Examination of Longitudinal Alterations in Alzheimer’s Disease-Related Neurogenesis in an APP/PS1 Transgenic Mouse Model, and the Effects of P33, a Putative Neuroprotective Agent Thereon
Abstract
1. Introduction
2. Results
2.1. Hippocampal Neurogenesis Is Impaired by Aging in APP/PS1 and Wild-Type Mice
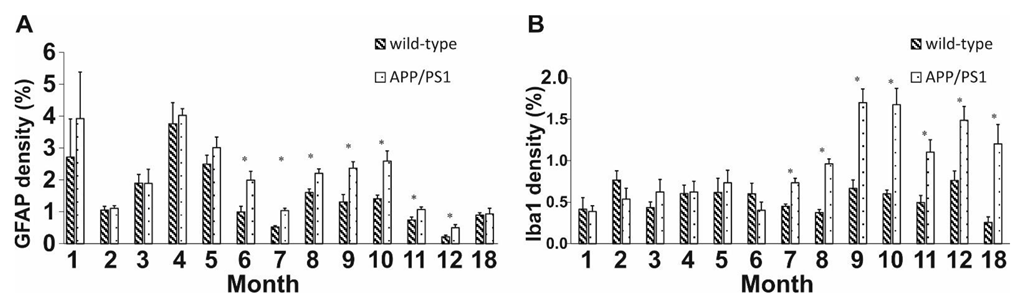
2.2. Neuroinflammation Increases with Age in APP/PS1 Mice
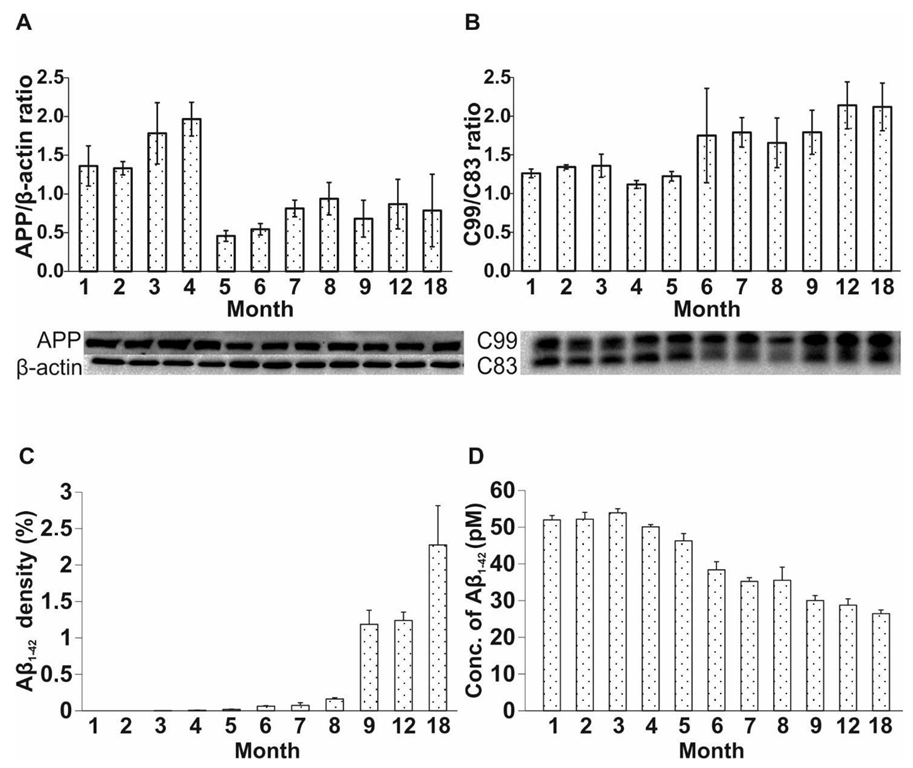
2.3. Age-Related Modulation of APP Processing Pathways in APP/PS1 Mice
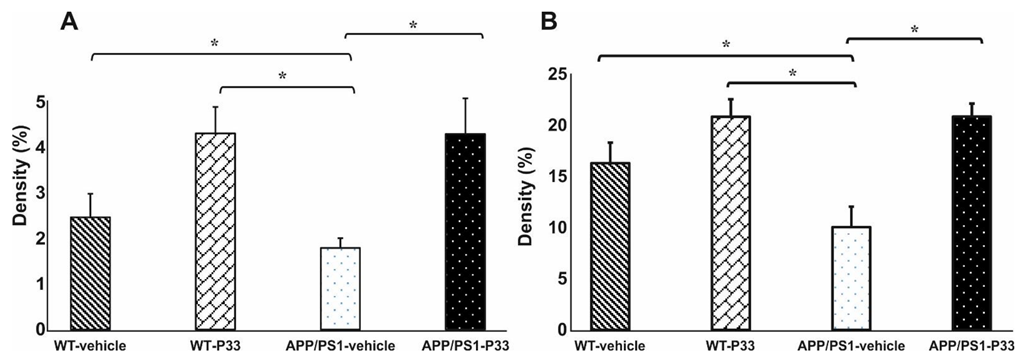
2.4. Elongated Treatment with Pentapeptide P33 Improves Neurogenesis in APP/PS1 Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Immunohistochemistry
4.3. ELISA
4.4. WB
4.5. Quantification of Immunohistochemistry Data
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AICD | APP’s intracellular domain |
| APP | amyloid precursor protein |
| APP/PS1 APPSwe/PS1dE9 | double transgenic mice |
| Aβ | amyloid β |
| BrdU | 5-Bromo-2′-Deoxyuridine |
| BSA | bovine serum albumin |
| CTX | cortex |
| DAB | 3,3′-diaminobenzidine |
| DCX | doublecortin |
| DG | dentate gyrus |
| DPX | dibutyl phthalate xylene |
| ELISA | enzyme-linked immunoassay |
| FAD | familiar form of Alzheimer’s disease |
| GCL | granular cell layer |
| GFAP | glial fibrillary acidic protein |
| HC | hippocampus |
| HRP | horseradish peroxidase |
| ICV | intracerebroventricular |
| i.p. | intraperitoneal |
| Iba1 | ionized calcium-binding adapter molecule 1 |
| LPS | lipopolysaccharide |
| NPCs | neural progenitor cells |
| PBS | phosphate-buffered saline |
| PFA | paraformaldehyde |
| PS1 | presenilin-1 |
| PS2 | presenilin-2 |
| ROI | regions of interest |
| RT | room temperature |
| sAPPβ | soluble APPβ |
| SEM | standard error of the mean |
| SGZ | subgranular zone |
| STZ | streptozotocin |
| SVZ | subventricular zone |
| WB | Western blot |
| WT C57BL/6J | wild-type mice |
References
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef]
- Chen, Q.; Nakajima, A.; Choi, S.H.; Xiong, X.; Sisodia, S.S.; Tang, Y.P. Adult neurogenesis is functionally associated with AD-like neurodegeneration. Neurobiol. Dis. 2008, 29, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, O.; Marr, R.A. Of mice and men: Neurogenesis, cognition and Alzheimer’s disease. Front. Aging Neurosci. 2013, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G. Adult Neurogenesis: An Evolutionary Perspective. Cold Spring Harb. Perspect. Biol. 2015, 8, a018986. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H. Adult neurogenesis in mammals. Science 2019, 364, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Bruel-Jungerman, E.; Davis, S.; Laroche, S. Brain plasticity mechanisms and memory: A party of four. Neuroscientist 2007, 13, 492–505. [Google Scholar] [CrossRef]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005, 28, 223–250. [Google Scholar] [CrossRef]
- Chuang, T.T. Neurogenesis in mouse models of Alzheimer’s disease. Biochim. Biophys. Acta 2010, 1802, 872–880. [Google Scholar] [CrossRef]
