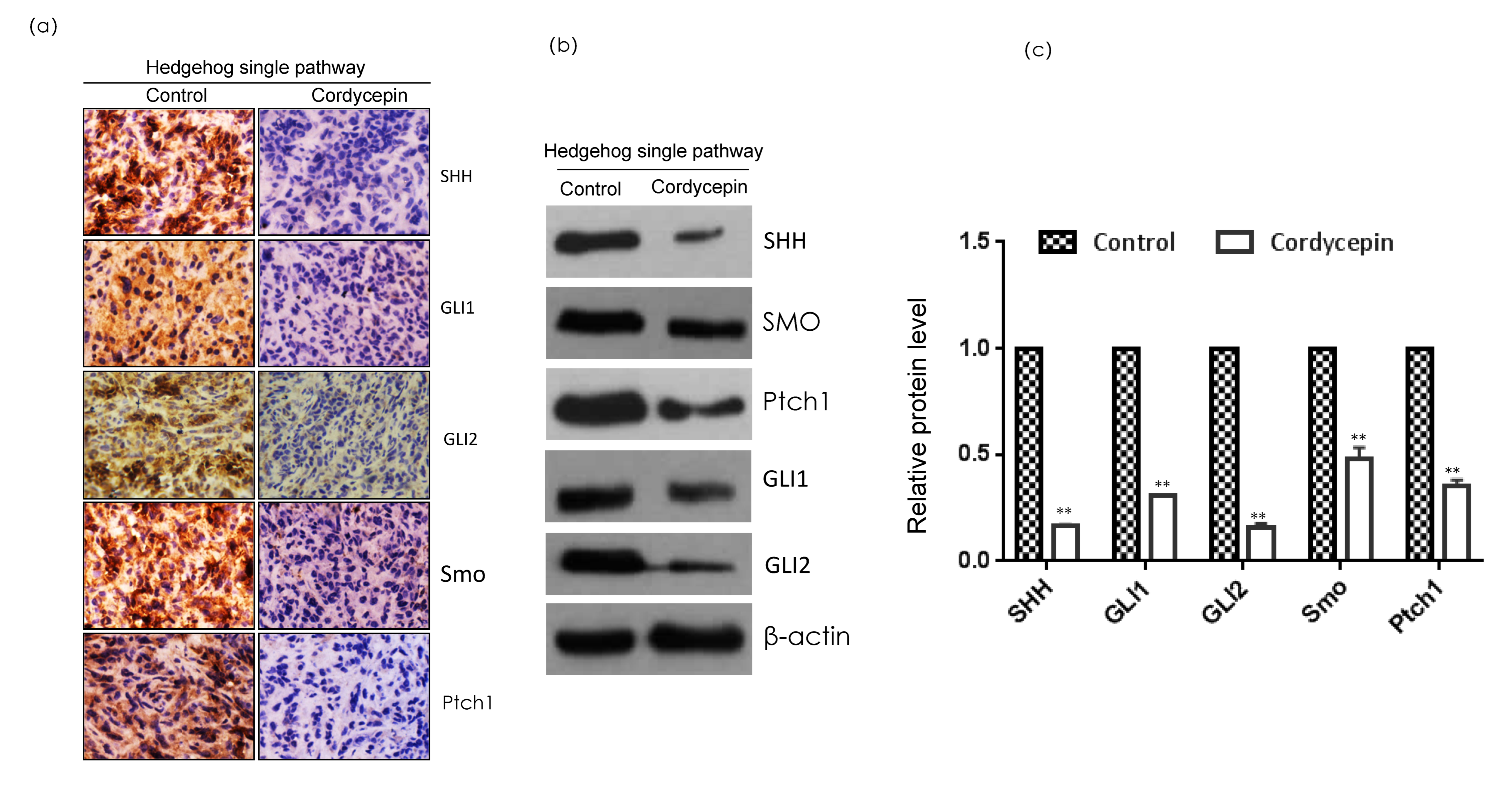
2.8. Cordycepin Inhibits Hedgehog Pathway Markers in Triple-Negative Breast Cancer
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Effect of Cordycepin on the Growth of MDA-MB-231 Cell Xenografts
4.3. RNA Isolation
4.4. Western Blotting
4.5. Immunohistochemistry
4.6. Oncomine Database Analysis
4.7. Kaplan–Meier Analysis
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoo, H.S.; Shin, J.W.; Cho, J.H.; Son, C.G.; Lee, Y.W.; Park, S.Y.; Cho, C.K. Effects of Cordyceps militaris extract on angiogenesis and tumor growth. Acta Pharmacol. Sin. 2004, 25, 657–665. [Google Scholar]
- Liu, X.C.; Zhu, Z.Y.; Liu, Y.L.; Sun, H.Q. Comparisons of the anti-tumor activity of polysaccharides from fermented mycelia and cultivated fruiting bodies of Cordyceps militaris in Vitro. Int. J. Biol. Macromol. 2019, 130, 307–314. [Google Scholar] [CrossRef]
- Chiu, C.P.; Liu, S.C.; Tang, C.H.; Chan, Y.; Mohamed, E.S.; Lee, C.L.; Du, Y.C.; Wu, T.Y.; Chang, F.R.; Wu, Y.C. Anti-inflammatory Cerebrosides from Cultivated Cordyceps militaris. J. Agric. Food Chem. 2016, 64, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- He, B.L.; Zheng, Q.W.; Guo, L.Q.; Huang, J.Y.; Yun, F.; Huang, S.S.; Lin, J.F. Structural characterization and immune-enhancing activity of a novel high-molecular-weight polysaccharide from Cordyceps militaris. Int. J. Biol. Macromol. 2020, 145, 11–20. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q.; Zhang, J.H.; Liu, J.; Qian, K.; Wang, D.Y. Research progress on Cordyceps militaris degeneration strains. North Seric. 2021, 42, 1–5. [Google Scholar]
- Han, Y.Y.; Yu, Y.; Che, Y.; Xiao, L. The research progress of artificial cultivation and applications of Cordyceps militaris. Shandong Chem. Ind. 2019, 48, 68–69. [Google Scholar]
- Xia, Y.L.; Luo, F.F.; Shang, Y.F.; Chen, P.L.; Lu, Y.Z.; Wang, C.S. Fungal Cordycepin Biosynthesis Is Coupled with the Production of the Safeguard Molecule Pentostatin. Cell Chem. Biol. 2017, 24, 1479–1489.e4. [Google Scholar] [CrossRef]
- Mahesh, B.; Singh, S.K. Prospects of Cordycepin and Polysaccharides Produced by Cordyceps; Springer Nature: Singapore, 2022; pp. 93–107. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chen, C.H.; Lin, W.C.; Tung, C.W.; Chen, Y.C.; Yang, S.H.; Huang, B.M.; Chen, R.J. The Role of Autophagy in Anti-Cancer and Health Promoting Effects of Cordycepin. Molecules 2021, 16, 4954. [Google Scholar] [CrossRef]