- Lazarov, O.; Mattson, M.P.; Peterson, D.A.; Pimplikar, S.W.; van Praag, H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010, 33, 569–579. [Google Scholar] [CrossRef]
- Kempermann, G.; Jessberger, S.; Steiner, B.; Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004, 27, 447–452. [Google Scholar] [CrossRef]
- Shruster, A.; Melamed, E.; Offen, D. Neurogenesis in the aged and neurodegenerative brain. Apoptosis 2010, 15, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Dard, R.F.; Dahan, L.; Rampon, C. Targeting hippocampal adult neurogenesis using transcription factors to reduce Alzheimer’s disease-associated memory impairments. Hippocampus 2019, 29, 579–586. [Google Scholar] [CrossRef]
- Sung, P.S.; Lin, P.Y.; Liu, C.H.; Su, H.C.; Tsai, K.J. Neuroinflammation and Neurogenesis in Alzheimer’s Disease and Potential Therapeutic Approaches. Int. J. Mol. Sci. 2020, 21, 701. [Google Scholar] [CrossRef] [PubMed]
- Horgusluoglu, E.; Nudelman, K.; Nho, K.; Saykin, A.J. Adult neurogenesis and neurodegenerative diseases: A systems biology perspective. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2017, 174, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Winkler, J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb. Perspect Biol. 2015, 7, a021287. [Google Scholar] [CrossRef]
- Mu, Y.; Gage, F.H. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 85. [Google Scholar] [CrossRef]
- Lazarov, O.; Marr, R.A. Neurogenesis and Alzheimer’s disease: At the crossroads. Exp. Neurol. 2010, 223, 267–281. [Google Scholar] [CrossRef]
- Hollands, C.; Bartolotti, N.; Lazarov, O. Alzheimer’s Disease and Hippocampal Adult Neurogenesis; Exploring Shared Mechanisms. Front. Neurosci. 2016, 10, 178. [Google Scholar] [CrossRef]
- Zhou, Z.-d.; Chan, C.H.-s.; Ma, Q.-h.; Xu, X.-h.; Xiao, Z.-c.; Tan, E.-k. The roles of amyloid precursor protein (APP) in neurogenesis: Implications to pathogenesis and therapy of Alzheimer disease. Cell Adh. Migr. 2011, 5, 280–292. [Google Scholar] [CrossRef]
- Coronel, R.; Bernabeu-Zornoza, A.; Palmer, C.; Muñiz-Moreno, M.; Zambrano, A.; Cano, E.; Liste, I. Role of Amyloid Precursor Protein (APP) and Its Derivatives in the Biology and Cell Fate Specification of Neural Stem Cells. Mol. Neurobiol. 2018, 55, 7107–7117. [Google Scholar] [CrossRef]
- Zetterberg, H.; Blennow, K.; Hanse, E. Amyloid beta and APP as biomarkers for Alzheimer’s disease. Exp. Gerontol. 2010, 45, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Ma, Y. Molecular mechanisms of altered adult hippocampal neurogenesis in Alzheimer’s disease. Mech. Ageing Dev. 2021, 195, 111452. [Google Scholar] [CrossRef] [PubMed]
- Evin, G.; Li, Q.X. Platelets and Alzheimer’s disease: Potential of APP as a biomarker. World J. Psychiatry 2012, 2, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Gadadhar, A.; Marr, R.; Lazarov, O. Presenilin-1 regulates neural progenitor cell differentiation in the adult brain. J. Neurosci. 2011, 31, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Niidome, T.; Taniuchi, N.; Akaike, A.; Kihara, T.; Sugimoto, H. Differential regulation of neurogenesis in two neurogenic regions of APPswe/PS1dE9 transgenic mice. Neuroreport 2008, 19, 1361–1364. [Google Scholar] [CrossRef]
- Demars, M.; Hu, Y.S.; Gadadhar, A.; Lazarov, O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J. Neurosci. Res. 2010, 88, 2103–2117. [Google Scholar] [CrossRef]
- Scopa, C.; Marrocco, F.; Latina, V.; Ruggeri, F.; Corvaglia, V.; La Regina, F.; Ammassari-Teule, M.; Middei, S.; Amadoro, G.; Meli, G.; et al. Impaired adult neurogenesis is an early event in Alzheimer’s disease neurodegeneration, mediated by intracellular Aβ oligomers. Cell Death Differ. 2020, 27, 934–948. [Google Scholar] [CrossRef]
- Gray, S.C.; Kinghorn, K.J.; Woodling, N.S. Shifting equilibriums in Alzheimer’s disease: The complex roles of microglia in neuroinflammation, neuronal survival and neurogenesis. Neural. Regen. Res. 2020, 15, 1208–1219. [Google Scholar]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Ekdahl, C.T.; Claasen, J.H.; Bonde, S.; Kokaia, Z.; Lindvall, O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13632–13637. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Valero, J.; Mastrella, G.; Neiva, I.; Sánchez, S.; Malva, J.O. Long-term effects of an acute and systemic administration of LPS on adult neurogenesis and spatial memory. Front. Neurosci. 2014, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Bassani, T.B.; Turnes, J.M.; Moura, E.L.R.; Bonato, J.M.; Cóppola-Segovia, V.; Zanata, S.M.; Oliveira, R.M.M.W.; Vital, M.A.B.F. Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced rat model of dementia of Alzheimer’s type. Behav. Brain Res. 2017, 335, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Singh, S.; Shukla, S.; Shukla, R. Intracerebroventricular streptozotocin impairs adult neurogenesis and cognitive functions via regulating neuroinflammation and insulin signaling in adult rats. Neurochem. Int. 2018, 113, 56–68. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Li, N.; Huang, R.; Zheng, X.; Huang, L.; Hou, S.; Yuan, Q. Multiple inflammatory profiles of microglia and altered neuroimages in APP/PS1 transgenic AD mice. Brain Res. Bull. 2020, 156, 86–104. [Google Scholar] [CrossRef]
- McLoughlin, D.M.; Miller, C.C. The FE65 proteins and Alzheimer’s disease. J. Neurosci. Res. 2008, 86, 744–754. [Google Scholar] [CrossRef]
- Sabo, S.L.; Ikin, A.F.; Buxbaum, J.D.; Greengard, P. The Alzheimer amyloid precursor protein (APP) and FE65, an APP-binding protein, regulate cell movement. J. Cell Biol. 2001, 153, 1403–1414. [Google Scholar] [CrossRef]
- Sabo, S.L.; Ikin, A.F.; Buxbaum, J.D.; Greengard, P. The amyloid precursor protein and its regulatory protein, FE65, in growth cones and synapses in vitro and in vivo. J. Neurosci. 2003, 23, 5407–5415. [Google Scholar] [CrossRef]