- Gluz, O.; Liedtke, C.; Gottschalk, N.; Pusztai, L.; Nitz, U.; Harbeck, N. Triple-negative breast cancer—Current status and future directions. Ann Oncol. 2009, 20, 1913–1927. [Google Scholar] [CrossRef]
- Liu, C.Y.; Qi, M.; Li, L.; Yuan, Y.; Wu, X.P.; Fu, J.S. Natural cordycepin induces apoptosis and suppresses metastasis in breast cancer cells by inhibiting the Hedgehog pathway. Food Funct. 2020, 11, 2107–2116. [Google Scholar] [CrossRef]
- Scott, R.; Uri, T.; Cynthia, H. A comprehensive review of paediatric low-grade diffuse glioma: Pathology, molecular genetics and treatment. Brain Tumor Pathol. 2017, 34, 51–61. [Google Scholar] [CrossRef]
- Chen, L.; Lin, G.; Chen, K.; Liang, R.; Wan, F.; Zhang, C.; Tian, G.; Zhu, X. VEGF promotes migration and invasion by regulating EMT and MMPs in nasopharyngeal carcinoma. J. Cancer 2020, 11, 7291–7301. [Google Scholar] [CrossRef] [PubMed]
- Philip, A.B.; Sunil, S.K.; David, M.B. Tissue repair and stem cell renewal in carcinogenesis. Nature 2004, 432, 324–331. [Google Scholar] [CrossRef]
- Jon, H.C.; Fred, B.C. A loss-of-function mutation in PTCH1 suggests a role for autocrine hedgehog signaling in colorectal tumorigenesis. Oncotarget 2013, 4, 2208–2211. [Google Scholar] [CrossRef]
- Wang, L.; Joy, Q.J.; Zhou, Y.; Tian, Z.Q.; David, M.J.; He, B. Gli is activated and promotes epithelial- mesenchymal transition in human esophageal adenocarcinoma. Oncotarget 2018, 9, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Mitsuteru, N.; Kelly, A.W.; Shingo, K.; Koji, T.; Veronique, G.; Prasanna, M.C.; Apple, L.; Varun, S.; Douglas, S.D.; Jianwen, Q.; et al. Interplay between Notch1 and Notch3 promotes EMT and tumor initiation in squamous cell carcinoma. Nat. Commun. 2017, 8, 1758. [Google Scholar] [CrossRef]
- Gao, Y.P.; Yi, J.; Zhang, K.; Bai, F.; Feng, B.; Wang, R.; Chu, X.Y.; Chen, L.B.; Song, H.Z. Downregulation of MiR-31 stimulates expression of LATS2 via the hippo pathway and promotes epithelial-mesenchymal transition in esophageal squamous cell carcinoma. Exp. Clin. Cancer Res. 2017, 36, 161–181. [Google Scholar] [CrossRef]
- Ogden, S.K.; Ascano, M.; Stegman, M.A.; Robbins, D.J. Regulation of Hedgehog signaling: A complex story. Biochem. Pharmacol. 2004, 67, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.J.; Zhou, D.; Liu, Y.H. Signaling Crosstalk between Tubular Epithelial Cells and Interstitial Fibroblasts after Kidney Injury. Kidney Dis. 2016, 2, 136–144. [Google Scholar] [CrossRef]