- Santiard-Baron, D.; Langui, D.; Delehedde, M.; Delatour, B.; Schombert, B.; Touchet, N.; Tremp, G.; Paul, M.F.; Blanchard, V.; Sergeant, N.; et al. Expression of human FE65 in amyloid precursor protein transgenic mice is associated with a reduction in beta-amyloid load. J. Neurochem. 2005, 93, 330–338. [Google Scholar] [CrossRef]
- Bukhari, H.; Glotzbach, A.; Kolbe, K.; Leonhardt, G.; Loosse, C.; Mueller, T. Small things matter: Implications of APP intracellular domain AICD nuclear signaling in the progression and pathogenesis of Alzheimer’s disease. Prog. Neurobiol. 2017, 156, 189–213. [Google Scholar] [CrossRef] [PubMed]
- Feilen, L.P.; Haubrich, K.; Stier, G.; Sinning, I.; Wild, K.; Haubrich, K.; Simon, B.; Strecker, P.; Eggert, S.; Kins, S.; et al. Fe65-PTB2 Dimerization Mimics Fe65-APP Interaction. Front. Mol. Neurosci. 2017, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, O.; Demars, M.P. All in the family: How the APPs regulate neurogenesis. Front. Neurog. 2012, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Coronel, R.; Palmer, C.; Bernabeu-Zornoza, A.; Monteagudo, M.; Rosca, A.; Zambrano, A.; Liste, I. Physiological effects of amyloid precursor protein and its derivatives on neural stem cell biology and signaling pathways involved. Neural Regen. Res. 2019, 14, 1661–1671. [Google Scholar] [PubMed]
- Beckett, C.; Nalivaeva, N.N.; Belyaev, N.D.; Turner, A.J. Nuclear signalling by membrane protein intracellular domains: The AICD enigma. Cell Signal 2012, 24, 402–409. [Google Scholar] [CrossRef]
- Li Puma, D.D.; Piacentini, R.; Grassi, C. Does Impairment of Adult Neurogenesis Contribute to Pathophysiology of Alzheimer’s Disease? A Still Open Question. Front. Mol. Neurosci. 2020, 13, 578211. [Google Scholar] [CrossRef]
- Ghosal, K.; Stathopoulos, A.; Pimplikar, S.W. APP intracellular domain impairs adult neurogenesis in transgenic mice by inducing neuroinflammation. PLoS ONE 2010, 5, e11866. [Google Scholar] [CrossRef]
- Ma, Q.H.; Futagawa, T.; Yang, W.L.; Jiang, X.D.; Zeng, L.; Takeda, Y.; Xu, R.X.; Bagnard, D.; Schachner, M.; Furley, A.J.; et al. A TAG1-APP signalling pathway through Fe65 negatively modulates neurogenesis. Nat. Cell Biol. 2008, 10, 283–294. [Google Scholar] [CrossRef]
- Szögi, T.; Schuster, I.; Borbély, E.; Gyebrovszki, A.; Bozsó, Z.; Gera, J.; Rajkó, R.; Sántha, M.; Penke, B.; Fülöp, L. Effects of the Pentapeptide P33 on Memory and Synaptic Plasticity in APP/PS1 Transgenic Mice: A Novel Mechanism Presenting the Protein Fe65 as a Target. Int. J. Mol. Sci. 2019, 20, 3050. [Google Scholar] [CrossRef]
- Verret, L.; Trouche, S.; Zerwas, M.; Rampon, C. Hippocampal neurogenesis during normal and pathological aging. Psychoneuroendocrinology 2007, 32 (Suppl. S1), S26–S30. [Google Scholar] [CrossRef]
- Olesen, L.; Sivasaravanaparan, M.; Severino, M.; Babcock, A.A.; Bouzinova, E.V.; West, M.J.; Wiborg, O.; Finsen, B. Neuron and neuroblast numbers and cytogenesis in the dentate gyrus of aged APPswe/PS1dE9 transgenic mice: Effect of long-term treatment with paroxetine. Neurobiol. Dis. 2017, 104, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Taniuchi, N.; Niidome, T.; Goto, Y.; Akaike, A.; Kihara, T.; Sugimoto, H. Decreased proliferation of hippocampal progenitor cells in APPswe/PS1dE9 transgenic mice. Neuroreport 2007, 18, 1801–1805. [Google Scholar] [CrossRef] [PubMed]
- Borchelt, D.R.; Davis, J.; Fischer, M.; Lee, M.K.; Slunt, H.H.; Ratovitsky, T.; Regard, J.; Copeland, N.G.; Jenkins, N.A.; Sisodia, S.S.; et al. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet. Anal. 1996, 13, 159–163. [Google Scholar] [CrossRef]
- Borchelt, D.R.; Thinakaran, G.; Eckman, C.B.; Lee, M.K.; Davenport, F.; Ratovitsky, T.; Prada, C.M.; Kim, G.; Seekins, S.; Yager, D.; et al. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron 1996, 17, 1005–1013. [Google Scholar] [CrossRef]
- von Bohlen Und Halbach, O. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007, 329, 409–420. [Google Scholar] [CrossRef]
- Brinkmalm, G.; Brinkmalm, A.; Bourgeois, P.; Persson, R.; Hansson, O.; Portelius, E.; Mercken, M.; Andreasson, U.; Parent, S.; Lipari, F.; et al. Soluble amyloid precursor protein α and β in CSF in Alzheimer’s disease. Brain Res. 2013, 1513, 117–126. [Google Scholar] [CrossRef]
- Gabelle, A.; Roche, S.; Gény, C.; Bennys, K.; Labauge, P.; Tholance, Y.; Quadrio, I.; Tiers, L.; Gor, B.; Chaulet, C.; et al. Correlations between soluble α/β forms of amyloid precursor protein and Aβ38, 40, and 42 in human cerebrospinal fluid. Brain Res. 2010, 1357, 175–183. [Google Scholar] [CrossRef]
- Perneczky, R.; Alexopoulos, P.; Kurz, A. Soluble amyloid precursor proteins and secretases as Alzheimer’s disease biomarkers. Trends Mol. Med. 2014, 20, 8–15. [Google Scholar] [CrossRef]
- Deng, W.; Aimone, J.B.; Gage, F.H. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010, 11, 339–350. [Google Scholar] [CrossRef]
- Abbott, L.C.; Nigussie, F. Adult neurogenesis in the mammalian dentate gyrus. Anat. Histol. Embryol. 2020, 49, 3–16. [Google Scholar] [CrossRef]
- Epp, J.R.; Chow, C.; Galea, L.A. Hippocampus-dependent learning influences hippocampal neurogenesis. Front. Neurosci. 2013, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Beckervordersandforth, R.; Rolando, C. Untangling human neurogenesis to understand and counteract brain disorders. Curr. Opin. Pharmacol. 2019, 50, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zheng, M.; Zhang, T.; He, G. Hippocampal neurogenesis in the APP/PS1/nestin-GFP triple transgenic mouse model of Alzheimer’s disease. Neuroscience 2016, 314, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; He, J.; Zhang, Y.; Luo, H.; Zhu, S.; Yang, Y.; Zhao, T.; Wu, J.; Huang, Y.; Kong, J.; et al. Increased hippocampal neurogenesis in the progressive stage of Alzheimer’s disease phenotype in an APP/PS1 double transgenic mouse model. Hippocampus 2009, 19, 1247–1253. [Google Scholar] [CrossRef]
- López-Toledano, M.A.; Shelanski, M.L. Increased neurogenesis in young transgenic mice overexpressing human APP(Sw, Ind). J. Alzheimers Dis. 2007, 12, 229–240. [Google Scholar] [CrossRef]