- Jeong, M.H.; Lee, C.M.; Lee, S.W.; Seo, S.Y.; Seo, M.J.; Kang, B.W.; Jeong, Y.K.; Choi, Y.J.; Yang, K.M.; Jo, W.S. Cordycepin-enriched Cordyceps militaris induces immunomodulation and tumor growth delay in mouse-derived breast cancer. Oncol. Rep. 2013, 30, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.L.; Li, Y.; Xiao, H.W.; Luo, D.; Zhang, S.Q.; Zhu, C.C.; Jiang, M.; Cui, M.; Lu, L.; Fan, S.J. Cordycepin sensitizes breast cancer cells toward irradiation through elevating ROS production involving Nrf2. Toxicol. Appl. Pharmacol. 2019, 364, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, W.Y.; Jung, K.; Kwon, Y.S.; Kim, D.; Hwang, G.S.; Kim, C.E.; Lee, S.; Kang, K.S. The Inhibitory Effect of Cordycepin on the Proliferation of MCF-7 Breast Cancer Cells, and Its Mechanism: An Investigation Using Network Pharmacology-Based Analysis. Biomolecules 2019, 9, 414. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.F.; Lu, J.H.; Wang, Y.; Wang, J.Y.; Meng, Q.F.; Lee, R.J.; Teng, L.S. Cordycepin, a Natural Antineoplastic Agent, Induces Apoptosis of Breast Cancer Cells via Caspase-dependent Pathways. Nat. Prod. Commun. 2016, 11, 1934578X1601100119. [Google Scholar] [CrossRef]
- Wei, C.; Khan, M.A.; Du, J.; Cheng, J.; Tania, M.; Leung, E.L.; Fu, J. Cordycepin Inhibits Triple-Negative Breast Cancer Cell Migration and Invasion by Regulating EMT-TFs SLUG, TWIST1, SNAIL1, and ZEB1. Front Oncol. 2022, 12, 898583. [Google Scholar] [CrossRef] [PubMed]
- Miroslava, J.; Ľudovít, D.; Štefan, P.; Ivan, V. Ki67, PCNA, and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016, 118, 544–552. [Google Scholar] [CrossRef]
- John, R.R.; Malathi NRavindran, C.; Anandan, S. Mini review: Multifaceted role played by cyclin D1 in tumor behavior. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2017, 28, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Frederique, P.L.; Nina, R.R. Ki67 assessment in breast cancer: An update. Pathology 2016, 49, 166–171. [Google Scholar] [CrossRef]
- Zhao, J.F.; Shi, X.Q.; Wang, C.Z.; Yang, M.Y.; Liu, L.; Liao, M.M. Relationship between nicotine-induced drug resistance and Bcl-2 family proteins in lung cancer cells. China J. Mod. Med. 2018, 28, 42–46. [Google Scholar]
- Almudena, E.V.; Amelia, E.A.; Diego, F. Functional Role of Non-Coding RNAs during Epithelial-To-Mesenchymal Transition. Non-Coding RNA 2018, 4, 14. [Google Scholar] [CrossRef]
- Das, S.; Amin, S.A.; Jha, T. Inhibitors of gelatinases (MMP-2 and MMP-9) for the management of hematological malignancies. Eur. J. Med. Chem. 2021, 223, 113623. [Google Scholar] [CrossRef] [PubMed]