- Chevallier, N.L.; Soriano, S.; Kang, D.E.; Masliah, E.; Hu, G.; Koo, E.H. Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation. Am. J. Pathol. 2005, 167, 151–159. [Google Scholar] [CrossRef]
- Jin, K.; Galvan, V.; Xie, L.; Mao, X.O.; Gorostiza, O.F.; Bredesen, D.E.; Greenberg, D.A. Enhanced neurogenesis in Alzheimer’s disease transgenic (PDGF-APPSw,Ind) mice. Proc. Natl. Acad. Sci. USA 2004, 101, 13363–13367. [Google Scholar] [CrossRef]
- Gan, L.; Qiao, S.; Lan, X.; Chi, L.; Luo, C.; Lien, L.; Yan Liu, Q.; Liu, R. Neurogenic responses to amyloid-beta plaques in the brain of Alzheimer’s disease-like transgenic (pPDGF-APPSw,Ind) mice. Neurobiol. Dis. 2008, 29, 71–80. [Google Scholar] [CrossRef][Green Version]
- Hamilton, A.; Holscher, C. The effect of ageing on neurogenesis and oxidative stress in the APP(swe)/PS1(deltaE9) mouse model of Alzheimer’s disease. Brain Res. 2012, 1449, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.S.; Xu, P.; Pigino, G.; Brady, S.T.; Larson, J.; Lazarov, O. Complex environment experience rescues impaired neurogenesis, enhances synaptic plasticity, and attenuates neuropathology in familial Alzheimer’s disease-linked APPswe/PS1DeltaE9 mice. FASEB J. 2010, 24, 1667–1681. [Google Scholar] [CrossRef]
- Verret, L.; Jankowsky, J.L.; Xu, G.M.; Borchelt, D.R.; Rampon, C. Alzheimer’s-type amyloidosis in transgenic mice impairs survival of newborn neurons derived from adult hippocampal neurogenesis. J. Neurosci. 2007, 27, 6771–6780. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Goico, B.; Martin, M.; Csernansky, C.A.; Bertchume, A.; Csernansky, J.G. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience 2004, 127, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.S.; Marschallinger, J.; Kaindl, J.; Höfling, C.; Rossner, S.; Heneka, M.T.; Van der Linden, A.; Aigner, L. Early Changes in Hippocampal Neurogenesis in Transgenic Mouse Models for Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 5796–5806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, Y.; Wang, Y.; Song, L.; Zhang, R.; Du, Y. Long-term treadmill exercise attenuates Aβ burdens and astrocyte activation in APP/PS1 mouse model of Alzheimer’s disease. Neurosci. Lett. 2018, 666, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Martín, M.; Trejo, J.L. Multiple birthdating analyses in adult neurogenesis: A line-up of the usual suspects. Front. Neurosci. 2011, 5, 76. [Google Scholar] [CrossRef]
- Mandyam, C.D.; Harburg, G.C.; Eisch, A.J. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience 2007, 146, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, J.M.; Kee, N. BrdU assay for neurogenesis in rodents. Nat. Protoc. 2006, 1, 1399–1405. [Google Scholar] [CrossRef]
- Kuipers, S.D.; Schroeder, J.E.; Trentani, A. Changes in hippocampal neurogenesis throughout early development. Neurobiol. Aging 2015, 36, 365–379. [Google Scholar] [CrossRef]
- He, J.; Crews, F.T. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacol. Biochem. Behav. 2007, 86, 327–333. [Google Scholar] [CrossRef]
- Zhang, X.M.; Cai, Y.; Xiong, K.; Cai, H.; Luo, X.G.; Feng, J.C.; Clough, R.W.; Struble, R.G.; Patrylo, P.R.; Yan, X.X. Beta-secretase-1 elevation in transgenic mouse models of Alzheimer’s disease is associated with synaptic/axonal pathology and amyloidogenesis: Implications for neuritic plaque development. Eur. J. Neurosci. 2009, 30, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Rosene, D.L.; Moss, M.B.; Raju, S.; Hyman, B.T.; Irizarry, M.C. Beta-secretase activity increases with aging in human, monkey, and mouse brain. Am. J. Pathol. 2004, 164, 719–725. [Google Scholar] [CrossRef]
- Luo, G.; Xu, H.; Huang, Y.; Mo, D.; Song, L.; Jia, B.; Wang, B.; Jin, Z.; Miao, Z. Deposition of BACE-1 Protein in the Brains of APP/PS1 Double Transgenic Mice. Biomed. Res. Int. 2016, 2016, 8380618. [Google Scholar] [CrossRef] [PubMed]
- Kandalepas, P.C.; Sadleir, K.R.; Eimer, W.A.; Zhao, J.; Nicholson, D.A.; Vassar, R. The Alzheimer’s β-secretase BACE1 localizes to normal presynaptic terminals and to dystrophic presynaptic terminals surrounding amyloid plaques. Acta Neuropathol. 2013, 126, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Koffie, R.M.; Meyer-Luehmann, M.; Hashimoto, T.; Adams, K.W.; Mielke, M.L.; Garcia-Alloza, M.; Micheva, K.D.; Smith, S.J.; Kim, M.L.; Lee, V.M.; et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl. Acad. Sci. USA 2009, 106, 4012–4017. [Google Scholar] [CrossRef]
- Viola, K.L.; Klein, W.L. Amyloid β oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015, 129, 183–206. [Google Scholar] [CrossRef]
- Ashe, K.H. Learning and memory in transgenic mice modeling Alzheimer’s disease. Learn Mem. 2001, 8, 301–308. [Google Scholar] [CrossRef]
- Czeh, M.; Gressens, P.; Kaindl, A.M. The yin and yang of microglia. Dev. Neurosci. 2011, 33, 199–209. [Google Scholar] [CrossRef]
- Richetin, K.; Petsophonsakul, P.; Roybon, L.; Guiard, B.P.; Rampon, C. Differential alteration of hippocampal function and plasticity in females and males of the APPxPS1 mouse model of Alzheimer’s disease. Neurobiol. Aging 2017, 57, 220–231. [Google Scholar] [CrossRef]
- Lee, C.Y.; Landreth, G.E. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. 2010, 117, 949–960. [Google Scholar] [CrossRef]

| Markers | Age (Months) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 18 | Ref. | ||
| BrdU | Our study | L | H | H | L | H | L | L | L-S | L-S | L-S | L-S | L-S | NA | NA | NA | H | |
| Ref. | NA | L-S [26] | L-S [36,69,73] | NA | L-S [69], L [52] | NA | NA | NA | L-S [25,52] | L-S [69] | NA | NA | NA | NA | L [69] | NA | [25,26,36,52,69,73] | |
| DCX | Our study | L-S | H | H | L | H | H | L | L | L-S | L-S | L-S | L | NA | NA | NA | L | |
| Ref. | NA | L-S [26] | H [69,73], L-S [36] | NA | L [69], H [52] | L-S [36] | NA | NA | L-S [52] | L-S [36,69], L [73] | NA | L-S [36] | H [73] | NA | L-S [69] | NA | [26,36,52,69,73] | |
| NeuN | Our study | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NA | NA | NA | NC | |