- Daniel, R.R.; Yu, J.J.; Shanker, K.; Nandan, D.; Radhika, V.; Debashis, G.; Terrence, B.; Akhilesh, P.; Arul, M.C. ONCOMINE: A Cancer Microarray Database and Integrated Data-Mining Platform. Neoplasia Int. J. Oncol. Res. 2004, 6, 1–6. [Google Scholar] [CrossRef]
- Rhodes, D.R.; KSundaram, S.; Mahavisno, V.; Varambally, R.; Yu, J.J.; Briggs, B.B.; Barrette, T.R.; Anstet, M.J.; Colleen, K.B.; Kulkarni, P.; et al. Oncomine 3.0: Genes, Pathways, and Networks in a Collection of 18,000 Cancer Gene Expression Profiles. Neoplasia Int. J. Oncol. Res. 2007, 9, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Balázs, G.; Pawel, S.; Jan, B.; András, L. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS ONE 2017, 8, 12. [Google Scholar] [CrossRef]
- Ali, H.A. The hedgehog pathway in breast cancer. Chin. J. Cancer Res. 2012, 24, 261–262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, S.M.; Yuan, Y.; Yang, B.; Chen, M. Good method for isolating total RNA from Crataegi fructus at different developmental stages. Chin. J. Exp. Tradit. Med. Formulae 2016, 22, 39–43. [Google Scholar]


| Time | Control (g) | Cordycepin (g) |
|---|---|---|
| Week 1 | 19.42 ± 1.37 | 21.23 ± 0.58 |
| Week 2 | 20.02 ± 1.32 | 21.16 ± 0.71 |
| Week 3 | 20.46 ± 1.35 | 20.51 ± 0.72 |
| Week 4 | 21.17 ± 0.56 | 21.17 ± 0.48 |
| Week 5 | 21.27 ± 1.08 | 21.08 ± 0.56 |
| Week 6 | 21.33 ± 1.22 | 21.53 ± 0.68 |
| Week 7 | 21.43 ± 0.78 | 21.31 ± 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Li, X.; Qi, M.; Hu, X.; Cao, F.; Wu, X.; Fu, J. Cordycepin Inhibits Growth and Metastasis Formation of MDA-MB-231 Xenografts in Nude Mice by Modulating the Hedgehog Pathway. Int. J. Mol. Sci. 2022, 23, 10362. https://doi.org/10.3390/ijms231810362
Wu W, Li X, Qi M, Hu X, Cao F, Wu X, Fu J. Cordycepin Inhibits Growth and Metastasis Formation of MDA-MB-231 Xenografts in Nude Mice by Modulating the Hedgehog Pathway. International Journal of Molecular Sciences. 2022; 23(18):10362. https://doi.org/10.3390/ijms231810362
Chicago/Turabian StyleWu, Wenya, Xiaomin Li, Meng Qi, Xin Hu, Fenghua Cao, Xiaoping Wu, and Junsheng Fu. 2022. "Cordycepin Inhibits Growth and Metastasis Formation of MDA-MB-231 Xenografts in Nude Mice by Modulating the Hedgehog Pathway" International Journal of Molecular Sciences 23, no. 18: 10362. https://doi.org/10.3390/ijms231810362
APA StyleWu, W., Li, X., Qi, M., Hu, X., Cao, F., Wu, X., & Fu, J. (2022). Cordycepin Inhibits Growth and Metastasis Formation of MDA-MB-231 Xenografts in Nude Mice by Modulating the Hedgehog Pathway. International Journal of Molecular Sciences, 23(18), 10362. https://doi.org/10.3390/ijms231810362