| Ref. | NA | NA | NA | NA | NA | NA | NA | NA | NA | NC [74] | NA | NC [36] | NA | NA | NA | NA | [36,74] | |
| GFAP | Our study | H | H | L | H | H | H-S | H-S | H-S | H-S | H-S | H-S | H-S | NA | NA | NA | H | |
| Ref. | NA | NA | L [36] | NA | NA | H-S [36] | NA | NA | NA | H-S [36,74] | NA | H-S [36] | NA | NA | NA | NA | [36,74] | |
| Iba1 | Our study | L | L | H | H | L | H | H-S | H-S | H-S | H-S | H-S | H-S | NA | NA | NA | H-S | |
| Ref. | NA | NA | H [73], L [36] | NA | NA | H-S [36] | NA | NA | NA | H-S [36,74] | NA | H-S [36] | NA | NA | NA | NA | [36,73,74] | |
| Aβ plaque | Our study | - | - | - | + | + | + | + | + | + | NA | NA | + | NA | NA | NA | + | |
| Ref. | NA | NA | − [36], + [29], + [69] | + [73] | + [52,69] | + [36] | + [52] | NA | + [25,52] | + [36,69,73,74] | NA | + [36] | + [73] | + [29] | + [69] | NA | [25,29,36,52,69,73,74] | |
| soluble Aβ | Our study | + | + | + | + | - | - | - | - | - | NA | NA | - | NA | NA | NA | - | |
| Ref. | NA | + [26] | NA | NA | + [52,69] | NA | + [52] | NA | + [52] | NA | NA | NA | NA | NA | NA | NA | [26,52,69] | |
| APP level | Our study | H | H | H | H | L | L | L | L | L | NA | NA | L | NA | NA | NA | L | |
| Ref. | NA | H [26] | NA | NA | NA | NA | NA | NA | NA | H [74] | NA | NA | NA | NA | NA | NA | [26,74] | |
| C99/C83 ratio | Our study | L | L | L | L | L | H | H | H | H | NA | NA | H | NA | NA | NA | H | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szögi, T.; Borbély, E.; Schuster, I.; Bozsó, Z.; Sántha, M.; Tóth, M.E.; Penke, B.; Fülöp, L. Examination of Longitudinal Alterations in Alzheimer’s Disease-Related Neurogenesis in an APP/PS1 Transgenic Mouse Model, and the Effects of P33, a Putative Neuroprotective Agent Thereon. Int. J. Mol. Sci. 2022, 23, 10364. https://doi.org/10.3390/ijms231810364
Szögi T, Borbély E, Schuster I, Bozsó Z, Sántha M, Tóth ME, Penke B, Fülöp L. Examination of Longitudinal Alterations in Alzheimer’s Disease-Related Neurogenesis in an APP/PS1 Transgenic Mouse Model, and the Effects of P33, a Putative Neuroprotective Agent Thereon. International Journal of Molecular Sciences. 2022; 23(18):10364. https://doi.org/10.3390/ijms231810364
Chicago/Turabian StyleSzögi, Titanilla, Emőke Borbély, Ildikó Schuster, Zsolt Bozsó, Miklós Sántha, Melinda E. Tóth, Botond Penke, and Lívia Fülöp. 2022. "Examination of Longitudinal Alterations in Alzheimer’s Disease-Related Neurogenesis in an APP/PS1 Transgenic Mouse Model, and the Effects of P33, a Putative Neuroprotective Agent Thereon" International Journal of Molecular Sciences 23, no. 18: 10364. https://doi.org/10.3390/ijms231810364
APA StyleSzögi, T., Borbély, E., Schuster, I., Bozsó, Z., Sántha, M., Tóth, M. E., Penke, B., & Fülöp, L. (2022). Examination of Longitudinal Alterations in Alzheimer’s Disease-Related Neurogenesis in an APP/PS1 Transgenic Mouse Model, and the Effects of P33, a Putative Neuroprotective Agent Thereon. International Journal of Molecular Sciences, 23(18), 10364. https://doi.org/10.3390/ijms231810364








